Abstract
Across individuals, risk for poor health varies inversely with socioeconomic position (SEP). The pathways by which SEP affects health have been viewed from many epidemiological perspectives. Central to these perspectives is the notion that socioeconomic health disparities arise from an interplay between nested, recursive, and cumulative environmental, social, familial, psychological, behavioral, and physiological processes that unfold over the life span. Epidemiological perspectives on socioeconomic health disparities, however, have not yet formally integrated emerging findings from neuropharmacological, molecular genetic, and neuroimaging studies demonstrating that indicators of SEP relate to patterns of brain neurotransmission, brain morphology, and brain functionality implicated in the etiology of chronic medical conditions and psychological disorders. Here, we survey these emerging findings and consider how future neurobiological studies in this area can enhance our understanding of the pathways by which different dimensions of SEP become embodied by the brain to influence health throughout life.
Keywords: brain-body medicine, health neuroscience, lifecourse neuroepidemiology, neuroimaging, neurogenetics, social health disparities
INTRODUCTION
Privilege, power, prestige. How does the brain come to represent these and other dimensions of socioeconomic position (SEP)? When do these brain representations emerge in life? How do they change with age, and how do they become embodied by the cumulative life experiences from which they are formed? Why would these representations differ so appreciably when we reference them to ourselves, to our family and friends, and to others in the social and economic hierarchies in which we stand? How do these representations come to affect meaningful social, cognitive, and emotional facets of our lives? More importantly, could these representations, in part, beget socially stratified patterns of behavior and biology that undermine equities in physical health, psychological well-being, and even longevity across individuals? If so, would this inform the design of brain-based preventative strategies, interventions, or social policies aimed at reducing the human cost of chronic medical conditions and psychological disorders that track a socioeconomic gradient? These questions are neither new nor exhaustive, but they remain open and pressing, as developing nations and those with economies of scale confront ever-increasing challenges in allocating limited resources to the public and widening socioeconomic health disparities that are arguably unjust (1).
With these broad questions in mind, this survey highlights recent findings from neuropharmacological, molecular genetic, and neuroimaging studies that hold the potential to enhance our understanding of how the brain links socioeconomic factors to health throughout life. Contextually, this survey complements theoretical perspectives advocating the integration of neurobiological and molecular research approaches to epidemiological studies of socioeconomic health disparities (2). This survey also builds on a renewed interest in applying brain-body medicine and health neuroscience approaches to understand how environmental, social, psychological, behavioral, and biological factors interact to affect human health and disease (3,4).
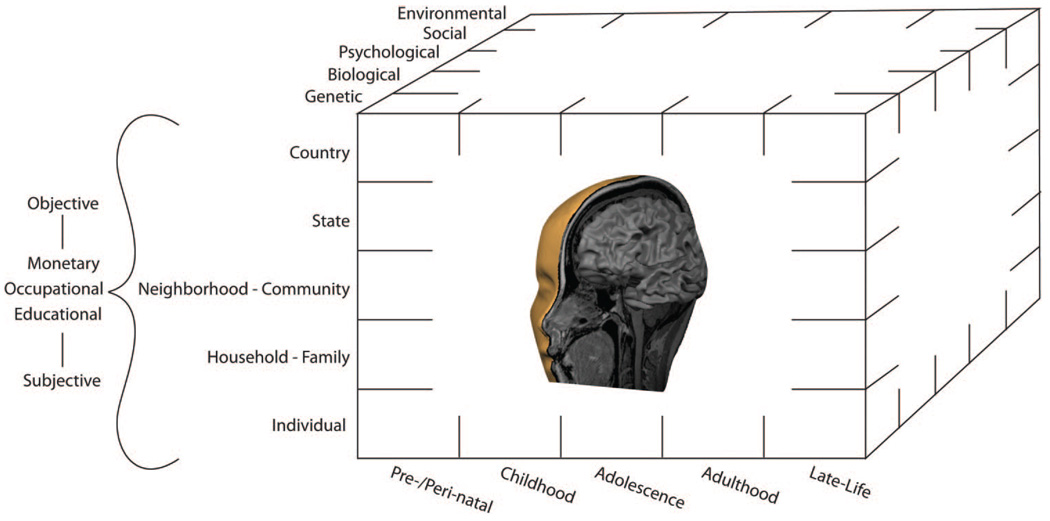
Before our empirical survey, we emphasize the established necessity of conceptualizing SEP as a multidimensional and multilevel construct (Fig. 1). In doing so, we underscore the longstanding argument that indicators of SEP can relate to diverse health outcomes by different, but often, interacting pathways (5–7). Next, we review findings from the few studies that have begun to identify specific neurobiological pathways that might plausibly link dimensions of SEP to physical and mental health outcomes. More precisely, these findings link indicators of SEP to the functionality of monoamine neurotransmitter systems and to the activity and morphology of brain circuitries that are instrumental for i) peripheral physiological regulation; ii) emotion, mood, and stress processes; and iii) behavioral attributes implicated in risk for health conditions for which there are established socioeconomic disparities at the population level. In view of this concentrated survey, we defer to other reviews for treatments of neurobiological studies of socioeconomic factors and cognitive functioning in humans (8) and social hierarchies and health in nonhuman animals (9,10). We close by considering open lines of inquiry on how socioeconomic factors may become embodied by the brain to influence health and longevity.
Figure 1.
Conceptual schematic illustrating the multidimensional and multilevel aspects of socioeconomic position (SEP). By convention, objective and subjective indicators of monetary, occupational, educational, and other dimensions of SEP discussed in the present article can be measured at the individual and higher levels of social organization. In the context of epidemiological research, these SEP indicators can be linked to disparities in i) health damaging behaviors adopted by an individual, an individual’s family, or an individual’s proximal social contacts; ii) putative biomediators of disease risk; iii) risk factors for psychological disorders; iv) risk factors for often comorbid chronic medical conditions; and v) markers of pathophysiology and preclinical conditions that are predictive of disease end points. The interacting mechanisms by which SEP affects health disparities are thought to encompass those spanning from genetic to environmental levels of analysis. The present survey emphasizes the role of stress, emotion, and mood circuitries and modulatory neurotransmitter systems of the brain as candidate neurobiological pathways that may embody socioeconomic factors and link genetic to environmental mechanisms to health disparities.
Neurobiological Pathways Linking SEP and Health: Multidimensional, Multilevel, and Life Span Considerations
As reviewed elsewhere (5,10,11), there are widely documented socioeconomic health disparities that are not entirely explained by absolute thresholds of income, education, and occupational status, or the restricted availability of health care among those of lower SEP. Rather, the graded association between indicators of relative SEP and aspects of health, as well as longevity, seems to be attributable to manifold processes, particularly those by which disfavored life circumstances and opportunities engendered by holding a comparatively lower SEP than others affect the regulation of central and peripheral nervous system functions that are important for emotional experience and expression, for mood regulation, for cognition, for social information processing, and for adaptively coping with life stressors at the levels of behavior and physiology (5,11–14). SEP is a multidimensional and multilevel construct that is traditionally measured by different indicator variables. Furthermore, different SEP indicator variables can be referenced to individuals, families, communities, counties, electoral wards, provinces, and even higher levels of social organization (e.g., entire countries) (5–7). Historically, indicators of SEP have been labeled as “objective” or “subjective” (15). Objective SEP indicators often assess aspects of educational attainment, employment grade, income, accumulated wealth, and asset ownership. Subjective SEP is most commonly measured by variants of a single-item, self-anchoring scale presented to respondents as a visual “ladder” of ordered rungs of socioeconomic ranking—rungs that are often also referenced to aspects of education, income, and employment. This ladder, termed the MacArthur Scale of Subjective Social Status, is considered to assess a “cognitive averaging” of multiple dimensions of SEP, many of which may not be adequately assessed by any single indicator of objective SEP (16). These additional dimensions include an individual’s valuation of i) current, prior, and anticipated financial security; ii) qualitative dimensions of educational and occupational histories; iii) comparative standards of living and housing; and iv) possibly social prestige or influence (17,18). It is noteworthy that the extent to which subjective and objective indicators of SEP correlate with one another may vary appreciably among individuals, ethnic populations, cultures, and countries (19). Moreover, our understanding of the differences and commonalities between objective and subjective SEP indicators continues to evolve—particularly with respect to how these types of SEP indicators may relate to neurobiological measures assessed in the context of socioeconomic health disparities.
