Abstract
When another person's actions are observed it appears that these actions are simulated, such that similar motor processes are triggered in the observer. Much evidence suggests that such simulation concerns the achievement of behavioural goals, such as grasping a particular object, and is less concerned with the specific nature of the action, such as the path the hand takes to reach the goal object. We demonstrate that when observing another person reach around an obstacle, an observer's subsequent reach has an increased curved trajectory, reflecting motor priming of reach path. This priming of reach trajectory via action observation can take place under a variety of circumstances: with or without a shared goal, and when the action is seen from a variety of perspectives. However, of most importance, the reach path priming effect is only evoked if the obstacle avoided by another person is within the action (peripersonal) space of the observer.
Keywords: Mirror neurons, Peripersonal space, Reaching, Obstacle avoidance, Action priming
For more than a decade there has been a substantial amount of research investigating how the actions of other individuals are encoded. A key idea is that one means of understanding the behaviour of others is by simulating their actions. Thus, the motor representations that would be activated when undertaking a task are active when one is merely observing another person undertake the same action. Perhaps the key to motivating this research approach was the discovery of mirror cells. These were neurons in ventral premotor region F5 in the macaque that responded when the monkey grasped an object, but also responded in a similar way when the same action was observed (e.g., di Pellegrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992). Later research has also found neurons with mirror properties in the inferior parietal lobe (IPL) of monkeys (Fogassi, Gallese, Fadiga, & Rizzolatti, 1998; Fogassi et al., 2005).
After the initial discovery of mirror systems via single unit recording in the monkey, subsequent work has revealed similar systems in humans. Via functional magnetic resonance imaging (fMRI), regions around the dorsal and ventral premotor cortices and in the inferior parietal lobe have been identified that become active when observing another person's behaviour (e.g., Binkofski et al., 1999; Gazzola, Rizzolatti, Wicker, & Keysers, 2007; Iacoboni et al., 1999). Furthermore, activation of visual–motor systems via observation predicts that one's own actions are primed by this activation. Indeed, observing an action such as grasping an object facilitates the production of the same action shortly afterwards (e.g., Castiello, Lusher, Mari, Edwards, & Humphreys, 2002; Craighero, Fadiga, Rizzolatti, & Umiltà,1998; Edwards, Humphreys, & Castiello, 2003). Similarly, Bach and Tipper (2007) showed that when actions such as kicking a soccer ball or typing are observed, subsequent foot and hand responses, respectively, were facilitated.
A key issue is what form of information is encoded when another individual's actions are observed. It is clear that there are two components to prehensile actions: reach and grasp. These appear to be encoded by different neural systems (e.g., Jeannerod, 1988). It might be the case that both of these motor processes are simulated, such that the path the hand takes to reach an object and the highly specific patterns of the finger movements as they shape to grasp the target are reflected in neural responses. However, in single-cell studies of mirror systems sensitive to the form of grasp, the specific reach path as the hand approaches an object has not been reported as critical to neural activity (e.g., Fogassi et al., 2001; Murata, Gallese, Luppino, Kaseda, & Sakata, 2000). It is therefore possible that the mirror-like system's primary role is to understand the goal of the action (pick up a glass), with little concern for the specific way the goal was achieved (how the hand reached the glass; van Elk, van Schie, &Bekkering, 2008).
There is clear evidence to support the idea that the action simulation processes are concerned with encoding the behavioural goal, rather than the low-level specific reach properties of the action: When observing a reach, some parietal cells respond selectively when the final grasp is for eating but not when it is for placing (Fogassi et al., 2005; and in humans, Iacoboni et al., 2005). In fact the action goal, such as pressing down on an object, can produce facilitation priming effects when repeated, even if the observed and subsequently produced action is undertaken by a different effector (e.g., hand then foot; Costantini, Committeri & Galati, 2008). Similarly, the discovery of acoustic mirror neurons that respond to the sound of an action that cannot be seen (Kohler et al., 2002) and cells that respond when action cannot be directly observed when grasping an object behind an occluder (Umiltà et al., 2001), also suggest that the mirror system may code actions at an abstract level in terms of the goal to be achieved rather than the specific form of the action (e.g., Gallese & Lakoff, 2005). Similarly, in human participants, Castiello (2003) showed that the motor system of a participant can be primed when no action is directly viewed. That is, observing another person looking towards target and distractor objects activates the intention to act, which in turn stimulates similar motor representations in the observer. That actions can be primed when no action is observed is a strong demonstration that motor simulation is driven by abstract goals and that the specific properties of an action, such as reach trajectory, are not necessary for action priming (see also Bach & Tipper, 2006).
In the current study we attempt to find evidence that not only is the goal of an action encoded via grasp information, but in fact in some circumstances more specific properties of action, such as reach trajectory, are also encoded (see also Hamilton & Grafton, 2006). Clearly prehensile actions directed towards objects require these two reach and grasp processes, and we believe that in some situations the reach, as well as action goal of grasp, is simulated. For example, consider the task of reaching around an obstacle to grasp a target object. In this situation we hypothesize that the reach component is now relevant to understanding the other person's behaviour and hence will be simulated. Therefore, although grasp- and goal-related simulation has been detected thus far, the key component of how the hand reaches to a target should also be simulated in some situations for a full understanding of another person's behaviour.
To investigate whether the reach of another person can be implicitly simulated we employed priming methods. As noted above, a large number of studies have investigated how the implicit encoding of observed, goal-orientated, grasping actions affects subsequent action by the observer (e.g., Brass, Bekkering, & Prinz, 2001; Craighero et al., 1998; Stürmer, Aschersleben, & Prinz, 2000). Other studies have focused on related issues—that is, situations where actions have to be directed to target objects in the presence of distractors. For example, Tipper, Lortie, and Baylis (1992) and Tipper, Howard, and Jackson (1997) demonstrated action-centred selection processes in a selective reaching task. That is, when a person was reaching for a target, distractors closer to the hand produced significant interference and were associated with inhibition, as revealed by curved trajectories and subsequently slowed responses (i.e., negative priming effects). Thus, selective action directed towards a target was achieved in part by inhibition of the competing distractor, and in other studies (e.g., Schuch & Tipper, 2007) we have provided some evidence that another person's inhibition of an action can be simulated by an observer (see also Frischen, Loach, & Tipper, 2009).
In the current work, to investigate simulation of selective reaching, we utilized the methods developed by Jax and Rosenbaum (2007). They reported that, after avoidance of an obstacle, subsequent reaches to targets when there was no obstacle present showed curved trajectories. That is, prior obstacle avoidance primed the motor system such that those representations were accessed for subsequent reaches, resulting in curved trajectories when a straight reach was more appropriate. Therefore the obstacle avoidance priming effect would seem to be an ideal technique to examine whether the processes involved—that is, the avoidance reach path—are simulated. We adopted the methods of Jax and Rosenbaum because they produce robust effects and because the avoidance of an obstacle is a salient property of another person's reach trajectory, and hence it is a suitable approach to examine whether the obstacle avoidance priming effects generalize between people.
A series of experiments are reported. As a preview, in a new procedure we replicate the basic effects reported by Jax and Rosenbaum (2007) where a reach is more curved when a previous reach by the same person avoided an obstacle. In a further two experiments, when two people undertook alternate trials we did not find any evidence for the obstacle avoidance of one individual affecting the reach trajectory of a second person on the next trial. Although this suggested that obstacle avoidance is not simulated, we noted that in these experiments the obstacle avoided was outside the peripersonal space of the observer. In previous research investigating response to targets in the presence of distractors, effects appeared to be influenced by the distance between the distractor object and responding hand (e.g., Howard & Tipper, 1997; Meegan & Tipper, 1999; Tipper et al., 1992). Indeed, a subsequent study showed that when the observed obstacle was within a viewer's peripersonal action space, another person's reach path was simulated and influenced the observer's subsequent reach trajectory. In final studies we show that these peripersonal reach simulation/priming effects generalize to situations where two people are in a variety of different spatial positions relative to one another, whether they respond to the same target object, avoid the same distractor obstacle, or even when they respond to completely separate targets and obstacles in different tasks. A final study employing a transparent barrier between an observer and another person's reach confirmed the effect when the obstacle was within the peripersonal space of the viewer.
