Abstract
Background.
Neopterin, a GTP metabolite expressed by macrophages, is a marker of immune activation. We hypothesize that levels of this serum marker alter with donor age, reflecting increased chronic immune activation in normal aging. In addition to age, we assessed gender, race, body mass index (BMI), and percentage of body fat (%fat) as potential covariates.
Methods.
Serum was obtained from 426 healthy participants whose age ranged from 18 to 87 years. Anthropometric measures included %fat and BMI. Neopterin concentrations were measured by competitive ELISA. The paired associations between neopterin and age, BMI, or %fat were analyzed by Spearman's correlation or by linear regression of log-transformed neopterin, whereas overall associations were modeled by multiple regression of log-transformed neopterin as a function of age, gender, race, BMI, %fat, and interaction terms.
Results.
Across all participants, neopterin exhibited a positive association with age, BMI, and %fat. Multiple regression modeling of neopterin in women and men as a function of age, BMI, and race revealed that each covariate contributed significantly to neopterin values and that optimal modeling required an interaction term between race and BMI. The covariate %fat was highly correlated with BMI and could be substituted for BMI to yield similar regression coefficients.
Conclusion.
The association of age and gender with neopterin levels and their modification by race, BMI, or %fat reflect the biology underlying chronic immune activation and perhaps gender differences in disease incidence, morbidity, and mortality.
Keywords: Neopterin, Immune activation, Inflammation, BMI, Homeostasis
CHRONIC immune activation is involved in a number of diverse pathologies, including AIDS (1), atherosclerosis (2), autoimmune disease (3), cancer (4), obesity, and metabolic syndrome (5,6). Importantly, immune system activation has also been implicated in the aging process in normal healthy individuals (7,8). Neopterin, a metabolite of GTP, is produced in macrophages upon stimulation by interferon gamma released by activated T cells (9). In humans, neopterin is a marker of macrophage activation (10). This marker has been used clinically in the assessment of bacterial and viral infections (11,12), autoimmune diseases (13,14), sleep apnea (15), and in malignant conditions (16).
Individual reports have been equivocal regarding correlations of serum neopterin levels with age or gender (17–19). However, these studies typically employed comparison groups with discontinuous age (ie, young adult vs old adult) and did not exclude potential confounding conditions (ie, smoking, hypertension, pulmonary disease, and other chronic inflammatory disease). In addition, a number of recent studies with relatively small numbers of participants (n <50) have suggested that neopterin levels can be influenced by body mass index (BMI) values (20,21). The current study was undertaken to carefully assess the relationship of neopterin with age, BMI, and percentage of body fat (%fat) and to see whether gender and race modulate those relationships.
METHODS
Participants
Sera from 426 clinically defined healthy participants were obtained under institutional review board-approved protocols from a serum bank (SeraCare Life Sciences Inc., Oceanside, CA) as well as from the Johns Hopkins Bayview Medical Center Clinical Research Unit. For this study, inclusion criteria as a healthy serum donor included measures within the normal range for fasting glucose (<100 mg/dL) and thyroid-stimulating hormone (0.5–2.1 mIU/mL), as well as a clinical assessment by a physician. Patients with a history of smoking, angina, myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass surgery, congestive heart failure, and stroke were ineligible. Other exclusionary criteria included a history of diabetes mellitus, pulmonary disease, renal or hepatic dysfunction, dementia, cancer, any chronic inflammatory condition (eg, rheumatoid arthritis), and use of anti-inflammatory agents (eg, steroids). Approval for the study protocol was acquired from the local institutional review board, and informed consent was obtained from all patients.
Clinical Measures
Anthropometric measures included height, weight, and waist circumference. Foot-to-foot bioimpedance analysis was conducted to estimate %fat using a Tanita scale (Tanita Corporation of America, Arlington Heights, IL). A fasting blood sample was obtained in a resting and fasting state in the morning. All venous samples were placed at 4°C prior to serum separation. After centrifugation at 1000g for 20 minutes, serum was stored at −80°C. Serum neopterin was measured using a commercially available competitive ELISA (ALPCO Diagnostics, Inc., Salem, NH). In the laboratory, this enzyme immunoassay had a sensitivity of 0.8 nM and an interassay coefficient of variance of 5.50%.
