Abstract
1α,25-Dihydroxyvitamin D3 (1α,25(OH)2D3) inhibits the expression of an immediate-early gene, IEX-1, which is involved in the regulation of cellular growth and apoptosis in a variety of cells. 1α,25(OH)2D3 alters the subcellular localization of IEX-1 by causing an efflux of IEX-1 from the nucleus, and the sterol decreases the expression of IEX-1 messenger RNA in cells via a novel DR3 repeat-type DNA response element.
Introduction
The role and mechanism of action of vitamin D3, and specifically 1α,25(OH)2D3, in calcium transport is well established (Beckman and DeLuca 1998; DeLuca and Zierold 1998; Haussler et al. 1998; Jones et al. 1998; Jurutka et al. 2001; Kumar 1995; Norman and Silva 2001; Norman et al. 1999). 1α,25(OH)2D3 is known to increase active calcium transport in the intestine by regulating the expression of intestinal calcium-binding protein (9 K and/or 28 K calbindin), the plasma membrane calcium pump, and epithelial calcium channels (Gross and Kumar 1990; Hoenderop et al. 2001, 2002; Johnson and Kumar 1994a, 1994b, Kumar 1991; Wasserman et al. 1992; Wood et al. 2001). 1α,25(OH)2D3 also plays an important role in regulating bone calcium and phosphorus resorption, mainly by increasing osteoclast activity and osteoclastogenesis (Gurlek and Kumar 2001; Suda et al. 1990, 1992, 1995). In addition, 1α,25(OH)2D3 also has direct effects on osteoblast function (Gurlek and Kumar 2001; Gurlek et al. 2002).
The role of vitamin D and its metabolites in the control of cellular growth has been examined less thoroughly. In general, 1α,25(OH)2D3 inhibits the growth of cells in culture in physiological concentrations when serum is present in the cell culture medium (Gross et al. 1986; Haugen et al. 1996). When absent, or when present in low concentrations of serum, the hormone enhances cellular proliferation when added to cell cultures in physiological amounts, and it inhibits cell proliferation at high concentrations of hormone (Gross et al. 1986; Haugen et al. 1996). In vivo, 1α,25(OH)2D3 has antiproliferative effects in various cell types such as keratinocytes and in hyperproliferative states such as psoriasis, and it has variable effects on the growth of various cancers in vivo and in vitro (Bikle 1992; Brown 2001; Holick et al. 1996; Kragballe 1992; Lamprecht and Lipkin 2001; Mehta and Mehta 2002; Reichrath 2001; van den Bemd and Chang 2002).
1α,25(OH)2D3 Alters Cellular Growth Via Diverse Mechanisms
Vitamin D3 and active D3 metabolites such as 1α,25(OH)2D3 alter the growth rate of cells in diverse ways which include: increases in the uptake of substrates such as amino acids into cells (Bellido and Boland 1989; Birge and Haddad 1975; Dabbagh et al. 1990), changes in the synthesis of various autocrine or paracrine growth factors (Gurlek and Kumar 2001; Gurlek et al. 2002), alterations in growth factor receptor number (Gurlek and Kumar 2001; Gurlek et al. 2002), and alterations in the expression of intracellular growth regulatory molecules. 1α,25(OH)2D3 has been shown to alter protein kinase C expression (Bellido et al. 1993; Khare et al. 1999; Obeid et al. 1990; Schwartz et al. 2002a, b; Simboli-Campbell et al. 1994; Wali et al. 1996) as well as polyamine synthesis in intestinal and other cells (Chida et al. 1984; Ittel et al. 1986; Shinki et al. 1985; Somjen et al. 1983; Steeves and Lawson 1985). C-myc expression is decreased when 1α,25(OH)2D3 is added to cells grown in serum-containing medium (Brelvi et al. 1986; Huh et al. 1987; Manolagas et al. 1987; Reitsma et al. 1983; Simpson et al. 1987). This is associated with a decrease in cellular proliferation. Expression of the Cdk inhibitor, p21, is increased by 1α,25(OH)2D3 (Liu et al. 1996; Munker et al. 1998; Muto et al. 1999). An increase in cyclin expression occurs in association with G1 cell cycle arrest (Jensen et al. 2001; Rots et al. 1999). Raf kinase expression and activities increases when hepatic cell proliferation increases following the addition of 1α,25(OH)2D3 (Lissoos et al. 1993).
Given the role of vitamin D in the regulation of early gene expression, we analyzed keratinocytes following treatment of these cells with 1α,25(OH)2D3. We used differential display PCR to search for changes in the expression of messenger RNAs for growth factor or intracellular growth-modulatory molecules following treatment of cells with 1α,25(OH)2D3. We identified a gene, IEX-1, whose mRNA is consistently reduced following treatment of cells with 1α,25(OH)2D3 (Kobayashi et al. 1998). IEX-1 has recently been identified as a radiation and differentiation-induced, and pituitary adenylate cyclase activating protein (PACAP) and cholecystokinin-induced gene (Kondratyev et al. 1996; Kumar et al. 1998; Pietzsch et al. 1997, 1998; Schafer et al. 1999a). The structure and amino acid sequence of IEX-1 and the sites at which it might undergo posttranslational modification are shown in Fig. 1. The product of the gene, which is widely distributed in cells, has been shown to increase cellular growth and to alter the rate of apoptosis in cells (Arlt et al. 2001; Feldmann et al. 2001; Grobe et al. 2001; Kobayashi et al. 1998; Kumar et al. 1998; Schilling et al. 2001; Wu et al. 1998; Zhang et al. 2002).