EXPERIMENT 1: SINGLE PERSON REACHING
In this initial experiment we sought to replicate the carry-over effect found by Jax and Rosenbaum (2007) and to establish whether this approach, placed into a real-world setting, would provide a methodology that could be used to investigate between-person effects. Note that in Jax and Rosenbaum's work, targets and distractors were presented on a computer screen, and reaches were made by moving a pen across a graphics tablet oriented 90° to the display and in a different spatial location. In sharp contrast, our current experiment requires participants to reach for and lift up target blocks directly in front of them. When an obstacle was present they were asked to avoid that obstacle by reaching vertically over it.
Method
Participants
A total of 24 right-handed students (20 female), with a mean age of 20.3 years, participated in this study in return for course credits. All participants had normal or corrected-to-normal vision. Participants gave informed consent, and the study received the approval of the School of Psychology's ethics committee.
Materials and apparatus
Each participant had a retro reflective marker placed on their right wrist. Participants’ movements were tracked using a Qualisys ProReflex motion capturing system (Qualisys AB, Gothenburg, Sweden), and the data were recorded using Qualisys Track Manager (QTM) software (Qualisys AB). The target object to be reached for was 3 × 2 cm, and it was 9 cm high. The obstacle to be avoided was 4.5 × 4.5 cm, and it was 18 cm high. These materials and apparatus were constant throughout the series of experiments.
Procedure and design
Participants sat at a desk with the chair adjusted so that the arm to be used to reach to the target rested comfortably on the desk with the forearm at right angles to the upper arm and the hand approximately 20 cm from the trunk. The far edge of the target block (which participants have to reach to in order to grasp) was 40 cm from the starting position of the participant's reaching hand. This distance was selected as a comfortable reach distance, which was nevertheless close to the full extent of reach. The near edge of the obstacle, when present, was 20 cm directly ahead of the hand. The distances of the participant's target and obstacle blocks remained constant throughout all the experiments reported here. The experimenter sat directly in front of the participant. The obstacle, when not in place, was kept out of view. See Figure 1 for details.
Figure 1.
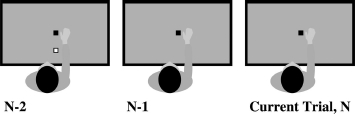
Experiment 1: Single person reaching. This diagram demonstrates an example series of reaches, where the same person performs on every trial. n-1 is the trial that preceded the current trial. n-2 is the last but one trial. Black square is the target; white square is the obstacle. This example shows an O–No–No trial sequence.
The experiment sought to investigate the effect of n-1 and n-2 trials on the current trial n. The order of obstacle presentation was therefore counterbalanced for n-1 and n-2 trials. Trials could be with or without an obstacle; counterbalancing for the current and previous two trials gave eight possible trial orders. Each of these occurred 10 times for the participant, in random order; two additional trials that were not further analysed were added to the start of the experiment to provide an n-2 condition history for the first trials of relevance.
Participants carried out 10 practice reaches. They were instructed to close their eyes at the end of a reach, opening them again on the instruction to initiate movement. This ensured that participants did not observe the experimenter's arm movements. They were instructed to reach out, lift up, and place the target block back down again in the same spot. If the obstacle was present they were instructed to reach over the obstacle in the vertical plane.
Results
For each trial we measured the maximum height reached on the outward movement. Unlike Jax and Rosenbaum's (2007) work we considered and compared only the n trials without an obstacle. With the use of real-world stimuli we found that participants were careful to avoid knocking over the obstacle and thus cleared the obstacle block with great care. This meant that during reaches over an obstacle there was very little variation in height between the trials; the obstacle trials had significantly smaller standard deviations than the non-obstacle trials [F(1, 13) = 41.05, p < .001]. Due to this lack of variability we observed no effects of the presence or absence of an obstacle in previous trials (n-2 and n-1) when analysing reach path on the critical trial (n). Therefore we do not discuss data from obstacle avoidance trials in the rest of the paper (though they are shown in the Appendix); rather we only discuss effects of prior trials on reaches where no obstacle was presented on trial n.
For the analysed trials where no obstacle was present in trial n there were four conditions (No = no obstacle, and O = obstacle):
| 1. | n-2 = No | n-1 = No | n = No |
| 2. | n-2 = O | n-1 = No | n = No |
| 3. | n-2 = No | n-1 = O | n = No |
| 4. | n-2 = O | n-1 = O | n = No |
We excluded a number of trials where the participant knocked over one of the blocks, and the two trials that followed such errors were removed (2.08% of trials). We have also excluded a number of trials where part of the trajectory was not properly tracked by the equipment.
Figure 2 represents the results. The heights were analysed using a two-way within-subjects analysis of variance (ANOVA), with two factors: n-1 trial type (with or without obstacle) and n-2 trial type. This analysis revealed a main effect of both the n-1 trial [F(1, 23) = 14.57, p < .001], and the n-2 trial [F(1, 23) = 10.36, p = .004]. That is, when participants have just avoided an obstacle, response on the next reach (n-1 effect), or the second reach (n-2 effect), are higher than if the previous trials had not contained an obstacle. There was no significant interaction between n-1 and n-2 [F(1, 23) = 2.39, ns]. Figure 2 also illustrates the results of planned comparisons between conditions. These comparisons compared the baseline No–No–No condition with O–No–No showing the n-2 effect to be significant [t = 2.127, p < .05], and No–No–No compared to No–O–No showing that the n-1 effect was significant [t = 2.522, p < .02].
Figure 2.
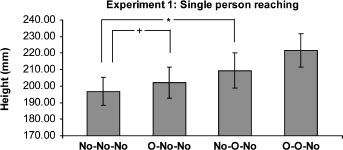
Means of the maximum height reached for non-obstacle reach trials (trial n), with their standard errors. The bars marked ∗ show a significant t test result. These are one-tailed t tests, as are those in the following experiments, as they examine a priori predictions. The bars are labelled with the trial type (n-2, n-1, n), where “No” represents non-obstacle trials and “O” where an obstacle was present.
Discussion
The results confirm the previous findings of Jax and Rosenbaum (2007) and van der Wel, Fleckenstein, Jax, and Rosenbaum (2007). The trial after obstacle avoidance is more curved as measured by the highest point of the reach trajectory. Although for our aims we did not do the thorough analysis of Jax and Rosenbaum, we have shown that prior obstacle avoidance influences actions two reaches in the future (n-2 effects), as reflected by the additive effects in the O–O–No condition. Therefore this procedure enables us to examine the effects when two people undertake alternate trials. We are able to examine the effects of one person on another (n-1) as well as examine the effects of an individual's own behaviour on their subsequent reach trajectories (n-2; see Schuch & Tipper, 2007, for similar approaches).
EXPERIMENT 2: SHARED TARGET, SEATED OPPOSITE, SAME HANDS
This experiment employed a very similar procedure to that of Experiment 1, except that two people, seated opposite each other, participated in the reaching to grasp task. The participants alternated their reaching actions, such that the possible priming effects produced by observing another person's reach could be observed on the next trial.
Method
Participants
A total of 24 right-handed students (17 female), with a mean age of 18.9 years, participated in this study in return for course credits. All participants had normal or corrected-to-normal vision.
Procedure and design
Having established that the Jax and Rosenbaum (2007) method elicited within-person obstacle priming effects, we modified the procedure for use with two persons. Our participants alternated reaching and observing while seated opposite each other. The shared target block was the same distance away from each person's reaching hand, as in Experiment 1 (40 cm). The experimenter sat at right angles to the participants. The obstacle block was placed at the same distance from each participant's reaching hand, as in Experiment 1. That is, the obstacle was 20 cm from each person's hand, and each participant had their own obstacle and never reached over the other person's obstacle. See Figure 3, Panel A for details.
Figure 3.
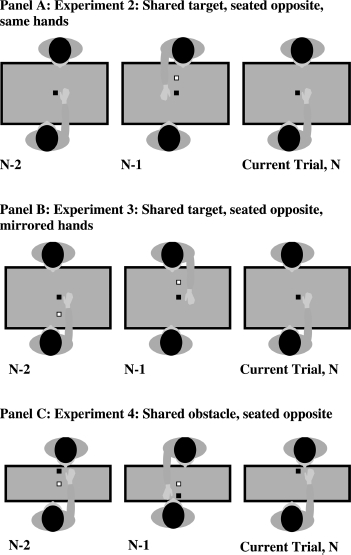
Experiments 2—4, seated opposite. Panel A: Experiment 2: shared target, seated opposite, same hands. Black square is the target; white square is the obstacle. This example shows a No — O—No trial. Panel B: Experiment 3: shared target, seated opposite, mirrored hands. This example shows an O —O —No trial. Panel C: Experiment 4: shared obstacle, seated opposite, same hands. This example shows an O —O —No trial. Experiments 2 and 3 share the same layout and differ only in hand use. Experiment 4 uses a narrower table, allowing participants to be close enough to share the same obstacle and to reach into each other's peripersonal space.