As illustrated in Figure 1, health disparities that track a socioeconomic gradient can be measured from early to later periods of life, and the pathways that proximally link dimensions of SEP to observable health disparities over the lifecourse extend from genetic to environmental levels of analysis. The conceptual argument adopted here and elsewhere (2,7) is that the expression of socioeconomic health disparities depends on the embodiment by the brain of socially stratified biological, psychological, social, and environmental factors linked to health and mortality across individuals, particularly in interaction with predisposing genetic risk and epigenetic plasticity (20). We note that the concept of embodiment has been defined similarly from both epidemiological and cognitive neuroscience frameworks. From an ecosocial perspective of disease distribution, for example, Krieger (21) defined embodiment as “… a concept referring to how we literally incorporate, biologically, the material and social world in which we live, from in utero to death” (p. 352). Similarly, from a cognitive neuroscience perspective, Marshall (22) defined embodiment as an organizing concept that “… places the mind within the body and brain of an active organism which is deeply embedded in the world” (p. 113). Considering these converging definitional frameworks, embodiment can be viewed as a core concept or supposition for neurobiological studies of socioeconomic health disparities, insofar as most health outcomes of interest in such studies are the distal end points of patterns of behavior and peripheral physiology that are orchestrated by the brain, which itself is the target of environmental and social influences throughout life (23). A corollary of this supposition is that the brain’s embodiment of socioeconomic factors can be quantified by measurement methods in human neuroscience, including those of neuropharmacology, molecular genetics, and neuroimaging. In addition to others, major measures from these fields include quantitative aspects of i) the functionality of neurotransmitter systems; ii) the morphology of brain regions and neural circuitries; and iii) the coordinated engagement and coupling of these regions and circuitries by cognitive and psychosocial stimuli. Moreover, these neurobiological measures can be readily studied in association with genetic variation, providing a basis to probe for the interacting influence of heredity and environment on the expression of socioeconomic health disparities that emerge from brain-dependent mechanisms. Specific neurobiological measures referred to above have been reviewed elsewhere, particularly for researchers in the biobehavioral health sciences (3,4). As yet, however, little research has incorporated these or other neurobiological measures in studies of socioeconomic health disparities. By comparison, use of such measures in the field of cognitive neuroscience has yielded early success in understanding the neurobiology of socioeconomic disparities in cognitive development, particularly as they relate to intellectual functioning and educational achievement (24 –27). Next, we selectively survey studies that have similarly begun working toward understanding the neurobiology of socioeconomic health disparities.
Neurobiological Pathways Linking SEP and Health: A Putative Role for Stress and Mood Systems of the Brain
Reviewed below are recent studies that have used neuropharmacological, molecular genetic, and neuroimaging approaches to understand the neurobiology of socioeconomic health disparities. These studies emphasize the functional and morphological (structural) characteristics of emotion, mood, and stress circuitries of the brain, along with their major modulatory neurotransmitter systems. The rationale behind this emphasis is that lower SEP reliably occasions adverse environmental exposures, as well as social and psychological hardships arising from the challenges of daily living, uncertainties of future prospect, and (for some) social exclusion, marginalization, and internalized states of demoralization that are not buffered by supportive social ties or protective psychosocial resources (5,11,14,28,29). Furthermore, to the extent that lower SEP precipitates exposure to chronic forms of environmental and psychosocial adversities, the brain and neurotransmitter systems linking dimensions of SEP to health most plausibly include those that are jointly involved in i) supporting emotional and social information processing and related behaviors; and ii) coregulating autonomic, metabolic, neuroendocrine, and immune functions associated with disease processes (23). Accordingly, the particular brain systems emphasized in the studies below are cardinal components of a corticolimbic circuitry that is instrumental for coordinating behavior with peripheral physiology in the service of adaptively coping with potentially adverse environmental and psychosocial challenges. Also emphasized below is the monoamine neurotransmitter, serotonin, which modulates corticolimbic functionality and morphology in strong association with genetic influence (30). For the interested reader, there are detailed treatments elsewhere of methods to assess the functionality and morphology of corticolimbic brain systems, their modulation by neurotransmitters and neuropeptides, and their suspected roles in the etiology of medical conditions and psychological disorders (3,4).
Neuropharmacological and Molecular Genetic Studies of SEP
A neuropharmacological approach to studying the neurobiology of socioeconomic health disparities is illustrated by research on the monoamine neurotransmitter, serotonin. Serotonin-releasing neurons originate in the raphe nuclei of the midbrain, and they project to nearly all areas of the central nervous system, including much of the cerebral cortex and numerous subcortical cell groups (e.g., amygdala, hippocampus, basal ganglia, thalamus, and hypothalamus), as well as sensory and motor neurons of the spinal cord and brain stem governing peripheral autonomic discharge (31). As a result, serotonin is capable of modulating diverse biological and behavioral systems. One method for assessing brain serotonergic function and its variability involves the measurement of neuroendocrine reactions to drugs that act on serotonergic neurons or neurons with serotonin receptors (32). One such neuropharmacological challenge, acute administration of fenfluramine, induces neuronal release of serotonin and inhibits its reuptake. Subsequent activation of serotonin receptors in the hypothalamus stimulates pituitary release of the hormone, prolactin, into circulation, so that the resulting increase in plasma prolactin concentration is interpreted as an index of central serotonergic responsivity, at least along the hypothalamic-pituitary axis (33,34).
People vary markedly in serotonergic responsivity, as evaluated by fenfluramine or related neuropharmacological challenges, and this variation reflects a moderately stable dimension of individual differences (35,36). With respect to behavior, persons of an antagonistic disposition and prone to aggression (particularly impulsively aggressive acts) and those with histories of major depression or suicidal tendencies commonly show attenuated serotonergic responsivity (e.g., a blunted prolactin response to fenfluramine), compared with individuals without these attributes (31,34,37). In addition, low central serotonergic responsivity has many health-related correlates, including central adiposity and physical inactivity, elevated blood pressure, dyslipidemia, insulin resistance (hence, the metabolic syndrome), and preclinical vascular disease (carotid artery atherosclerosis) (38–41). These converging observations have encouraged speculation that dysregulation of the brain’s serotonergic system may help explain why multiple behavioral and biological risk factors for ill health (even those suspected to have independent etiologies) often covary in populations and aggregate (cluster) among susceptible individuals, particularly those of lower SEP (42,43).
