Abstract
Flavivirus vaccines based upon the ChimeriVax™ technology contain the nonstructural genes of the yellow fever (YF) vaccine and the prM and E genes of heterologous flaviviruses such as Japanese encephalitis (JE) and West Nile (WN) viruses. These chimeric vaccines induce both humoral and cell mediated immunity. Mice were immunized with YF, YF/JE, or YF/WN vaccines followed by secondary homologous or heterologous immunization and the hierarchy and function of CD8+ T-cell responses to a variable envelope epitope were analyzed and compared to those directed against a conserved immunodominant YF NS3 epitope. Sequential immunization with heterologous chimeric flaviviruses generated a broadly cross-reactive CD8+ T-cell response, dependent on both the sequence of infecting viruses and epitope variant. The enhanced responses to variant epitopes following heterologous immunization were not related to pre-existing antibody or to higher virus titers. These results demonstrate that immunization sequence impacts the expansion of flavivirus cross-reactive CD8+ T-cells upon heterolgous challenge.
Keywords: Japanese encephalitis virus, West Nile virus, flavivirus, CD8+ T-cell, heterologous immunity, yellow fever vaccine, sequential immunization
INTRODUCTION
Flaviviruses are arthropod-borne, RNA viruses of the Flaviviridae family. Members of the Japanese Encephalitis (JE) virus serocomplex including JE, West Nile (WN) and Saint Louis encephalitis (SLE) viruses, cause neurological disease, while the four serotypes of dengue virus (DENV1–4) and YF result in hemorrhagic fever (1–5). The envelope (E) and NS3 proteins are known to induce adaptive immune responses (1, 6–7).
Flavivirus antigen-specific CD8+ T-cells are important in clearing virus from tissues and preventing virus persistence (8–11). Primary exposure to a virus primesCD8+ T-cells by virus-derived immunodominant peptides, presented in the context of MHC class-I by antigen presenting cells (APCs), lead to clonal differentiation and proliferation of effector cells followed by contraction and memory generation (12–15). Memory T-cells generated against subdominant epitopes of one virus may demonstrate plasticity in antigen-recognition upon secondary heterologous challenge leading to protective immunity or immunopathology (16–19). We previously demonstrated that sequential infection with different DENV serotypes induces sequence-specific expansion of DENV cross-reactive CD8+ T-cells (20). Prime-boost immunizations using recombinant DNA vaccines or virus-like particles similarly enhance immune responses to inserted heterologous epitopes (21–24).
The co-circulation and emergence of flaviviruses in many regions of the world point to the need for effective vaccination strategies against these viruses (1,2). Novel, live-attenuated chimeric flavivirus vaccines have been produced on a platform based on the nonstructural and core backbone of the YF 17D vaccine and the prM and E of heterologous flaviviruses, including JE, WN, and DENV. These virus vaccines have demonstrated protection against homologous virus challenge in animal models and generation of adaptive B and T cell responses in human clinical trials (25–30). Neutralizing antibody responses to chimeric vaccine viruses have been shown to be specific to the virus from which the E gene is derived (31,32). CD8+ T cells have been found to be necessary for protection from primary dengue, WNV and JE virus infections in mouse models; WNV envelope-specific CD8+ T cells afford protection against lethal WNV challenge (8,9,11,33,34).
The potential of chimeric vaccines to generate cross-reactive CD8+ T-cell responses to heterologous flaviviruses has not been studied. Here, we evaluated quantitatively and qualitatively the CD8+ T-cell responses to a subdominant flavivirus cross-reactive T-cell epitope on the E protein and to a conserved, immunodominant YF NS3 epitope, following primary or heterologous secondary infection. We found that secondary immunization with heterologous chimeric flavivirus vaccines generated an enhanced cross-reactive CD8+ T-cell response that was dependent on the sequence of the infecting vaccine viruses. These results suggest that controlled exposure to multiple related flavivirus vaccines may lead to enhanced protection against related flaviviruses.
MATERIALS AND METHODS
Mice, viruses and immunization
All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. 5–6 week old female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were immunized intra-peritoneally (ip) with 1×106 PFU ChimeriVax™-JE (YF/JE) or ChimeriVax™-WN (YF/WN) (from Acambis, Inc., now a part of Sanofi Pasteur), or YF (YF-Vax, from Connaught Laboratories, now a part of Sanofi Pasteur). Viruses were prepared and titered in Vero-81 cells (ATCC) (35). Kb and Db knockout mice (Taconic) were used to confirm MHC restriction of CD8+ T-cell epitopes.
For primary immunizations, splenocytes were harvested at serial days post immunization (dpi) for acute responses and on day 28 for memory responses. To determine secondary CD8+ T-cell responses, 4–6 weeks following primary immunization, groups of 3 to 4 mice from each primary virus immunization were immunized with either homologous or heterologous viruses ip with 1×106 PFU of virus.
Peptides
Crude peptides (20mer overlapping by 10 amino acids) spanning the envelope region of JE SA14-14-2 (GenBank Accession number AAK11279) and peptide truncations were synthesized by the University of Massachusetts Medical School Peptide Core Facility. WNE1 is a Db-restricted CD8+ T-cell epitope (11). Biology Workbench blast search revealed homologous sequences for other flaviviruses (Table 1). Variant E and YF NS3 peptides were synthesized at > 90% purity by AnaSpec, Inc.
Table 1.