In order for each person to carry out each type of trial 10 times there were 164 trials per experiment, with a 5-minute break half way through. Participants alternated reaching and were called by name at the beginning of each trial. They were instructed that between trials their eyes would be closed. On hearing the name of the person who would act they were told to both open their eyes. The participant whose turn it was executed the reach and lift, returned her hand to the starting position, and then closed her eyes. The other participant was instructed to observe the scene, passively watching the other person's reach. Each participant carried out 10 practice reaches at the start of the experiment.
Results
As previously described, error trials (e.g., obstacle collision) were removed from analysis. Furthermore, where participants failed to open their eyes on an observation trial their following action trial was discounted. We removed 0.99% of trials.
The heights were again analysed using a two-way within-subjects ANOVA for each person's results, with two factors: n-1 trial, the effect of the other person's reach; and n-2 trial, the effect of her own movement (each factor being with and without obstacle). This analysis revealed no main effects for the within-subject n-2 effect [F(1, 23) = 2.33, ns], the between-subject n-1 effect [F(1, [F(1, 23) = 0.64, ns], or the interaction 23) = 0.06, ns]. See Figure 4, Panel A for the graph of height means. Further analysis with t tests comparing n-1 (No–O–No) and n-2 (O–No–No) with baseline (No–No–No) revealed no significant effects [t (23) = 0.43 and t(23) = 0.78, respectively].
Figure 4.
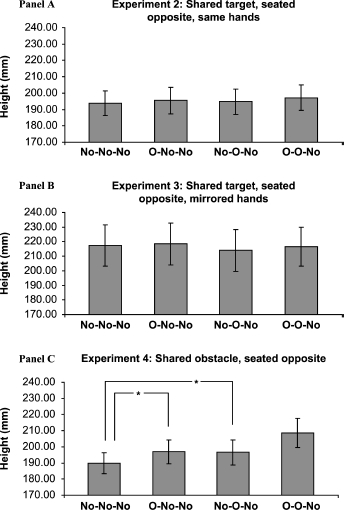
Means of the maximum height reached for non-obstacle reach trials (n), with their standard errors, for Experiments 2—4. The bars indicated by ∗ show a significant t test result atp < .01. The bars are labelled with the trial type (n-2, n-1, n), where “No” represents non-obstacle trials and “O” where an obstacle was present.
Discussion
Somewhat surprisingly, we did not detect any evidence for the idea that the reach trajectory of an observed person is simulated. That is, there was no evidence for a participant's reach to be higher just after they had observed another person reach over an obstacle. This lack of obstacle priming effect between people may be taken as evidence that indeed action simulation processes are somewhat abstract. Motor systems represent the goal of the action, in this case grasping the wooden block, but the specific manner of the action is not encoded. Such a result would be in line with previous work where mirror systems can encode actions even when they are not directly observed (e.g., Umiltà et al., 2001).
A second finding in this study is that the person's own prior reach has no significant effect on their subsequent reach path. That is, n-2 effects are not observed, which suggests that observation of another person undertaking the reach-to-grasp task interferes with the representation of one's own previous action.
To confirm the lack of reach path priming effects it was necessary to replicate and extend these results. Therefore in a follow-on study the same between-person priming study was undertaken, except that one participant reached with the right hand, whilst the other reached with the left (see Figure 3, Panel B). Previous work by Koski, Iacoboni, Dubeau, Woods, and Mazziotta (2003) has shown that inferior frontal mirror cells respond more when participants imitate as in a mirror (right hand–left hand) than when there is anatomical matching (right hand — right hand), and developmental studies show that early in life children tend to imitate as in a mirror, copying another person's right-hand actions with their own left hand (e.g., Bekkering, Wohlschlager, & Gattis, 2000; Wapner & Cirillo, 1968). Therefore this “mirror” condition may result in greater compatibility between observed and produced actions in that they were both presented in the same visual field, as compared to the opposite visual fields in Experiment 2 (compare Panels A and B in Figure 3).
EXPERIMENT 3: SHARED TARGET, SEATED OPPOSITE, MIRRORED HANDS
Method
Participants
A total of 24 right-handed students (18 female), with a mean age of 20.2 years, participated in this study in return for course credits. All participants had normal or corrected-to-normal vision.
Procedure and design
This experiment is the same as Experiment 2 except that one of the participants in each pair used their left hand, while the other used their right hand, so that the participants mirrored each other (see Figure 3, Panel B).
Results
In this experiment 1.72% of trials were removed. As in Experiment 2 there were no significant main effects [n-1: F(1, 23) = 2.10, ns; n-2: F(1, 23) = 0.42, ns] or interactions [F(1, 23) = 0.07, ns]. Neither the participants’ own previous actions (n-2) nor the observed actions (n-1) affected their current action (see Figure 4, Panel B). Further comparison between O–No–No and No–O–No and the baseline condition, No–No–No, gave no significant result [t (23) = 0.49 and t(23) = 1.39, respectively]. To increase power, the data from Experiments 2 and 3 were combined. This confirmed the lack of n-1 [F (1, 47) = 0.19, ns], and n-2 [F(1, 47) = 1.68, ns], effects and any interaction [F(1, 47) = 0.13, ns].
Discussion
This experiment replicated the results of Experiment 2 and seems to provide evidence that, as might be the case with the monkey mirror neuron system, the reach trajectory by which a target is approached is not encoded. However, it is necessary to be cautious in generalizing the results from any given set of experiments, and concluding that observed reach trajectories are never encoded might be premature. Certainly under the conditions described in these two experiments trajectory is not encoded, and the fact that in both experiments the person's own carry-over effect (n-2) also disappeared gave concern. This lack of own reach priming is discussed further in the General Discussion.
We thus sought to design an experiment in which the participants might engage more in the experiment and feel that the actions of the other person are more relevant to them. Note that in Experiments 2 and 3 during action observation the other person's obstacle was 54 cm (near edge) from the reaching hand and approximately 74 cm from the trunk of the observer. This resulted in the observed obstacle being just outside comfortable action (peripersonal) space for our participants. That is, without further actions such as leaning forward and raising the upper body, a reach action could not be achieved. Certainly, a reach over the other person's 18-cm-tall obstacle was not possible.
Therefore we hypothesized that perhaps the observation of avoidance of an obstacle outside peripersonal space, on which the participant could not act, might have made the action less relevant. This lack of relevance could have prevented the activation of simulation processes. Previous research has indeed shown that during selective reaching tasks, the distance of an irrelevant to-be-ignored distractor object from the reaching hand was critical for the obtained interference and priming effects. For example, Tipper et al. (1997; Tipper et al., 1992) revealed action-centred selection processes in a selective reaching task. That is, when reaching for a target, distractors closer to the hand produced significantly higher interference and negative priming.
Clear evidence for the existence of separate coding of peripersonal space comes from studies of neuronal activity in monkeys. Two areas involved in processing information in peripersonal space are the ventral intraparietal sulcus (VIP; Colby, Duhamel, & Goldberg, 1993; Duhamel, Colby, & Goldberg, 1998; Graziano & Gross, 1994) and the ventral premotor cortex, F4 (Fogassi et al., 1992, 1996; Graziano, Yap, & Gross, 1994) with the VIP having projections to F4 (Luppino, Murata, Govoni, & Matelli, 1999). F4 itself has projections to the primary motor cortex (Barbas & Pandya, 1987; Graziano & Gross, 1998). Therefore there is clearly a network in place in monkeys that specifically processes peripersonal space and allows this information to influence action.
That the brain makes a distinction between peripersonal and extrapersonal space in humans has been part of a number of theories. Previc (1998), for example, has proposed distinct cortical networks dealing with near and far space. He proposes that the dorsal visual pathway is involved with peripersonal space and actions carried out within it, whereas the ventral processing stream is concerned with extraperipersonal or far space. Similarly, the results of imaging studies by Weiss and colleagues (Weiss et al., 2000; Weiss, Marshall, Zilles, & Fink, 2003) support the differential involvement of these two streams. That near and far space might be dissociable is further indicated by the patients of Vuilleumier, Valenza, Mayer, Reverdin, and Landis (1998) and Halligan and Marshall (1991), the former having a patient who suffered from lateral neglect in far but not near peripersonal space, and the latter showing neglect for peripersonal near but not far space.
