Abstract
Most human T cell leukemia virus type 1 (HTLV-1) infected subjects remain asymptomatic throughout their lives, with a few individuals developing HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) or adult T cell leukemia. Lymphocytes from about half of HTLV-1 infected subjects spontaneously proliferate in vitro, and how this phenomenon relates to symptomatic disease outcome and viral burden is poorly understood. Spontaneous proliferation was measured in lymphocyte subsets, and these findings were correlated with HTLV-1 proviral load and Tax expression in peripheral blood mononuclear cells (PBMCs). We found that in addition to previously described vigorous CD8+ T cell spontaneous proliferation, natural killer (NK) cells spontaneously proliferated to a similar high level, resulting in expansion of CD56-expressing NK cells. Spontaneous NK cell proliferation positively correlated with HTLV-1 proviral load but not with Tax expression or the presence of HAM/TSP. The strongest correlate with clinical outcome in this cohort was the ability of cells to express Tax, while HTLV-1 proviral load was more closely related to spontaneous NK cell proliferation. These results demonstrate that spontaneous proliferation, Tax expression, and proviral load are inter-related but not equivalent, and that spontaneous lymphocyte proliferation is not restricted to T cells, the targets of HTLV-1 infection.
Key words: HTLV, T cell, NK cell, Tax, HAM/TSP
Introduction
HTLV-1 causes chronic infection of humans via mother to child, sexual and parenteral transmission.1 HTLV-1 provirus integrates into the genome of the infected cell, predominantly CD4+ lymphocytes. During the chronic phase of infection there is little production of viral RNA, and propagation of infection is by means of clonal proliferation of infected lymphocytes.2 Approximately 2–4% of those infected will develop adult T cell leukemia, presumably because of additional mutations accumulating within proliferating clones.3,4 A different pathophysiology is thought to underlie HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP), a chronic progressive neurologic disease with pathology affecting the thoracic spinal cord.5,6 Affected individuals (1–4% of all HTLV-1 carriers)7 have high levels of HTLV-1 provirus in the peripheral blood,8 as well as a vigorous CD8+ cytotoxic T cell response directed against HTLV-1 antigens.9 Rare cases of HTLV-1 associated pneumonitis have also been described and may have a similar pathophysiology.10,11
In spite of serious morbidity associated with HTLV-1 in a minority of subjects, most infected individuals appear to be in general good health, including many with high levels of provirus in peripheral blood.12 However, some subtle immune defects are apparent even in the otherwise healthy HTLV-1 infected population. Compared to individuals without HTLV-1 infection, parasite load is higher and chronic carriage more common in subjects with strongyloidiasis and HTLV-1 co-infection (reviewed in ref. 13), Mycobacterium avium complex infection causes more extensive pulmonary lesions in HTLV-1 infected subjects,14 and HTLV-1 infected subjects are at increased risk for bladder or kidney infections, possibly related to neurological dysfunction.15,16 A detailed understanding of the immunopathophysiology underlying HTLV-1 disease associations is lacking, in part because many studies have been narrowly focused. Similarly, prognostic markers to predict which chronically infected individuals will develop hematologic, neurologic or pulmonary disease have not been identified. These would be useful because antiviral or immune modulator treatment could be considered in those at high risk. Once immunopathology has been established such as in HAM/TSP, the early establishment of fibrosis has proved resistant to treatment strategies.17
Over 20 years ago it was noted that lymphocytes from HTLV-1 infected subjects can spontaneously proliferate in vitro18 and that HTLV-1 infection of T cell clones leads to cellular proliferation that is independent of stimulation with cognate antigen.19 PBMCs from approximately half of HLTV-1 infected subjects exhibit spontaneous lymphocyte proliferation.20 In subjects with HAM/TSP, it has been shown that a higher percentage of CD8+ than CD4+ T cells proliferates spontaneously in vitro, with the hypothesis that proliferation of HTLV-1-specific CD8+ T cells may account for the more vigorous proliferation of this subset.21 Most work to date examining spontaneous lymphocyte proliferation in HTLV-1 infection has focused on mechanisms of induction of T cell proliferation, with little attention paid to other lymphocyte subsets.
We therefore utilized specimens from a long-standing prospective cohort of HTLV-1 infection to characterize markers of the HTLV-1 immunologic response. In particular, we concentrated on in vitro spontaneous lymphocyte proliferation in multiple lymphocyte subsets and correlated these with Tax mRNA expression and HTLV-1 proviral load. We hypothesize that a better understanding of the immunology of spontaneous lymphocyte proliferation and its relation to proviral load and Tax expression might yield etiologic and prognostic information useful for the prediction of HAM/TSP and other immunopathology associated with HTLV-1.
Results
Study subject characteristics.
The study group was comprised of 21 subjects with HTLV-1 infection, including 15 HTLV-1 carriers and 6 subjects with HAM/TSP. The HAM/TSP subjects had all presented with neurological symptoms consistent with HAM/ TSP prior to donation of the samples studied in the current experiments. HAM was further classified as mild moderate or severe. Mild cases had spasticity and hyperreflexia but minimal impairment in daily functioning; moderate cases had impaired activities of daily living but were still able to ambulate with or without crutches; and severe cases were wheelchair-bound. Study subjects ranged in age from 19 to 81 years and had been followed in the cohort and known positive an average of 12 years (Table 1). As a comparison group PBMCs were obtained from 11 healthy HTLV-1 seronegative control subjects, 8 males and 3 females, aged 30-56 years (median age = 38).
Table 1.
Subject characteristics.