The heterologous envelope variants and conserved NS3 epitopes.
| Virus | Sequence | Homology | Accession No. |
|---|---|---|---|
| JE SA14–14–2 | LGMGNRDFI | 100% | AAK11279 |
| WNV NY 99 | LGMSNRDFL | 78% | AAW50577 |
| SLE | LGTSNRDFV | 67 % | ACA28960.1 |
| DENV 1 | VGIGNRDFV | 67 % | ACC68715.1 |
| DENV 2 | IGISNRDFV | 56 % | ACC68732.1 |
| DENV 3 | VGVGNRDFV | 67 % | ACC68732.1 |
| DENV 4 | VGVGNRDFV | 67 % | ACC68765.1 |
| YF17D | IGITDRDFI | 56 % | ACN41908.1 |
| YF17D NS3 | ATLTYRML | * | ACN41908.1 |
The first 7 sequences are from the envelope region (amino acid residues 4–12) of the respective flavivirus genome. The last sequence is from the nonstructural region (NS3 268–275) of the YF17D. The differences in amino acid sequence are shown in italics.
The homology is with reference to JEE1 sequence.
represents the absence of homology as it is not from the envelope region of the YF17D.
The MHC specificity for all the envelope peptides is H2-Db and for YF NS3 it is H2-Kb. JE = Japanese encephalitis virus; WNV = West Nile virus; SLE = St. Louis encephalitis virus; DENV = dengue virus; YF17D = yellow fever 17D vaccine strain
Generation of T-cell lines and cytotoxic T-cell assay
Spleens were obtained 7 days following homologous boost from mice immunized with YF/WN or YF/JE. Single cell suspensions were prepared as previously (20). Cells were cultured in the presence of peptide (10μg/mL) in RPMI-1640 containing 10% FCS, 1% penicillin/streptomycin, 5 × 10−5 M mercaptoethanol and recombinant human IL-2 (BD Biosciences; 25 U/mL) at 37°C. Every 14 days, γ-irradiated naïve splenocytes were pulsed with peptide (10 μg/mL) and added at a 5:1 ratio of stimulators:responders. 51Chromium release assays were performed as previously described (36). Percent specific lysis was calculated as [(sample 51Cr release − spontaneous 51Cr release)/(maximum 51Cr release − spontaneous 51Cr release)] × 100.
IFN-γ ELISPOT assays
IFN-γ ELISPOT assays were performed according to the manufacturer's protocol (Mabtech) as we have described (36). Briefly, 96-well Multiscreen-IP plates (Millipore) were coated with 15 μg/mL rat anti-mouse IFN-γ mAb (AN-18) overnight at 4°C. Splenocytes (2.5 × 105/well) were incubated in triplicate with peptides (2 μg/mL), Con A (5 μg/mL), or media alone at 37°C for 18–20 hrs. Plates were washed, incubated with biotinylated rat anti-mouse IFN-γ mAb (R4-6A2), washed, and further incubated with streptavidin-HRP. Plates were developed by addition of substrate (Vector NovaRED Substrate kit, Vector Laboratories). Precursor frequency was calculated as: (number of spots in experimental well − number of spots in medium control)/total number of cells per well) × 106.
Intracellular IFN-γ detection assay
Peptide-specific IFN-γ- and TNF-α- producing CD3+ CD8+ T-cells were quantified by intracellular cytokine staining (ICS) assay (20). Briefly, 1×106 splenocytes per well were seeded in 96 well plates for 5–6 hrs with 10 μg/mL peptide, medium alone or PMA (50 ng/mL) plus ionomycin (500 ng/mL) in the presence of 5 μL/mL of brefeldin A (GolgiPlug, BD Biosciences). Following wash and Fc blocking, cells were incubated with anti-mouse CD3ε-PerCP (145-2C11, BD Biosciences) and anti-mouse CD8α-FITC (5.3-C711, eBioscience). Following fixation with BD Cytofix/Cytoperm, wash with perm-wash buffer, and staining with PE conjugated IFN-γ (eBioscience) and APC conjugated TNF-α (BD Biosciences), cells were washed and resuspended in 1% paraformaldehyde and data acquired on a FACSCalibur flow cytometer. Data were analyzed with FlowJo Software (Treestar, Inc.). Background media frequencies were subtracted from those of peptide-stimulated cells.
Surface marker and pentamer staining
MHC-peptide pentamers and RPE label (ProImmune, Inc.) were used according to the manufacturer's protocol. Briefly, 1×106 splenocytes were incubated with pentamer, washed and Fc blocked with 2.4G2 (BD). Cells were surface stained with RPE, PerCP-conjugated CD3 and FITC-labeled CD8. Following wash and fixation with 0.1% paraformaldehyde, the samples were acquired on a FACSCaliber flow cytometer and data were analyzed using FlowJo Software.
Adoptive transfer of serum
Mice were immunized with 1×106 PFU YF/JE. On 28 dpi, serum was collected from YF/JE-immune or naïve mice and heat inactivated. Naïve mice were injected ip with 150 μL of undiluted or diluted (1:10 in PBS) immune or naïve serum and immunized ip with YF/WN 24 hr later. On 8 dpi, splenocytes were harvested and analyzed for IFN-γ response.
Measurement of virus replication in affected organs
Mice were immunized with YF/JE and 3 and 7 dpi spleen, liver, brain, and serum were collected and stored at −70°C. Remaining mice were administered YF/WN on 28 dpi and the same organs were collected and frozen 3 and 7 dpi. Organs were homogenized in MEM, sonicated, and centrifuged. Virus titers were measured in supernatants by plaque assay as previously described (35).