The fundamental importance of object distance for action is clear. That specific neural systems are dedicated to encoding peripersonal space, which enable immediate reach-to-grasp actions, while other systems encode objects in far space that require other motor processes (e.g., walking) before action can be produced, makes sense in terms of computational efficiency. It therefore remains a reasonable hypothesis that simulation processes of observed action may also be influenced by the distinction between peripersonal and far space. Therefore in Experiment 4 we replicated Experiments 2 and 3, but crucially, when observing obstacle avoidance the obstacle was within the peripersonal space of the observer; that is, it was 20 cm from the observer's hand.
EXPERIMENT 4: SHARED OBSTACLE, SEATED OPPOSITE, SAME HANDS
Method
Participants
A total of 24 right-handed students (18 female), with a mean age of 23.2 years, participated in this study in return for course credits. All participants had normal or corrected-to-normal vision.
Procedure and design
Both participants used their right hands and sat opposite each other. Unlike the previous two experiments, participants shared the obstacle, which remained 20 cm from their reaching hand. Therefore the obstacle was now in the peripersonal space of both the participants. In this new task the participants now reached for separate target objects, which were 40 cm from their reaching hand (see Figure 3, Panel C), with the other's target now 5 cm from their hand.
Results
We removed 1.20% of trials due to collision with the obstacle or failed recording.
Unlike the previous two experiments, this experiment showed a significant main effect for the influence of the other participants’ action on their current reach [n-1: F(1, 23) = 14.983, p < .01], showing that the priming effect, previously only observed within a participant's own previous actions, had transferred between people. Interestingly, also in contrast to Experiments 2 and 3, observing an action does not remove the effect of a person's own previous reach [n-2: F(1, 23) = 13.259, p < .01] (see Figure 4, Panel C). These two effects, within and between people, appear to be independent, and no interaction was found [F(1, 23) = 1.21, ns]. Further planned contrast t tests revealed that reaches in each of the obstacle conditions (n-2 and n-1) were significantly higher (p < .01) than the baseline condition (No–No–No) [that is, O–No–No, t(23) = 2.80; No–O–No, t(23) = 2.86].
In addition to the analysis mentioned above we compared the results from Experiment 4 with those of the previous two experiments. Comparing Experiment 4 with Experiment 2 in a mixed two-way ANOVA revealed a significant interaction between experiment and n-1 obstacle priming [F(1, 46) = 7.193, p < .05], and between experiment and n-2 priming [F(1, 46) = 6.423, p < .05]. Similarly, contrasts between Experiment 4 with Experiment 3 revealed significant interactions between experiment and n-1 obstacle priming [F(1, 46) = 15.809, p < .01], and between experiment and n-2 obstacle priming [F(1, 46) = 4.147, p < .05]. These results further confirm the distinction between the results of Experiment 4, where we find the obstacle priming effect, and Experiments 2 and 3, where no priming effect occurs.
As Experiment 4 is the first discovery of reach path priming between people and to further investigate the nature of the trajectory differences between Experiments 2 and 4, we carried out further analysis on several points along the trajectories, in addition to the comparison of maximum heights previously described. The Panels in Figure 5 illustrate the n-2 effect, that is, the comparison between No–No–No and O–No–No trials (Panels A and B), and the n-1 effect, that is, No–No–No versus No–O–No trials (Panels C and D). These figures show the qualitative distinction between experiments. The vertical lines show the points of comparison along the trajectories where we have carried out analysis in a 2 (obstacle condition) ? location (6 loci at 5-cm steps) ANOVA. For Experiment 4 there was a significant n-2 obstacle priming effect [F(1, 22) = 19.96, p < .001], which, as would be expected, interacted with location [F(1, 22) = 8.99, p < .001], declining as the hand approached the target. A similar pattern of effects was observed for n-1 obstacle avoidance [F(1, 22) = 14.85, p < .001], and interaction with location [F(1, 22) = 3.63, p < .01]. Confirming previous findings, there were no significant obstacle avoidance priming effects in Experiment 2 (all Fs < 2.01).
Figure 5.
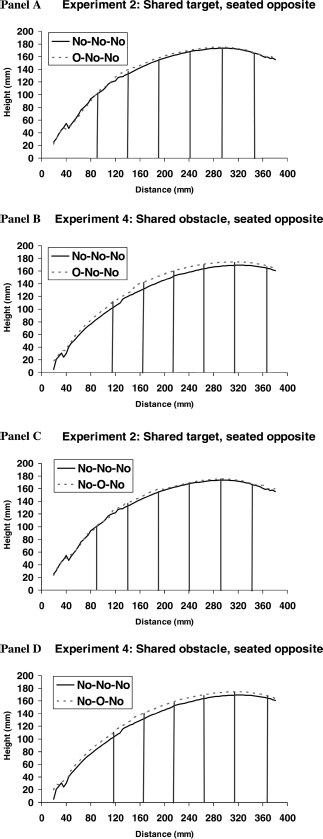
Panel A: Experiment 2: shared target, seated opposite, No–No–No vs. O–No–No trials. Panel B: Experiment 4: shared obstacle, seated opposite, No–No–No vs. O–No–No trials. Panel C: Experiment 2: shared target, seated opposite, No–No–No vs. No–O–No trials. Panel D: Experiment 4: shared obstacle, seated opposite, No–No–No vs. No–O–No trials. The trajectories shown are the aggregate of each participant's average reach in that condition. The last 5 cm of the reach path have been removed due to the amount of noise at the end of the reach as participants adjust their hand for grasping. The vertical lines mark the points of the curve used in the ANOVA analysis described in the results section of Experiment 4. The far left of the curve was not analysed due to missing data from some participants from the early part of their reaches.
Discussion
For the first time we have shown that observing another person's actions appears to evoke simulations of the reach path of the hand. Thus, after observing another person reach over an obstacle, the observer's subsequent reach is higher. Such an effect implies that prior activation of a reach simulation remains active, or can be retrieved from memory, to affect a subsequent reach.
Importantly, we have identified a critical boundary condition to such a reach trajectory simulation effect. That is, the contrasting data between Experiments 2 and 3 and Experiment 4 support our proposal that reach path simulation only takes place when objects are within peripersonal action space. We also ran a control experiment to confirm that the obstacle avoidance priming effects were indeed produced by observing another person's reach and not just due to the presence of the objects. In a new version of Experiment 4 a single participant was required to reach for targets only on alternate trials and to merely look at the display on the other trials. That is, participants were asked to merely look at the n-1 display and then undertake the next reach trial (n). In this situation the same objects were viewed on n-1 trials, but there was no other person making a reach. We found no hint of an obstacle avoidance priming effect, confirming that it is the observation of another person's action that mediates the effect.
However, note that there are other contrasts between Experiments 2 and 3 and Experiment 4 that could influence when reach simulation processes are activated. For example, in Experiments 2 and 3 where no simulation/priming effects were observed, participants reached towards and grasped the same target, while they avoided completely different obstacles. In contrast, in Experiment 4 where reach simulation effects were observed, participants shared an obstacle, but reached to completely different targets. Although we have no a priori reasons for predicting that these circumstances could mediate our contrasting effects, we needed to investigate more formally the roles of sharing a target as compared to sharing an obstacle.
Therefore in Experiments 5a and 5b we developed a new task to examine three issues: First, in these experiments the obstacle was always within the action/peripersonal space of both participants, and hence we hoped to replicate reach simulation/priming effects. Second, in Experiment 5a both participants reach over the same obstacle while responding to different targets. This is similar to the procedure of Experiment 4 and hence should replicate those reach simulation/priming effects. In contrast, Experiment 5b required participants to reach to the same target while avoiding different obstacles. This procedure is similar to that of Experiments 2 and 3. If we observe reach simulation/priming in this latter condition, the prior results cannot be explained by whether or not obstacles are shared. Third, we examined a new interpersonal spatial layout in this experiment. Rather than two people sitting opposite each other, they were oriented 90° (see Figure 6, Panels A and B). We predicted that reach simulation processes were not constrained by specific viewpoints of other people, and effects would be detected in this new design.
Figure 6.
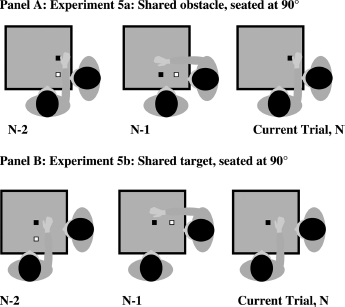
Seated at 90°. Panel A: Experiment 5a: shared obstacle, seated at 900°. Panel B: Experiment 5b: shared target, seated at 90°. In Experiment 5a participants avoided the same obstacle. In Experiment 5b participants grasped the same target. Black square is the target; white square is the obstacle.