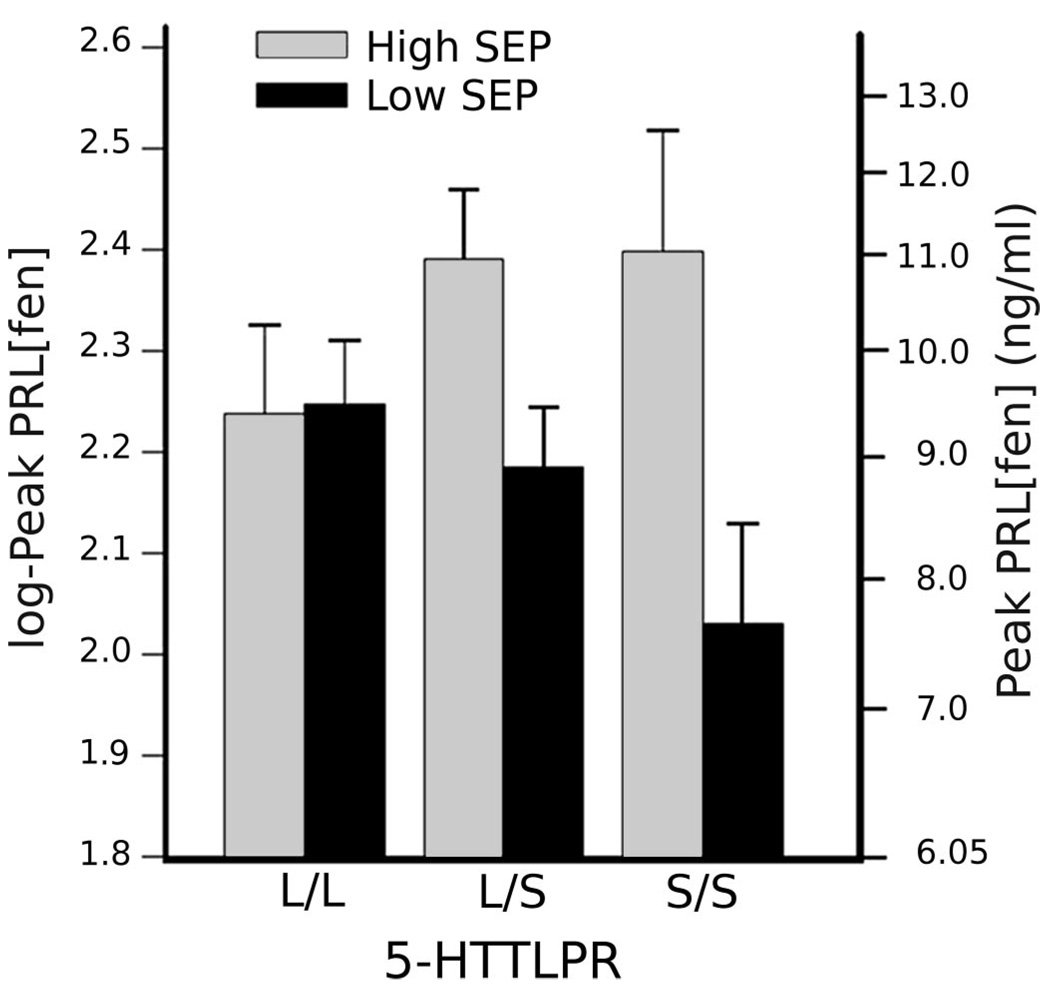
Less explored, however, are the origins of individual differences in brain serotonergic functional activity, which—like most complex neurobehavioral phenotypes—may include environmental and genetic influences. Similar to humans, rhesus monkeys (Macaca mulatta) vary in serotonergic activity, as indexed by the cerebral spinal fluid concentration of serotonin’s principal metabolite, 5-hyrdoxyindoleacetic acid (5-HIAA), and low 5-HIAA in these animals is associated with aggressiveness, low social affiliation, and a propensity for impulsive, high-risk behaviors (44). In experimental studies, moreover, monkeys raised with peers and in the absence of their mothers exhibit lower cerebral spinal fluid 5-HIAA levels than mother-reared controls (45). Yet, the influence of early maternal deprivation on serotonergic function is not seen in all peer-reared monkeys, but only among those carrying the “short” variant of a biallelic length polymorphism in the regulatory region of the gene that encodes the serotonin transporter (46). Analogous observations do not exist in humans, but two so-called objective indicators of personal (individual-level) SEP—income and educational attainment— have been shown to covary positively with serotonergic responsivity in nonpatient sample of midlife, community volunteers (47). Hence, under fenfluramine challenge, individuals of lower SEP exhibit an attenuated increase in plasma prolactin concentration relative to individuals of more advantaged SEP. Similar to findings in rhesus monkeys, moreover, the covariation of serotonergic responsivity and SEP is modulated by orthologous genetic variation in the human serotonin transporter (48). In particular, individuals of comparatively lesser income and education only show a diminished prolactin response to fenfluramine if they possess at least one “short” (or deletion) allele of this polymorphism, which reduces transcriptional efficiency of the transporter gene (49) (Fig. 2). In sum, functional genetic variation in one component of serotonergic neurotransmission, the serotonin transporter, moderates the influence of both early adversity (in monkeys) and lower objective SEP (in humans) on brain serotonergic activity.
Figure 2.
Allelic variation in the regulatory region of the serotonin transporter gene moderates an association between personal socioeconomic indicators and central nervous system serotonergic responsivity. In this study, 139 adults (n = 75 men and 64 women) were administered a neuroendocrine challenge to assess central serotonergic responsivity (plasma prolactin [PRL] response to the serotonin releasing agent, fenfluramine [fen]). Objective socioeconomic position (SEP) was estimated by income and years of education. Regression analyses showed serotonergic responsivity to be predicted by the interaction of serotonin transporter genotype and SEP (p = .018). Hence, individuals of lower income and lesser education had lower peak PRL concentrations post administration of fenfluramine than those higher on these dimensions, but only if they possessed at least one “short” allele of the serotonin transporter gene. Mean baseline-adjusted, log-peak PRL[fen] concentrations are shown as a function of long/long (L/L), long/short (L/S), and short/short (S/S) genotypes among individuals of higher and lower SEP, as defined by median division of the distribution of SEP scores. For reference, comparison values of PRL concentration, back-transformed to the unit of PRL measurement (ng/mL), are listed on the right ordinate (here, standard error bars can be interpreted only with respect to the scale of log-transformed scores). 5-HTTLPR = serotonin-transporter-linked promoter region. Reprinted with permission from Manuck and colleagues (48).
As noted earlier, it is increasingly recognized that the SEP of individuals, as typically inferred from indicators of income, education, and occupational grade, only partially reflects the spectrum of socioeconomic disparities of populations (50). Additional information is captured when SEP is referenced to higher levels of social organization, including communities, census tracts, and electoral wards (51–53). Such “community-level” or “area-level” indicators of SEP vary widely, but among those most often studied are median household incomes, area-aggregated rates of poverty and unemployment, vehicle or home ownership, density of housing or overcrowding; as indicators of social “fragmentation,” the following are studied: percentage of single-person households, pensioners, or unmarried individuals residing in a given community. Thus, to the extent that indicators of objective SEP at the individual level have been found to covary with brain serotonergic function, it may be asked whether similar covariation exists with respect to community-level SEP.
In testing this hypothesis in the same research program linking individual-level SEP and serotonergic function, an indicator of community-level SEP was computed as the aggregate of several variables derived from the U.S. Census for tracts of residence, including median residential income, proportion of households beneath the federally designated poverty level of income, percentage of the workforce unemployed, median value of owner-occupied housing units, median gross rent (as a percentage of income), and proportion of the population aged > 25 years lacking a high school diploma (54). Like the findings for indicators of objective SEP measured at the individual level (income and education), participants residing in less-advantaged communities showed lower serotonergic responsivity (blunted prolactin response) than residents of more advantaged communities. Individual- and community-level indicators of SEP are moderately correlated, yet when adjusted for individual-level SEP, SEP at the community level continued to predict fenfluramine-stimulated prolactin increases and, unlike individual-level SEP, did so independently of genetic variation in the serotonin transporter. This association was likewise independent of age, sex, diet, or any other measured “person” variable related to community of residence, such as cognitive ability (intelligence quotient) and trait conscientiousness. Limited statistical power in this study, however, precluded mixed-effects modeling of serotonergic responsivity among individuals nested within different communities and stratified by serotonin transporter status.