Statistical analysis
Data were analyzed by Mann-Whitney test using SigmaPlot/SigmaStat, and p values of ≤ 0.05 were considered significant. The correlation between ICS and pentamer data was analyzed using Pearson rank correlation using Minitab program. The median values were obtained using Microsoft Excel.
RESULTS
Heterologous and conserved peptides used for in-vitro stimulation
T-cell epitopes against JE virus have not been previously identified in C57Bl/6 mice. We utilized IFN-γ ELISPOT assay to screen peptide pools containing overlapping JE envelope peptides in splenocytes from YF/JE-immunized. We identified an optimal epitope, JEE1, a homologue of a Db-restricted WNV envelope epitope (8). Using JEE1 as the base sequence, homologous envelope sequences were identified for 7 other flaviviruses (Table 1). JEE1-stimulated bulk cultures from YF/JE immune mice showed recognition of JEE1 and WNE1 epitopes in chromium release assays (data not shown). Immunization of Kb and Db knockout mice with chimeric YF/JE confirmed that recognition of all eight E variants was Db restricted (data not shown). We selected an immunodominant H-2kb-restricted YFNS3-specific CD8+ T-cell epitope (7) whose sequence is conserved among all three viruses for comparison to the variable epitope.
Kinetics of epitope-specific T-cell IFN-γ responses during primary and secondary flavivirus immunizations
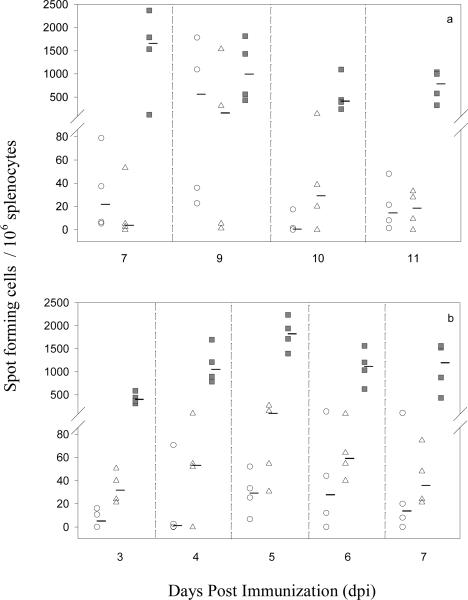
Epitope-specific T-cell responses may differ during primary and secondary virus infection (20–23). We therefore the kinetics of T-cell responses following primary and secondary infections. The peak IFN-γ responses to the conserved NS3 and variable E epitopes after primary immunization were observed at 7 and 9 dpi for NS3, and 9 dpi for E (Figure 1a). The peak IFN-γ responses to both E and NS3 were observed on 5 dpi following secondary immunization (Figure 1b). For subsequent experiments, primary and secondary responses were therefore analyzed at 8 and 5 dpi, respectively. Despite greater sequence homology of the dengue E variants than the YFE1 to JEE1, we found that in vitro stimulation with DENV E variants (56 to 67% homology) led to lower IFN-γ responses compared to YFE1 (56% homology) (not shown). Therefore, we included the JEE1, WNE1, YFE1, and SLEE1 peptides, and the conserved NS3 peptide for subsequent studies.
Figure 1.
Kinetics of IFN-γ production following primary or secondary flavivirus immunization. C57BL/6 mice were immunized with 1×106 PFU YF/JE or YF17D. Splenocytes were harvested on indicated days and in vitro stimulated with all E variant peptides and the conserved NS3 epitope and IFN-γ production measured by ELISPOT assay. Four to six weeks after primary immunization, mice were immunized with 1×106 PFU YF, YF/JE or YF/WN and at 3, 4, 5, 6, and 7 days post secondary immunization splenocytes were harvested and ELISPOT assay performed as above. Figure shows representative data for (a) primary YF/JE and (b) primary YF/JE followed by secondary YF/WNV immunization. Each data point represents an individual mouse. Thick dash symbols (−) represent median values for each group. (○) WNE1, (Δ) JEE1, (■) YFNS3.
Cytokine production by CD8+ T-cells following primary and secondary flavivirus immunization
In order to determine whether the effects of sequential infection differ for variable vs. conserved epitopes, we assessed IFN-γ and TNF-α producing cells in response to stimulation with YFNS3 peptide and the variable flavivirus E peptides following primary and secondary homologous/heterologous immunization with YF, YF/JE, and YF/WN. The TNF-α producing cellular response to E and NS3 sequences was negligible in both primary and secondary infections (not shown). However, an increase in the number of IFN-γ producing cells was observed in response to E and YFNS3 epitopes when a particular primary immunization was followed by heterologous immunization (Figures 2a, 2b, Table 2). Similar to DENV, the effect of sequential infection on the epitope-specific T-cell response was dependent on the specific sequence of immunizations (20). For example, primary immunization with YF/JE elicited increased IFN-γ responses upon heterologous YF or YF/WN challenge (Figure 2a). In contrast, there was little change in IFN-γ production from CD8+ T-cells in mice immunized with primary YF/WN followed by heterologous YF or YF/JE. These changes were not only virus-sequence dependent, but also epitope-variant dependent. Although the overall increase in responses in mice that received primary YF followed by YF/JE or YF/WN were modest for WNE1, JEE1, YFE1, and YFNS3, there was a significant increase in the response to the SLEE1 epitope (Figures 2a, 2b, Table 2).