EXPERIMENT 5A: SHARED OBSTACLE, SEATED AT 90°
Method
Participants
A total of 24 right-handed students (17 female), with a mean age of 21.63 years, participated in this study in return for course credits. All participants had normal or corrected-to-normal vision.
Procedure and design
Both participants used their right hands. They were seated at 90° to each other on two sides of a table. They both shared the obstacle, which was 20 cm from each of them. They responded to separate targets, which were 40 cm from the reaching hand (see Figure 6, Panel A).
Results
We removed 0.68% of obstacle collision and failed recording trials.
Results are shown in Figure 7, Panel A. As with the previous experiment significant main effects were found for the influence of the other participant on the reach [n-1: F(1, 23) = 9.086, p < .01] and of an individual's previous reach [n-2: F(1, 23) = 7.402, p < .05], again with no significant interaction [F(1, 23) = 4.05, ns]. Further planned contrast t tests revealed that reaches in each of the obstacle conditions were significantly higher (p < .01) than those in the baseline condition (No–No–No) [that is, O–No–No, t(23) = 3.37; No–O–No, t(23) = 3.53].
Figure 7.
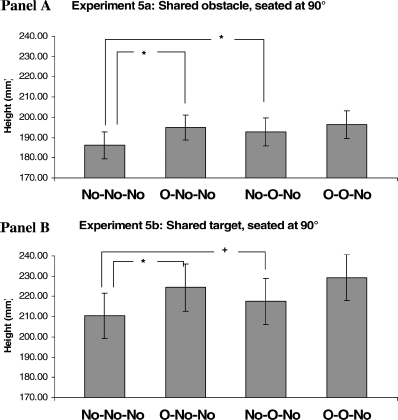
Means of the maximum height reached for non-obstacle reach trials (n), with their standard errors, for Experiments 5a and 5b. The bars indicated by ∗ show a significant t test result at p < .01; + represents a p value < .05. The bars are labelled with the trial type (n-2, n-1, n), where “No” represents non-obstacle trials and “O” where an obstacle was present.
Discussion
This experiment replicated Experiment 4, showing again that reaches within peripersonal space, with a shared obstacle, are encoded. It additionally shows that our results are not view dependent and generalize to other perspectives. It is an important companion experiment to the following Experiment 5b, which investigated the effect of shared targets.
EXPERIMENT 5B: SHARED TARGET, SEATED AT 90°
Method
Participants
A total of 24 right-handed students (16 female), with a mean age of 21.00 years, participated in this study in return for course credits. All participants had normal or corrected-to-normal vision.
Procedure and design
Both participants used their right hands. They were seated at 90° to each other on two sides of a table. They both shared the target object, which was 40 cm from each of their hands. Their separate obstacles were 20 cm from their reaching hands (see Figure 6, Panel B). The other person's obstacle was 42 cm from their hand and was within peripersonal reach space.
Results
We removed 0.57% of obstacle collision and failed recording trials.
This experiment showed significant main effects for the influence of the other participant's reach [n-1: F(1, 23) = 8.549, p < .01] and of the individual's previous reach [n-2: F(1, 23) = 46.026, p < .001] (see Figure 7, Panel B). There was no interaction between these two factors [F(1, 23) = 0.13, ns]. Further planned contrast t tests revealed that reaches in each of the key obstacle conditions were significantly higher than those in the baseline condition (No–No–No) [that is, O–No–No, t(23) = 3.76, p < .01; No–O–No, t(23) = 1.85, p < .05].
Discussion
This result further extends and clarifies the circumstances under which observation of action primes the trajectory of a person's future actions, showing that in addition to occurring when participants avoid the same shared obstacle it can also occur when the participants are sharing a target object, and an obstacle is not shared. This demonstrates that the lack of significant results from Experiments 2 and 3 did not occur because the participants shared the target rather than obstacle, but it lends weight to our argument that the factor influencing whether or not priming occurs is in fact whether or not observed action takes place in the peripersonal space of the viewer. In Experiment 5a the observed obstacle was 20 cm, and in Experiment 5b it was 42 cm, from the observer's reaching hand, both within action space.
EXPERIMENT 6: SEATED ADJACENT, NO OBJECTS SHARED
So far our experiments have all involved the sharing of either the target or obstacle blocks. Humans posses the capability of joint action to achieve goals; this has been demonstrated in various settings (see Sebanz, Bekkering, & Knoblich, 2006, for a review). It is a plausible assumption that simulation of another's action may be tempered by how involved the participant feels in the other's action, and thus simulation might be limited to those scenarios in which objects as well as space are directly shared.
To investigate whether such a limitation was the case we designed Experiment 6. This task is represented in Figure 8, Panel A. Participants were adjacent to each other facing in the same direction. Each participant reached for their own target presented directly in front of them and also reached over their own obstacle. In essence each person was now undertaking their own individual reaching task, while the other person's reaches were to objects that were irrelevant. Importantly, although the other person's obstacle was irrelevant and never near the observer's reach path, it was within 40 cm of the observer's hand, so within peripersonal reaching space.
Figure 8.
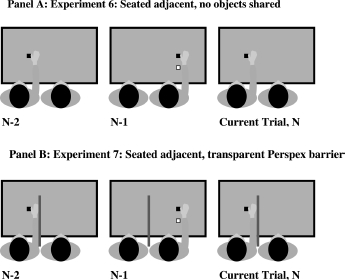
Seated adjacent. Panel A: Experiment 6: seated adjacent, no objects shared. Panel B: Experiment 7: seated adjacent, transparent Perspex barrier. In Experiments 6 and 7 neither the target nor the obstacle is shared by the participants, and they reach to different locations. The black square is the target; the white square is the obstacle. Importantly in Experiment 6 the other person's obstacle was within reaching peripersonal space of the observer. In Experiment 7 the obstacle was visually within peripersonal space; however, the Perspex barrier (the dark grey vertical line), prevented the participants from being able to actually reach to the other's obstacle. The barrier was 50 cm high and 55 cm long. It extended over the edge of the table between the participants by 5 cm.
Method
Participants
A total of 24 right-handed students (17 female), with a mean age of 18.9 years, participated in this study in return for course credits. All participants had normal or corrected-to-normal vision.
Procedure and design
In this experiment participants sat adjacent to each other. They both used their right hands and did not share each other's blocks. As in the previous experiments the obstacle when present was 20 cm from their hand, and the target block was 40 cm. The other participant's obstacle was 40 cm away from their reaching hand.
Results
We removed 1.62% of obstacle collision and failed recording trials.
The results from this experiment replicate and extend the results from Experiments 4, 5a, and 5b. Again there was a significant effect of own previous movements [n-2: F(1, 23) = 6.55; p < .05] and of the other person's movements [n-1: F(1, 23) = 8.43; p < .01] on the current reach trajectory (see Figure 9, Panel A). Again there was no significant interaction [F(1, 23) = 0.15, ns]. Further planned contrast t tests revealed that reaches in each of the obstacle conditions (n-2 and n-1) were significantly (p < .01) higher than those in the baseline condition (No–No–No) [that is, O–No–No, t(23) = 2.37; No–O–No, t(23) = 2.38].
Figure 9.
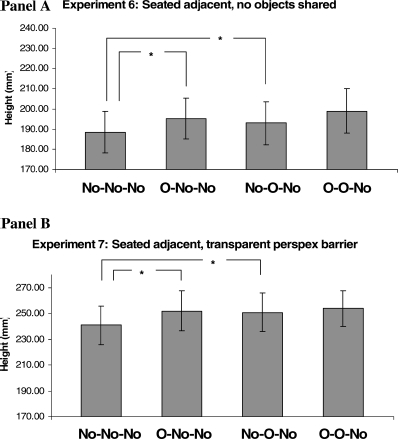
Means ofthe maximum height reached for non-obstacle reach trials, with their standard errors, for Experiments 6 and 7. The bars indicated by ∗ show a significant t test result at p < .01. The bars are labelled with the trial type (n-2, n-1, n), where “No” represents non-obstacle trials and “O” where an obstacle was present.
Discussion
Here we show that it is not necessary for there to be joint action on shared objects in order for priming to occur, merely that the actions of the other person take place in peripersonal space. Furthermore, the viewpoint of observed actions has been changed again, revealing that these reach path priming effects are robust in a range of settings, as long as the obstacles are within peripersonal space.