| Subject # | Classification | Age | Gender | Race | Co-morbiditiesa |
| 1 | HTLV-1 | 19 | F | Asian | healthy |
| 2 | HTLV-1 | 73 | M | Black | osteoarthritis |
| 3 | HTLV-1 | 47 | F | White | healthy |
| 4 | HTLV-1 | 59 | F | Asian | healthy |
| 5 | HTLV-1 | 53 | F | White | healthy |
| 6 | HTLV-1 | 63 | F | White | lupus, arthritis |
| 7 | HTLV-1 | 68 | F | Black | arthritis, diabetes |
| 8 | HTLV-1 | 56 | F | Hispanic | osteoporosis |
| 9 | HTLV-1 | 63 | F | Asian | healthy |
| 10 | HTLV-1 | 60 | M | White | DJDb, osteoarthritis |
| 11 | HTLV-1 | 74 | M | Asian | healthy |
| 12 | HTLV-1 | 49 | F | White | healthy |
| 13 | HTLV-1 | 52 | F | White | healthy |
| 14 | HTLV-1 | 81 | F | White | CHFb, hiatal hernia |
| 15 | HTLV-1 | 76 | F | Asian | healthy |
| 16 | HAM/TSP | 57 | F | White | moderate HAM |
| 17 | HAM/TSP | 59 | F | White | severe HAM |
| 18 | HAM/TSP | 55 | F | White | mild HAM |
| 19 | HAM/TSP | 68 | M | Black | severe HAM |
| 20 | HAM/TSP | 54 | F | White | severe HAM |
| 21 | HAM/TSP | 48 | F | Asian | severe HAM |
| 22 | Healthy control | 56 | M | White | |
| 23 | Healthy control | 30 | F | White | |
| 24 | Healthy control | 30 | F | White | |
| 25 | Healthy control | 44 | M | White | |
| 26 | Healthy control | 33 | M | White | |
| 27 | Healthy control | 47 | M | White | |
| 28 | Healthy control | 31 | M | Asian | |
| 29 | Healthy control | 46 | F | White | |
| 30 | Healthy control | 32 | M | White | |
| 31 | Healthy control | 38 | M | White | |
| 32 | Healthy control | 53 | M | Asian |
Co-morbidities were not recorded for control subjects.
DJD, degenerative joint disease; CHF, congestive heart failure.
Preferential spontaneous proliferation of NK cells and CD8+ T cells from HTLV-1 infected subjects.
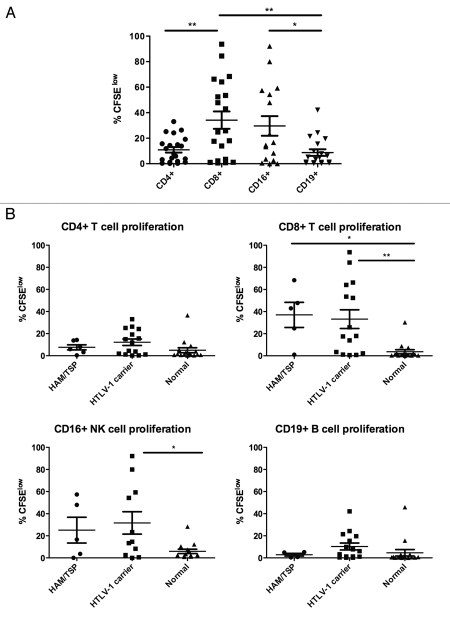
Spontaneous lymphocyte proliferation has been described in HTLV-1 infection, primarily in the CD8+ T cell subset of lymphocytes.21 The development of flow cytometry-based proliferation assays has allowed a much better understanding of which lymphocyte subsets respond to antigenic or other stimulation.24 We sought to understand whether spontaneous lymphocyte proliferation also occurred in non-T cell subsets of lymphocytes and measured proliferation by carboxyfluorescein succinimidyl ester (CFSE) dilution in unstimulated PBMCs at seven days in CD4+ and CD8+ T cells, NK cells (CD3−/CD16+), and B cells (CD3−/CD19+). The gating strategy and representative results are shown in Figure 1. Our results confirmed prior observations that CD8+ T cells show higher levels of spontaneous lymphocyte proliferation than CD4+ T cells in HTLV-1 infected subjects (Fig. 2A). Unexpectedly, we also observed vigorous spontaneous proliferation of CD16+ NK cells, which was significantly higher than that found in CD19+ B cells. Spontaneous proliferation in CD8+ T cells and CD16+ NK cells was greater in HTLV-1 infected subjects compared to normal control donors (p = 0.0004 and p = 0.009, respectively, data not shown), while the proliferation levels were not significantly different for CD4+ T cells or CD19+ B cells. We next explored whether HTLV-1 disease status correlated with the degree of spontaneous lymphocyte proliferation. Spontaneous lymphocyte proliferation in CD8+ T cells was significantly greater in HAM/TSP subjects and HTLV-1 carriers than normal controls, but there was no significant difference in spontaneous proliferation between the HAM/TSP subjects and HTLV-1 carriers (Fig. 2B). Similarly, spontaneous proliferation within CD4+ T cell, CD19+ B cell and CD16+ NK cell subsets were not different between the HAM/TSP subjects and HTLV-1 carriers, though NK cells from HTLV-1 carriers proliferated significantly more vigorously than those from normal control subjects (Fig. 2B). These data reveal that NK cells as well as CD8+ T cells show increased spontaneous proliferation in HTLV-1 infection, but in the current relatively small study spontaneous proliferation was not associated with HAM/TSP.
Figure 1.
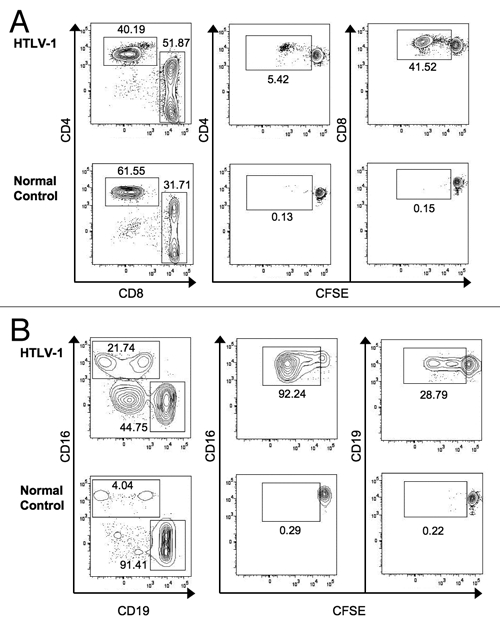
Detection of spontaneous lymphocyte proliferation in T, NK and B cell populations. (A) Cells were first gated to identify single cells that were Aqua-amine reactive dye negative (viable) and CD3+. Gated cells were plotted as CD4 vs. CD8 (left column). Gated CD4+ or CD8+ T cells were plotted vs. CFSE (middle and right columns, respectively), with proliferating cells falling in the CFSElow gate. (B) cells were gated to include viable single cells that were CD3−. Gated cells were plotted as CD16 vs. CD19 (left column). Gated CD16+ NK cells or CD19+ B cells were plotted vs. CFSE (middle and right columns, respectively), with proliferating cells falling in the CFSElow gate. Representative data are shown for HTLV-1 infected (upper rows) and normal control subjects (lower rows).
Figure 2.
Spontaneous proliferation of PBMC subsets. Proliferation was measured by CFSE dilution in cells cultured for seven days without exogenous stimulation. (A) Among HTLV-1 infected subjects CD8+ T cells and CD16+ NK cells showed the highest levels of spontaneous proliferation. (B) Spontaneous proliferation was measured in subjects with HAM/TSP (n = 6), HTLV-1 carriers (n = 15), and normal controls (n = 16). A minimum of 30,000 live lymphocytes was collected for each subject, and cell subsets with at least 100 gated events were included for analysis of proliferation (%CFSElow). *p < 0.05, **p < 0.01.