Figure 2.
IFN-γ production by CD8+ T-cells following simulation with E variants (2a) and YFNS3 (2b) epitopes. C57BL/6 mice were administered sequential heterologous and homologous immunization with 1×106 PFU YF/JE, YF/WN and YF17D viruses as described in Materials and Methods. IFN-γ responses to the WNE1 (leftmost column), JEE1 (second column), YFE1 (third column), and SLEE1 (rightmost column) peptides were measured 8 dpi post primary, 28 days after primary immunization for memory and 5 dpi post secondary immunization. X axis represents the sequence of infection that the mice received (J=YF/JE, W=YF/WN, Y=YF17D). For example, J+W refers to mice that received a primary YF/JE followed by secondary YF/WN. `m' indicates responses at 28 days after primary infection. All 9 potential sequences were tested; data for secondary infections are grouped by the primary serotype. Data from each primary infection is included in each graph for reference. Y axis represents the percent of IFN-γ+ CD3+ CD8+ cells. Each open circle (○) represents an individual animal. Thick dash symbols (−) represent median values for each group. P values for the comparisons between different groups were calculated using the Mann-Whitney test. P values < 0.05 are shown.
Table 2.
Summary of IFN-γ responses and MHC pentamer binding to the variant E and conserved YF NS3 epitopes.
| Primary Virus | Secondary Virus | N | Reference Group | IFN-γ Response | Pentamer Binding | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WNE1 | JEE1 | YFE1 | SLEE1 | YFNS3 | WNE1 | JEE1 | YFNS3 | ||||
| YF/JE | YF/JE | 12 | memory YF/JE | * | |||||||
| 12 | acute YF/JE | * | |||||||||
| YF/WN | 11 | memory YF/JE | * | * | * | * | * | ||||
| 12 | acute YF/WN | * | * | * | * | * | * | * | |||
| YF | 12 | memory YF/JE | * | * | * | * | * | nd | |||
| 12 | acute YF | * | * | * | * | * | * | * | |||
| YF/WN | YF/WN | 7 | memory YF/WN | ||||||||
| 12 | acute YF/WN | ||||||||||
| YF/JE | 7 | memory YF/WN | * | * | |||||||
| 12 | acute YF/JE | * | |||||||||
| YF | 8 | memory YF/WN | |||||||||
| 12 | acute YF | * | |||||||||
| YF | YF | 8 | memory YF | * | * | ||||||
| 12 | acute YF | * | * | ||||||||
| YF/JE | 8 | memory YF | * | * | * | ||||||
| 12 | acute YF/JE | * | * | * | |||||||
| YF/WN | 8 | memory YF | * | * | |||||||
| 12 | acute YF/WN | * | * | * | |||||||
Groups of mice were immunized with YF/JE, YF/WN or YF17D (YF). Secondary immunizations with homologous or heterologous virus were performed on day 28 post-primary immunization. Splenocytes were examined for IFN-γ production and pentamer binding at 8 and 28 days post-immunization for acute and memory primary responses, respectively, and at day 5 post-secondary immunization.
indicates a statistically significant different response when compared to the reference group.
Variant epitopes: WNE1 = West Nile E1; JEE1 = Japanese encephalitis virus E1; YFE1 = Yellow fever E1; SLE E1 = St. Louis encephalitis virus E1; YF NS3 = Yellow fever NS3. N = number of mice, nd = no data
Frequency of CD8+ T-cells specific for variable (E) and conserved (NS3) epitopes upon primary and secondary flavivirus immunization
To determine whether the frequency of IFN-γ producing cells represented the majority of epitope-specific cells, we measured epitope-specific cells with pentamers of MHC loaded with WNE1, JEE1, or YFNS3. Following primary immunization with YF/JE or YF/WN, there was an increase in the frequency of pentamer binding cells from day 8 to weeks 4–6. A similar increase was not seen after primary YF immunization (Figure 3, Table 2). We also observed a boost in the frequency of epitope-specific cells upon secondary heterologous virus immunization. This boost appeared to be virus sequence-specific. For example, primary immunization with YF/JE or YF followed by heterologous virus immunization boosted WNE1 and JEE1 epitope-specific T-cell frequencies, whereas primary immunization with YF/WN did not show a similar effect. We also detected epitope-specific differences in T-cell frequencies to the same immunization regimen. For example, primary YF/WN immunization followed by heterologous immunization with YF/JE induced a significant increase in the frequency of YFNS3-specific CD8+ T-cells (p=0.011) but did not demonstrate any increase in WNE1- or JEE1-specific CD8+ T-cells (Figure 3, Table 2).
Figure 3.
Pentamer specificity for WNE1, JEE1, and YFNS3. C57BL/6 mice were administered sequential heterologous and homologous immunization with 1×106 PFU YF/JE, YF/WN and YF17D viruses as described in Materials and Methods. Pentamer binding to WNE1 (left most column) JEE1 (second column), YFNS3 (rightmost column) were measured 8 dpi post primary and 5 dpi post secondary immunization. X axis represents the sequence of infection that the mice received (J=YF/JE, W=YF/WN, Y=YF17D). For example, J+W refers to mice that received primary YF/JE followed by secondary YF/WN. `m' indicates responses at 28 days after primary infection. All 9 potential sequences were tested; data for secondary infections are grouped by the primary serotype. Data from each primary infection is included in each graph for reference. The Y axis represents the percent pentamer specific CD3+ CD8+ cells. The dotted lines represent the background pentamer binding for splenocytes in naïve mice. Each open circle (○) represents an individual animal. Thick dash symbols (−) represent median values for each group. P values for the comparisons between different groups were calculated using the Mann-Whitney test. P values < 0.05 are shown.
