Abstract
A thiophene-based macrocycle containing four secondary and two tertiary amines has been synthesized and its binding affinity has been investigated toward sulfate anion in solution and solid states. Structural analysis of the sulfate salt suggests that the ligand in its hexaprotonated form, is capable of encapsulating one sulfate within the cavity through cooperative NH⋯O and CH⋯O interactions. As investigated by 1HNMR titrations, the lignad forms a 1:1 complex with sulfate in water at pH 2.1, showing a binding constant (K) of 3200 M-1. The formation of the complex has been further confirmed by ESI-MS, indicating that the complex can exist in solution with a considerable stability.
Anion binding is a natural process that occurs in numerous biochemical systems.1 In biology, sulfate is known to play a key role in biosynthesis.2 A protein-bound sulfate complex has been structurally identified in which all of the sulfate oxygens except one are held by two hydrogen bonds with adjacent amino acid residues, resulting in a seven coordinate anion complex.3 Because of the directionality of the lone pairs on the oxygens, sulfate is an attractive anion as a potential template in the formation of a number of molecular devices including macrocycles, helixes and molecular capsules.4 Sulfate is also prevalent in environment that is a known contaminant in water and soil.5 Therefore, the design of new hosts capable of encapsulating sulfate anion still remains an important area of research in supramolecular chemistry.
It is known that sulfate can form upto twelve coordination bonds via hydrogen bonding or the combination of hydrogen bonding and electrostatic interactions with synthetic receptors.6 The first structural evidences of encapsulated sulfate complexes were observed with cryptand-based receptors in which the divalent sulfate was held by five hydrogen bonds with a polyamine cryptand or eight hydrogen bonds with a polyamide cryptand.7 Sulfate encapsulation was also observed in the crystal lattice of metal–organic framework,6 self-assembled metal–organic cage host8 or monocyclic polyamide.9 Although, azamacrocycles are good hosts for a variety of inorganic anions,10 forming monotopic to ditopic complexes with different binding modes,11 to the best of our knowledge, there is no structural report of encapsulated sulfate within monocycle-based polyamines. Herein, we report a new receptor with thiophene spacers that encapsulates sulfate through NH⋯O as well as CH⋯O interactions.
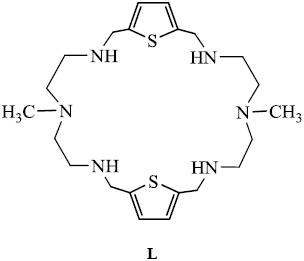
The ligand L was synthesized form the reaction of an equimolar amount of N-methyl-2,2’-diaminodiethylamine and 2,5-thiophenedicarbaldehyde under high dilution condensation in CH3OH followed by the reduction with NaBH4. The sulfate salt of L was obtained as a white powder from the reaction of the ligand (20 mg, 0.044 mM) with a few drops of concentrated H2SO4 in CH3OH (2mL). X-ray quality crystals were grown from the sulfate salt dissolved in H2O/CH3OH (5:1, v/v) under slow evaporation at room temperature.
Single-crystal X-ray diffraction analysis12 of the sulfate salt reveals that the macrocycle is hexaprotonated with encapsulated sulfate within the macrocycle. In the macrocycle two protons on tertiary amines (N1 and N14) and two protons on secondary amines (N11 and N11i) are pointed towards the cavity and are involved in hydrogen bonding interactions with the internal sulfate via short NH⋯O bonds (<3 Å).7 As shown in Figure 1A and Table 1, the encapsulated sulfate is coordinated with the macrocycle with relatively strong four NH⋯O bonds (2.633(7) to 2.852(7)Å) and also with one CH⋯O (3.10 Å).13
Figure 1.