Fig. 1.
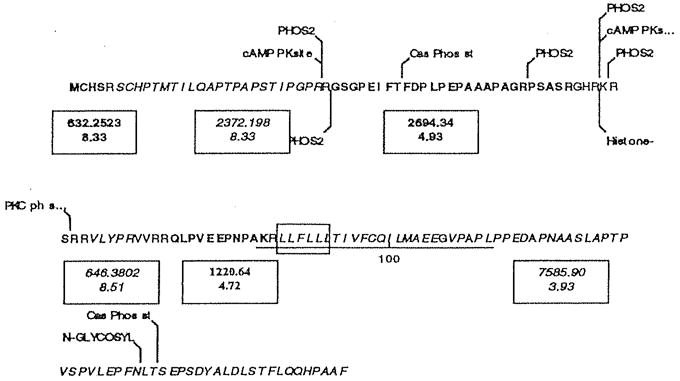
Amino acid sequence of IEX-1 (Swiss Prot Acc #P46695) and sites at which the protein might undergo posttranslational modification. The underlined sequence from residue 86 to 111 has homology with rhodopsin-like G protein coupled receptors, and the boxed amino acids from 88 to 93 display the LLXLL motif characteristic of coactivator proteins. A nuclear localization signal, RKRSRR partially overlaps the PKC phosphorylation site. Also shown, in alternating bold and italics, are the tryptic fragments greater than 500 Da, with the monoistopic mass and pl of each unmodified fragment. These fragments represent approximately 93% of the 156-amino acid protein. Calculated molecular weight, 16,927.48 kDa; estimated pl, 8.83
Regulation of IEX-1 Expression by 1α,25(OH)2D3
We examined the expression of IEX-1 mRNA in subconfluent cultures of normal human keratinocytes following treatment with 10−8 M 1α,25(OH)2D3 (Kobayashi et al. 1998). 1α,25(OH)2D3 caused a rapid reduction in IEX-1 mRNA expression. The effect of 1α,25(OH)2D3 on IEX-1 mRNA expression in confluent cultures of keratinocytes was similar to that found in the case of subconfluent keratinocytes (Kobayashi et al. 1998).
Effect of Radiation and Other Agents on IEX-1 Expression
We examined the effect of ultraviolet light β and γ radiation on IEX expression (Kumar et al. 1998). We found that ultraviolet β caused a prompt increase in messenger RNA concentrations for IEX-1 in keratinocytes. We also found that γ radiation induced IEX-1 expression, although the increase was less marked with that seen with UVB radiation (Fig. 5). IEX-1 mRNA expression is increased by epidermal growth factor, tumor-promoting phorbol ester, and cycloheximide.
Fig. 5.
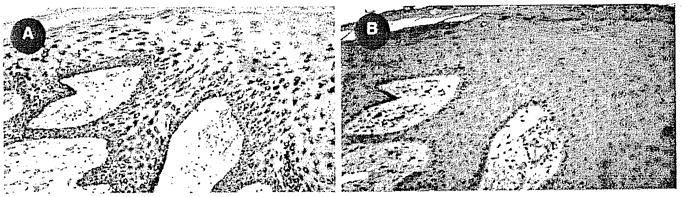
A, B IEX-1 Immunostaining in squamous cell carcinoma. A IEX-1 staining within hyperplastic tumor fronds. B Negative control.
Distribution of IEX-1 RNA and Protein in Various Cells
Western blot analysis of protein from keratinocytes using a polyclonal antibody against IEX-1 (Feldmann et al. 2001) showed two forms of the IEX-1 protein, an unglycosylated form with an Mr of approximately 17.4 kDa and a glycosylated form with an Mr of approximately 30,000 kDa. We used this antibody to examine the expression of IEX-1 in various tissues, and found that IEX-1 was highly expressed in genitourinary tissues, gastrointestinal tissues, skin and its appendages, as well as in cultured keratinocytes (Fig. 2). Other tissues such as pancreas expressed abundant amounts of IEX-1 in islets but not in ductal and acinar cells. Messenger RNA distribution of IEX-1 in various tissues was similar to that observed for IEX-1 protein using polyclonal antibodies against IEX-1.
Fig. 2.
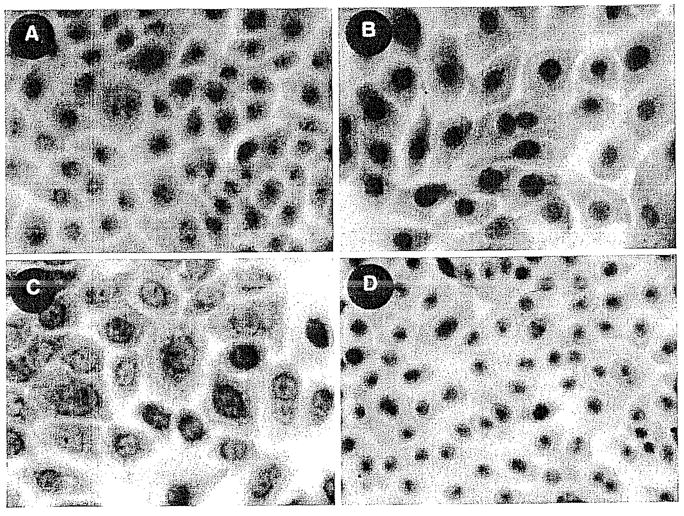
A–D. IEX-1 Immunostaining in normal keratinocytes. A IEX-1 localized within nucleus. B Negative control. C IEX-1 localized in nuclear and perinuclear regions. D Negative control. All cells were fixed with methanol-acetone.