Role of T-cell functional avidity
The avidity of the T-cell receptor (TCR) for peptide-MHC complexes affects both the quality and quantity of a given T-cell response. We hypothesized that the differences seen in JEE1 and WNE1 pentamer binding and cytokine induction might be a result of differences in avidity. We therefore measured IFN-γ responses to different concentrations of JEE1, WNE1, and YFNS3 peptides (functional avidity). Primary immunization with YF/JE as well as primary YF/JE followed by secondary YF/WN exhibited similar patterns of functional avidity for both envelope epitopes with peak responses occurring at peptide concentrations of 0.1 μg/mL and 1 μg/mL, respectively, and reaching a nadir at a concentration of 0.001 μg/mL after primary immunization and 0.01 μg/mL after secondary heterologous immunization (Figures 4a, 4b). For both primary and secondary immunization, IFN-γ responses to the NS3 epitope did not diminish, even at 0.001 μg/mL, indicating a higher functional avidity compared to the JEE1 and WNE1 epitopes.
Figure 4.
Functional avidity to variant E and YF NS3 epitopes is not enhanced after heterologous immunization. Mice were immunized with YF/JE followed by YF/WN and 8 dpi after primary (a) and 5 dpi post secondary immunization (b) splenocytes were harvested and in-vitro stimulated with different concentrations of WNE1, JEE1 and YFNS3 peptides (x-axis) and examined for IFN-γ response from CD8+ T cells (y-axis) by ICS as described in Materials and Methods. (○) WNE1, (Δ) JEE1, (■) YFNS3.
Antibody dependent enhancement is not responsible for the increased T-cell response following sequential heterologous flavivirus immunizations
IFN-γ ICS and pentamer binding results indicated that when primary immunization with YF/JE was followed by heterologous immunization with YF/WN or YF17D, there was an increase in frequency and cytokine production by epitope specific CD8+ T-cells. In heterologous dengue virus infection, pre-existing antibody from the primary infection is theorized to enhance infection of FcγR-expressing cells leading to increased viremia and disease severity upon secondary infection. To evaluate the possible role of pre-existing anti-flavivirus antibodies in enhancing T-cell responses (37), we conducted passive immunization studies with YF/JE-immune or naïve serum followed by YF/WN immunization. We found no significant difference in the IFN-γ response by splenocytes from mice that received YF/JE-immune serum (undiluted or at 1:10 dilution) compared to splenocytes from mice that received naïve serum (Figure 5).
Figure 5.
Adoptive transfer of flavivirus-immune serum does not enhance epitope-specific T-cell responses following flavivirus immunization. C57Bl/6 mice were administered undiluted (a) or 1:10 diluted (b) serum from YF/JE immune (hatched bars) or naïve mice (open bars). After 24 hrs, mice were immunized ip with 1×106 PFU of YF/WN virus. At 8 dpi, splenocytes were harvested and in vitro stimulated with the envelope variants and the conserved NS3 peptide and IFN-γ production measured in ICS. Error bars represent the standard error of the mean. Each bar represents mean data from single mice (n=8) run in two separate experiments.
Viral titers in primary and secondary infections
We collected serum, spleen, liver, and brain from mice on days 3 and 7 post-primary and post-secondary immunization. We were unable to detect infectious virus from any of these tissues at either time point, suggesting that the increased epitope-specific T-cell frequencies were not related to increased virus antigen (data not shown).
DISCUSSION
Secondary infection/immunization with related or unrelated pathogens can result in either protective immunity or immunopathology (16, 17, 38–40). There have been several studies exploring immune responses to chimeric flaviviruses (8, 9), cross-reactive immunity (26), and the benefits of recombinant vaccines in prime-boost strategies (21–24). However, the effect of sequential immunization on CD8+ T-cell responses using chimeric viruses for JE and WN has not previously been studied. We identified and characterized a new JE envelope-specific CD8+ T-cell epitope which is a variant of an H-2Db restricted WNV epitope (8). Using this epitope as a reference, we identified additional flavivirus variant E epitopes. The majority of these variants fit the H-2Db binding motif, which comprises of asparagine at P5, and methionine, isoleucine or leucine at P9, although amino acid changes at other positions, especially P2, may adversely affect peptide binding (41–42). Surprisingly, YFE1, which shared the lowest homology to JEE1 with only 5 of 9 amino acids conserved and with a non-conservative change at P5 from asparagine to aspartic acid still retained substantial functional cross-reactivity whereas the more closely homologous dengue epitopes did not. (43, 44).
The peak responses to E and NS3 epitopes were detected on day 5 post-secondary immunization. Given the more rapid increase in epitope-specific cells when compared to that seen following primary immunization, these findings suggest that cross-reactive memory CD8+ T-cells are responsible for this early response. Circulating memory cells have been shown to have a lower activation threshold than naïve T-cells, and may therefore be preferentially activated at a much faster rate, when presented with the same or cross-reactive epitope in the context of the appropriate MHC (12–14). Primary immunization with YF/JE resulted in memory CD8+ T-cells that were able to recognize both novel E variants and the conserved YF NS3 epitopes following homologous and heterologous secondary immunizations, suggesting that this type of sequential immunization strategy can generate a memory pool with broad spectrum immunity (CD8+ T-cell as well as neutralizing antibody responses) as seen in other flavivirus animal model systems (20, 43). Similarly in humans, administration of chimeric YF dengue-2 vaccine to YF-immune subjects led not only to enhanced levels of dengue-2 specific neutralizing antibodies, but also induced a greater breadth of cross-reactive neutralizing antibody directed against other dengue serotypes (32).