Statistical Analysis
Univariate statistics of the study group were individually examined. Given the nonnormal distribution of serum neopterin levels (15), comparisons between genders or races were performed using a Mann–Whitney U test. The relationship between neopterin and age, BMI, and %fat was initially investigated by LOWESS (locally weighted scatterplot smoothing) modeling. The correlation of neopterin with age, BMI, or %fat was analyzed using the Spearman rank correlation test. The association of neopterin with age, gender, race, BMI, and %fat was modeled and analyzed by stepwise multiple linear regression after log transformation of neopterin levels. Age, BMI, and %fat were continuous independent variables, whereas race and gender were dummy-coded independent variables. For race, black = 0 and white = 1; for gender, female = 0, male = 1. A post hoc t test determined if the regression weight of each independent variable differed significantly from zero. All statistical calculations were carried out using Prism and InStat software (GraphPad, Inc., La Jolla, CA) and StatView 5 (SAS Institute, Inc., Cary, NC).
RESULTS
Characteristics of the healthy cohort of 426 participants are listed in Table 1. The descriptive statistics exhibit well-documented anthropometric gender differences. The men were leaner with less %fat. Analysis of race differences for each gender indicated that BMI and %fat were significantly higher in black women versus white women as well as higher in black men versus white men. The %fat (mean ± SD) of the black women was 10% higher, and their mean BMI was 7 kg/m2 higher than that of white women. Gender differences in body composition within race were also evident. BMI and %fat were significantly higher in black women versus black men (p < .001 and p < .0001, respectively), whereas only %fat was significantly different between white women and men (p < .0001). Median values for neopterin were significantly higher in blacks compared with whites by gender. White women had higher median levels of neopterin compared with white men, whereas black men had a significantly higher median neopterin value than any other group.
Table 1.
Male and Female Sample Characteristics Contrasted by Race
| Women |
Men |
|||
| Black | White | Black | White | |
| n | 49 | 152 | 48 | 184 |
| Age | 44.8 ± 15.3 | 48.9 ± 15.1 | 41.2 ± 15.5 | 50.0 ± 14.9 |
| BMI | 37.8 ± 8.7 | 27.5 ± 8.9* | 30.8 ± 8.0 | 26.6 ± 6.2* |
| %Fat | 43.3 ± 8.2 | 33.6 ± 8.7* | 26.7 ± 9.7 | 22.0 ± 8.6* |
| Neopterin | ||||
| Mean (SD) | 7.32 ± 1.5 | 6.39 ± 1.71 | 8.24 ± 1.82 | 6.04 ± 1.67 |
| Median (range) | 6.83 (4.91–11.37) | 6.16 (2.39–11.88)† | 7.92 (4.73–12.81) | 5.74 (2.59–11.34)† |
Notes: Values for age, body mass index (BMI), and %body fat (%fat) reflect the mean ± SD. Comparisons were performed between races.
p < .005 by t test.
p < .005 by Mann–Whitney U test.
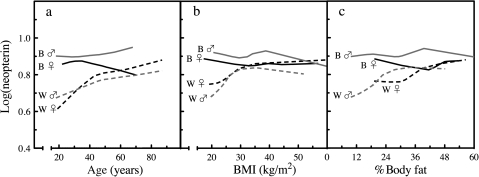
Exploratory LOWESS curves were generated, segregating samples by race and gender (Figure 1). The LOWESS curves suggested that neopterin values in blacks of both genders were relatively flat regardless of age, BMI, or %fat levels. In contrast, neopterin values in white men and women increased with increasing values of age, BMI, and %fat. The increase in neopterin appeared to slow down when the covariate age reached high levels (ie, >45 years) in white women. Similarly, the LOWESS plots suggested that a nonlinear association of neopterin values with BMI that was distinct between whites of both genders at BMI <25 kg/m2 and reached a plateau after 30 kg/m2.
Figure 1.