Subcellular Distribution of IEX-1 in Keratinocytes
We showed that IEX-1 is normally present in a nuclear location in keratinocytes maintained in culture, not only using a polyclonal antibody (Fig. 2) but also in cells expressing IEX-1 green fluorescent protein (Fig. 3C). We found that 1α,25(OH)2D3 caused an efflux of IEX-1 from nuclei to the cytoplasm (Kumar et al. 1998).
Fig. 3.
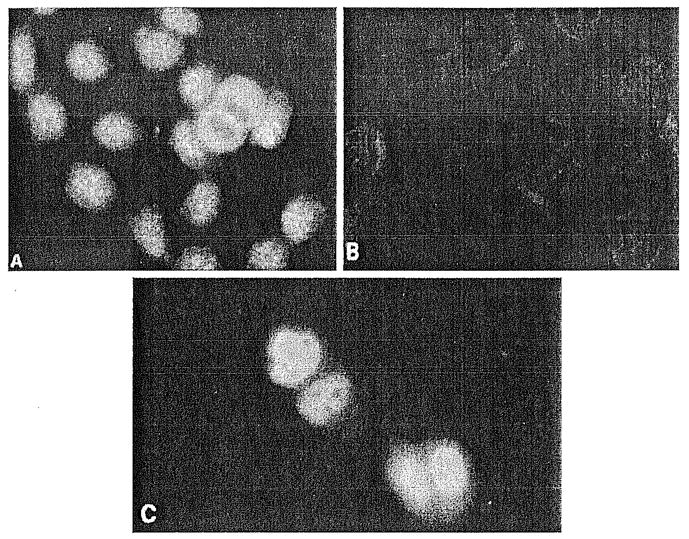
A–C Fluorescent microscopy of HaCaT cells localizing IEX-1 protein. Replicating HaCaT cells stained with: A IEX-1-1-specific antibody; B preimmune serum; C HaCaT cells transfected with GFP-IEX-1 chimeric plasmid and visualized directly by fluorescent microscopy 48 h later. (From Kumar et al. 1998, with permission)
Distribution of IEX-1 in Pathological Tissues
We found mat IEX-1 immunostaining was considerably increased in psoriatic tissues (Fig. 4). In addition, IEX-1 immunostaining was abnormal in squamous cell carcinomas of the skin (Fig. 5). Interestingly, IEX-1 expression was increased in ductal cells in chronic pancreatitis and in ductal carcinomas of the pancreas (Fig. 6).
Fig. 4.
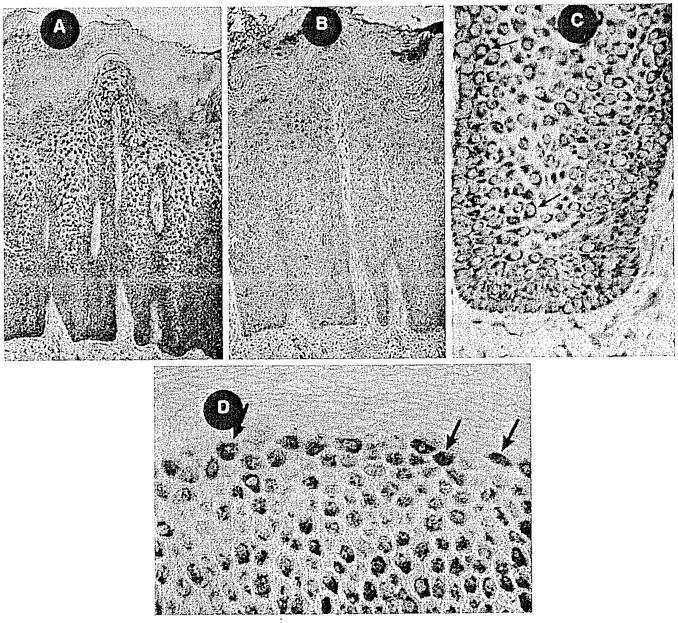
A–D IEX-1 immunostaining in psoriatic skin. A Hyperplastic and acanthotic epidermis showing intense IEX-1 staining within basal cell and suprabasal layers. B Negative control, no primary Ab. C High-power rete peg showing strong perinuclear staining. D Upper granular layer with strong nuclear localization of IEX-1 protein.
Fig. 6.