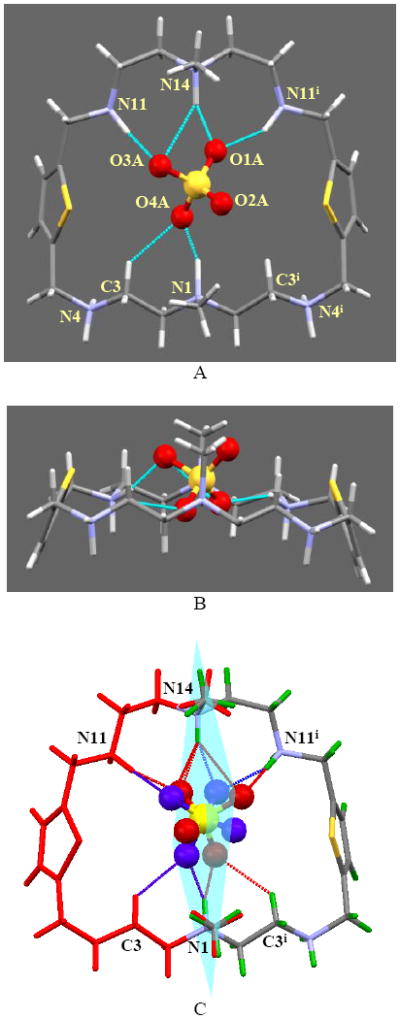
Crystal strucrure of H6L(SO4)]4+ motif showing encapsulated sulfate: (A) side view, (B) view along the tertiary N-N axis and (C) two symmetry related mirror images with disordered sulfate ion. External sulfates and water molecules are not shown for clarity.
Table 1.
Hydrogen bonding parameters (A, °) for SO42− binding in L.
| D—H⋯O | H⋯O | D⋯O | ∠DHO |
|---|---|---|---|
| N1—H1⋯4OA | 1.53 | 2.633(7) | 159.7 |
| C3—H3A ⋯O4A | 2.325 | 3.10 | 134.3 |
| N11i—H11Bi ⋯O1A | 1.82 | 2.644(7) | 137.7 |
| N14—H14⋯O1A | 1.69 | 2.716(7) | 157.8 |
| N14—H14⋯O3A | 2.557 | 3.366 | 156 |
| N11—H11B··O3A | 1.87 | 2.852(7) | 165.7 |
The distance of H⋯O in CH⋯O bond is 2.325 Å which is much shorter than the sum of van der Waals radii for H and O (2.72 Å), suggesting a strong CH⋯anion interactions.14 Indeed, the CH⋯O bond is known to be prevalent in many natural systems, playing an important role in protein-nucleic acid interactions and drug binding.13 In addition, the contact between N14 and O3A (3.36 Å) which is less than the upper limit (3.5 Å) for hydrogen bonding, could be considered as a weak hydrogen bond.6 Therefore, except one oxygen atom (O2A) which is directed outside the cavity, each oxygen atom accepts two hydrogen bonds either from two NH or one NH and one CH groups. This is in agreement to the SO42- binding by the two macrocyclic amides, in which each oxygen atom acts as two hydrogen bond acceptors.15
A close inspection of the structure suggests that the macrocyclic cation lies almost on a plane with N1⋯N14 distance of 7.218 Å (Figure 1B).16 The methyl groups on the tertiary amines sit on the same side of the macrocycle. The macrocycle, the encapsulated sulfate and three of the water molecules lie on a crystallographic mirror plane. The orientation of oxygens of the internal sulfate is such that they are disordered in a 50:50 ratio at mirror symmetry-related positions (Figure 1C). The water O4S is too close to one oxygen of the sulfate, so the occupancy of O4S was set to 1/2, the same occupancy as that of the oxygens in internal sulfate. The protons on (N4 and N4i) are directed outside the cavity and involved in coordinating two external sulfates (see supplementary materials).