In aggregate, two multilevel (individual and community) indicators of objective SEP covary with interindividual variation in serotonergic responsivity, even though interpreting the directionality of such covariation is precluded by cross-sectional evidence in samples that are appreciably smaller in size than many epidemiological studies. Furthermore, we note that, at the time the above-mentioned studies were conducted, recently validated indicators of subjective SEP, which are discussed below, were not available. Hence, the extent to which indicators of subjective SEP covary with serotonergic responsivity and serotonin transporter status remains unknown. Finally, we recognize that serotonergic neurotransmission and its variability among individuals (and undoubtedly, variation in other neurotransmitter systems) likely affect health and disease risk only to the extent that they modulate (bias) the activities of functional brain circuitries supporting psychological and biological processes, such as emotional experience and expression, mood regulation, behavioral motivations, and autonomic, metabolic, immune, neuroendocrine, and cardiovascular regulation (43). Because neuroimaging methods provide investigational access to these circuitries, it is possible to study the neurobiological factors associated with indicators of SEP more directly than permitted by neuropharmacologic and molecular genetic approaches alone.
Neuroimaging Studies of SEP
One neuroimaging approach useful for studying the neurobiology of socioeconomic health disparities is illustrated by research on SEP and corticolimbic brain morphology. There is longstanding evidence from animal models that chronic social stressors can remodel the structure of several brain regions—particularly corticolimbic regions within the medial prefrontal cortex, amygdala, and hippocampus (23,55). At the cellular level, and depending on the particular region, this remodeling can involve alterations in the branching complexity of neuronal dendrites, the expression of dendritic spines and spine synapses, the acetylation of microtubule subunits within neurons, the phosphorylation of microtubule-associated neural proteins, the proliferation of new neurons (neurogenesis), and the loss of neurons (55). Many of these cellular and structural changes—broadly referred to as forms of neuroplasticity—have been related not only to alterations in gross brain morphology (e.g., the regional volume or concentration of gray matter) but also disruptions in higher-order cognitive abilities, mood states, and bidirectional signaling between brain circuits and major peripheral physiological stress response axes (23). Although the mechanisms by which chronic stressors affect forms of neuroplasticity are incompletely understood, they are thought to reflect alterations in central levels of glucocorticoids, extracellular excitatory and inhibitory amino acids (e.g., glutamate and γ-aminobutyric acid), neurotrophic factors (e.g., brain-derived neurotrophic factor and insulin-like growth factor), adrenal steroids, and corticotrophin-releasing factor—all of which interact with serotonergic and other monoaminergic transmitter systems implicated in cognitive processes, emotion- and mood-related behaviors, as well as stress regulatory functions (56).
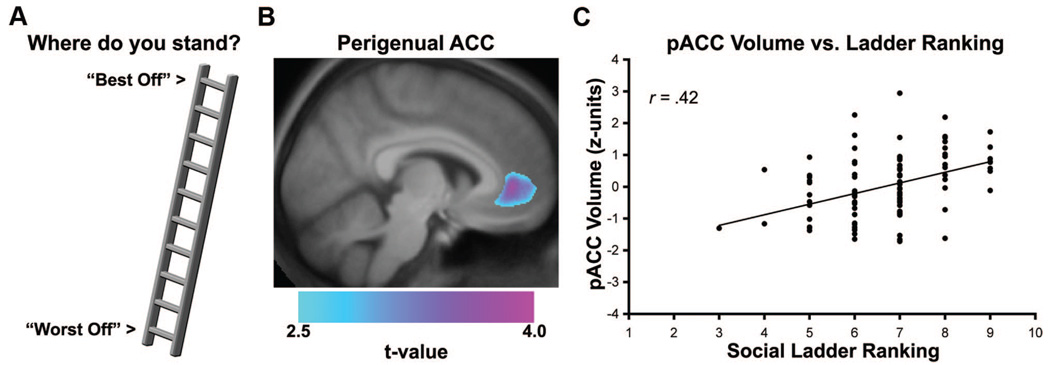
Hence, in the translational context of animal research on chronic stress and neuroplasticity, it is plausible that dimensions of comparatively lower SEP could covary with quantitative aspects of regional brain morphology. In support of this speculation, a recent cross-sectional structural neuroimaging study (57) demonstrated that individuals who subjectively report holding a lower SEP—as reflected by lower scores on the MacArthur Scale of Subjective Social Status—show a reduced gray matter volume in the rostral area of the anterior cingulate cortex, a corticolimbic brain region involved in experiencing and regulating emotions and orchestrating behavioral and physiological reactivity to psychosocial stressors (3). In this study, a neuroanatomical method called voxel-based morphometry (58) was applied to structural brain images obtained in a healthy community sample of 100 adults (88% Caucasian, 56% women). Primary results from this study, illustrated in Figure 3, showed that lower subjective SEP was associated with reduced cingulate gray matter volume after accounting for several demographic factors, depressive symptoms, dispositional forms of negative emotionality, and even individual- and community-level indicators of objective SEP (assessed by family income, educational attainment, and U.S. Census Bureau variables, respectively). Interestingly, neither individual- nor community-level indicators of objective SEP themselves covaried with cingulate gray matter volume, providing provisional evidence that objective and subjective indicators of SEP may show unique patterns of association with neurobiological measures at the level of the brain. Moreover, neither individual- nor community-level indicators of objective SEP showed an association with regional gray matter volume in whole-brain analyses, which were executed with correction for multiple statistical testing. In aggregate, this evidence seems to parallel epidemiological findings that different indicators of SEP are not interchangeable and that they may reflect different pathways by which socioeconomic factors relate to behavioral, biological, and health-related outcomes (6). Furthermore, it is noteworthy that reduced volume of the anterior cingulate cortex has been linked to stress and mood-related functions of relevance to understanding socioeconomic health disparities, including stressor-evoked cardiovascular reactivity (59), hypothalamic-pituitary-adrenal axis functioning (60,61), and depressive symptomatology (62,63).
Figure 3.
Lower subjective socioeconomic position (SEP), as reflected by a lower self-reported ranking on the MacArthur Scale of Subjective Social Status, was associated with reduced gray matter volume in the perigenual area of the anterior cingulate cortex (pACC) in a cross-sectional neuroimaging study discussed in this article. For illustration, panel A shows the 10-point MacArthur social ladder scale used to assess subjective SEP. In panel B, a statistical parametric map of color-scaled t values is overlaid on an anatomical template brain. This map illustrates the pACC area where lower subjective SEP was associated with reduced gray matter volume across individuals. Plotted along the y-axis in panel C is the standardized (Z score) gray matter volume values for pACC area profiled in panel B. Plotted along the x-axis are ladder rankings from the scale illustrated in panel A (1 = “Worst Off”; 10 = “Best Off”). *p < .001. Reprinted with permission from Gianaros and colleagues (57).
Although consistent with the general notion that some dimensions of comparatively lower SEP (e.g., as reflected by lower subjective SEP) covary with quantitative aspects of brain morphology, limitations of these structural neuroimaging observations are that they were obtained from modally Caucasian, well-educated, higher-income, and healthy individuals without psychiatric, neurological, cerebrovascular, or cardiovascular diseases. Therefore, the racial composition, socioeconomic distribution, and general health of this sample constrain extrapolations to the general population. Moreover, it may be problematic to detect a relationship between subjective SEP and volumetric differences in other putatively stress-sensitive corticolimbic brain areas, such as the amygdala and hippocampus, when studying otherwise healthy individuals compressed at the upper end of the socioeconomic distribution (as reflected by objective indicator variables). In extension, it is likewise possible that restricted variance in objective SEP indicator variables measured at the individual and community levels could partly explain an apparent absence of association between these dimensions of SEP and regional brain morphology. Finally, it is conceivable that future longitudinal studies could better capture the cumulative aspects of subjective and objective SEP over time, which may better predict regional volumetric changes across the brain. In point, cumulative longitudinal reports of chronic psychological stress have been associated with reduced hippocampal gray matter volume in our prior work (64).