Exploratory LOWESS (locally weighted scatterplot smoothing) profiles of neopterin, age, body mass index (BMI), and %body fat. LOWESS modeling of log-transformed neopterin values segregated by gender and race were determined as a function of (a) age, (b) BMI, and (c) %body fat. Black dotted lines represent white women, gray dotted lines represent white men, solid black lines represent black women, and gray solid lines black men.
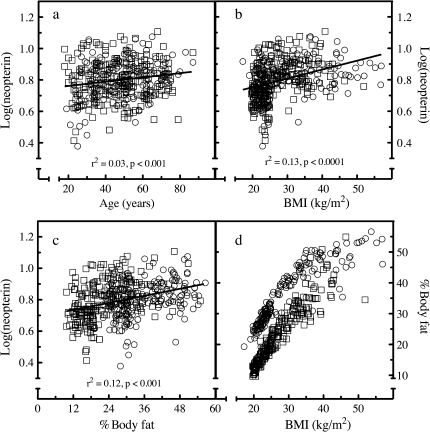
Correlation analysis was used to test the bivariate relationship between neopterin and age, and a statistically significant relationship was observed with a Spearman correlation coefficient (r) of .15, p < .0005. Examining the bivariate relationship between neopterin and BMI by Spearman’s rank correlation also yielded a significant correlation with a Spearman r of .43, p < .0001. Rank correlation analysis also revealed a significant relationship between neopterin and %fat with a Spearman r of .35, p < .0001. The nature of the relationship (linear vs nonlinear) between neopterin and age, BMI, or fat was assessed by linear regression of log-transformed neopterin values (Figure 2). Age and %fat exhibited a linear pattern throughout their range, whereas BMI appeared nonlinear at high BMI values, though this could arise from the fewer number of participants at the high end. The bivariate relationship between BMI and %fat was also investigated, separating the values by gender (Figure 2d). The relationship between BMI and %fat has been characterized in a number of different populations where deviation from linearity was observed at high BMI and %fat values (22–24). Data from 426 participants of the current study exhibited the same pattern. Data from both genders were combined, and %fat was modeled by multiple linear regression. The optimal equation was [%fat] = (1.00 ± 0.03) × BMI − (10.3 ± 0.4) × gender + (0.10 ± 0.02) × age + (5.4 ± 1.1), accounted for 85% of the variance, and the variable race did not contribute significantly to the model. The beta coefficients were all consistent with previous studies that modeled %fat as a function of age, gender, and BMI (24–26).
Figure 2.
Correlations between serum neopterin, age, body mass index (BMI), and %body fat. The levels of neopterin in 426 healthy participants were determined by commercial sandwich ELISA. The levels of log-transformed neopterin were plotted as a function of (a) participant’s age, (b) BMI value, and (c) %body fat. Correlation was assessed by linear regression analysis. (d) The association between BMI and %body fat was graphically investigated, contrasting by gender. Open circles = female; open squares = male.
Multiple regression models were constructed to examine the effect of age, race, and BMI on log(neopterin) values. The data for women and men were analyzed separately. Five stepwise models were analyzed and are given in Table 2. Model 1 contained the regression equation for log(neopterin) and age. Model 2 added the dummy-coded variable race to the model, and it was statistically significant in both gender models. The negative beta coefficient for race indicates that whites (coded as “1”) have lower neopterin values than blacks (coded as “0”) for both women and men. The variable BMI (Model 3) was added next, and it was also statistically significant in both genders, BMI accounted for and additional 7% female and 9% male variance in log(neopterin). As a way to address the apparent nonlinear association between BMI and log(neopterin) values, two slopes at different age ranges were modeled by adding the variable BMI25up (Model 4). For BMI ≤25 kg/m2, BMI25up = 0, so the BMI term in the model is βBMI × BMI, and for BMI >25 kg/m2, BMI25up = BMI, so the BMI terms are (βBMI + βBMI25up) × BMI, where βBMI25up reflects the difference in slopes before and after a BMI value of 25 kg/m2. The variable was significant in both genders, accounting for an additional 8% female and 10% male variance in log(neopterin).