In order to compare the sulfate binding in solution with that in solid state, 1H NMR titrations were performed using [H6L]·6Ts (5 mM) with an increasing amount of sodium sulfate solution (50 mM) in D2O at pH 2.1 (see supplementary materials for the synthetic procedure of [H6L]·6Ts). As shown in Figure 2, the addition of the anion resulted in a significant downfield shift of the macrocyclic protons. It is remarkable that the large shifts were observed for the aliphatic protons, on CH2 (b, Δδ=0.73 ppm) and CH3 (a, Δδ=0.69 ppm) which are directly linked to the tertiary nitrogen centers. The observation suggests the possible interactions between sulfate with protons of central nitrogens, supporting the results obtained in the crystal structure. The involvement of the protons on tertiary amines were in recently in a thiophene-based cryptand binding one chloride17a and three nitrate17b ions in aqueous solution. The protons (c and d, see the Figure 2) on the methylene groups linked with secondary amines, shifted downfield by 0.25 and 0.08 ppm, respectively. On the other hand, there was no significant shift observed in the aromatic resonances of L. We also assume that the large shift in the aliphatic protons as compared to the related compounds,7,11,17 could be due to the effect of the added charge in the divalent sulfate. The observed shift changes for several protons were plotted against the anion concentration which provided the best fit for a 1:1 binding model18 (Figure 3), yielding in a binding constant (K) of 3200 M-1 which is considerable higher than those reported for other receptors in polar solvents, for example, 30 to 170 M-1 with tren based amides or sulfonamides in acetonitrile,19 or 68 M-1 with a amide-based cryptand in DMSO-d67 determined by NMR titrations. Clearly, the binding of L with sulfate is enhanced by electrostatic interactions of charged ligand.
Figure 2.
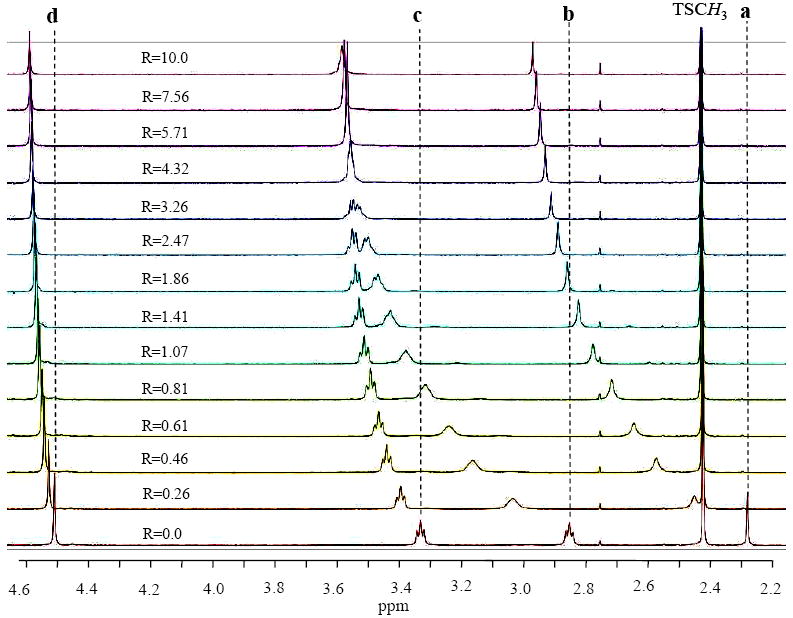
Partial 1H NMR spectra of tosylated salt of L (5mM) with the increasing amount of sodium sulfate (50 mM) in D2O at pH 2.1. a = NCH3, b = NCH2, c = NCH2CH2 and d = ArCH2.
Figure 3.
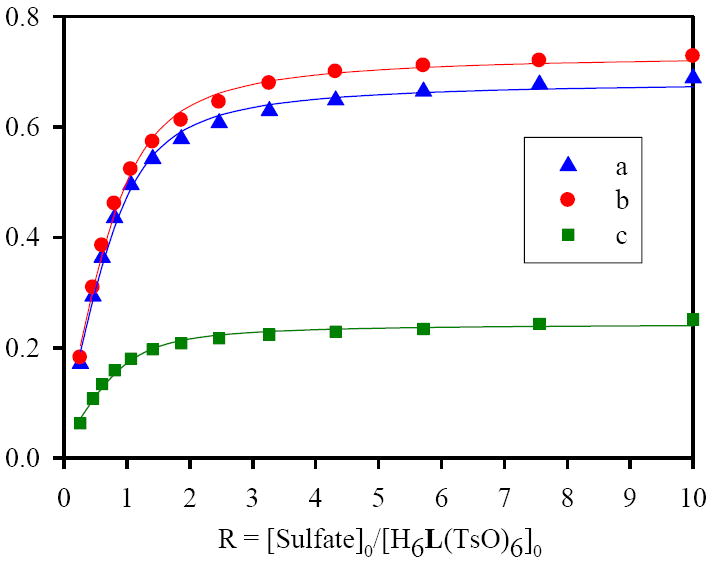
1H NMR titration curves for sulfate binding with H6L(OTs)6 in D2O at pH = 2.1. Net changes in the chemical shifts of a = NCH3, b = NCH2 and c = NCH2CH2 are shown against the anion concentration.