Phenotype of proliferating NK cells.
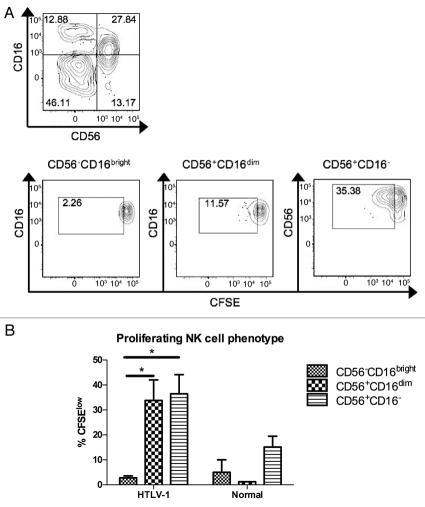
The finding that NK cells underwent increased spontaneous proliferation in HTLV-1 infected subjects was surprising, particularly given a prior report of decreased NK cell frequency in HTLV-1 infection.25 NK cells have been grouped into functional subsets based on expression of CD16 (FcγRIIIA, a low-affinity receptor for the Fc portion of IgG) and CD56 (an adhesion molecule), with CD56dimCD16+ NK cells possessing vigorous cytotoxic activity and CD56brightCD16-/dim NK cells biased toward cytokine secretion (reviewed in refs. 26 and 27). In a subset of subjects, spontaneous proliferation of NK cells was further explored by adding an antibody to CD56 to the spontaneous lymphocyte flow cytometry panel and measuring NK cell phenotype after seven days' culture. Proliferation was measured by CFSE dilution in NK cells, which fell into three populations: CD56-CD16bright, CD56+CD16dim and CD56+CD16− (Fig. 3A). Among HTLV-1 infected subjects spontaneous proliferation of both CD56+ NK cell subsets was statistically significantly higher than for the CD56-subset of NK cells (Fig. 3B). These data demonstrate that NK cells bearing CD56 preferentially proliferate in unstimulated cultures ex vivo, or alternatively that CD56 is upregulated in NK cells undergoing spontaneous proliferation.
Figure 3.
Phenotype of proliferating NK cells after seven days' culture ex vivo. (A) NK cells were first identified as viable CD3− lymphocytes based on FSC/SSC, aqua amine-reactive dye, and anti-CD3 staining. The NK cells were then gated into three subsets based on CD56 and CD16 expression (upper) and CFSE dilution was measured within each subset (lower). (B) For each of the three defined NK cell phenotypes, proliferating cells were measured in HTLV-1 infected and normal control subjects. A minimum of 30,000 live lymphocytes was collected for each subject, and cell subsets with at least 50 gated events were included for analysis of proliferation (%CFSElow). Error bars represent standard error; *p < 0.05.
Dynamics of Tax expression.
Tax mRNA expression was quantified by RT-PCR using three different sets of primers (Table 2). Three different primer sets were utilized to increase precision of detection, as Tax mRNA expression was low in most samples where it was detected. The cycle number at which each positive assay became detectable ranged from 27 to 38, with a median positive cycle number of 33. To maximize the sensitivity of Tax mRNA detection, we developed a “Tax score,” and if a PCR reaction scored positive in either the forward or reverse direction for each set of primers the sample would receive one point, for a total possible score of six points per sample. We examined the correlation between the Tax score and the average cycle number for those reactions that were positive and found a good correlation (Fig. 4A). Because Tax expression can increase following in vitro culture of PBMCs from HTLV-1 infected subjects, we measured whether sensitivity of detection of Tax mRNA expression would be increased by first incubating PBMCs for two or seven days prior to RNA extraction. Incubation of cells prior to RNA extraction resulted in a modest increase in detection of Tax expression at days 2 and 7 vs. day 0 (Fig. 4B and C), and the difference was statistically significantly higher for day 2 vs. day 0 (p < 0.01). Tax was also quantified by staining cells for intracellular Tax protein expression. In spite of clear Tax staining in MT-2 cells, Tax protein detection in PBMCs was inconsistent in our hands, even after in vitro culture (data not shown). We next examined whether or not Tax expression correlated with HTLV-1 proviral load. Tax expression at both days 2 and 7 after incubation correlated positively with proviral load, with the stronger correlation found for Tax mRNA levels at day 7 (Fig. 4D). Based on these observations it is clear that Tax expression was linked to HTLV-1 viral burden, though the relative contribution of each of these to disease manifestations and spontaneous T cell and NK cell proliferation remained to be determined.
Table 2.
Primers for Tax mRNA and HTLV-1 proviral load
| Tax expression | |
| HTLV-1TaxF | 5′-TGT TTG GAG ACT GTG TAC AAG GCG-3′ |
| HTLV-1TaxR | 5′-GTT GTA TGA GTG ATT GGC GGG GTA A-3′ |
| RPX3 | 5′-ATC CCG TGG AGA CTC CTC AA-3′ |
| RTTAXB1 | 5′-AGA GGT TCT CTG GGT GGG GAA G-3′ |
| 3HTLVTAXF | 5′-TGT TTG GAG ACT GTG TAC AAG GCG-3′ |
| 3HTLVTAXR | 5′-GTT GTA TGA GTG ATT GGC GGG GTA A-3′ |
| -actinF | 5′-CAA GAG ATG GCC ACG GCT GCT-3′ |
| -actinR | 5′-TCC TTC TGC ATC CTG TCG GCA-3′ |
| Proviral load | |
| HTV-F5 | 5′-CGG ATA CCC IGT CTA CGT GTT T-3′ |
| HTV-R4 | 5′-CTG AGC IGA IAA CGC GTC CA-3′ |
| DQ- GH26 | 5′-GTG CTC CAG GTG TAA ACT TGT ACC AG-3′ |
| DQ- GH27 | 5′-CAC GGA TCC GGT AGC AGC GGT AGA GTT G-3′ |
Figure 4.