Table 2.
Multiple Linear Regression Models by Gender Examining the Effect of Age, Race, BMI, and Interaction Terms on Neopterin Values
| Female models |
|||||
| 1 | 2 | 3 | 4 | 5 | |
| Intercept | 0.70 ± 0.03* | 0.74 ± 0.03* | 0.60 ± 0.04* | 0.75 ± 0.05* | 0.66 ± 0.06* |
| Age | 0.0023 ± 0.0005* | 0.0027 ± 0.0005* | 0.0027 ± 0.0005* | 0.0026 ± 0.0005* | 0.0053 ± 0.0013* |
| Race | −0.085 ± 0.019* | −0.046 ± 0.021† | −0.026 ± 0.020 | −0.029 ± 0.020 | |
| BMI | 0.0037 ± 0.0008* | 0.0042 ± 0.0019† | −0.0041 ± 0.0019† | ||
| BMI25up | −0.0042 ± 0.0009† | −0.0015 ± 0.0006† | |||
| age45up | 0.0049 ± 0.0009* | ||||
| r2 | .08 | .17 | .24 | .32 | .35 |
| Δr2 | .09 | .07 | .02 | .08 | |
| Male models |
|||||
| 1 | 2 | 3 | 4 | 5 | |
| Intercept | 0.7 ± 0.03* | 0.83 ± 0.02* | 0.65 ± 0.04* | 0.87 ± 0.05* | 1.00 ± 0.06* |
| Age | 0.0006 ± 0.0005 | 0.0019 ± 0.0005† | 0.0021 ± 0.0004* | 0.0019 ± 0.0004* | 0.0022 ± 0.0004* |
| Race | −0.162 ± 0.019* | −0.140 ± 0.018* | −0.134 ± 0.017* | −0.356 ± 0.066* | |
| BMI | 0.0055 ± 0.0010* | −0.0056 ± 0.0020† | −0.0094 ± 0.0022* | ||
| BMI25up | 0.0052 ± 0.0008* | 0.0045 ± 0.0008* | |||
| Race × BMI | 0.0074 ± 0.0022† | ||||
| r2 | .01 | .25 | .34 | .44 | .47 |
| Δr2 | .24 | .09 | .10 | .03 | |
Notes: Each p value compares the full model with a simpler model omitting that variable.
p < .0001.
p < .05.
Similarly, the apparent nonlinear association between age and neopterin was modeled by introducing the variable age45up, where age45up = 0 for age ≤45 years and age45up = age at values >45 years. The variable age45up was significant in women (female Model 5) but not in men, consistent with the LOWESS analysis. In contrast, the interaction term Race × BMI did not add significantly to female models, whereas it accounted for an additional 3% male variance (male Model 5). For the optimal Model 5, age contributed more to neopterin levels in women than in men (the age coefficient in women was approximately twice that of men), whereas race and BMI values contributed more to male neopterin values. The negative coefficient for race and BMI in the final model indicates that whites of both genders will have a greater subtraction from the neopterin calculation.
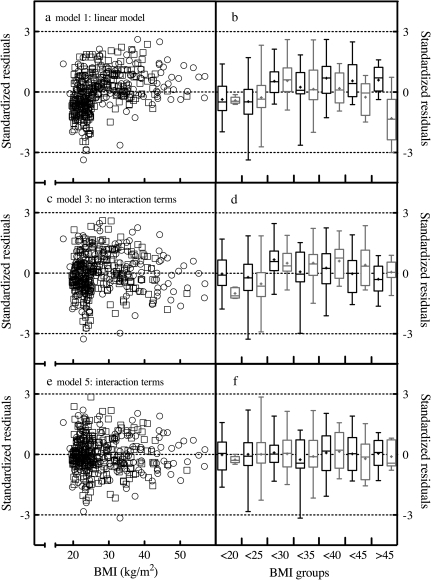
The robustness of the models was profiled by plotting standardized residuals stratified by gender for Models 1, 3, and 5 as a function of BMI (Figure 3). The residuals for Model 1 (linear regression model) exhibited an asymmetric distribution. For a more precise assessment of the model fit, standardized residual values were grouped by BMI range (<20, 20 to <25, 25 to <30, 30 to <35, 35 to <40, 40 to <45, and >45 kg/m2), segregated by gender and box-and-whisker plots were generated (Figure 3b). In general, the linear model overestimated male and female neopterin values at lean BMI while underestimating neopterin values in the overweight to obese range. The multiple regression model that included age, race, and BMI, but no interaction terms (Model 3) reduced the asymmetry in female values, though male values still exhibited runs above and below zero by BMI group. The final model, which included the interaction terms age45up, BMI25up, and Race × BMI exhibited standardized residuals for both women and men that were centered around zero (neopterin values were minimally under- or overestimated).