The evidence of the complex formation between L and sulfate was also further confirmed by ESI-MS experiments. As shown in Figure 4, the complex displays a cationic peak at m/z 549.0 for the monovalent [H3LSO4]+ and m/z 274.8 for the divalent [H4LSO4]2+. The peak at m/z 451.2 corresponds to monovalent free ligand [HL]+ while the peak at m/z 226.3 for divalent [H2L]2+. This data further support that sulfate is tightly held with the macrocyclic ligand, which is consistence to the 1:1 complexation observed in the solid and solution states.
Figure 4.
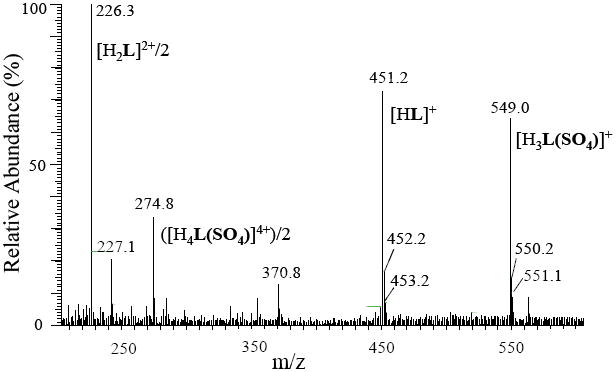
ESI-MS (positive ion mode) spectrum of the sulfate complex. The solution was prepared from the sulfate salt of L (1.0×10-5 M) in MeOH/H2O (50:50).
In conclusion, we have synthesized a new thiophene-based azamacrocycle containing both secondary and tertiary amines which is found to encapsulate sulfate anion via strong NH⋯O and CH⋯O interactions. Although the conventional hydrogen bonds are the primary binding forces, the involvement of CH⋯O bond provides enhanced stability of the sulfate complex. To the best of our knowledge, structural evidence of an encapsulated sulfate in polyamine-based macrocycle has not been reported before. The results from 1HNMR and ESI-MS studies further confirmed the formation of the sulfate complex which can exist in solution with a considerable stability.
Supplementary Material
Acknowledgments
This research sponsored by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, US Department of Energy, under contract with Oak Ridge National Laboratory (MAH). The project described was supported by Grant Number G12RR013459 from the National Center for Research Resources. This material is based upon work supported by the National Science Foundation under CHE-0821357. Purchase of the diffractometer was made possible by grant No. LEQSF (1999-2000)-ENH-TR-13, administered by the Louisiana Board of Regents. The authors thank the National Science Foundation (CHE-0130835) and the University of Oklahoma for funds to acquire the diffractometer used in this work.