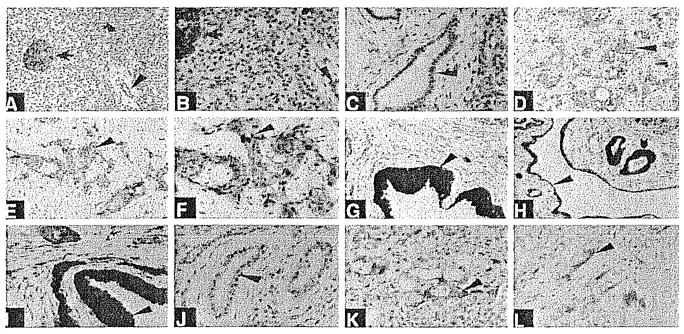
A–L. Immunohistochemistry of pancreatic tissues using a specific antigen-affinity purified antibody to IEX-1 (A–I, K). Immunostaining in J and L is with IEX-1 antibody preabsorbed with antigen. A normal pancreatic tissue (×200). Arrow shows intense staining of an islet of Langerhans. Arrowhead shows modest staining of ductal epithelium. Blocked arrow shows some staining of acinar cells. B Normal pancreatic tissue (×400). Arrows indicate same tissue as in A. C Normal pancreatic tissue (×400). Note immunostaining of some ductal epithelial cells. D, E Pancreatic acinar adenocarcinoma (×200). Note immunostaining of tumor cells (arrowhead). F Pancreatic acinar adenocarcinoma (×400). Note immunostaining of tumor cells (arrowhead). G Chronic pancreatitis (×200). Note immunostaining of hyperplastic ductal epithelial cells (arrowhead). Nuclear and cytoplasmic staining is evident. H Chronic pancreatitis (×200). Note immunostaining of hyperplastic ductal-epitheliai cells (blocked arrow and arrowhead). Nuclear and cytoplasmic staining is evident. Not all ductal cells are as intensely stained. I Chronic pancreatitis (×400). Note immunostaining of hyperplastic ductal epithelial cells (arrowhead). Nuclear and cytoplasmic staining is evident. Not all ductal cells are as intensely stained. J Chronic pancreatitis (×200). Preabsorbed serum. K Pancreatic carcinoma metastatic to the liver (×400). Note immunostaining of carcinoma cells (arrowhead). L Pancreatic acinar adenocarcinoma (×400). Preadsorbed antiserum.
Biological Activity of IEX-1
We biosynthesized an expression plasmid for IEX-1 and showed that there was an increase in IEX-1 expression in cells transfected with this plasmid. Cells transfected with the IEX-1 plasmid grew at a faster rate than cells transfected with a plasmid not containing the IEX-1 insert. There has been considerable interest in the ability of IEX-1 to alter the rate of apoptosis in cells grown in culture and in vivo (Arlt et al. 2001; Grobe et al. 2001; Schafer et al. 1999b; Wu et al. 1998; Zhang et al. 2002). In recent experiments in which the IEX-1 transgene was overexpressed in mice, the rate of apoptosis appeared to be reduced in lymphocytes expressing IEX-1 (Zhang et al. 2002). We have found that overexpression of IEX-1 in HaCaT cells was associated with no change in the rate of apoptosis in keratinocytes in the basal state (unstressed state) (Schilling et al. 2001). However, cellular stress caused by treatment of cells with ultraviolet light or camptothecin or following serum withdrawal was associated with an increase in the rate of apoptosis (Schilling et al. 2001). These data are supported by information from other laboratories (Arlt et al. 2001; Grobe et al. 2001).
Regulation of the IEX-1 Promoter by 1α,25(OH)2D3
We examined the IEX-1 promoter for various substances that might play a role in its regulation (Im et al. 2002a). The organization of the promoter and binding sites for various nuclear proteins are shown (Fig. 7). We localized a vitamin D response element in the promoter region of the IEX-1 gene using deletion analysis and electrophoretic gel mobility assays. A vitamin D response element that down-regulated the expression of the IEX-1 gene in response to vitamin D was found from nucleotide −405 to nucleotide −390 in the IEX-1 promoter (Im et al. 2002b). The vitamin D response element is: 5′ TGAACC ACG GAGTCA3′. The complement of the first hormone response element sequence is identical to the half-site hormone response element of the osteopontin, rat 24-hydroxylase, p21, and parathyroid hormone promoter vitamin D response elements. The 3′ hexameric motif conforms to the canonical HRE-RRKNSA, where R=A or G, K=G or T, S=C or G.
Fig. 7.