EXPERIMENT 7: SEATED ADJACENT, TRANSPARENT PERSPEX BARRIER
In the final experiment we examined further properties of the peripersonal action priming effects: first, whether they were determined by the geometrical distance of the obstacle to the participant's hand and, second, whether they were determined by the object's potential for action. The approach is based on our previous studies of selective reaching (Meegan & Tipper, 1999; Tipper, Meegan, & Howard 2002).1 We had previously demonstrated that when reaching for target keys to be depressed, irrelevant to-be-ignored distractors interfered with (slowed) response to the target and were associated with inhibition, as measured via negative priming effects (e.g., Tipper et al., 1992).
These distractor interference and negative priming effects were hand centred, in that they were larger when the distractors were closer to the participant's reaching hand. We argued that such hand-centred effects were due to the near distractor winning the race for the control of action. This race model predicted that if we could slow down response encoding of the distractor, while maintaining the same visual information, interference and negative priming effects would be reduced. To this end we presented transparent obstacles over the distractor object, which made the key depression response to them, when they were targets on other trials, more difficult. The results were very clear. Although the visual properties of the distractor were held constant, making the response more difficult with the transparent obstacle placed over the key greatly reduced how much the distractor interfered, and it abolished the inhibition associated with it.
Therefore we replicated Experiment 6, but now presented a transparent Perspex barrier between the two participants (see Figure 8, Panel B). If it is simply the metric distance of the obstacle to the participant's hand that determines whether simulation of reach path is evoked, then we should see similar action priming to that observed in Experiment 6. However, following the findings of Meegan and Tipper (1999) and Tipper et al. (2002), we predicted that even though the other person's reach over an obstacle could be clearly seen, because it was not a potential obstacle for the viewing participant, it would not be simulated and hence would have no effect on the participant's subsequent reach.
Method
Participants
A total of 24 right-handed students (14 female), with a mean age of 24.4 years, took part in this study in return for course credits. All participants had normal or corrected-to-normal vision.
Procedure and design
The seating arrangement and block placement in this experiment were identical to those of Experiment 6, with participants seated adjacent to each other. However, in this experiment participants were separated from each other by a clear Perspex screen (see Figure 8, Panel B). The screen was 50 cm high and 55 cm long. The screen extended from the table, between the participants, by 5 cm. The screen was 5 cm from the blocks of the participant seated on the left side.
Results
We removed 1.24% of obstacle collision and failed recording trials.
The results from this experiment replicate and extend the results from Experiments 4, 5a, 5b, and 6. Again there was a significant effect of own previous movements [n-2: F(1, 23) = 8.09; p < .01] and of the other person's movements [n-1: F(1, 23) = 4.987; p < .05] on the current reach trajectory (see Figure 9, Panel B). Furthermore there was a significant interaction between n-2 and n-1 [F(1, 23) = 4.38, p < .05], revealing that obstacle priming effects were more potent for n-2. Further planned contrast t tests revealed that reaches in each of the key n-2 and n-1 obstacle conditions were significantly (p < .01) higher than those for the baseline condition (No–No–No) [that is, O–No–No, t(23) = 4.59; No–O–No, t(23) = 3.46].
Discussion
This experiment tested two alternative accounts of the between-person obstacle priming effect. In one, the metric distance of the obstacle from the participant's responding hand was computed, and if this was perceived to be within peripersonal action distance, simulation of the other person's obstacle avoidance processes was activated. The alternative hypothesis was that the potential for action was encoded. Thus, although the obstacle avoidance of the other person's reach could easily be seen through the transparent barrier, because the obstacle could not be directly acted upon by the viewer the simulation processes would not be activated.
As noted above, our expectation was that the latter account would be supported. When viewing the other person's reach over an obstacle through a barrier, simulation would not take place, and hence no n-1 reach path priming effects would be detected. Clearly this was not confirmed, as significant n-1 obstacle priming was detected. Thus after observing through a transparent barrier a person reach over an obstacle, the participant's subsequent reach was higher. This result contrasts with our previous work (Meegan & Tipper, 1999; Tipper et al., 2002). However, we note that in the previous studies the obstacle was placed over the target, so it did not influence much of the reach path, but affected the final adjustment of the hand as it depressed the target key. In contrast, in the current study the obstacle was placed midway between the hand and target and influenced the reach aspect of the prehension system. These different findings perhaps reveal a further contrast between reach and the final stages of action such as grasp and key depression. Certainly the present results of Experiment 7 support the notion that the simulation of another person's obstacle avoidance reach path is determined by the metric distance of the obstacle from the observers’ hand, and not higher level factors such as the potential for action.
GENERAL DISCUSSION
Previous studies of mirror systems in monkey and humans have found evidence for the simulations of another individual's actions. This work has shown that a particular object–effector interaction is encoded in the understanding of the goals that another individual is attempting to achieve. This form of action simulation does not appear to consider the specific forms of the action, as simulation can take place even when the action cannot be directly seen, or is only heard. However, the current studies have provided evidence that in addition to encoding and simulating goal-orientated actions, the means by which the goal is achieved is also simulated. We have shown that viewing another person avoiding an obstacle primes the following actions of the observer, such that their own reach trajectory deviates more strongly as a consequence.
Our experiments have shown that the reach simulation/priming effects are quite general, occurring in a number of different scenarios: Thus they generalize across a range of different interpersonal viewpoints (e.g., allocentric in Experiment 4 and egocentric in Experiments 6 and 7); they appear to be independent of whether the participants jointly act on the same objects (Experiments 4, 5a, and 5b) or share no objects (Experiments 6 and 7); and, perhaps most surprisingly, simulation of another's obstacle avoidance takes place when it is viewed through a transparent barrier (Experiment 7). However, the key determining factor in our experiments appears to be the distance between the obstacle and the viewer's acting hand. When the obstacle was beyond the comfortable reach space of the observer (Experiments 2 and 3), no simulation of the reach path appeared to take place.
At the time of submitting this article for publication we were not aware of any investigations that had examined the possibility that monkey mirror neuron activity may be mediated by whether or not certain actions take place in peripersonal space. However, since submitting our article we recently became aware of the work of Caggiano, Fogassi, Rizzolatti, Their, and Casile (2009). They demonstrated that mirror cells in monkey F5 can be sensitive to the location of grasped objects. That is, when viewing another individual grasp an object, some cells only respond when the object is within peripersonal reach space, while others respond only when it is in far extrapersonal space.
However, two contrasts between Caggiano et al. (2009) and our current data are of note. First, although some mirror cells are encoding the distance of the object, the overall population of mirror cells is not restricted to only peripersonal space, many respond to far space, and indeed the majority of cells are unaffected by object distance. In contrast, our study of reach trajectory suggests the encoding of distance plays a more fundamental role, where reach path is only simulated when observed obstacles are in peripersonal space. Second, in a study similar to our Experiment 7, F5 mirror cells appear to compute the potential for action, where peripersonal cells no longer respond when viewing an object in peripersonal space through a transparent surface; whereas we found viewing obstacle avoidance reaches through a transparent surface had no effect on reach path simulation.
To account for apparently discrepant results, we hypothesize that whether or not observed actions are within peripersonal space is critical for the specific kinematic properties of the reach, but it may not always be so important for more general action goals. That is, priming effects examining achievement of general goals, such as whether a hand or foot response was observed, whether a peanut or apple was grasped, whether the object is visible or occluded, or whether the action is viewed or only heard, are not necessarily determined by the distance of the object from the viewer. Thus the simulations are of the general behavioural goals, where specific microdetails of the action are of less relevance. In contrast, specific effects of how a hand negotiates its way around an obstacle are far more detailed, and these are only simulated when relevant objects are within peripersonal action space.
As discussed, if observing a particular reach path primes the participant's own reach trajectory, then the details of the actual observed reach trajectories must be encoded. Whilst our study can give no indication as to where in the brain these trajectories are simulated, one can speculate that these observed actions are most likely being encoded in the same areas that encode one's own reach trajectory. Such areas implicated in one's own reaches, and which would most plausibly respond to observation of that action, include the inferior frontal gyrus, which Hamilton and Grafton (2007) have identified as being involved in the encoding of kinematics (in humans at least). Single unit recording studies in the macaque have identified a region in the intra-parietal sulcus (IPS), the parietal reach region
(PRR), which includes the medial intraparietal sulcus (MIP; e.g. Batista & Anderson, 2001). There is still speculation concerning where the human homologue of this region might be, and candidates include the medial occipito-parietal junction and the medial intraparietal sulcus (Culham & Kanwisher, 2001; Culham & Valyear, 2006). Of particular note, the superior parieto-occipital cortex (SPOC) selectively computes whether objects are within peripersonal action space (e.g., Culham, Gallivan, Cavina-Pratesi, & Quinlan, 2008), and encoding within this region is likely to be key to evoking the simulation of obstacle avoidance reach path.