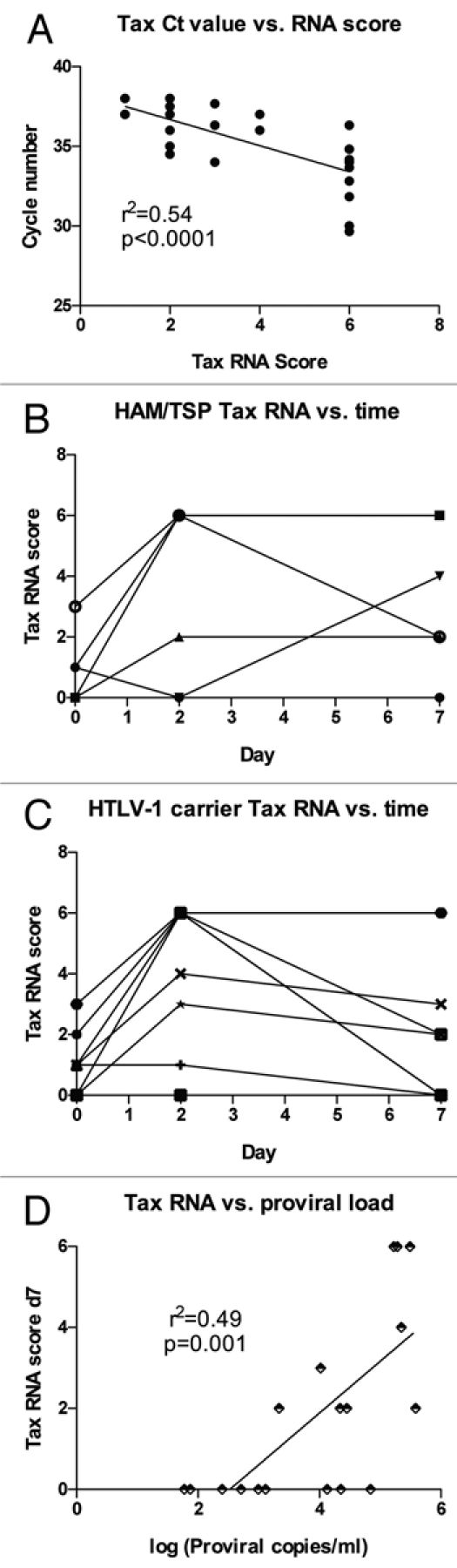
Dynamics of Tax mRNA expression and relationship to HTLV-1 proviral load. (A) RNA was extracted from PBMC aliquots and was tested by RT-PCR using three sets of forward and reverse primers specific for Tax (see Table 2). A Tax score was calculated, with each positive primer set increasing the score by one (for a maximum value of six). The average cycle number for positive reactions was correlated with the calculated Tax score. (B and C) RNA was harvested from cells ex vivo or after two or seven days' in vitro culture, and results are shown for (B) HAM/TSP subjects (n = 5) or (C) HTLV-1 carriers (n = 13). (D) HTLV-1 proviral load was determined in ex vivo PBMCs (n = 13) and paired with Tax scores from PBMCs after 7 days' in vitro culture, showing a positive linear correlation.
Influence of Tax expression and HTLV-1 proviral load on NK cell proliferation.
Tax expression has been linked to activation of HTLV-1-specific CD8+ T cells and adverse consequences of infection such as HAM/TSP.9 Both Tax expression at day 7 and HTLV-1 proviral load appeared higher in HAM/ TSP subjects compared to those with asymptomatic HTLV-1 infection (Fig. 5A and C), though the differences only reached statistical significance for Tax expression (p < 0.05 and p = 0.07, respectively). It should be noted that the HTLV-1 carriers were selected for relatively high proviral load to increase the proportion of subjects with Tax expression, which decreased the power to detect a significant effect of proviral load on disease outcome. Interestingly, there was no correlation between disease outcome and Tax expression at days 0 or 2 (data not shown), implying that cells with the ability to sustainably upregulate Tax expression are associated with HAM/TSP.
Figure 5.
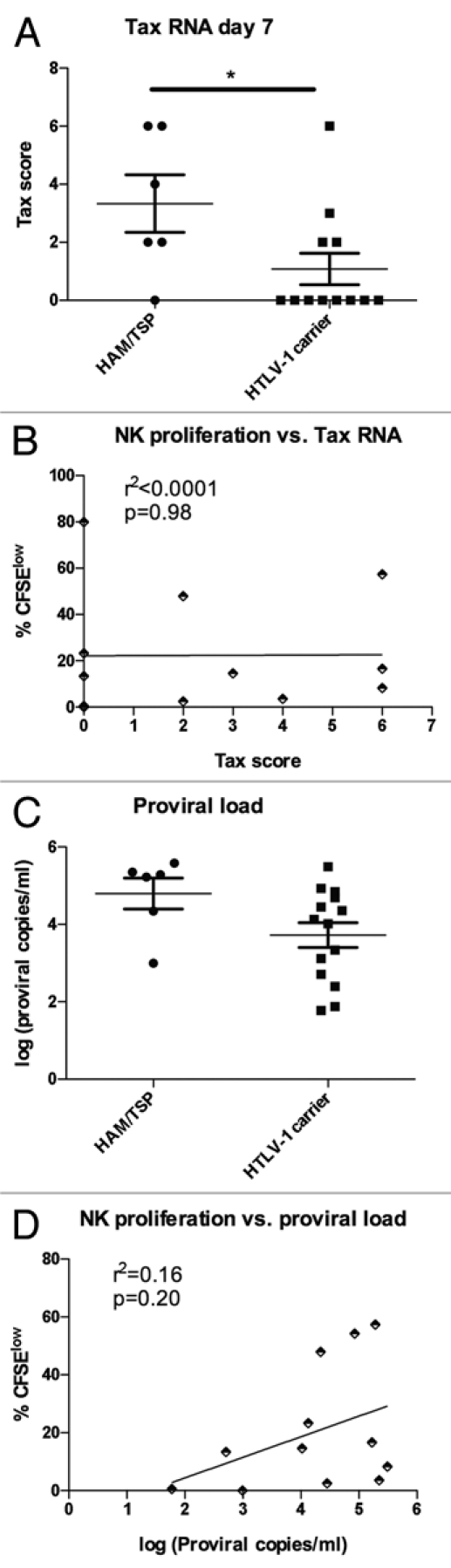
Relationship between Tax mRNA level, proviral load and spontaneous NK cell proliferation. (A) The Tax score to quantify Tax mRNA expression was calculated as in Figure 4 and compared in subjects with HAM/TSP (n = 6) and HTLV-1 carriers (n = 12), with HAM/TSP subjects showing higher Tax scores from PBMCs after 7 days' in vitro culture. (B) proliferation of CD8+ T cells at day 7 (%CFSElow) was compared to Tax expression at day 7 in HTLV-1 infected subjects (n = 13) and analyzed by linear regression. (C) proviral load was measured in ex vivo PBMCs in subjects with HAM/TSP (n = 6) and HTLV-1 carriers (n = 15). (D) Proliferation of CD8+ T cells at day 7 (%CFSElow) was compared to HTLV-1 proviral load ex vivo in HTLV-1 infected subjects (n = 15) and analyzed by linear regression.