Despite the above limitations, reduced gray matter volume in the anterior cingulate cortex seems to be a structural neural correlate of one dimension of SEP (lower subjective SEP), which may plausibly relate to mental or physical health outcomes via mechanisms of neuroplasticity. Conceptually, though, a relationship between lower subjective SEP and reduced cingulate gray matter volume could be interpreted from an alternative vulnerability perspective (56). Specifically, genetically and/or developmentally altered regional gray matter volume in corticolimbic brain areas could bias individuals to view themselves subjectively as holding a lower SEP than others. There is evidence that exposure to early life stressors predicts reduced cingulate volume in adulthood (65), possibly increasing psychological vulnerability and sensitivity to perceived adverse psychosocial conditions or life stressors. In addition, it has recently been found that individuals carrying the short allele of the promoter polymorphism of the serotonin transporter gene show an approximate 25% reduction in cingulate gray matter volume in comparison with carriers of the alternate, long allele (66), an effect that seems to be conserved across primates (67). Hence, it will be important for future studies to account for the interactive influence of developmental and genetic factors on normative variation in regional gray matter volume in association with subjective and objective dimensions of SEP—particularly in light of the observations reviewed earlier on the influence of genetic variation in the human serotonin transporter on the association between individual SEP and serotonergic responsivity.
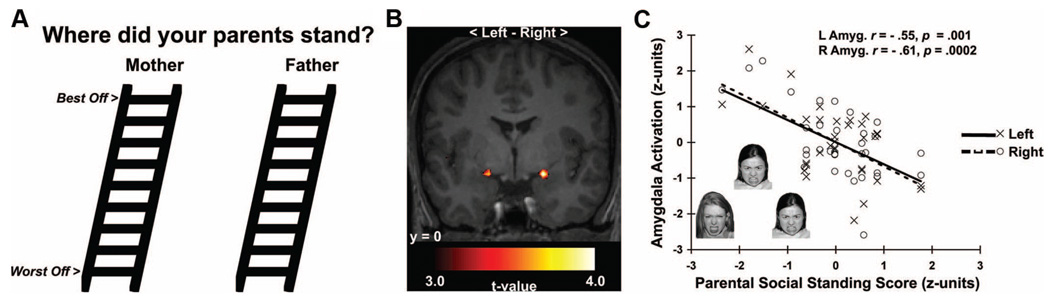
Extending the structural imaging work above, there is recent functional neuroimaging evidence that another corticolimbic area subject to serotonergic modulation, the amygdala, may link stress-related processes to health within the context of childhood socioeconomic factors. In particular, social information processing models emphasizing a lifecourse perspective posit that individuals who mature in disadvantaged socioeconomic environments may develop an early sensitivity to social threats, leading to dysregulated forms of emotional processing and recurrent stress responses that increase risk for ill health in later life (68 –70). This postulate parallels the notion that risk trajectories for ill health may be developmentally “embedded” in the brain and in biobehavioral stress-response systems by early and unfavorable socioeconomic circumstances (2,71,72). Consistent with this notion, a recent neuroimaging study (73) showed that low subjective parental SEP, a putative indicator of socioeconomic disadvantage during childhood and adolescence, is uniquely associated with greater amygdala reactivity to threatening (angry) facial expressions but not to neutral or surprised facial expressions (Fig. 4). Notably, this association was observed among individuals who had not yet attainted an adult SEP (i.e., had not yet finished schooling or entered an occupation), and it was not explained by several potential confounding factors, including sex, ethnicity, dispositional emotionality, recent symptoms of depression and anxiety, parental educational attainment, and the participants’ own ratings of their subjective SEP.
Figure 4.
Lower subjective parental socioeconomic position (SEP) predicted greater amygdala reactivity to angry faces in a functional neuroimaging study of young adults. A) Modified versions of the MacArthur Scales of Subjective Social Status used to assess subjective parental SEP. B) Statistical parametric maps projected onto an anatomical template. The maps profile amygdala areas where lower subjective parental SEP predicted greater reactivity to angry faces. C) Plots depicting standardized subjective parental SEP scores (x-axis) and mean-centered, standardized reactivity values derived from left (L, open circles, dashed line) and right (R, closed circles, solid line) amygdala areas in B. Inset in C illustrates exemplar trial of angry faces used to elicit amygdala reactivity. Reprinted with permission from Gianaros and colleagues (73).
Given that the amygdala is i) instrumental for registering the emotional salience of social and environmental information, ii) critical for regulating the neuroendocrine and autonomic stress-response axes, and iii) sensitive to early life stress, increased amygdala reactivity to angry or otherwise threat-related facial expressions could reflect a neural “embedding” or “embodiment” of experiences associated with early socioeconomic disadvantage that influence sensitivity to perceived social threats—possibly affecting stress regulatory peripheral systems influencing health or disease vulnerability in later life. Consistent with such speculation, amygdala reactivity has been linked to stressor-evoked cardiovascular reactivity (59) and preclinical atherosclerosis (74).
Most recently, amygdala reactivity has been shown to covary with concurrent changes in the neural representation of social hierarchies in humans. In a study by Zink and colleagues (75), functional magnetic resonance imaging was used to identify neural responses correlated with perceived social rank within the context of an interactive social context involving exposure to both stable and unstable social hierarchies. Interestingly, in the context of an unstable social hierarchy, viewing a superior ranking individual engaged the amygdala and areas of the medial prefrontal cortex involved in social information processing. Here, it is interesting to note that chronic exposure to unstable social groups has been found to accelerate the development of coronary artery atherosclerosis among otherwise well-protected (normotensive, normocholesterolemic) male cynomolgus monkeys; however, it is equally important to note that the atherogenic effects of social instability among these monkeys pertained to social groupings and not to social hierarchies themselves, as relative social ranks were conserved in the groups of redistributed (unstable) memberships (76). Hence, the above human neuroimaging findings are significant in that they are beginning to translate animal studies on the role of the amygdala and networked brain areas in potentially linking brain-dependent stress processes to candidate neurobiological mechanisms associated with socioeconomic gradients in mental and physical health.
CONCLUSIONS
Recent neuropharmacological, molecular genetic, and neuroimaging studies are starting to contribute to our neurobiological understanding of socioeconomic health disparities. Future work building on these studies will require interdisciplinary collaborations between neuroscientists, behavioral geneticists, social and biological psychologists, epidemiologists, and policy and intervention researchers who share an interest in the pathways by which socioeconomic factors may become “embodied” by the brain to influence health throughout life (2). Below, we consider some open lines of inquiry for which there are immediate and promising opportunities to pursue this shared interest.
First, higher-level social factors, social interactions, and forms of social information processing that are important for health are presumably represented and enabled by brain circuitries that are instrumental not only for adaptive interpersonal behaviors but also peripheral physiological regulation. These brain circuitries, collectively referred to as the “social brain,” include areas of the prefrontal cortex (particularly cingulate, orbital and medial areas), insula, amygdala, hippocampus, and temporoparietal junction, along with networked cell groups in the midbrain and brain stem (77). The development and functioning of these circuitries are modulated by a complex interplay of endogenous neuropeptides, monoaminergic and cholinergic neurotransmitters, and genetic and epigenetic substrates explicated in animal and some human studies (2,23,55). What is unknown, however, is how the life experiences engendered by dimensions of SEP over the life span affect the functionality and morphology of these circuitries and their underlying molecular substrates, particularly in the context of delineating the neurobiological pathways by which SEP relates to emergent and brain-dependent social processes. These processes could include brain-dependent representations of social networks, the availability of social support and psychosocial resources, and the neural encoding of potentially threatening social information (e.g., racial or gender stereotyping, discrimination, social exclusion, or marginalization)—all of which are implicated in health and longevity via peripheral physiological mechanisms. As examples, the perceived availability of psychosocial resources has been associated with a blunted neuroendocrine (cortisol) response to stress, which may be mediated by a down-regulation of amygdala or subcortical limbic activity via prefrontal control mechanisms (78,79). Building on this evidence, it could be asked whether dimensions of SEP relate to i) the neural correlates or representations of psychosocial resources and related aspects of social information processing, and ii) associated peripheral physiological stress responses.