Figure 3.
Residual plots and model fits. Standardized residuals were calculated for multiple regression models using the formula standardized residual = (residual)/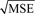 , where MSE is the mean squared error. Scatter plots of standardized residuals as a function of continuous body mass index (BMI) values were generated for (a) the linear model, model 1, (c) the multiple regression model with no interaction terms, model 3, and (e) the final multiple regression model with interaction terms, model 5. Open circles = female; open squares = male. Box-and-whisker plots of standardized residuals where values were grouped in 5 U intervals of BMI were also generated to profile runs above and below zero for (a) model 1, (d) model 3, and (f) model 5. In these box-and-whisker plots, black bars represent women, gray bars represent men, the box frame defines the lower and upper quartile, the whiskers depict maximum and minimum, the line within the box marks the median value, and the “+” symbol marks the mean value.
, where MSE is the mean squared error. Scatter plots of standardized residuals as a function of continuous body mass index (BMI) values were generated for (a) the linear model, model 1, (c) the multiple regression model with no interaction terms, model 3, and (e) the final multiple regression model with interaction terms, model 5. Open circles = female; open squares = male. Box-and-whisker plots of standardized residuals where values were grouped in 5 U intervals of BMI were also generated to profile runs above and below zero for (a) model 1, (d) model 3, and (f) model 5. In these box-and-whisker plots, black bars represent women, gray bars represent men, the box frame defines the lower and upper quartile, the whiskers depict maximum and minimum, the line within the box marks the median value, and the “+” symbol marks the mean value.
When %fat was introduced in place of BMI in the stepwise linear regression model building for females and male neopterin values, the beta coefficients were nearly identical to models using BMI. For example, the corresponding coefficients for a Model 3 for women was β0 = 0.56 ± 0.05, βage = 0.0027 ± 0.0005, βrace = −0.0434 ± 0.0205, βfat = 0.0041 ± 0.0009, with an r2 of .26, and for men was β0 = 0.72 ± 0.03, βage = 0.0020 ± 0.0005, βrace = −0.137 ± 0.018, βfat = 0.0040 ± 0.0007, with an r2 of .34. The variable %fat accounted for an additional 9% female and 9% male variance in log(neopterin) values. A Race × %Fat interaction term was found to contribute significantly to the models (data not shown) explaining an additional 4% and 7% of the variance in female and male neopterin values, respectively.
Stepwise multiple linear regression was also applied to the complete data set of women and men combined, incorporating age, gender, race, BMI, and Race × BMI as well as Gender × BMI interaction terms. The optimized model accounted for 40% of the variance and yielded coefficients of log(neopterin) = (0.98 ± 0.05) + (0.0035 ± 0.0008) × age − (0.082 ± 0.035) × gender − (0.320 ± 0.048) × race − (0.0098 ± 0.0016) × BMI + (0.0040 ± 0.0006) × BMI25up (0.0072 ± 0.0014) × race × BMI + (0.0023 ± 0.001) × gender × BMI. Similarly, a model of the complete data set using %fat as a variable yielded optimized model coefficients of log(neopterin) = (0.78 ± 0.04) + (0.0025 ± 0.0003) × age + (0.041 ± 0.011) × gender − (0.315 ± 0.036) × race − 0.0010 ± 0.0008 × [%fat] + 0.0066 ± 0.0009 × [race × %fat] and accounted for 35% of the variance. The well-known high degree of correlation between %fat and BMI as well as gender differences in %body fat allow the variable %fat to account for most the outcome variability explained by the variable BMI and the interaction term Gender × BMI. The beta coefficients for models using BMI or %fat were not significantly different for age (0.0098 vs 0.0010, respectively), race (0.320 vs 0.315, respectively), and the interaction term with race (0.0072 vs 0.0066, respectively).