It is also noteworthy that our obstacle avoidance priming effects are implicit. That is, participants were not required to make decisions about the other person's actions. Rather they were required to simply undertake their own reach to target task, while not making any judgements about the other person's actions. Such automatic encoding would seem to be necessary for coherent interpersonal behaviour. When interacting with others during everyday encounters a person rarely has to explicitly focus on the specific detailed forms of another person's individual reach-to-grasp actions. Rather, such processes are undertaken automatically to facilitate interpersonal interactions, enabling the limited capacity of conscious awareness to be focused on other task demands.
In contrast, many of the fMRI studies mentioned above often require more explicit encoding of actions, as when judging the weight of a box requires attention to be focused on how the box is lifted (e.g., Hamilton, Joyce, Flanagan, Frith, & Wolpert, 2007) or participants are asked to imagine themselves imitating the viewed action (e.g., Lestou, Pollick, & Kourtzi, 2008). In sharp contrast, our behavioural priming effects are implicit, in that the action of the other person is irrelevant to a participant's task. Whether the same neural systems mediate both implicit and explicit action simulation processes is an open issue.
Finally, a further interesting result from our study was the observation that in Experiments 2 and 3, in addition to the lack of priming by the observation of the other's action (n-1), participants’ within-person (n-2) priming effect no longer occurred. That is, a person's previous actions no longer affected their current action. This is surprising in the light of Jax and Rosenbaum's (2007) study, which showed that the reach trajectory priming effect carried over a number of trials, and in light of the robustness of this within-person (n-2) effect in all of our other experiments.
We speculate that the maintenance and retrieval of prior reach trajectories is undertaken while relevant to the ongoing task. However, when a participant observes a reach over an obstacle that is outside their peripersonal space, and hence is never an object they have to reach over, retrieval of prior reach programmes is vetoed. That is, as Jax and Rosenbaum (2007) note, producing a reach that is more curved than is necessary is costly, but this is usually outweighed by speeded processing when prior reach programmes can be reaccessed, rather than computing new reaches on each trial. However, when those prior (observed) reaches are irrelevant to a participant's actions, because they are outside action space it is more efficient to recompute a reach on each trial rather than retrieve irrelevant actions from memory. Admittedly these are speculations to account for this unexpected finding, and future work is necessary to provide a stronger explanation.
In sum, our visuomotor systems have evolved to cope with complex environments. A central problem is directing action to relevant objects in the presence of irrelevant objects that compete for the control of action. In particular, the avoidance of objects is of fundamental importance, and constant collisions would become extremely costly (Graziano & Cooke, 2006). Thus the retrieval of previous motor programmes containing obstacle avoidance routines would be an efficient means of selectively guiding behaviour. Our current data suggest that even the mere observation of another person avoiding an obstacle can activate corresponding motor representations and facilitate a person's own obstacle avoidance behaviour by evoking more curved reaches. That these reach path effects are only evoked when the obstacle in the observed action is within the peripersonal space of the viewer suggests that such reach priming is only engaged when of relevance.
Acknowledgments
This work was supported by a Wellcome Trust Programme Grant awarded to Steven P. Tipper.
APPENDIX
Mean heights (mm) for trials with an obstacle
| Experiment | No–No–O | O–No–O | No–O–O | O–O–O | |
| Experiment 1 | Single person reaching | 307.59 | 308.82 | 309.20 | 312.15 |
| Experiment 2 | Shared target, seated opposite, same hands | 318.11 | 325.79 | 322.59 | 323.65 |
| Experiment 3 | Shared target, seated opposite, mirrored hands | 325.51 | 326.60 | 323.37 | 325.93 |
| Experiment 4 | Shared obstacle, seated opposite | 314.58 | 315.77 | 312.64 | 315.93 |
| Experiment 5a | Shared obstacle, seated at 90° | 312.74 | 318.56 | 313.73 | 317.03 |
| Experiment 5b | Shared target, seated at 90° | 320.52 | 325.03 | 322.11 | 328.07 |
| Experiment 6 | Seated adjacent, no objects shared | 317.38 | 317.22 | 316.66 | 319.82 |
| Experiment 7 | Seated adjacent, transparent Perspex barrier | 336.16 | 336.82 | 337.48 | 338.72 |
Footnotes
1 We thank Paul Downing for reminding us of this approach.
References
- Bach P., Tipper S. P. Bend it like Beckham: Embodying the motor skills of famous athletes. Quarterly Journal of Experimental Psychology. 2006;59(12):2033–2039. doi: 10.1080/17470210600917801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P., Tipper S. P. Implicit action encoding influences personal-trait judgements. Cognition. 2007;102(2):151–178. doi: 10.1016/j.cognition.2005.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H., Pandya D. N. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. Journal of Comparative Neuropsychology. 1987;256(2):211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Batista A. P., Andersen R. A. The parietal reach region codes the next planned movement in a sequential reach task. Journal of Neurophysiology. 2001;85:539–544. doi: 10.1152/jn.2001.85.2.539. [DOI] [PubMed] [Google Scholar]
- Bekkering H., Wohlschlager A., Gattis M. Imitation of gestures in children is goal-directed. Quarterly Journal of Experimental Psychology. 2000;53:153–164. doi: 10.1080/713755872. [DOI] [PubMed] [Google Scholar]
- Binkofski F., Buccino G., Posse S., Seitz R. J., Rizzolatti G., Freund H. J. A frontoparietal circuit for object manipulation in man: Evidence from an fMRI-study. European Journal of Neuroscience. 1999;11(9):3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Brass M., Bekkering H., Prinz W. Movement observation affects movement execution in a simple response task. Acta Psychologica. 2001;106:3–22. doi: 10.1016/s0001-6918(00)00024-x. [DOI] [PubMed] [Google Scholar]
- Caggiano V., Fogassi L., Rizzolatti G., Their P., Casile A. Mirror neurons differentially encode the peripersonal and extrapersonal space of monkeys. Science. 2009;324:403–406. doi: 10.1126/science.1166818. [DOI] [PubMed] [Google Scholar]
- Castiello U. Understanding other people's actions: Intention and attention. Journal of Experimental Psychology: Human Perception & Performance. 2003;29:416–430. doi: 10.1037/0096-1523.29.2.416. [DOI] [PubMed] [Google Scholar]
- Castiello U., Lusher D., Mari M., Edwards M., Humphreys G. Observing a human or a robotic hand grasping an object: Differential motor priming effects. In: Prinz W., Hommel B., editors. Attention and Performance XIX: Common mechanisms in perception and action. New York: Oxford University Press; 2002. pp. 315–333. [Google Scholar]
- Colby C. L., Duhamel J. R., Goldberg M. E. Ventral intraparietal area of the macaque: Anatomic location and visual response properties. Journal of Neurophysiology. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Costantini M., Committeri G., Galati G. Effector- and target-independent representation of observed actions: Evidence from incidental repetition priming. Experimental Brain Research. 2008;188(3):341–351. doi: 10.1007/s00221-008-1369-x. [DOI] [PubMed] [Google Scholar]
- Craighero L., Fadiga L., Rizzolatti G., Umiltà C. Visuomotor priming. Visual Cognition. 1998;5:109–125. [Google Scholar]
- Culham J. C., Gallivan J., Cavina-Pratesi C., Quinlan D. J. fMRI investigations of reaching and ego space in human superior parieto-occipital cortex. In: Klatsky R. L., MacWhinney B., Behrmann M., editors. Embodiment, ego-space and action. New York: Psychology Press; 2008. pp. 247–274. [Google Scholar]