While Tax expression and HTLV-1 proviral load are strongly correlated, it is possible that one or the other has a larger influence on spontaneous NK cell proliferation. Given the role of Tax in cell cycle activation,28,29 we hypothesized that Tax expression would correlate more strongly with NK cell proliferation. In fact, we found no evidence that Tax expression influenced spontaneous proliferation, while HTLV-1 proviral load showed a weak effect on spontaneous NK cell proliferation (Fig. 5B and D). The same trends were seen for CD4+ and CD8+ T cells (data not shown). These data show that while Tax expression and the level of HTLV-1 proviral load are strongly correlated, the former is more closely tied to disease manifestation while the latter is associated with spontaneous NK cell proliferation.
Discussion
HTLV-1 causes subtle changes in immune cell function, including spontaneous lymphocyte proliferation, in a large percentage of infected subjects. We sought to understand which subsets of lymphocytes spontaneously proliferate in HTLV-1 infection, the factors associated with proliferation, and how these related to serious disease outcome (HAM/TSP). Surprisingly, NK cells showed levels of spontaneous proliferation that were comparable to CD8+ T cells in HTLV-1 infected subjects, which has implications for understanding the mechanism of spontaneous lymphocyte induction. Tax mRNA expression and HTLV-1 proviral load were correlated, and Tax mRNA expression was associated with the presence of HAM/TSP, consistent with a prior study.9 We further demonstrated that HTLV-1 primarily drove expansion of CD56+ NK cells.
The most intriguing finding of the current study was that NK cells from HTLV-1 infected subjects undergo clear spontaneous proliferation. NK cell frequencies have been reported as low in HAM/TSP subjects, though the differences were primarily driven by CD3+CD16+ cells,25 more consistent with decreased natural killer-like T cells than NK cells.30 A small study of in vivo proliferation of NK cells measured using deuterium-enriched glucose revealed that HTLV-1 infected subjects did not have a significantly different rate of NK cell proliferation from normal control donors.31 In HTLV-2 infection subjects who exhibit spontaneous lymphocyte proliferation have decreased proportions of NK cells and a trend to decreased absolute NK cell numbers.32 It seems unlikely that the mechanism of NK cell stimulation is through direct infection by HTLV-1, as DNA is not detected in NK cells by PCR ex vivo.33 However, it has been shown that NK cells can be infected with HTLV-1 if first activated with antiCD16 antibody, and infected NK cells proliferate more vigorously and survive longer in vitro, though they are not immortalized.34 Our findings of vigorous in vitro spontaneous proliferation of NK cells suggests that re-examination of NK cell frequencies in HTLV-1 infection may be warranted. It is possible that CD8+ T cell proliferation would predominate in HTLV-1 infected subjects, filling the immunological niche and preventing NK cell expansion in vivo.
A number of etiologies have been proposed as driving spontaneous lymphocyte proliferation of T cells. Tax protein has been linked to transformation of HTLV-1 infected T cells in adult T cell leukemia cells,35 which likely involves a different set of processes from spontaneous proliferation. Tax protein expression has been shown to correlate with proliferation of CD8+ T cells at day 13 but not at earlier time points in one study,29 consistent with this study and prior work that showed no correlation between Tax expression and spontaneous proliferation up to day 7 of in vitro culture.9 Furthermore, neutralization of Tax protein with a monoclonal antibody does not affect spontaneous lymphocyte proliferation, and exposure of PBMCs to HTLV-1 infected cells that have been irradiated or fixed will induce spontaneous proliferation in a cell contact dependent fashion.36,37 It appears that HTLV-1 infected T cells present antigens and costimulatory molecules on their cell surface that interact with CD8+ T cells to induce proliferation. Studies using blocking antibodies have shown that important pathways for spontaneous lymphocyte proliferation include IL-2 and IL-2 receptor,37,38 costimulatory molecules CD80 and CD86,39 adhesion molecules CD2/LFA-3 and possibly LFA-1/ICAM-1,36,37 and the class I HLA molecule.40 HTLV-1 infected B cells are not sufficient to stimulate lymphocyte proliferation,41 and the presence of adherent antigen presenting cells enhances proliferation.42 More recent work has implicated HTLV-1 basic leucine zipper factor (HBZ) in promotion of proliferation of transformed T cells43 and in pathogenesis of adult T cell leukemia and HAM/TSP.44 Whether or not these pathways would be sufficient to activate NK cells is unknown and requires further investigation.
As has been demonstrated previously, we found that CD8+ T cells proliferated spontaneously to higher levels than CD4+ T cells,21 and we additionally showed that NK cells underwent brisk spontaneous proliferation in HTLV-1 infected subjects. This implies that there may be a common mechanism causing proliferation of both CD8+ T cells and NK cells. Mechanisms that might drive spontaneous proliferation of both NK and CD8+ T cells include paracrine IL-2 driven expansion and antigen-specific expansion. Supporting a role for IL-2, only the CD56+ subset of NK cells expanded in vitro in our study of spontaneous proliferation, and CD56+ NK cells express the IL-2 receptor while CD56 NK cells do not.45,46 HTLV-1-specific expansion of NK cells could also play a role in both CD8+ T cell and NK cell expansion, as NK cells have recently been demonstrated to preferentially expand in response to a mouse cytomegalovirus antigen presented by a major histocompatibility complex class I protein.47 While HTLV-1 infected cells do not appear to express NK cell activating ligands NCR and NKG2D, they do express the co-activating receptors NTB-A and 2B4,48 which may partially explain NK cell stimulation by infected cells.
In summary, we have shown that NK cells can undergo significant spontaneous proliferation in HLTV-1 infected subjects, broadening this process to more than a T cell-based phenomenon. This finding raises a number of new research questions about the immune modulation caused by HTLV-1 infection. It is not clear if other PBMC subsets such as dendritic cells or monocytes will also undergo spontaneous proliferation. Understanding which cells are stimulated by HTLV-1 will help define the mechanism of stimulation. Another area of new research will be to identify whether the same activation pathways operate to stimulate NK cells and CD8+ T cells, or whether they respond to distinct stimuli. Understanding how HTLV-1 interacts with the human immune system will potentially yield new prognostic parameters for those in the chronic, stable phase of infection and will allow development of new immunotherapeutic interventions to disrupt development of complications of HTLV-1 infection.