DISCUSSION
Neopterin (6-D-erythro-1′,2′,3′-trihydroxypropyl-pterin) is synthesized from GTP by GTP-cyclohydrolase I in response to interferon gamma stimulation of human monocytes/macrophages (27,28). Increased concentrations of neopterin in serum have been found during viral infections, various malignant disorders, and autoimmune diseases (13). The current study demonstrates for the first time that in a well-defined healthy population, serum neopterin levels vary with donor age, gender, race, and BMI. The observation of an increase in neopterin production with increasing age is consistent with a number of other studies (18,19). In general, these previous study populations compared discrete groups of young (<40 years of age) with old (>60 years of age). In addition, entry/enrollment criteria in those studies did not necessarily exclude diseases associated with immune activation and increased neopterin concentrations in the elderly participants, such as atherosclerosis (29) or dementia (30).
The current study models neopterin along a continuous aging trajectory with at least 10 participants per gender per decade spanning the 20s to the 80s. The study cohort had extensive exclusion criteria to minimize confounding due to age-related conditions. It is possible that in the older participants, pathological processes may have already started that were clinically latent. However, changes were observed in neopterin between relatively young ages (eg, third and fourth decades). A novel finding of the current study is that the changes in neopterin with age were associated with gender and race differences.
Median values of neopterin were significantly different between healthy lean male and female participants. The effects of gender on serum neopterin may be linked to gender differences in chronic immune activation. Gender differences in susceptibility to autoimmune diseases have been observed, with women at greater risk than men of rheumatoid arthritis and multiple sclerosis (31). Race appears to contribute to neopterin values with blacks (especially men) exhibiting higher neopterin levels than whites. However, the relatively small black sample size (n = 97) may be reflecting sample bias. While this is possible, the participants from the current study exhibited body composition distributions and correlations between BMI and %fat that match other larger studies (22–26), suggesting that at least in those parameters sampling bias did not occur. The current results found a race effect for both women and men, and it interacted with BMI (or %fat). A potential hypothesis to address gender and racial differences in morbidity and mortality is the stress or “weathering” hypothesis, which states that greater exposure to stressors over the life course increases susceptibility to morbidity and mortality among members of specific groups (32). Stress is a well-characterized immunomodulator, causing immune activation (33). Further investigation with a much larger samples size will be necessary for the influence of race on a serum marker of immune action to be robustly defined.
Serum neopterin levels are elevated in a number of pathologies involving chronic inflammation, including alcoholic hepatitis, hepatitis B and C virus, rheumatoid arthritis, atherosclerosis, diabetes, and inflammatory bowel diseases (10). Previous studies on serum neopterin levels found an association of neopterin values with BMI (15,34). The inclusion of the variable %fat, which is related to BMI and has well-known gender differences in distribution, into models of neopterin yielded similar model parameters. Both elevated BMI and %fat are associated with altered cytokines and a proinflammatory state (35–37). Aging also has been associated with low-level inflammation [“inflammaging” (38)], thought to lead to or exacerbate many chronic medical conditions (39,40).
Immune activation is a sentinel homeostatic mechanism. Instead of maintaining organ and organismal optimal function, a dysregulated homeostatic mechanism can contribute to tissue pathology and disease progression. Chronic immune activation, through its cytokine production and/or tissue remodeling program, alters the cellular microenvironment, which can contribute to altered cellular function (41). In conclusion, age and gender are covariates that contribute to neopterin levels, and these covariates are, in turn, modified by BMI (or %fat) and race presumably through altered chronic immune activation load. The relative contribution of age and gender to modulating neopterin levels (a surrogate for immune activation) in normal physiological events may reflect the biology underlying aging, late-age onset diseases, and perhaps gender differences in morbidity and mortality.