Second, particular dimensions of SEP have long been associated with more proximal determinants of health behaviors, psychosocial and biological disease risk factors, and clinical outcomes and events. In early development, childhood, and adolescence, these determinants include parental resources and nurturance patterns, early environmental exposures, enriching educational experiences, and peer group affiliations—all of which can affect brain development and plasticity within the broader context of genetic influence (80). In later life, proximal determinants linked to dimensions of SEP presumably include aspects of financial security, employment, autonomy, dispositional attributes of self-esteem, mood, and impulse control, health literacy and knowledge, and supportive social ties or networks (11). To the extent that these proximal determinants mediate or link dimensions of SEP with health behaviors, risk factors, and clinical outcomes, then their measurement should be included in multilevel and longitudinal neurobiological studies of socioeconomic health disparities. In this way, novel targets for interventions and more mechanistic accounts of SEP ⇒ health associations could be established.
Third, much of the research on socioeconomic health disparities has emphasized associations between dimensions of SEP and markers of autonomic, metabolic, neuroendocrine, and immune activity (81). It is likely that alterations in peripheral physiology play a primary role in initiating or exacerbating many pathophysiological changes predictive of adverse health outcomes. Importantly, there are strong bidirectional (efferent and afferent) feedback loops between the brain and peripheral physiological regulatory systems, which have recently been conceptualized as brain-body “information transfer systems” (3,4). It is well established from animal models that alterations in these feedback loops affect not only the function but also the morphology of several corticolimbic brain systems, as well as the cognitive, emotional, mood, and stress processes they support (23,55). Unknown at present, however, are i) how dimensions of SEP affect patterns of feedback and feedforward signaling between the brain and peripheral physiological systems; and ii) how altered forms of these signaling patterns relate to particular health outcomes over the life span. Addressing these gaps in existing knowledge will require future neurobiological research to integrate multidimensional and multilevel indicators of SEP and peripheral physiology in the context of predicting disease risk and development (3).
Fourth, SEP is by no means a static construct, insofar as many individuals will experience upward or downward shifts in their socioeconomic circumstances throughout life. Asked from a lifecourse perspective, how might mobility changes in SEP affect the functionality of the neurotransmitter and brain systems reviewed herein? Would the impact of these mobility changes depend on genetic or environmental influences that have been studied previously in association with particular health disparities? Moreover, beyond individual-level socioeconomic factors that may change with time, economic and social inequalities at the level of communities, which may also change over time, strongly predict a range of health outcomes, including all-cause mortality and age-adjusted deaths attributable to such specific causes as cardiovascular and respiratory diseases, stroke, some forms of cancers, accidents, suicide, and violence (51,53). And short of death, the socioeconomic aspects of communities are associated with many health-related risks, such as sedentary life-styles, cigarette smoking and alcohol abuse, as well as cardiometabolic risk factors, systemic inflammation, and preclinical atherosclerosis. Disadvantaged communities engender many adversities germane to disease risk, such as restricted access to medical care, incommodious housing, nutritional deficiencies and exposure to environmental toxins, and social norms promoting the acquisition of health-impairing habits. Yet, because there is presently little direct evidence that these factors entirely account for community-level effects on disease and mortality (51,82), it remains unclear how cross-sectional or longitudinal variation in the socioeconomic aspects of communities influence the health of their residents. One arguable possibility is that the pathogenicity of community environments is exerted through neurobiological mechanisms more closely reflecting psychological, as opposed to material, burdens of relative deprivation. In extension, we noted earlier in this review that serotonergic neurotransmission and its variability among individuals (and undoubtedly, variation in other neurotransmitter systems) affect psychological and physical health risk by modulating activities of functional corticolimbic circuitries subserving psychological and biological processes, such as emotional experience, behavioral motivation (e.g., in relation to health-impairing attributes of habit and life-style), and autonomic, metabolic, neuroendocrine, and immune functions. In view of this assumption, it could be asked in future neuroimaging work with sufficient sampling for mixed-statistical modeling (e.g., individuals nested within communities) whether community-level indicators of SEP associated with serotonergic function relate to the activity or morphology of corticolimbic circuitries important for social information processing and peripheral physiological regulation, particularly in the context longitudinal disease prediction.
Fifth, we believe that future questions regarding the neurobiology of socioeconomic health disparities should rely on a methodological integration of neuropharmacology and neuroimaging approaches. Hence, it could be asked whether the reduced serotonergic responsivity observed among individuals of lower SEP might explain some of differential corticolimbic (e.g., amygdala) reactivity seen in the neuroimaging work reviewed above. In point, the amygdala plays a key role in detecting stimuli of biological significance, including threat-related cues, and is readily engaged by facial displays of negative affect (83). Moreover, heightened activation of the amygdala in response to such stimuli is associated with personality traits that predispose individuals to experience states of negative affect and aroused or dysphoric mood (e.g., trait anxiety, neuroticism) (84,85). It is noteworthy that socioeconomic disadvantage also increases vigilance for threat (86), the neural representation of which might similarly involve a relative hyperresponsivity of the amygdala. In addition to the observation that lower subjective parental SEP predicts greater amygdala reactivity to anger-related facial expressions in young adults (87), there is corroborating evidence that adolecents’ subjective ratings of their families’ social status covaries not only with threat-related amygdala reactivity, but also reactivity of two brain systems implicated in the processing of social distress and exclusion: the dorsal anterior cingulate cortex and anterior insula (88). Recall, however, that elsewhere we have found both individual- and community-level indicators of low objective SEP to be associated with a blunted central serotonergic responsivity, as indexed by neuropharmacologic challenge (48,54). Serotonin-releasing neurons project in abundance to the amygdala, and pharmacologic interventions that enhance serotonergic neurotransmission dampen the amygdala response to affective stimuli in both healthy and depressed individuals (89 –95). Conversely, lowering the central availability of serotonin transiently by acute tryptophan depletion increases amygdala reactivity to fear-related facial expressions in threat-sensitive individuals (96), as does regulatory variation in the serotonin transporter gene—the same polymorphic variation that we found to modulate serotonergic activity as a function of individual-level indicators of objective SEP (97). Hence, we speculate that the covariation of SEP with brain serotonergic responsivity and the association of serotonergic responsivity with aspects of cardiovascular risk may be partly mediated via the influences of this neuromodulatory system on components of emotional processing and ensuing autonomic and neuroendocrine responses. Again, such speculation could only be tested by the integration of neuropharmacology and neuroimaging approaches nested within larger epidemiological designs.