Material and Methods
Study subjects.
The study subjects were selected from a cohort of 151 HTLV-1 infected subjects enrolled in the HTLV Outcome Study (HOST). Study subjects were followed longitudinally with study visits once every two years, at which point questionnaires detailing disease related symptoms were administered and blood was drawn with cryopreservation of PBMCs and plasma. Subjects with neurological disease (HAM/TSP) were selected for the current study and HTLV-1 carriers were chosen from the remaining cohort based on sample availability and the presence of relatively high proviral load, in an effort to maximize the number of subjects with detectable Tax production (Table 1). All samples were collectedafter written informed consent under protocols approved by the University of California, San Francisco Committee on Human Research.
Sample preparation.
Samples were stored in LN2 vapor prior to testing. Frozen PBMCs were thawed into and washed once in RPMI-1640 (Gibco) supplemented with 20% heat inactivated FBS (Hyclone). Cells were then resuspended in RPMI-1640 supplemented with 10% heat inactivated human serum (Sigma). Cells were aliquoted into individual Falcon 2058 culture tubes (BD Biosciences) containing l ml with 1 × 106 cells each.
Spontaneous lymphocyte proliferation.
Samples were divided into four Falcon 2058 tubes, two used for surface staining and IgG2a isotype control for intracellular staining (tube 1) and two for all test antibodies with anti-Tax antibody added for intracellular staining (tube 2). Two of these tubes were for testing on day two with the remaining two tubes for testing on day seven. These four tubes to assess spontaneous lymphocyte proliferation along with one extra tube of normal control PBMC to be used as a compensation control were stained with 5 µg/ml CFSE (Sigma). Normal donor cells from a single donor in each test batch were thawed to prepare cells for unstained and single color compensation controls at the same time. All test sample and compensation control tubes were incubated in a slant rack for two or seven days at 37°C with 5% CO2. For a subset of samples a third tube was added to the panel for testing on day seven, which included an additional marker for NK cells (CD56) and CD11a. Anti-Tax antibody was not included in the third tube.
Flow cytometry antibodies and staining.
Anti-Tax antibody was produced and purified from a Balb/c mouse splenocyte/P3X Ag8.453 hybridoma (AIDS Research and Reference Reagent Program, contributed by Beatrice Langton). The antibody is IgG2a isotype and was used unconjugated. After two or seven days in culture, the cells were washed twice in PBS and resuspended in 100 µl PBS for immunofluorescent staining. Cells were first stained with LIVE/DEAD® Fixable Dead Cell Aqua Stain (Molecular Probes) at a final dilution of 1:1,000 for 10 minutes followed by a surface cocktail of antibodies for lymphocyte subsets for an additional 20 minutes at room temperature protected from light. The surface cocktail included fluorochrome conjugated antibodies to CD3-Pacific Blue, CD4-Alexafluor700, CD8-APC, CD16-PE-Cy5, CD19-PE-Cy7 (all from BD Biosciences, Pharmingen) which were used at an appropriately titered concentration. The surface cocktail for NK cell studies included CD3-Pacific Blue, CD16-PE-Cy5, CD56-PE-Cy7 and CD11a-APC (all from BD Biosciences). Surface stained cells were fixed and permeabilized with the Fix & Perm® Cell Permeabilization Reagent Kit (Caltag Laboratories) following the manufacturer's recommended protocol but with the addition of 7% goat serum (Sigma) to the PBS wash buffer. Fixed cells were resuspended in 100 µl of permeabilization solution B with 7% goat serum to which 10 µl FcR Block Reagent (Miltenyi Biotech) was added and incubated for 10 minutes at room temperature protected from light. Appropriately titered anti-Tax antibody was added to one tube of each sample set and unconjugated IgG2a isotype (BD Bioscience, Pharmingen) to the other tube of each set and incubated for an additional 20 minutes at room temperature protected from light. All cells were washed twice with PBS containing 7% goat serum. After the last wash, the cell pellets were resuspended in 100 µl permeabilization solution B with 7% goat serum prior to addition of PE-labeled goat anti-mouse IgG2a (Southern Biotech) second step reagent. Cells were incubated for 20 minutes at room temperature protected from light followed by two washes in PBS with 7% goat serum. Cell pellets were resuspended in 1% paraformaldehyde in PBS and incubated for at least 30 minutes at 4°C prior to FACS analysis. MT-2 cells were used as a positive Tax protein control and H9 cells were used as a Tax protein negative control. MT-2 and H9 cells were stained and processed the same as test samples except the surface cocktail only contained CD3-Pacific Blue and CD4-Alexafluor700.
FACS analysis.
All samples were acquired on a calibrated LSR II flow cytometer (BD Biosciences) using DiVa v5.0.2 software (BD Biosciences). The FCS 3.0 data files were analyzed with Flowjo v8.7.2 software (Tree Star). For spontaneous proliferation, the percent of each lymphocyte subset that proliferated was averaged from tubes 1 and 2. The percent non-specific staining obtained with the IgG2a isotype control was subtracted from the Tax-stained samples. At least 30,000 gated viable lymphocytes were collected for each sample.
Tax mRNA quantification.
RNA was extracted from PBMCs using the QIAamp RNA Blood Mini Kit (Qiagen) per manufacturer's instructions. DNase was added to the extracted RNA and incubated for 30 min at 37°C and then for 10 min at 100°C to inactivate the DNase. The RNA was reverse transcribed into cDNA using three downstream primers in separate reactions (Table 2). The samples were also reverse transcribed using a primer for β-actin, a housekeeping gene. For each sample 5 µl of cDNA was added to the PCR mixture with the appropriate primer pair. Real-time PCR was performed using a GeneAmp 5700 machine (Applied Biosystems) under the following cycle conditions: 10 min at 95°C followed by 45 cycles of: 30 sec at 95°C, 30 sec at 64°C and 45 sec at 72°C. Reactions were performed in duplicate.
HTLV-1 proviral load detection.