Primary immunization with YF/WN did not seem to generate a similar pattern of cross-reactivity in response to the E variants as seen with primary YF/JE. A possible explanation for the virus sequence-specific differences in the generation of epitope-specific responses may relate to differing pMHC avidity among different epitope variants. However, this did not seem to be the case as the functional avidity for the JEE1 and WNE1 peptides were similar following primary infection with YF/JE or primary YF/JE followed by secondary YF/WN. Alternatively, differences in the frequencies of cross-reactive memory CD4+ T-cells generated after primary immunization with YF/JE and YF/WN may alter the ability to provide help to CD8+ T-cells upon secondary heterologous infection as has been suggested in our previous studies in dengue (46). Using mathematical modeling, Zhou et al. have suggested that in order to generate a more broadly cross-reactive immune response against all four dengue serotypes, the optimal dengue vaccine to afford tetravalent protection would be a polytopic vaccine that includes subdominant epitopes for priming (47).
We found a discrepancy in the frequency and functionality of the antigen specific T-cells in which the frequency of cells measured by pentamer binding was generally higher than that seen in IFN-γ ICS (Table 2). These differences likely result from additional unmeasured effector functions (e.g., cytolytic function or other cytokines). We have previously shown that CD8+ T-cells from humans immunized with live-attenuated dengue virus vaccines are able to secrete varying quantities of IFN-γ, TNF-α and MIP-1β (15). In this model, we were not able to measure appreciable amounts of TNF-α in response to peptide stimulation, and a reliable murine anti-MIP-1β antibody was not available. Although we were able to measure cross-reactive cytotoxic responses to both JEE1 and WNE1 in JEE1 and WNE1 bulk cultures raised from YF/JE and YF/WN immunized mice (not shown), we did not examine cytolytic function directly ex vivo. The avidity of T-cells for the peptide MHC complex has been shown to play a key role in the type of response by the epitope specific T-cells (48–50). However, results of peptide dose response experiments do not suggest that the differences in the memory response to E and NS3 are related to differences in T-cell avidity for variant peptide MHC complexes.as both E epitopes had similar and lower functional avidity compared to that of the conserved YFNS3 epitope. Previous studies have demonstrated that variant epitopes of low avidity are more likely to be cross-reactive whereas immunodominant epitopes such as the YF NS3 tend to have tight binding to pMHC (49, 50).
In vitro enhancement of dengue virus infection in the presence of heterologous dengue virus-specific antibody leads to increased uptake by FcγR-expressing cells (37). In vivo support of the role of enhancing antibodies in the pathogenesis of dengue virus infection remains controversial, but a resultant increase in virus burden might enhance antigen presentation to memory cross-reactive T-cells leading to activation and increased cytokine production (37). Our passive transfer studies using YF/JE immune serum followed by YF/WN immunization do not support this mechanism for the enhanced IFN-γ response in heterologous immunization after primary YF/JE infection (Figure 5). The absence of detectable infectious virus in the serum and tissues of mice immunized with YF/JE followed by secondary YF/WN virus also do not support a role for differences in virus burden as a mechanism for enhanced T-cell responses.
Our findings suggest that cross-reactive CD8+ T-cells generated during sequential immunization with heterologous chimeric flavivirus vaccines depend on the primary vaccine virus as well as on the sequence of immunization. Mice that received primary YF/JE had greater numbers of epitope specific CD8+ T-cells and these cells further expanded in frequency and effector function following secondary immunization with the heterologous virus vaccines YF/WN and YF17D. Therefore, the above strategy of immunization may result in the generation of increased epitope-specific cross-reactive T-cells that are protective. While CD8+ T-cell responses in humans differ from those in mice, this study indicates that certain sequences of immunization may augment T-cell responses more than others. Further research to examine the role of other viral and host factors may provide useful insight into the significance of sequential immunization with chimeric flaviviruses.
Acknowledgments
We would like to thank Dr. Guy Bruno and Dr. Anuja Mathew for their review and insightful comments on the original manuscript. Sincere thanks are due to Jane Nowicki and Dr. Liyan Yang, and the staff of the UMMS FACS Core facility for technical assistance, and Marcia Woda for assistance with flow cytometry assays and analyses.
Financial support: This project was supported by NIH grants U19 AI057319 and P30 DK032520 and NIH contract N01 AI25490. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations used
- YF
Yellow fever 17D vaccine virus
- JE
Japanese encephalitis virus
- WN
West Nile virus
- YF/JE
chimeric Japanese encephalitis virus
- YF/WN
chimeric West Nile virus
- DENV
Dengue virus
- SLE
Saint Louis encephalitis virus
- E
envelope
- NS
nonstructural
- IFN-γ
interferon gamma
- dpi
days post immunization
- ADE
antibody dependent enhancement
- ELISPOT
enzyme-linked immunospot
Footnotes
Potential conflict of interest: F.G. is an employee of Sanofi-Pasteur, and a former employee of Acambis, Inc. which have been developing the chimeric Japanese encephalitis and West Nile virus vaccines. S.G., A.L.R. and F.A.E have received some financial support as part of a contract with Acambis and/or Sanofi-Pasteur. This statement is being made in the interest of full disclosure and not because the author considers this to be a conflict of interest. The remaining authors have no commercial associations that might pose a conflict of interest.