Footnotes
Crystallographic file in CIF format and synthetic procedures. Supplementary data associated with this article can be found in the online version, at doi:xxxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Bowman-James K. Acc Chem Res. 2005;38:671–678. doi: 10.1021/ar040071t. [DOI] [PubMed] [Google Scholar]; (b) Gale PA. Acc Chem Res. 2006;39:465–475. doi: 10.1021/ar040237q. [DOI] [PubMed] [Google Scholar]; (c) Hossain MA. Curr Org Chem. 2008;12:1231–1256. [Google Scholar]; (d) Caltagirone C, Gale PA. Chem Soc Rev. 2009;38:520–563. doi: 10.1039/b806422a. [DOI] [PubMed] [Google Scholar]
- 2.Young RW. J Cell Biol. 1973;57:175–189. doi: 10.1083/jcb.57.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflugrath JW, Quiocho FA. Nature. 1985;314:257–260. doi: 10.1038/314257a0. [DOI] [PubMed] [Google Scholar]
- 4.Mullen KM, Beer PD. Chem Soc Rev. 2009;38:1701–1713. doi: 10.1039/b806041j. [DOI] [PubMed] [Google Scholar]
- 5.(a) Sessler JL, Katayev E, Pantos GD, Ustynyuk YA. Chem Commu. 2004:1276–1277. doi: 10.1039/b403665d. [DOI] [PubMed] [Google Scholar]; (b) Fowler CJ, Haverlock TJ, Moyer BA, Shriver JA, Gross DE, Marquez M, Sessler JL, Hossain MA, Bowman-James K. J Am Chem Soc. 2008;130:14386–14387. doi: 10.1021/ja806511b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Custelcean R, Moyer BA, Hay BP. Chem Commun. 2005:5971–5973. doi: 10.1039/b511809c. [DOI] [PubMed] [Google Scholar]
- 7.Kang SO, Hossain MA, Powell D, Bowman-James K. Chem Commun. 2005:328–330. doi: 10.1039/b411904e. [DOI] [PubMed] [Google Scholar]
- 8.Wu B, Liang J, Yang J, Jia C, Yang X-J, Zhang H, Tang N, Janiak C. Chem Commun. 2008:1762–1764. doi: 10.1039/b719019k. [DOI] [PubMed] [Google Scholar]
- 9.Kang SO, Day VW, Bowman-James K. Org Lett. 2009;11:3654–3657. doi: 10.1021/ol9014249. [DOI] [PubMed] [Google Scholar]
- 10.(a) Llinares JM, Powell D, Bowman-James K. Coord Chem Rev. 2003;240:57–75. [Google Scholar]; (b) García-España E, Díaz P, Llinares JM, Bianchi A. Coord Chem Rev. 2006;250:2952–2986. [Google Scholar]; (c) Clifford T, Danby A, Llinares JM, Mason S, Alcock NW, Powell D, Aguilar JA, Garcìa-España E, Bowman-James K. Inorg Chem. 2001;40:4710–4720. doi: 10.1021/ic010135l. [DOI] [PubMed] [Google Scholar]
- 11.Hossain MA, Saeed MA, Fronczek FR, Wong BM, Dey KR, Mendy JS, Gibson D. Cryst Growth Des. 2010;10:1478–1481. doi: 10.1021/cg100110f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crystal data for 10020: C22H44N6S2•3(O4S)•4(H2O), M = 817.00, orthorhombic, a = 11.901(12) Å, b = 13.752(16) Å, c = 21.64(2) Å, V = 3542(6) Å3, T = 100(2) K, space group Pnma, Z = 4, 21410 reflections measured, 1941 independent reflections (Rint = 0.0786). The final R1 values were 0.0444 (I > 2σ(I)). The final wR(F2) values were 0.1154 (I > 2σ(I)).
- 13.Scheiner S, Kar T, Gu Y. J Biol Chem. 2001;276:9832–9837. doi: 10.1074/jbc.M010770200. [DOI] [PubMed] [Google Scholar]
- 14.Juwarker H, Lenhardt JM, Pham DM, Craig SL. Angew Chem Int Ed. 2008;120:3800–3803. doi: 10.1002/anie.200800548. [DOI] [PubMed] [Google Scholar]
- 15.Hossain MA, Llinares JM, Powell D, Bowman-James K. Inorg Chem. 2001;40:2936–2937. doi: 10.1021/ic015508x. [DOI] [PubMed] [Google Scholar]
- 16.We also isolated crystals of sulfate complex of L grown in different environment which provided the same structure but with five molecules of water. The cell parameters and the nature of disorder were exactly the same as in the structure reported herein.
- 17.(a) Saeed MA, Fronczek FR, Hossain MA. Chem Commun. 2009:6409–6411. doi: 10.1039/b916099j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saeed MA, Fronczek FR, Huang M-J, Hossain MA. Chem Commun. 2010;46:404–406. doi: 10.1039/b923013k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider HJ, Kramer R, Simova S, Schneider U. J Am Chem Soc. 1988;110:6442–6448. [Google Scholar]
- 19.Valiyaveettil S, Engbersen JFJ, Verboom W, Reinhoudt DN. Angew Chem Int Ed. 1993;32:900–901. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