FUNDING
This research was supported by National Institutes of Health (NIH) grants CA 87311 and 113865 (N.S.F.). M.E.S. was supported by grant T35 AG026758 and the Medical Student Training in Aging Research Program run jointly by American Federation on Aging Research and the John A. Hartford Foundation. N.-Y.W. was supported by grant UL1 RR 025005 and A.M. by grant T32 AG000120. The Johns Hopkins Bayview Medical Center Clinical Research Unit is supported by grant UL1 RR 025005 from the National Center for Research Resources, a component of the NIH and NIH Roadmap for Medical Research.
References
- 1.Cadogan M, Dalgleish AG. HIV induced AIDS and related cancers: chronic immune activation and future therapeutic strategies. Adv Cancer Res. 2008;101:349–395. doi: 10.1016/S0065-230X(08)00409-0. [DOI] [PubMed] [Google Scholar]
- 2.Blasi C. The autoimmune origin of atherosclerosis. Atherosclerosis. 2008;201:17–32. doi: 10.1016/j.atherosclerosis.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Vasto S, Candore G, Balistreri CR, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Dalgleish AG, O'Byrne KJ. Chronic immune activation and inflammation in the pathogenesis of aids and cancer. Adv Cancer Res. 2002;84:231–276. doi: 10.1016/s0065-230x(02)84008-8. [DOI] [PubMed] [Google Scholar]
- 5.Brandacher G, Hoeller E, Fuchs D, Weiss HG. Chronic immune activation underlies morbid obesity: is IDO a key player? Curr Drug Metab. 2007;8:289–295. doi: 10.2174/138920007780362590. [DOI] [PubMed] [Google Scholar]
- 6.Duncan BB, Schmidt MI. Chronic activation of the innate immune system may underlie the metabolic syndrome. Sao Paulo Med J. 2001;119:122–127. doi: 10.1590/S1516-31802001000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly: scientific basis and clinical implications. Drugs Aging. 2005;22:589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- 8.Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner ER, Werner-Felmayer G, Fuchs D, et al. Biochemistry and function of pteridine synthesis in human and murine macrophages. Pathobiology. 1991;59:276–279. doi: 10.1159/000163662. [DOI] [PubMed] [Google Scholar]
- 10.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 11.Denz H, Fuchs D, Hausen A, et al. Value of urinary neopterin in the differential diagnosis of bacterial and viral infections. Klin Wochenschr. 1990;68:218–222. doi: 10.1007/BF01662720. [DOI] [PubMed] [Google Scholar]
- 12.Sheldon J, Riches PG, Soni N, et al. Plasma neopterin as an adjunct to C-reactive protein in assessment of infection. Clin Chem. 1991;37:2038–2042. [PubMed] [Google Scholar]
- 13.Berdowska A, Zwirska-Korczala K. Neopterin measurement in clinical diagnosis. J Clin Pharm Ther. 2001;26:319–329. doi: 10.1046/j.1365-2710.2001.00358.x. [DOI] [PubMed] [Google Scholar]
- 14.Nasonov EL, Samsonov M, Tilz G, Fuchs D. [Neopterin: new immunological marker of autoimmune rheumatic disease] Klin Med (Mosk) 2000;78:43–46. [PubMed] [Google Scholar]
- 15.Punjabi NM, Beamer BA, Jain A, Spencer ME, Fedarko N. Elevated levels of neopterin in sleep-disordered breathing. Chest. 2007;132:1124–1130. doi: 10.1378/chest.07-0743. [DOI] [PubMed] [Google Scholar]
- 16.Reibnegger G, Fuchs D, Fuith LC, et al. Neopterin as a marker for activated cell-mediated immunity: application in malignant disease. Cancer Detect Prev. 1991;15:483–490. [PubMed] [Google Scholar]
- 17.Currie MS, Rao MK, Blazer DG, Cohen HJ. Age and functional correlations of markers of coagulation and inflammation in the elderly: functional implications of elevated crosslinked fibrin degradation products (d-dimers) J Am Geriatr Soc. 1994;42:738–742. doi: 10.1111/j.1532-5415.1994.tb06534.x. [DOI] [PubMed] [Google Scholar]