References
- 1.Gubler D, Kuno G, Markoff L. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Vol 1. Lippincott William & Wilkins Press; Philadelphia: 2007. pp. 1153–1253. [Google Scholar]
- 2.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and Dengue viruses. Nat Med. 2004;10(12):S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 3.Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351:370–378. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- 4.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–42. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 5.Tesh RB. Viral hemorrhagic fevers of South America. Biomedica. 2002;22(3):287–95. [PubMed] [Google Scholar]
- 6.Mukhopadhyay S, Kuhn RJ, Rossman MG. A Structural Perspective of the Flavivirus Life Cycle. Nature Reviews/Microbiology. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 7.van der Most RG, Harrington LE, Giuggio V, Mahar PL, Ahmed R. Yellow fever virus 17D envelope and NS3 proteins are major targets of the antiviral T cell response in mice. Virology. 2002;296(1):117–24. doi: 10.1006/viro.2002.1432. [DOI] [PubMed] [Google Scholar]
- 8.Purtha WE, Myers N, Mitaksov V, et al. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. Eur J Immunol. 2007;37(7):1845–54. doi: 10.1002/eji.200737192. [DOI] [PubMed] [Google Scholar]
- 9.Shrestha B, Diamond MS. Role of CD8+ T Cells in Control of West Nile Virus Infection. J Virol. 2004;78(15):8312–21. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas SJ, Hombach J, Barrett A. Scientific consultation on cell mediated immunity (CMI) in dengue and dengue vaccine development. Vaccine. 2009;1427(3):355–68. doi: 10.1016/j.vaccine.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 11.Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol. 2007;37(7):1855–63. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 12.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27(3):393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harty JT, Tvinnereim, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Antov LC, Bennink JR, Yewdell JW. Dissecting the multifactorial causes of immunodominance in MHC Class I restricted T cell responses to viruses. Immunity. 2000;12:83–93. doi: 10.1016/s1074-7613(00)80161-2. [DOI] [PubMed] [Google Scholar]
- 15.Bashyam HS, Green S, Rothman AL. Dengue virus-reactive CD8+ T cells display quantitative differences in their response to variant epitopes of heterologous viral serotypes. J Immunol. 2006;176(5):2817–24. doi: 10.4049/jimmunol.176.5.2817. [DOI] [PubMed] [Google Scholar]
- 16.Selin LK, Cornberg M, Bhrem MA, et al. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin Immunol. 2004;16(5):335–47. doi: 10.1016/j.smim.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh RM, Rothman AL. Dengue immune response: low affinity, high febrility. Nat Med. 2003;9(7):820–2. doi: 10.1038/nm0703-820. [DOI] [PubMed] [Google Scholar]
- 18.Monath TP, Myers GA, Beck RA, et al. Safety testing for neurovirulence of novel live, attenuated flavivirus vaccines: infant mice provide an accurate surrogate for the test in monkeys. Biologicals. 2005;33(3):131–44. doi: 10.1016/j.biologicals.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Mongkolsapaya J, Duangchinda T, Dejnirattisai W, et al. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal. J Immunol. 2006;176(6):3821–9. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- 20.Beaumier CM, Mathew A, Bashyam HS, Rothman AL. Cross-reactive memory CD8(+) T cells alter the immune response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. J Infect Dis. 2008;197(4):608–17. doi: 10.1086/526790. [DOI] [PubMed] [Google Scholar]
- 21.Schneider J, Gilbert SC, Hannan CM, et al. Induction of CD8+ T cells using heterologous prime-boost immunization strategies. Immunol Rev. 1999;170:29–38. doi: 10.1111/j.1600-065x.1999.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 22.Radosević K, Rodriguez A, Lemckert A, Goudsmit J. Heterologous prime-boost vaccination for poverty-related disease: advantages and future prospects. Expert Rev Vaccines. 2009;8(5):577–92. doi: 10.1586/erv.09.14. [DOI] [PubMed] [Google Scholar]
- 23.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177(2):831–9. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 24.Deng Y, Zhang K, Tan W, et al. A recombinant DNA and vaccinia virus prime-boost regimen induces potent long-term T-cell responses to HCV in BALB/c mice. Vaccine. 2009;27(15):2085–8. doi: 10.1016/j.vaccine.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers TJ, Nestorowicz A, Mason PW, Eckels KH, Rice CM. Yellow fever/Japanese encephalitis chimeric viruses: Construction and biological properties. J. Virol. 1999;73:3095–3101. doi: 10.1128/jvi.73.4.3095-3101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugachev KV, Guirakhoo F, Mitchell F, et al. Construction of yellow fever/St. Louis encephalitis chimeric virus and the use of chimeras as a diagnostic tool. Am J Trop Med Hyg. 2004;71(5):639–45. [PubMed] [Google Scholar]
- 27.Monath TP, Levenbook I, Soike K, et al. Chimeric yellow fever virus 17D-Japanese encephalitis virus vaccine: dose-response effectiveness and extended safety testing in rhesus monkeys. J Virol. 2000;74(4):1742–51. doi: 10.1128/jvi.74.4.1742-1751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monath TP, Guirakhoo F, Nichols R, et al. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis. 2003;188(8):1213–30. doi: 10.1086/378356. [DOI] [PubMed] [Google Scholar]