- Culham J.C., Kanwisher N.G. Neuroimaging of cognitive functions in human parietal cortex. Current Opinionin Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Culham J. C., Valyear K. F. Human parietal cortex in action. Current Opinion in Neurobiology. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G., Fadiga L., Fogassi L., Gallese V., Rizzolatti G. Understanding motor events: A neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Duhamel J. R., Colby C. L., Goldberg M. E. Ventral intraparietal area of the macaque: Congruent visual and somatic response properties. Journal of Neurophysiology. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Edwards M. G., Humphreys G. W., Castiello U. Motor facilitation following action observation: A behavioural study in prehensile action. Brain and Cognition. 2003;53:495–502. doi: 10.1016/s0278-2626(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Ferrari P. F., Gesierich B., Rozzi S., Chersi F., Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Gallese V., Buccino G., Craighero L., Fadiga L., Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey. Brain. 2001;124(3):571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Gallese V., di Pellegrino G., Fadiga L., Gentilucci M., Luppino G., et al. Space coding by premotor cortex. Experimental Brain Research. 1992;89:686–690. doi: 10.1007/BF00229894. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Gallese V., Fadiga L., Luppino G., Matelli M., Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4). Journal of Neurophysiology. 1996;76:141–157. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Gallese V., Fadiga L., Rizzolatti G. Neurons responding to the sight of goal directed hand/arm actions in the parietal area PF (7b) of the macaque monkey. Society of Neuroscience Abstracts. 1998;24:257. [Google Scholar]
- Frischen A., Loach D., Tipper S. P. Seeing the world through another person's eyes: Simulating selective attention via action observation. Cognition. 2009;111:212–218. doi: 10.1016/j.cognition.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Gallese V., Lakoff G. The brain's concepts: The role of the sensory-motor system in conceptual knowledge. Cognitive Neuropsychology. 2005;22(2-3):455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Gazzola V., Rizzolatti G., Wicker B., Keysers C. The anthropomorphic brain: The mirror neuron system responds to human and robotic actions. NeuroImage. 2007;35:1674–1684. doi: 10.1016/j.neuroimage.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Graziano M. S. A., Cooke D. F. Parietofrontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44:845–859. doi: 10.1016/j.neuropsychologia.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Graziano M. S.A., Gross C. G. The representation of extrapersonal space: A possible role for bimodal visual-tactile neurons. In: Gazzaniga M. S., editor. The cognitive neurosciences. Cambridge, MA: MIT Press; 1994. pp. 1021–1034. [Google Scholar]
- Graziano M. S. A., Gross C. G. Spatial maps for the cortical control of movement. Current Opinion in Neurobiology. 1998;8:195–201. doi: 10.1016/s0959-4388(98)80140-2. [DOI] [PubMed] [Google Scholar]
- Graziano M. S. A., Yap G. S., Gross C. G. Coding of visual space by premotor neurons. Science. 1994;266:1054–1057. doi: 10.1126/science.7973661. [DOI] [PubMed] [Google Scholar]
- Halligan P. W., Marshall J. C. Left neglect for near but not far space in man. Nature. 1991;350:498–500. doi: 10.1038/350498a0. [DOI] [PubMed] [Google Scholar]
- Hamilton A. F. C., Grafton S. T. Goal representation in human anterior intraparietal sulcus. Journal of Neuroscience. 2006;26(4):1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. F. C., Grafton S. T. The motor hierarchy: From kinematics to goals and intentions. In: Rosetti Y., Kawato M., Haggard P., editors. Attention & Performance XXII. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- Hamilton A. F. C., Joyce D. W., Flanagan J. R., Frith C. D., Wolpert D. M. Kinematic cues in perceptual weight judgments and their origins in box lifting. Psychological Research. 2007;71:13–21. doi: 10.1007/s00426-005-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L. A., Tipper S. P. Hand deviations away from visual cues: Indirect evidence for inhibition. Experimental Brain Research. 1997;113:144–152. doi: 10.1007/BF02454150. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Molnar-Szakacs I., Gallese V., Buccino G., Mazziotta J. C., Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLOS Biology. 2005;3:529–535. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M., Woods R. P., Brass M., Bekkering H., Mazziotta J. C., Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jax S. A., Rosenbaum D. A. Hand path priming in manual obstacle avoidance: Evidence that the dorsal stream does not only control visually guided actions in real time. Journal of Experimental Psychology: Human Perception & Performance. 2007;33(2):425–441. doi: 10.1037/0096-1523.33.2.425. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The neural and behavioral organization of goal-directed movements. Oxford, UK: Clarendon Press; 1988. [Google Scholar]
- Kohler E., Keysers C., Umiltà M. A., Fogassi L., Gallese V., Rizzolatti G. Hearing sounds, understanding actions: Action representation in mirror neurons. Science. 2002;297:846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Koski L., Iacoboni M., Dubeau M. C., Woods R. P., Mazziotta J. C. Modulation of cortical activity during different imitative behaviors. Journal of Neurophysiology. 2003;189:460–471. doi: 10.1152/jn.00248.2002. [DOI] [PubMed] [Google Scholar]
- Lestou V., Pollick F. E., Kourtzi Z. Neural substrates for action understanding at different description levels in the human brain. Journal of Cognitive Neuroscience. 2008;20:324–341. doi: 10.1162/jocn.2008.20021. [DOI] [PubMed] [Google Scholar]
- Luppino G., Murata A., Govoni P., Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Experimental Brain Research. 1999;128(1-2):181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Meegan D. V., Tipper S. P. Visual search and target-directed action. Journal of Experimental Psychology: Human Perception and Performance. 1999;25(5):1347–1362. [Google Scholar]
- Murata A., Gallese V., Luppino G., Kaseda M., Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. Journal of Neurophysiology. 2000;83(5):2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Previc F. H. The neuropsychology of 3-D space. Psychological Bulletin. 1998;124:123–164. doi: 10.1037/0033-2909.124.2.123. [DOI] [PubMed] [Google Scholar]
- Schuch S., Tipper S. P. On observing another person's actions: Influences of observed inhibition and errors. Perception and Psychophysics. 2007;69(5):828–837. doi: 10.3758/bf03193782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebanz N., Bekkering H., Knoblich G. Joint action: Bodies and minds moving together. Trends in Cognitive Sciences. 2006;10(2):70–76. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Stürmer B., Aschersleben G., Prinz W. Correspondence effects with manual gestures and postures: A study of imitation. Journal of Experimental Psychology: Human Perception and Performance. 2000;26(6):1746–1759. doi: 10.1037//0096-1523.26.6.1746. [DOI] [PubMed] [Google Scholar]
- Tipper S. P., Howard L. A., Jackson A. S. R. Selective reaching to grasp: Evidence for distractor interference effects. Visual Cognition. 1997;4:1–38. [Google Scholar]
- Tipper S. P., Lortie C., Baylis G. C. Selective reaching: Evidence for action-centered attention. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:891–905. doi: 10.1037//0096-1523.18.4.891. [DOI] [PubMed] [Google Scholar]
- Tipper S. P., Meegan D., Howard L. A. Action-centred negative priming: Evidence for reactive inhibition. Visual Cognition. 2002;9:591–614. [Google Scholar]
- Umiltà M. A., Kohler E., Gallese V., Fogassi L., Fadiga L., Keysers C., et al. I know what you are doing: A neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- van der Wel R. P. R. D., Fleckenstein R. M., Jax S. A., Rosenbaum D. A. Hand path priming in manual obstacle avoidance: Evidence for abstract spatiotemporal forms in human motor control. Journal of Experimental Psychology: Human Perception and Performance. 2007;33(5):1117–1126. doi: 10.1037/0096-1523.33.5.1117. [DOI] [PubMed] [Google Scholar]
- Van Elk M., van Schie H. T., Bekkering H. Conceptual knowledge for understanding other's actions is organized primarily around action goals. Experimental Brain Research. 2008;189:99–107. doi: 10.1007/s00221-008-1408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P., Valenza N., Mayer E., Reverdin A., Landis T. Near and far visual space in unilateral neglect. Annals of Neurology. 1998;43:406–410. doi: 10.1002/ana.410430324. [DOI] [PubMed] [Google Scholar]
- Wapner S., Cirillo L. Imitation of a model's hand movements: Age changes in transposition of left-right relations. Child Development. 1968;39:887–894. [PubMed] [Google Scholar]
- Weiss P. H., Marshall J. C., Wunderlich G., Tellmann L., Halligan P. W., Freund H. J., et al. Neural consequences of acting in near versus far space: A physiological basis for clinical dissociations. Brain. 2000;123:2531–2541. doi: 10.1093/brain/123.12.2531. [DOI] [PubMed] [Google Scholar]
- Weiss P. H., Marshall J. C., Zilles K., Fink G. R. Are action and perception in near and far space additive or interactive factors? NeuroImage. 2003;18:837–846. doi: 10.1016/s1053-8119(03)00018-1. [DOI] [PubMed] [Google Scholar]