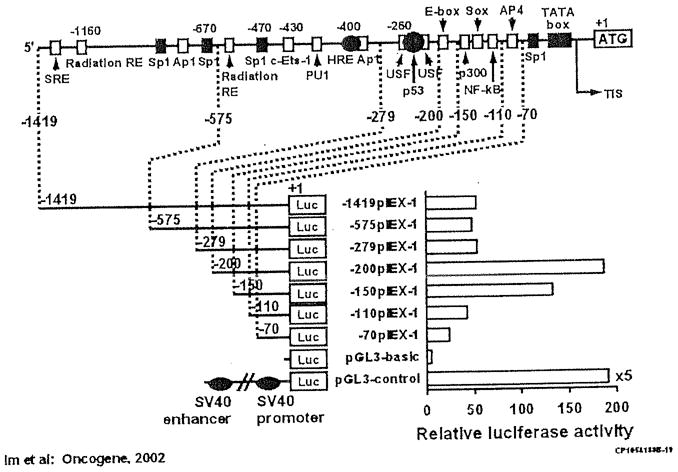
Positions of putative transcription factor binding sites discussed in the text are indicated. Putative HRE (VDRE) is shown as black oval. A TATA box is located 25 bp upstream from the transcription initiation site (TIS). Promoter activities of the IEX-1 promoter deletion constructs fused to firefly luciferase reporter gene transiently transfected in HaCaT cells. Left, the length of the tested promoter fragments is shown. Numbers indicate the relative positions with respect to the transcription start site. Right, luciferase activity is shown as n-fold value compared to cells transfected with the promoterless pGL3-basic vector (which was assigned an activity value of 1.0). Data represent the means of three independent experiments in duplicates, with at least two different plasmid preparations
Summary
We have shown that IEX-1 is an immediate-early gene that is repressed by 1α,25(OH)2D3. It is induced by a variety of growth factors that induce growth in cells and is repressed by others that inhibit growth. It is highly expressed in rapidly growing tissues and it is overexpressed in certain tumors. It stimulates growth in normally growing cells, but induces apoptosis in cells that have been stressed. 1α,25(OH)2D3 alters IEX-1 gene expression via a novel vitamin D response element. It also alters the nuclear localization of the protein. Investigation of the cellular role of IEX-1 in growth and differentiation and its regulation by vitamin D analogs and steroids is a further fruitful area of research.
References
- Arlt A, Grobe O, Sieke A, Kruse ML, Folsch UR, Schmidt WE, Schafer H. Expression of the NF-kappa B target gene IEX-1 (p22/PRG1) does not prevent cell death but instead triggers apoptosis in Hela cells. Oncogene. 2001;20:69–76. doi: 10.1038/sj.onc.1204061. [DOI] [PubMed] [Google Scholar]
- Beckman MJ, DeLuca HF. Modern view of vitamin D3 and its medicinal uses. Prog Med Chem. 1998;35:1–56. doi: 10.1016/s0079-6468(08)70033-x. [DOI] [PubMed] [Google Scholar]
- Bellido T, Boland R. Stimulation of myoblast membrane protein synthesis by 25-hydroxy-vitamin D3. Zeitschrift fur Naturforschung. Section C. J Biosci. 1989;44:807–812. doi: 10.1515/znc-1989-9-1019. [DOI] [PubMed] [Google Scholar]
- Bellido T, Morelli S, Fernandez LM, Boland R. Evidence for the participation of protein kinase C and 3′,5′-cyclic AMP-dependent protein kinase in the stimulation of muscle cell proliferation by 1,25-dihydroxy-vitamin D3. MolCell Endocrinol. 1993;90:231–28. doi: 10.1016/0303-7207(93)90156-e. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Clinical counterpoint: vitamin D: new actions, new analogs, new therapeutic potential. Endocr Rev. 1992;13:765–784. doi: 10.1210/edrv-13-4-765. [DOI] [PubMed] [Google Scholar]
- Birge SJ, Haddad JG. 25-hydroxycholecalciferol stimulation of muscle metabolism. J Clin Invest. 1975;56:1100–1107. doi: 10.1172/JCI108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelvi ZS, Christakos S, Studzinski GP. Expression of monocyte-specific oncogenes c-fos and c-fms in HL60 cells treated with vitamin D3 analogs correlates with inhibition of DNA synthesis and reduced calmodulin concentration. Lab Invest. 1986;55:269–275. [PubMed] [Google Scholar]
- Brown A. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001;38(Suppl 5):S3–S19. doi: 10.1053/ajkd.2001.28111. [DOI] [PubMed] [Google Scholar]
- Chida K, Hashiba H, Suda T, Kuroki T. Inhibition by 1α,25-dihydroxyvitamin D3 of induction of epidermal ornithine decarboxylase caused by 12-O-tetradecanoylphorbol-13-acetate and teleocidin B. Cancer Res. 1984;44:1387–1391. [PubMed] [Google Scholar]
- Dabbagh S, Gusowski N, Padilla M, Theissen M, Chesney RW. Perturbation of renal amino acid transport by brush border membrane vesicles in the vitamin D-deficient rat. Biocheml Med Metab Biol. 1990;44:64–76. doi: 10.1016/0885-4505(90)90046-4. [DOI] [PubMed] [Google Scholar]
- DeLuca HF, Zierold C. Mechanisms and functions of vitamin D. Nutr Rev. 1998;56:S4–10. doi: 10.1111/j.1753-4887.1998.tb01686.x. discussion S 54–75. [DOI] [PubMed] [Google Scholar]
- Feldmann KA, Pittelkow MR, Roche PC, Kumar R, Grande JP. Expression of an immediate early gene, IEX-1, in human tissues. Histochem Cell Biol. 2001;115:489–497. doi: 10.1007/s004180100284. [DOI] [PubMed] [Google Scholar]
- Grobe O, Arlt A, Ungefroren H, Krupp G, Folsch UR, Schmidt WE, Schafer H. Functional disruption of IEX-1 expression by concatemeric hammerhead ribozymes alters growth properties of 293 cells. FEBS Lett. 2001;494:196–200. doi: 10.1016/s0014-5793(01)02344-4. [DOI] [PubMed] [Google Scholar]