- 29.Arroyo J, Miller C, Catalan J, et al. ChimeriVax-West Nile virus live-attenuated vaccine: preclinical evaluation of safety, immunogenicity, and efficacy. J Virol. 2004;78(22):12497–507. doi: 10.1128/JVI.78.22.12497-12507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monath TP, Liu J, Kanesa-Thasan N, et al. A live attenuated recombinant west nile virus vaccine. Proc Natl Acad Sci USA. 2006;103:6694–6699. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monath TP, McCarthy K, Bedford P, et al. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20:1004–1018. doi: 10.1016/s0264-410x(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 32.Guirakhoo F, Kitchener S, Morrison D, et al. Live attenuated chimeric yellow fever dengue type 2 ChimeriVax™-DEN2 vaccine: Phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all. Hum Vaccine. 2006;2(2):60–67. doi: 10.4161/hv.2.2.2555. [DOI] [PubMed] [Google Scholar]
- 33.Murali-Krishna K, Ravi V, Manjunath R. Protection of adult but not newborn mice against lethal intracerebral challenge with Japanese encephalitis virus by adoptively transferred virus-specific cytotoxic T lymphocytes: requirement for L3T4+ T cells. J Gen Virol. 1996;77(Pt 4):705–714. doi: 10.1099/0022-1317-77-4-705. [DOI] [PubMed] [Google Scholar]
- 34.Yauch LE, Zellweger RM, Kotturi MF, et al. A protective role for dengue virus-specific CD8+ T cells. J Immunol. 2009;182:4865–4873. doi: 10.4049/jimmunol.0801974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puig-Basagoiti F, Tilgner M, Forshey B, et al. Triaryl pyrazoline compound inhibits flavivirus RNA replication. Antimicrob Agents Chemother. 2006;50:1320–1329. doi: 10.1128/AAC.50.4.1320-1329.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew A, Terajima M, West K, et al. Identification of murine poxvirus-specific CD8+ CTL epitope with distinct functional profiles. J Immunol. 2005;174(4):2212–9. doi: 10.4049/jimmunol.174.4.2212. [DOI] [PubMed] [Google Scholar]
- 37.Konty U, Kurane I, Ennis FA. Gamma interferon augments Fcγ receptor-mediated dengue virus infection of human monocytic cells. J Virol. 1988;62(11):3928–3933. doi: 10.1128/jvi.62.11.3928-3933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selin LK, Brehm MA, Naumov YN, et al. Memory of mice and men: CD8+ T-cell cross-reactive and heterologous immunity. Immunol Rev. 2006;211:164–81. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim CK, Takashi T, Kotaki A, Kurane I. Vero cell inactivated west nile (WN) vaccine induces protective immunity against lethal WN virus infection in mice and shows a facilitated neutralizing antibody response in mice previously immunized with Japanese encephalitis vaccine. Virology. 2008;374(1):60–70. doi: 10.1016/j.virol.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 40.Blattman JN, Sourdive DJ, Murali-Krishna K, Ahmed R, Altman JD. Evolution of the T cell repertoire during primary, memory, and recall responses to viral infection. J Immunol. 2000;165:6081–6090. doi: 10.4049/jimmunol.165.11.6081. [DOI] [PubMed] [Google Scholar]
- 41.Falk K, Rötzschke O, Deres K, Metzger J, Jung G, Rammensee HG. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J Exp Med. 1991;174:425–434. doi: 10.1084/jem.174.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigal LJ, Goebel P, Wylie DE. Db-binding peptides from influenza A virus: effect of non-anchor residues on stability and immunodominance. Mol Immunol. 1995;32(9):623–632. doi: 10.1016/0161-5890(95)00031-9. [DOI] [PubMed] [Google Scholar]
- 43.Trojan A, Witzen M, Schultze JL, et al. Generation of cytotoxic T lymphocytes against naïve and altered peptides of human leukocyte antigen-A* 0201 restricted epitopes from the human epithelial cell adhesion molecule. Cancer Res. 2001;61:4761–4765. [PubMed] [Google Scholar]
- 44.Keogh E, Fikes J, Southwood S, Celius E, Chestnut R, Sette A. Identification of new epitopes from four different tumors associated-antigens: recognition of naturally processed epitopes correlates with HLA-A* 0201-binding affinity. J Immunol. 2001;167:787–796. doi: 10.4049/jimmunol.167.2.787. [DOI] [PubMed] [Google Scholar]
- 45.Guy G, Barban V, Mantel N, et al. Evaluation of interferences between dengue virus serotypes in a monkey model. AJTMH. 2009;80(2):302–311. [PubMed] [Google Scholar]
- 46.Beaumier C, Rothman AL. Cross-reactive memory CD4+ T cells alter the CD8+ T-cell response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. Viral Immunol. 2009;22(3):215–219. doi: 10.1089/vim.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou H, Deem MW. Sculpting the immunological response to dengue fever by polytopic vaccination. Vaccine. 2006;24(14):2451–2459. doi: 10.1016/j.vaccine.2005.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone JD, Chervin AS, Kranz DM. T cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126(2):165–76. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alam SM, Davis GM, Lin CM, et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–37. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 50.Rosette C, Werlen G, Daniels MA, et al. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]