- 18.Diamondstone LS, Tollerud DJ, Fuchs D, et al. Factors influencing serum neopterin and beta 2-microglobulin levels in a healthy diverse population. J Clin Immunol. 1994;14:368–374. doi: 10.1007/BF01546321. [DOI] [PubMed] [Google Scholar]
- 19.Ledochowski M, Murr C, Jager M, Fuchs D. Dehydroepiandrosterone, ageing and immune activation. Exp Gerontol. 2001;36:1739–1747. doi: 10.1016/s0531-5565(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 20.Bozdemir AE, Barutcuoglu B, Dereli D, Kabaroglu C, Habif S, Bayindir O. C-reactive protein and neopterin levels in healthy non-obese adults. Clin Chem Lab Med. 2006;44:317–321. doi: 10.1515/CCLM.2006.055. [DOI] [PubMed] [Google Scholar]
- 21.Ursavas A, Karadag M, Oral AY, Demirdogen E, Oral HB, Ege E. Association between serum neopterin, obesity and daytime sleepiness in patients with obstructive sleep apnea. Respir Med. 2008;102:1193–1197. doi: 10.1016/j.rmed.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Deurenberg P, van der Kooy K, Leenen R, Weststrate JA, Seidell JC. Sex and age specific prediction formulas for estimating body composition from bioelectrical impedance: a cross-validation study. Int J Obes. 1991;15:17–25. [PubMed] [Google Scholar]
- 23.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- 24.Jackson AS, Stanforth PR, Gagnon J, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26:789–796. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 26.Jackson AS. Research design and analysis of data procedures for predicting body density. Med Sci Sports Exerc. 1984;16:616–622. [PubMed] [Google Scholar]
- 27.Huber C, Batchelor JR, Fuchs D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werner ER, Werner-Felmayer G, Fuchs D, et al. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem. 1990;265:3189–3192. [PubMed] [Google Scholar]
- 29.Weiss G, Willeit J, Kiechl S, et al. Increased concentrations of neopterin in carotid atherosclerosis. Atherosclerosis. 1994;106:263–271. doi: 10.1016/0021-9150(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 30.Leblhuber F, Walli J, Demel U, Tilz GP, Widner B, Fuchs D. Increased serum neopterin concentrations in patients with Alzheimer’s disease. Clin Chem Lab Med. 1999;37:429–431. doi: 10.1515/CCLM.1999.070. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy M. The “Gender gap” in autoimmune disease. Lancet. 2000;356:1088. doi: 10.1016/S0140-6736(05)74535-9. [DOI] [PubMed] [Google Scholar]
- 32.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2:207–221. [PubMed] [Google Scholar]
- 33.Petticrew M, Hunter D. Stress-induced immunomodulation. JAMA. 1999;282:2209–2210. doi: 10.1001/jama.282.23.2209. [DOI] [PubMed] [Google Scholar]
- 34.Ledochowski M, Murr C, Widner B, Fuchs D. Association between insulin resistance, body mass and neopterin concentrations. Clin Chim Acta. 1999;282:115–123. doi: 10.1016/s0009-8981(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 35.Festa A, D’Agostino R, Jr, Williams K, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;25:1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 36.Saito I, Yonemasu K, Inami F. Association of body mass index, body fat, and weight gain with inflammation markers among rural residents in Japan. Circ J. 2003;67:323–329. doi: 10.1253/circj.67.323. [DOI] [PubMed] [Google Scholar]
- 37.Sites CK, Toth MJ, Cushman M, et al. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil Steril. 2002;77:128–135. doi: 10.1016/s0015-0282(01)02934-x. [DOI] [PubMed] [Google Scholar]
- 38.Giunta S. Is inflammaging an auto[innate]immunity subclinical syndrome? Immun Ageing. 2006;3:12. doi: 10.1186/1742-4933-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85:473–483. doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]