- Gross M, Kumar R. Physiology and biochemistry of vitamin D-dependent calcium binding proteins. Am J Physiol. 1990;259:F195–F209. doi: 10.1152/ajprenal.1990.259.2.F195. [DOI] [PubMed] [Google Scholar]
- Gross M, Kost SB, Ennis B, Stumpf W, Kumar R. Effect of 1,25-dihydroxyvitamin D3 on mouse mammary tumor (GR) cells: evidence for receptors, cellular uptake, inhibition of growth and alteration in morphology at physiologic concentrations of hormone. J Bone Miner Res. 1986;1:457–467. doi: 10.1002/jbmr.5650010510. [DOI] [PubMed] [Google Scholar]
- Gurlek A, Kumar R. Regulation of osteoblast growth by interactions between transforming growth factor-β and 1α,25-dihydroxyvitamin D3. Crit Rev Eukaryot Gene Expr. 2001;11:299–317. [PubMed] [Google Scholar]
- Gurlek A, Pittelkow MR, Kumar R. Modulation of growth factor/cytokine synthesis and signaling by 1α,25-dihydroxyvitamin D3: implications in cell growth and differentiation. Endocr Rev. 2002;23:763–786. doi: 10.1210/er.2001-0044. [DOI] [PubMed] [Google Scholar]
- Haugen JD, Pittelkow MR, Zinsmeister AR, Kumar R. 1α,25-dihydroxyvitamin D3 inhibits normal human keratinocyte growth by increasing transforming growth factor β2 release. Biochem Biophys Res Comm. 1996;229:618–623. doi: 10.1006/bbrc.1996.1853. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Muller D, Van Der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, Van Os CH, Bindels RJ. Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol. 2001;12:1342–1349. doi: 10.1681/ASN.V1271342. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ. Molecular mechanism of active Ca2+ reabsorption in the distal nephron. Ann Rev Physiol. 2002;64:529–549. doi: 10.1146/annurev.physiol.64.081501.155921. [DOI] [PubMed] [Google Scholar]
- Holick MF, Chen ML, Kong XF, Sanan DK. Clinical uses for calciotropic hormones 1,25-dihydroxyvitamin D3 and parathyroid hormone-related peptide in dermatology: a new perspective. J Invest Dermatol. Symposium Proceedings. 1996;1:1–9. [PubMed] [Google Scholar]
- Huh N, Satoh M, Nose K, Abe E, Suda T, Rajewsky MF, Kuroki T. 1α,25-Dihydroxyvitamin D3 induces anchorage-independent growth and c-Ki-ras expression of BALB/3T3 and NIH/3T3 cells. Jpn Jf Cancer Res. 1987;78:99–102. [PubMed] [Google Scholar]
- Im HJ, Craig TA, Pittelkow MR, Kumar R. Characterization of a novel hexameric repeat DNA sequence in the promoter of the immediate early gene, IEX-1, that mediates 1α,25-dihydroxyvitamin D(3)-associated IEX-1 gene repression. Oncogene. 2002a;21:3706–3714. doi: 10.1038/sj.onc.1205450. [DOI] [PubMed] [Google Scholar]
- Im HJ, Pittelkow MR, Kumar R. Divergent regulation of the growth-promoting gene IEX-1 by the p53 tumor suppressor and Spl. J Biol Chem. 2002b;277:14612–1421. doi: 10.1074/jbc.M109414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittel TH, Ross FP, Norman AW. Activity of ornithine decarboxylase and creatine kinase in soft and hard tissue of vitamin D-deficient chicks following parenteral application of 1,25-dihydroxyvitamin D3 or 24R,25-dihydroxyvitamin D3. J Bone Miner Res. 1986;1:23–31. doi: 10.1002/jbmr.5650010106. [DOI] [PubMed] [Google Scholar]
- Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J. Inhibitory effects of 1α,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol. 2001;15:1370–1380. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Kumar R. Renal and intestinal calcium transport: roles of vitamin D and vitamin D-dependent calcium binding proteins. Semin Nephrol. 1994a;14:119–128. [PubMed] [Google Scholar]
- Johnson JA, Kumar R. Vitamin D and renal calcium transport. Curr Opin Nephrol Hypertens. 1994b;3:424–429. doi: 10.1097/00041552-199407000-00008. [DOI] [PubMed] [Google Scholar]
- Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2:203–216. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- Khare S, Bissonnette M, Wali R, Skarosi S, Boss GR, von Lintig FC, Scaglione-Sewell B, Sitrin MD, Brasitus TA. 1,25-dihydroxyvitamin D3 but not TPA activates PLD in Caco-2 cells via pp60(c-src) and RhoA. Am J Physiol. 1999;276:G1005–G1015. doi: 10.1152/ajpgi.1999.276.4.g1005. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Pittelkow MR, Warner GM, Squillace KA, Kumar R. Regulation of a novel immediate early response gene, IEX-1, in keratinocytes by 1α,25-dihydroxyvitamin D3. Biochem Biophys Res Comm. 1998;251:868–873. doi: 10.1006/bbrc.1998.9556. [DOI] [PubMed] [Google Scholar]
- Kondratyev AD, Chung KN, Jung MO. Identification and characterization of a radiation-inducible glycosylated human early-response gene. Cancer Res. 1996;56:1498–1502. [PubMed] [Google Scholar]