Finally, progress in our neurobiological understanding of socioeconomic health disparities will depend on advances in the measurement and interpretation of different indicators of SEP. More precisely, indicators of objective SEP have been interpreted as reflecting access to material and social resources, which can be indexed by quantitative or qualitative observations at multiple levels of social organization and over the life span. Indicators of subjective SEP may similarly reflect resource- and asset-based constructs, but they are thought to result from psychosocial appraisal processes that are incompletely understood. Importantly, the most common referents included in instruments for the measurement of subjective SEP (e.g., as included in variants of the MacArthur Scale of Subjective Social Status) are the same as those conventionally used to assess objective SEP. In other words, subjective SEP instruments often ask respondents to use aspects of education, income, and employment grade as referents for social comparisons. Thus, it may be that subjective indicators of SEP measure the same constructs captured by objective SEP indicators. However, measures of subjective SEP could conceivably also reflect socioeconomic attributes other than income, education, and occupation—particularly if respondents use referents other than those indicated in the instructional set of a given scale or instrument. If these referents include “objective” indicators that can be empirically verified or observed (e.g., accumulated wealth), it is possible that subjective SEP indicators are “better” measures of SEP than any objective indicator of SEP that is limited to just one or more of the conventional aspects of SEP (education, income, occupation) (19). In this case, one might expect subjective SEP indicators to predict health outcomes or covary with neurobiological measures of interest over-and-above or apart from objective SEP indicators—not because they are qualitatively different, but because subjective SEP indicators may aggregate over a greater number of “objective” referents than instruments of objective measurement tapping only one or a few aspects of SEP. However, subjective indicators of SEP also ask respondents for an appraisal of their social ranking compared with others, whereas objective SEP indicators can only be used to compare individuals within a range of measured observations in a particular sample. Hence, any single observation of objective SEP will not reflect individuals’ appraisals of their positional status as being “higher” or “lower” than others in terms of social ordering. Nor would a single indicator of objective SEP capture the kinds of asymmetrical socioeconomic relationships that people may perceive (or could potentially form) with others—appraisals that are perhaps based on a “cognitive averaging” of multiple objective SEP referents (e.g., occupations perceived as positions of power over others, or one’s sense of having financial resources to purchase goods and services that others cannot afford) (17). In this regard, subjective indicators of SEP may not entirely measure the same construct(s) as objective SEP indicators. In sum, these measurement and interpretive nuances need to be resolved, or at least refined, to better inform our understanding of the potentially distinguishing or overlapping neural correlates of SEP or of the putative neurobiological pathways linking SEP and health.
At minimum, addressing these interdependent questions and pursuing these lines of inquiry with the cross-disciplinary integration of epidemiological and health neuroscience methods holds promise for more than just adding new “biomarkers” to the study of socioeconomic health disparities. Rather, we believe that such integrative methodology can provide unique information about the embodiment of socioeconomic factors by the brain and the neurobiological pathways linking SEP to health throughout life.
Acknowledgments
This work was supported, in part, by Grants K01 MH070616 and R01 HL089850 (P.J.G.), and Grants P01 HL40962 and RO1 HL065137 (S.B.M.) from the National Institutes of Health.
Glossary
- 5-HIAA
5-hyrdoxyindoleacetic acid
- SEP
socioeconomic position
Footnotes
Drs. Rebecca Thurston and Gregory Miller provided constructive comments on a draft of this manuscript.
REFERENCES
- 1.Braveman P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health. 2006;27:167–194. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed] [Google Scholar]
- 2.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 3.Lane RD, Waldstein SR, Chesney MA, Jennings JR, Lovallo WR, Kozel PJ, Rose RM, Drossman DA, Schneiderman N, Thayer JF, Cameron OG. The rebirth of neuroscience in psychosomatic medicine, part I: historical context, methods and relevant basic science. Psychosom Med. 2009;71:117–134. doi: 10.1097/PSY.0b013e31819783be. [DOI] [PubMed] [Google Scholar]
- 4.Lane RD, Waldstein SR, Critchley HD, Derbyshire SW, Drossman DA, Wager TD, Schneiderman N, Chesney MA, Jennings JR, Lovallo WR, Rose RM, Thayer JF, Cameron OG. The rebirth of neuroscience in psychosomatic medicine, part II: clinical applications and implications for research. Psychosom Med. 2009;71:135–151. doi: 10.1097/PSY.0b013e318198a11f. [DOI] [PubMed] [Google Scholar]
- 5.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: the challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 7.Krieger N. Embodying inequality: a review of concepts, measures, and methods for studying health consequences of discrimination. Int J Health Serv. 1999;29:295–352. doi: 10.2190/M11W-VWXE-KQM9-G97Q. [DOI] [PubMed] [Google Scholar]
- 8.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan JR, Chen H, Manuck SB. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am J Primatol. 2009;71:732–741. doi: 10.1002/ajp.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapolsky R. Social status and health in humans and other animals. Annu Rev Anthropol. 2004;33:393–418. [Google Scholar]
- 11.Marmot M. The Status Syndrome: How Social Standing Affects Our Health and Longevity. New York: Henry Holt and Company; 2004. [Google Scholar]
- 12.Dohrenwend BP. The role of adversity and stress in psychopathology: some evidence and its implications for theory and research. J Health Soc Behav. 2000;41:1–19. [PubMed] [Google Scholar]
- 13.Kelly S, Hertzman C, Daniels M. Searching for the biological pathways between stress and health. Annu Rev Public Health. 1997;18:437–462. doi: 10.1146/annurev.publhealth.18.1.437. [DOI] [PubMed] [Google Scholar]
- 14.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 15.Adler N. When one’s main effect is another’s error: material vs. psychosocial explanations of health disparities. A commentary on Macleod et al., “is subjective social status a more important determinant of health than objective social status? Evidence from a prospective observational study of Scottish men” (61(9), 2005, 1916–1929) Soc Sci Med. 2006;63:846–850. doi: 10.1016/j.socscimed.2006.03.018. discussion, 851–7. [DOI] [PubMed] [Google Scholar]
- 16.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 17.Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the White-hall II study. Soc Sci Med. 2003;56:1321–1333. doi: 10.1016/s0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 18.Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom Med. 2005;67:855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- 19.Adler NA. Health disparities through a psychological lens. Am Psychol. 2009;64:663–673. doi: 10.1037/0003-066X.64.8.663. [DOI] [PubMed] [Google Scholar]
- 20.Manuck SB. The reaction norm in gene x environment interaction. Mol Psychiatry. doi: 10.1038/mp.2009.139. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger N. Embodiment: a conceptual glossary for epidemiology. J Epidemiol Community Health. 2005;59:350–355. doi: 10.1136/jech.2004.024562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall PJ. Relating psychology and neuroscience: taking up the challenges. Perspect Psychol Sci. 2009;4:113–125. doi: 10.1111/j.1745-6924.2009.01111.x. [DOI] [PubMed] [Google Scholar]
- 23.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 25.Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 26.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 27.Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev Sci. 2006;9:642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson RG. Health, hierarchy, and social anxiety. In: Adler NE, Marmot M, McEwen BS, Stewart J, editors. Socioeconomic Status and Health in Industrialized Nations: Social, Psychological, and Biological Pathways. Vol 896. New York: Annals of the New York Academy of Sciences; 1999. [DOI] [PubMed] [Google Scholar]
- 29.Christakis NA, Fowler JH. Connected: The Surprising Power of Our Social Networks and How They Shape Our Lives. Boston, MA: Little, Brown & Company; 2009. [Google Scholar]
- 30.Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manuck SB, Kaplan JR, Lotrich FE. Brain serotonin and aggressive disposition in humans and nonhuman primates. In: Nelson RJ, editor. Biology of Aggression. New York: Oxford University Press; 2006. [Google Scholar]
- 32.Yatham LN, Steiner M. Neuroendocrine probes of serotonergic function: a critical review. Life Sci. 1993;53:447–463. doi: 10.1016/0024-3205(93)90696-z. [DOI] [PubMed] [Google Scholar]