Quantitation of proviral DNA of HTLV-1 was performed using real-time PCR. PBMCs were digested in a PCR solution with proteinase K. To quantitate the cellular input for each reaction, HLA DQ-α copy number was measured separately. Primers were from highly conserved sequences of the HTLV-1 viral tax region designated as HTV-F5 (Table 2). For each sample 25 µl of DNA lysate was added to the PCR mixture (5 µl for the DQ-α reactions). Real-time PCR was performed as described above and reactions were performed in triplicate. Fluorescence intensity of Syber green incorporated into the amplified product was measured at every PCR cycle, and the cycle number at which fluorescence passed the baseline threshold was recorded for each replicate. For each run, a standard curve was generated from 1:10 serial dilutions of the MT-2 cell line (HTLV-1 infected, obtained from American Type Culture Collection, ATCC), with a range of 10−1 to 104 copies per reaction. Mean HTLV-1 copy numbers for MT-2 was 2.1 HTLV-1 copies per cell, as determined previously.22 The number of copies in the test sample was then calculated by interpolation of the experimentally determined threshold cycle number onto the control standard regression curves. The mean value of three replicates was calculated. To determine the viral load of each sample, the number of copies of HTLV-1 was divided by the DQ-α copy number. The lower limit of detection for the assay is 1 copy per 105 cells. Reproducibility of the assay was measured by testing 40 specimens in duplicate on two replicate assays (range 1 to 1,000 copies/ml). Interassay variability was approximately two-fold between plates, with r2 = 0.97 by linear regression.23
Statistical analyses.
Statistical comparisons were performed using Prism 5 software (GraphPad Software, Inc.). Comparisons made between two groups of individuals (e.g., proviral load for HAM/TSP subjects vs. HTLV-1 carriers) were made using an unpaired t test. For comparisons among multiple groups, one way ANOVA was performed with a Tukey post-test comparison to determine significance of differences among variables. For data that were not normally distributed the one way ANOVA was performed using the Kruskall-Wallis test with a Dunn's post-test comparison. Correlation of two potentially related parameters (e.g., spontaneous proliferation and proviral load) was analyzed by linear regression. Differences were considered significant for p < 0.05.
Acknowledgements
This research was supported by the National Institutes of Health, National Heart, Lung and Blood Institute, grant HL-062235.
P.J.N. designed research, analyzed and interpreted data, and wrote the manuscript; D.H.F. performed research and analyzed and interpreted data; D.D. collected data; T.H.L. designed research and analyzed data; E.M. designed research and wrote the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/9868
References
- 1.Murphy E, Biswas H. Human T-cell Lymphotropic Viruses Types I and II. Chapter 168. In: Mandelll G, Bennet J, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone; 2009. In press. [Google Scholar]
- 2.Wattel E, Vartanian JP, Pannetier C, Wain-Hobson S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol. 1995;69:2863–2868. doi: 10.1128/jvi.69.5.2863-2868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajima K, Kuroishi T. Estimation of rate of incidence of ATL among ATLV (HTLV-I) carriers in Kyushu, Japan. Jpn J Clin Oncol. 1985;15:423–430. [PubMed] [Google Scholar]
- 4.Murphy EL, Hanchard B, Figueroa JP, et al. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int J Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 5.Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 6.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 7.Orland JR, Engstrom J, Fridey J, et al. Prevalence and clinical features of HTLV neurologic disease in the HTLV Outcomes Study. Neurology. 2003;61:1588–1594. doi: 10.1212/01.wnl.0000096011.92542.da. [DOI] [PubMed] [Google Scholar]
- 8.Nagai M, Usuku K, Matsumoto W, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 9.Yamano Y, Nagai M, Brennan M, et al. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP) Blood. 2002;99:88–94. doi: 10.1182/blood.v99.1.88. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto M, Nakashima H, Matsumoto M, Uyama E, Ando M, Araki S. Pulmonary involvement in patients with HTLV-I-associated myelopathy: increased soluble IL-2 receptors in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1989;139:1329–1335. doi: 10.1164/ajrccm/139.6.1329. [DOI] [PubMed] [Google Scholar]
- 11.Yamazato Y, Miyazato A, Kawakami K, Yara S, Kaneshima H, Saito A. High expression of p40(tax) and pro-inflammatory cytokines and chemokines in the lungs of human T-lymphotropic virus type 1-related bronchopulmonary disorders. Chest. 2003;124:2283–2292. doi: 10.1378/chest.124.6.2283. [DOI] [PubMed] [Google Scholar]
- 12.Murphy EL, Glynn SA, Fridey J, et al. Increased prevalence of infectious diseases and other adverse outcomes in human T lymphotropic virus types I- and II-infected blood donors. Retrovirus Epidemiology Donor Study (REDS) Study Group. J Infect Dis 1997;176:1468–1475. doi: 10.1086/514143. [DOI] [PubMed] [Google Scholar]
- 13.Marsh BJ. Infectious complications of human T cell leukemia/lymphoma virus type I infection. Clin Infect Dis. 1996;23:138–145. doi: 10.1093/clinids/23.1.138. [DOI] [PubMed] [Google Scholar]
- 14.Matsuyama W, Mizoguchi A, Iwami F, et al. Clinical investigation of pulmonary Mycobacterium avium complex infection in human T lymphotrophic virus type I carriers. Thorax. 2000;55:388–392. doi: 10.1136/thorax.55.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy EL, Glynn SA, Fridey J, et al. Increased incidence of infectious diseases during prospective follow-up of human T-lymphotropic virus type II- and I-infected blood donors. Retrovirus Epidemiology Donor Study. Arch Intern Med. 1999;159:1485–1491. doi: 10.1001/archinte.159.13.1485. [DOI] [PubMed] [Google Scholar]
- 16.Murphy EL, Wang B, Sacher RA, et al. Respiratory and urinary tract infections, arthritis and asthma associated with HTLV-I and HTLV-II infection. Emerg Infect Dis. 2004;10:109–116. doi: 10.3201/eid1001.020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh U, Jacobson S. Treatment of HTLV-I-associated myelopathy/tropical spastic paraparesis: toward rational targeted therapy. Neurol Clin. 2008;26:781–797. doi: 10.1016/j.ncl.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Usuku K, Sonoda S, Osame M, et al. HLA haplotypelinked high immune responsiveness against HTLV-I in HTLV-I-associated myelopathy: comparison with adult T-cell leukemia/lymphoma. Ann Neurol. 1988;23:143–150. doi: 10.1002/ana.410230733. [DOI] [PubMed] [Google Scholar]
- 19.Popovic M, Flomenberg N, Volkman DJ, et al. Alteration of T-cell functions by infection with HTLV-I or HTLV-II. Science. 1984;226:459–462. doi: 10.1126/science.6093248. [DOI] [PubMed] [Google Scholar]