- Kragballe K. Treatment of psoriasis with calcipotriol and other vitamin D analogues. J Am Acad Dermatol. 1992;27:1001–1008. doi: 10.1016/0190-9622(92)70302-v. [DOI] [PubMed] [Google Scholar]
- Kumar R. Vitamin D and calcium transport. Kidney Int. 1991;40:1177–1189. doi: 10.1038/ki.1991.332. [DOI] [PubMed] [Google Scholar]
- Kumar R. Calcium transport in epithelial cells of the intestine and kidney. J Cell Biochem. 1995;57:392–398. doi: 10.1002/jcb.240570304. [DOI] [PubMed] [Google Scholar]
- Kumar R, Kobayashi T, Warner GM, Wu Y, Salisbury JL, Lingle W, Pittelkow MR. A novel immediate early response gene, IEX-1, is induced by ultraviolet radiation in human keratinocytes. Biochem Biophys Res Comm. 1998;253:336–341. doi: 10.1006/bbrc.1998.9692. [DOI] [PubMed] [Google Scholar]
- Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci. 2001;952:73–87. doi: 10.1111/j.1749-6632.2001.tb02729.x. [DOI] [PubMed] [Google Scholar]
- Lissoos TW, Beno DW, Davis BH. 1,25-Dihydroxyvitamin D3 activates Raf kinase and Raf perinuclear translocation via a protein kinase C-dependent pathway. J Biol Chem. 1993;268:25132–25138. [PubMed] [Google Scholar]
- Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Provvedini DM, Murray EJ, Murray SS, Tsonis PA, Spandidos DA. Association between the expression of the c-myc oncogene mRNA and the expression of the receptor protein for 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1987;84:856–860. doi: 10.1073/pnas.84.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Mehta R. Vitamin D and Cancer. J Nutr Biochem. 2002;13:252–264. doi: 10.1016/s0955-2863(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Munker R, Zhang W, Elstner E, Koeffler HP. Vitamin D analogs, leukemia and WAF1. Leuk Lymphoma. 1998;31:279–284. doi: 10.3109/10428199809059220. [DOI] [PubMed] [Google Scholar]
- Muto A, Kizaki M, Yamato K, Kawai Y, Kamata-Matsushita M, Ueno H, Ohguchi M, Nishihara T, Koeffler HP, Ikeda Y. 1,25-Dihydroxyvitamin D3 induces differentiation of a retinoic acid-resistant acute promyelocytic leukemia cell line (UF-1) associated with expression of p21(WAF1/CIP1) and p27(KIP1) Blood. 1999;93:2225–2233. [PubMed] [Google Scholar]
- Norman AW, Silva FR. Structure function studies: identification of vitamin D analogs for the ligand-binding domains of important proteins in the vitamin D-endocrine system. Rev Endocr Metab Disord. 2001;2:229–238. doi: 10.1023/a:1010067030049. [DOI] [PubMed] [Google Scholar]
- Norman AW, Song X, Zanello L, Bula C, Okamura WH. Rapid and genomic biological responses are mediated by different shapes of the agonist steroid hormone, 1α,25(OH)2vitamin D3. Steroids. 1999;64:120–128. doi: 10.1016/s0039-128x(98)00091-9. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Okazaki T, Karolak LA, Hannun YA. Transcriptional regulation of protein kinase C by 1,25-dihydroxyvitamin D3 in HL-60 cells. J Biol Chem. 1990;265:2370–2374. [PubMed] [Google Scholar]
- Pietzsch A, Buchler C, Aslanidis C, Schmitz G. Identification and characterization of a novel monocyte/macrophage differentiation-dependent gene that is responsive to lipopolysaccharide, ceramide, and lysophosphatidylcholine. Biochem Biophys Re Comm. 1997;235:4–9. doi: 10.1006/bbrc.1997.6715. [DOI] [PubMed] [Google Scholar]
- Pietzsch A, Buchler C, Schmitz G. Genomic organization, promoter cloning, and chromosomal localization of the Dif-2 gene. Biochem Biophys Res Comm. 1998;245:651–657. doi: 10.1006/bbrc.1998.8500. [DOI] [PubMed] [Google Scholar]
- Reichrath J. Will analogs of 1,25-dihydroxyvitamin D(3) (calcitriol) open a new era in cancer therapy? Onkologie. 2001;24:128–133. doi: 10.1159/000050299. [DOI] [PubMed] [Google Scholar]
- Reitsma PH, Rothberg PG, Astrin SM, Trial J, Bar-Shavit Z, Hall A, Teitelbaum SL, Kahn AJ. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983;306 (5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Rots NY, Iavarone A, Bromleigh V, Freedman LP. Induced differentiation of U937 cells by 1,25-dihydroxyvitamin D3 involves cell cycle arrest in G1 that is preceded by a transient proliferative burst and an increase in cyclin expression. Blood. 1999;93:2721–2729. [PubMed] [Google Scholar]
- Schafer H, Arlt A, Trauzold A, Hunermann-Jansen A, Schmidt WE. The putative apoptosis inhibitor IEX-1L is a mutant nonspliced variant of p22 (PRG1/IEX-1) and is not expressed in vivo. Biochem Biophys Res Comm. 1999a;262:139–145. doi: 10.1006/bbrc.1999.1131. [DOI] [PubMed] [Google Scholar]