- 33.Quattrone A, Tedeschi G, Aguglia U, Scopacasa F, Direnzo GF, Annunziato L. Prolactin secretion in man: a useful tool to evaluate the activity of drugs on central 5-hydroxytryptaminergic neurones. Studies with fenfluramine. Br J Clin Pharmacol. 1983;16:471–475. doi: 10.1111/j.1365-2125.1983.tb02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology. 1998;19:287–299. doi: 10.1016/S0893-133X(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 35.Flory JD, Manuck SB, Muldoon MF. Retest reliability of prolactin response to dl-fenfluramine challenge in adults. Neuropsychopharmacology. 2002;26:269–272. doi: 10.1016/S0893-133X(01)00330-X. [DOI] [PubMed] [Google Scholar]
- 36.Flory JD, Manuck SB, Perel JM, Muldoon MF. A comparison of d, l-fenfluramine and citalopram challenges in healthy adults. Psychopharmacology. 2004;174:376–380. doi: 10.1007/s00213-003-1763-9. [DOI] [PubMed] [Google Scholar]
- 37.Coccaro EF, Siever LJ, Klar HM, Maurer G, Cochrane K, Cooper TB, Mohs RC, Davis KL. Serotonergic studies in patients with affective and personality disorders. Correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry. 1989;46:587–599. doi: 10.1001/archpsyc.1989.01810070013002. [DOI] [PubMed] [Google Scholar]
- 38.Horacek J, Kuzmiakova M, Hoschl C, Andel M, Bahbonh R. The relationship between central serotonergic activity and insulin sensitivity in healthy volunteers. Psychoneuroendocrinology. 1999;24:785–797. doi: 10.1016/s0306-4530(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 39.Muldoon MF, Sved AF, Flory JD, Perel JM, Matthews KA, Manuck SB. Inverse relationship between fenfluramine-induced prolactin release and blood pressure in humans. Hypertension. 1998;32:972–975. doi: 10.1161/01.hyp.32.6.972. [DOI] [PubMed] [Google Scholar]
- 40.Muldoon MF, Mackey RH, Williams KV, Korytkowski MT, Flory JD, Manuck SB. Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity. J Clin Endocrinol Metab. 2004;89:266–271. doi: 10.1210/jc.2003-031295. [DOI] [PubMed] [Google Scholar]
- 41.Muldoon MF, Mackey RH, Sutton-Tyrrell K, Flory JD, Pollock BG, Manuck SB. Lower central serotonergic responsivity is associated with preclinical carotid artery atherosclerosis. Stroke. 2007;38:2228–2233. doi: 10.1161/STROKEAHA.106.477638. [DOI] [PubMed] [Google Scholar]
- 42.Brummett BH, Boyle SH, Kuhn CM, Siegler IC, Williams RB. Socioeconomic status moderates associations between CNS serotonin and expression of beta2-integrins CD11b and CD11c. J Psychiatr Res. 2010;44:373–377. doi: 10.1016/j.jpsychires.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams RB. Psychosocial and biobehavioral factors and their interplay in coronary heart disease. Annu Rev Clin Psychol. 2008;4:349–365. doi: 10.1146/annurev.clinpsy.4.022007.141237. [DOI] [PubMed] [Google Scholar]
- 44.Higley JD, Bennett AJ. Central nervous system serotonin and personality as variables contributing to excessive alcohol consumption in non-human primates. Alcohol. 1999;34:402–418. doi: 10.1093/alcalc/34.3.402. [DOI] [PubMed] [Google Scholar]
- 45.Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 46.Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- 47.Matthews KA, Flory JD, Muldoon MF, Manuck SB. Does socioeconomic status relate to central serotonergic responsivity in healthy adults? Psychosom Med. 2000;62:231–237. doi: 10.1097/00006842-200003000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Manuck SB, Flory JD, Ferrell RE, Muldoon MF. Socio-economic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter gene-linked polymorphic region. Psychoneuroendocrinology. 2004;29:651–668. doi: 10.1016/S0306-4530(03)00094-5. [DOI] [PubMed] [Google Scholar]
- 49.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 50.Lynch J, Kaplan G. Socioeconomic position. In: Berkman LF, Kawachi I, editors. Social Epidemiology. New York: Oxford University Press; 2000. [Google Scholar]
- 51.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91:1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martikainen P, Kauppinen TM, Valkonen T. Effects of the characteristics of neighbourhoods and the characteristics of people on cause specific mortality: a register based follow up study of 252,000 men. J Epidemiol Community Health. 2003;57:210–217. doi: 10.1136/jech.57.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robert SA. Socioeconomic position and health: the independent contribution of community socioeconomic context. Annu Rev Sociol. 1999;25:489–516. [Google Scholar]
- 54.Manuck SB, Bleil ME, Petersen KL, Flory JD, Mann JJ, Ferrell RE, Muldoon MF. The socio-economic status of communities predicts variation in brain serotonergic responsivity. Psychol Med. 2005;35:519–528. doi: 10.1017/s0033291704003757. [DOI] [PubMed] [Google Scholar]
- 55.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 56.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 57.Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, Critchley HD, Manuck SB, Hariri AR. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cog Affect Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 59.Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacLullich AM, Ferguson KJ, Wardlaw JM, Starr JM, Deary IJ, Seckl JR. Smaller left anterior cingulate cortex volumes are associated with impaired hypothalamic-pituitary-adrenal axis regulation in healthy elderly men. J Clin Endocrinol Metab. 2006;91:1591–1594. doi: 10.1210/jc.2005-2610. [DOI] [PubMed] [Google Scholar]
- 61.Cerqueira JJ, Catania C, Sotiropoulos I, Schubert M, Kalisch R, Almeida OF, Auer DP, Sousa N. Corticosteroid status influences the volume of the rat cingulate cortex—a magnetic resonance imaging study. J Psychiatr Res. 2005;39:451–460. doi: 10.1016/j.jpsychires.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, MacFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 66.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 67.Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Suomi SJ, Olsen AS, Porter JN, Lopresti BJ, Hariri AR, Bradberry CW. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry. 2010;15:512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen E, Matthews KA. Cognitive appraisal biases: an approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Ann Behav Med. 2001;23:101–111. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- 69.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: how and why do these relationships change with age? Psychol Bull. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 70.Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. J Pers. 2004;72:1376–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 71.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 72.Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom Med. 2007;69:402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 73.Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with Individual differences in reactivity and functional connectivity of the amygdala. Biol Psychiatry. 2009;65:943–950. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM, Miller EW. Social stress and atherosclerosis in normocholesterolemic monkeys. Science. 1983;220:733–735. doi: 10.1126/science.6836311. [DOI] [PubMed] [Google Scholar]
- 77.Lim MM, Bielsky IF, Young LJ. Neuropeptides and the social brain: potential rodent models of autism. Int J Dev Neurosci. 2005;23:235–243. doi: 10.1016/j.ijdevneu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. J Pers Soc Psychol. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- 80.Shonkoff JP, Phillips DA, editors. From Neurons to Neighborhoods: The Science of Early Childhood Development. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 81.Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Soc Sci Med. 2004;58:1511–1522. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 82.Davey Smith G. Health Inequalities: Lifecourse Approaches. Bristol: The Policy Press; 2003. [Google Scholar]
- 83.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 84.Fakra E, Hyde LW, Gorka A, Fisher PM, Muñoz KE, Kimak M, Halder I, Ferrell RE, Manuck SB, Hariri AR. Effects of HTR1A C(−1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci. 2007;121:249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- 86.Feldman PJ, Steptoe A. How neighborhoods and physical functioning are related: the roles of neighborhood socioeconomic status, perceived neighborhood strain, and individual health risk factors. Ann Behav Med. 2004;27:91–99. doi: 10.1207/s15324796abm2702_3. [DOI] [PubMed] [Google Scholar]
- 87.Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muscatell KA, Way BM, Eisenberger NI, Pfeifer JH, Dapretto M. Perceived familial social status modulates the neural response to viewing emotional facial expressions in adolescents. Presented at: 68th Annual Scientific Meeting; March 10–3, 2010; Portland, OR. Abstract 1743. [Google Scholar]
- 89.Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194:535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology. 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 92.Del-Ben CM, Deakin JF, McKie S, Delvai NA, Williams SR, Elliott R, Dolan M, Anderson IM. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30:1724–1734. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- 93.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 94.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 95.Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cools R, Calder AJ, Lawrence AD, Clark L, Bullmore E, Robbins TW. Individual differences in threat sensitivity predict serotonergic modulation of amygdala response to fearful faces. Psychopharmacology. 2005;180:670–679. doi: 10.1007/s00213-005-2215-5. [DOI] [PubMed] [Google Scholar]
- 97.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]