- 20.Prince HE, Lee H, Jensen ER, et al. Immunologic correlates of spontaneous lymphocyte proliferation in human T-lymphotropic virus infection. Blood. 1991;78:169–174. [PubMed] [Google Scholar]
- 21.Sakai JA, Nagai M, Brennan MB, Mora CA, Jacobson S. In vitro spontaneous lymphoproliferation in patients with human T-cell lymphotropic virus type I-associated neurologic disease: predominant expansion of CD8+ T cells. Blood. 2001;98:1506–1511. doi: 10.1182/blood.v98.5.1506. [DOI] [PubMed] [Google Scholar]
- 22.Albrecht B, Collins ND, Newbound GC, Ratner L, Lairmore MD. Quantification of human T-cell lymphotropic virus type 1 proviral load by quantitative competitive polymerase chain reaction. J Virol Methods. 1998;75:123–140. doi: 10.1016/s0166-0934(98)00087-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee TH, Chafets DM, Busch MP, Murphy EL. Quantitation of HTLV-I and II proviral load using real-time quantitative PCR with SYBR Green chemistry. J Clin Virol. 2004;31:275–282. doi: 10.1016/j.jcv.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Barchet W, Oehen S, Klenerman P, et al. Direct quantitation of rapid elimination of viral antigen-positive lymphocytes by antiviral CD8(+) T cells in vivo. European Journal of Immunology. 2000;30:1356–1363. doi: 10.1002/(SICI)1521-4141(200005)30:5<1356::AID-IMMU1356>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Yu F, Itoyama Y, Fujihara K, Goto I. Natural killer (NK) cells in HTLV-I-associated myelopathy/tropical spastic paraparesis-decrease in NK cell subset populations and activity in HTLV-I seropositive individuals. J Neuroimmunol. 1991;33:121–128. doi: 10.1016/0165-5728(91)90056-d. [DOI] [PubMed] [Google Scholar]
- 26.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 27.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ressler S, Morris GF, Marriott SJ. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J Virol. 1997;71:1181–1190. doi: 10.1128/jvi.71.2.1181-1190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sibon D, Gabet AS, Zandecki M, et al. HTLV-1 propels untransformed CD4 lymphocytes into the cell cycle while protecting CD8 cells from death. J Clin Invest. 2006;116:974–983. doi: 10.1172/JCI27198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanier LL, Kipps TJ, Phillips JH. Functional properties of a unique subset of cytotoxic CD3+ T lymphocytes that express Fc receptors for IgG (CD16/Leu-11 antigen) J Exp Med. 1985;162:2089–2106. doi: 10.1084/jem.162.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Wallace DL, de Lara CM, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121:258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prince HE, Jensen ER, York J. Lymphocyte subsets in HTLV-II-infected former blood donors: relationship to spontaneous lymphocyte proliferation. Clin Immunol Immunopathol. 1992;65:201–206. doi: 10.1016/0090-1229(92)90147-g. [DOI] [PubMed] [Google Scholar]
- 33.Richardson JH, Edwards AJ, Cruickshank JK, Rudge P, Dalgleish AG. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. 1990;64:5682–5687. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo KM, Vivier E, Rochet N, et al. Infection of human natural killer (NK) cells with replication-defective human T cell leukemia virus type I provirus. Increased proliferative capacity and prolonged survival of functionally competent NK cells. J Immunol. 1992;149:4101–4108. [PubMed] [Google Scholar]
- 35.Murata K, Yamada Y. The state of the art in the pathogenesis of ATL and new potential targets associated with HTLV-1 and ATL. Int Rev Immunol. 2007;26:249–268. doi: 10.1080/08830180701709817. [DOI] [PubMed] [Google Scholar]
- 36.Kimata JT, Palker TJ, Ratner L. The mitogenic activity of human T-cell leukemia virus type I is T-cell associated and requires the CD2/LFA-3 activation pathway. J Virol. 1993;67:3134–3141. doi: 10.1128/jvi.67.6.3134-3141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wucherpfennig KW, Hollsberg P, Richardson JH, Benjamin D, Hafler DA. T-cell activation by autologous human T-cell leukemia virus type I-infected T-cell clones. Proc Natl Acad Sci USA. 1992;89:2110–2114. doi: 10.1073/pnas.89.6.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tendler CL, Greenberg SJ, Blattner WA, et al. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: pathogenic implications and a rationale for immunotherapy. Proc Natl Acad Sci USA. 1990;87:5218–5222. doi: 10.1073/pnas.87.13.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lal RB, Rudolph DL, Dezzutti CS, Linsley PS, Prince HE. Costimulatory effects of T cell proliferation during infection with human T lymphotropic virus types I and II are mediated through CD80 and CD86 ligands. J Immunol. 1996;157:1288–1296. [PubMed] [Google Scholar]
- 40.Mann DL, Martin P, Hamlin-Green G, Nalewaik R, Blattner W. Virus production and spontaneous cell proliferation in HTLV-I-infected lymphocytes. Clin Immunol Immunopathol. 1994;72:312–320. doi: 10.1006/clin.1994.1147. [DOI] [PubMed] [Google Scholar]
- 41.Martin TC, Southern PJ. Infection and cellular activation by human T-cell leukemia viruses, types I and II. Virology. 1996;221:375–381. doi: 10.1006/viro.1996.0389. [DOI] [PubMed] [Google Scholar]
- 42.Prince HE, York J, Golding J, Owen SM, Lal RB. Spontaneous lymphocyte proliferation in human T-cell lymphotropic virus type I (HTLV-I) and HTLV-II infection: T-cell subset responses and their relationships to the presence of provirus and viral antigen production. Clin Diagn Lab Immunol. 1994;1:273–282. doi: 10.1128/cdli.1.3.273-282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnold J, Zimmerman B, Li M, Lairmore MD, Green PL. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T-lymphocyte proliferation. Blood. 2008;112:3788–3797. doi: 10.1182/blood-2008-04-154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito M, Matsuzaki T, Satou Y, et al. In vivo expression of the HBZ gene of HTLV-1 correlates with proviral load, inflammatory markers and disease severity in HTLV-1 associated myelopathy/tropical spastic para-paresis (HAM/TSP) Retrovirology. 2009;6:19. doi: 10.1186/1742-4690-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990;171:1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagler A, Lanier LL, Phillips JH. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med. 1990;171:1527–1533. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kielczewska A, Pyzik M, Sun T, et al. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J Exp Med. 2009;206:515–523. doi: 10.1084/jem.20080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banerjee P, Feuer G, Barker E. Human T-cell leukemia virus type 1 (HTLV-1) p12I down-modulates ICAM-1 and -2 and reduces adherence of natural killer cells, thereby protecting HTLV-1-infected primary CD4+ T cells from autologous natural killer cell-mediated cytotoxicity despite the reduction of major histocompatibility complex class I molecules on infected cells. J Virol. 2007;81:9707–9717. doi: 10.1128/JVI.00887-07. [DOI] [PMC free article] [PubMed] [Google Scholar]