- Schafer H, Lettau P, Trauzold A, Banasch M, Schmidt WE. Human PACAP response gene 1 (p22/PRG1): proliferation-associated expression in pancreatic carcinoma cells. Pancreas. 1999b;18:378–384. doi: 10.1097/00006676-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Schilling D, Pittelkow MR, Kumar R. IEX-1, an immediate early gene, increases the rate of apoptosis in keratinocytes. Oncogene. 2001;20:7992–7997. doi: 10.1038/sj.onc.1204965. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Ehland H, Sylvia VL, et al. 1α,25-Dihydroxyvitamin D3 and 24R,25-Dihydroxyvitamin D3 modulated growth plate chondrocyte physiology via protein kinase C-dependent phosphorylation of extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase. Endocrinology. 2002a;143 (7):2775–86. doi: 10.1210/endo.143.7.8889. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Sylvia VL, Larsson D, Nemere I, Casasola D, Dean DD, Boyan BD. 1α,25(OH)2D3 regulates chondrocyte matrix vesicle protein kinase C (PKC) directly via G-protein-dependent mechanisms and indirectly via incorporation of PKC during matrix vesicle biogenesis. J Biol Chem. 2002b;277:11828–11837. doi: 10.1074/jbc.M110398200. [DOI] [PubMed] [Google Scholar]
- Shinki T, Takahashi N, Kadofuku T, Sato T, Suda T. Induction of spermidine N1-acetyl-transferase by 1α,25-dihydroxyvitamin D3 as an early common event in the target tissues of vitamin D. J Biol Chem. 1985;260:2185–2190. [PubMed] [Google Scholar]
- Simboli-Campbell M, Gagnon A, Franks DJ, Welsh J. 1,25-Dihydroxyvitamin D3 translocates protein kinase C β to nucleus and enhances plasma membrane association of protein kinase Cα in renal epithelial cells. J Biol Chem. 1994;269:3257–3264. [PubMed] [Google Scholar]
- Simpson RU, Hsu T, Begley DA, Mitchell BS, Alizadeh BN. Transcriptional regulation of the c-myc protooncogene by 1,25-dihydroxyvitamin D3 in HL-60 promyelocytic leukemia cells. J Biol Chem. 1987;262:4104–4108. [PubMed] [Google Scholar]
- Somjen D, Binderman I, Weisman Y. The effects of 24R,25-dihydroxycholecalciferol and of 1α,25-dihydroxycholecalciferol on ornithine decarboxylase activity and on DNA synthesis in the epiphysis and diaphysis of rat bone and in the duodenum. Biochem J. 1983;214:293–298. doi: 10.1042/bj2140293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves RM, Lawson DE. Effect of 1,25-dihydroxyvitamin D on S-adenosylmethionine decarboxylase in chick intestine. Biochim Biophys Acta. 1985;841:292–298. doi: 10.1016/0304-4165(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Suda T, Shinki T, Takahashi N. The role of vitamin D in bone and intestinal cell differentiation. Ann Rev Nutr. 1990;10:195–211. doi: 10.1146/annurev.nu.10.070190.001211. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Abe E. Role of vitamin D in bone resorption. J Cell Biochem. 1992;49:53–58. doi: 10.1002/jcb.240490110. [DOI] [PubMed] [Google Scholar]
- Suda T, Udagawa N, Nakamura I, Miyaura C, Takahashi N. Modulation of osteoclast differentiation by local factors. Bone. 1995;17(2 Suppl):87S–91S. doi: 10.1016/8756-3282(95)00185-g. [DOI] [PubMed] [Google Scholar]
- Van den Bemd G, Chang G. Vitamin D and vitamin D analogs in cancer treatment. Curr Drug Targets. 2002;3:85–94. doi: 10.2174/1389450023348064. [DOI] [PubMed] [Google Scholar]
- Wali RK, Bissonnette M, Khare S, Aquino B, Niedziela S, Sitrin M, Brasitus TA. Protein kinase C isoforms in the chemopreventive effects of a novel vitamin D3 analogue in rat colonic tumorigenesis. Gastroenterology. 1996;111:118–126. doi: 10.1053/gast.1996.v111.pm8698190. [DOI] [PubMed] [Google Scholar]
- Wasserman RH, Chandler JS, Meyer SA, Smith CA, Brindak ME, Fullmer CS, Penniston JT, Kumar R. Intestinal calcium transport and calcium extrusion processes at the basolateral membrane. J Nutr. 1992;122(3 Suppl):662–671. doi: 10.1093/jn/122.suppl_3.662. [DOI] [PubMed] [Google Scholar]
- Wood RJ, Tchack L, Taparia S. 1,25-Dihydroxyvitamin D3 increases the expression of the CaT1 epithelial calcium channel in the Caco-2 human intestinal cell line. BMC Physiol. 2001;1:1.1. doi: 10.1186/1472-6793-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MX, Ao Z, Prasad KV, Wu R, Schlossman SF. IEX-1L, an apoptosis inhibitor involved in NF-kappaB-mediated cell survival. Science. 1998;281 (5379):998–100l. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schlossman SF, Edwards RA, et al. Impaired apoptosis, extended duration of immune responses, and a lupus-like autoimmune disease in IEX-1-transgenic mice. Proc Natl Acad Sci U S A. 2002;99:878–883. doi: 10.1073/pnas.022326699. [DOI] [PMC free article] [PubMed] [Google Scholar]


