Abstract
Elevations in systemic free fatty acids (FFA) contribute to insulin resistance. To determine the effects of an acute elevation in FFA on insulin action with aging, we infused saline or intralipid (IL) during a hyperinsulinemic–euglycemic clamp in three groups of rats: young ad libitum–fed (YAL), old ad libitum–fed (OAL), and old on lifelong calorie restriction (OCR). The OCR group was included to distinguish between aging per se and age-related changes in body fat distribution. IL induced marked insulin resistance in both YAL and OCR, but the onset of insulin resistance was approximately two to three times more rapid in OCR as compared with YAL. In response to IL infusion, plasminogen-activating inhibitor-1 (PAI-1) expression was increased in subcutaneous fat from OAL animals. In visceral fat, a marked increase in PAI-1 and interleukin-6 expression was observed in OAL and OCR rats, but not YAL, in response to IL treatment. Thus, aging per se increases the inflammatory response to excess nutrients and vulnerability to FFA-induced insulin resistance with aging.
Keywords: Aging, Calorie restriction, Lipids, Insulin resistance, Adipokines
AGING is associated with an increase in abdominal obesity (ie, visceral adiposity), which is an independent risk factor for metabolic decline (1,2), age-related disease risk (3,4), and all-cause mortality (5). The biologic underpinning(s) linking visceral fat accrual with metabolic dysfunction has been purported to involve increased circulating free fatty acids (FFA) and glycerol released by omental fat into the portal circulation (3). In addition, the endocrine effects of adipose tissue, especially with visceral fat accrual, results in the increased secretion of numerous cytokines from adipocytes and their associated macrophages (6), which can lead to a proinflammatory state and interfere with whole-body insulin action (7–9).
Elevated FFA are a common feature of aging in nondiabetic humans and rodents (10,11) and are associated with obesity-induced insulin resistance (12), decreased glucose transport in skeletal muscle (13–15), and hepatic insulin resistance (1,14,16). Furthermore, acutely elevating FFA can impair insulin action (17–19) and invoke the expression and secretion of proinflammatory cytokines from adipose tissue, which have been shown to impair insulin sensitivity (20). Likewise, we have shown that the acute administration of nutrients can provoke the expression of proinflammatory cytokines from fat, especially visceral fat (21), and this response is exaggerated in old animals (22). Furthermore, we were able to separate the effects of aging per se from changes in body fat distribution by establishing an experimental rodent model employing calorie restriction (22), which maintains a “youthful” phenotype in aged rodents, including the prevention of visceral fat accrual.
Several mechanisms may link a greater availability of nutrients with increased inflammation including enhanced intracellular nutrient flux through the hexosamine biosynthetic pathway (19,23). Indeed, energy substrates including glucose, amino acids, and FFA all lead to increased flux through hexosamine biosynthetic pathway and stimulate the expression of downstream effectors and inflammatory peptides that can contribute to the induction of insulin resistance (22,23). Furthermore, FFA have been shown to activate TLR4 receptors in several cell types, including macrophages and adipocytes, which can induce inflammatory signaling and impair insulin sensitivity (18). However, the effect of a lipid provocation on parameters of insulin action and inflammation with aging is unknown. Therefore, the purpose of this study was to determine if aging per se increases the susceptibility to FFA-induced insulin resistance utilizing our experimental paradigm (22).
METHODS
Animals
Adult F344 × BN rats (Harlan Worldwide, Somerville, NJ) were housed in individual cages and subjected to a standard photoperiod (12L:12D). Rats belonged to one of three groups: (a) ad libitum (AL)–fed young rats (YAL), which were 3 months old, (b) ad libitum–fed old rats (OAL), which were 17–21 months old, and (c) calorie-restricted old rats (OCR), which were also 17–21 months old. The diet for ad libitum–fed animals consisted of regular chow (64% carbohydrate, 30% protein, and 6% fat, 3.3 kcal/g). Beginning at 5 months of age, OCR animals were fed 55% of ad libitum–fed animals with a similar diet supplemented with extra vitamins. One week before the in vivo clamp study, rats were anesthetized by inhalation of isoflurane, and indwelling catheters were inserted into the right internal jugular vein and left carotid artery. Recovery was continued until body weight was within 3% of the pre-operative weight (∼5 to 6 days). Chronically catheterized rats were studied approximately 12 hours after their last feeding while awake, unrestrained, and unstressed.
In Vivo Studies
To test if intralipid (IL) infusion would differentially affect insulin resistance, three groups of animals, YAL, OAL, and OCR rats were assigned to receive either saline (control group) or IL infusion during a hyperinsulinemic–euglycemic clamp. Specifically, a 5-hour intravenous infusion of lipid emulsion (Intra lipid 20%; Baxter Healthcare Corporation, Deerfield, IL) in 1:2 suspension with heparinized saline (100 U/mL) at a rate of 1.5 mL/h along with insulin (3 mU/kg/min) was performed. Somatostatin (1.5 μg/kg/min) was infused to prevent insulin secretion. Infusion of 25% dextrose was titrated to maintain blood glucose at a basal level (∼7 mM) for the duration of the clamp study. A similar protocol was followed for control groups with the exception that saline was infused in place of IL at a rate of 1.5 mL/h during the clamp study. All rats received a primed continuous infusion (15–40 μCi/min) of high performance liquid chromatography-purified [3-3H] glucose (New England Nuclear, Boston, MA) throughout the study to determine glucose fluxes. Blood samples for determination of 3H-glucose–specific activity were obtained at 10-minute intervals throughout the insulin infusion, and samples for the determination of insulin concentrations were also obtained at predetermined intervals during the clamp study. The total volume of blood withdrawn was ∼5.0 mL per study and a solution (1:1 vol/vol) of ∼8.0 mL of fresh blood (obtained by heart puncture from a littermate of the test animal) and heparinized saline (10 U/mL) were infused to prevent volume depletion and anemia. At the conclusion of the study, the rats were sacrificed using pentobarbital sodium (60 mg/kg body weight) intravenously through catheters previously implanted. The abdomen was quickly opened, fat pads were weighed and were then snap frozen in liquid nitrogen, and stored at −80°C. The study protocol was reviewed and approved by the Animal Care and Use Committee of the Albert Einstein College of Medicine.
Quantification of Expression by Real-Time Polymerase Chain Reaction
Total RNA from subcutaneous fat and visceral fat depots were extracted following Clontech's protocol with some modifications as previously described (24). First-strand complementary DNA (cDNA) was synthesized with random primers and total RNA as a template using the SuperScript preamplification system (Gibco/Brl, Grand Island, NY). Primer sets for several cytokines including angiotensinogen (AT), interleukin (IL)-6, IL-18, plasminogen-activating inhibitor-1 (PAI-1), tumor necrosis factor-α (TNF-α), leptin, and resistin have been described in detail elsewhere (21). Quantitative real-time polymerase chain reaction was performed using the Roche Light Cycler 480 (Roche, Mannheim, Germany) using 10 ng of cDNA and Sybr green kit (Roche) for detection. Cytokine expression values were normalized to beta-2 microglobulin as this was the only housekeeping gene among several tested (tubulin, β-actin, 18S, glyceraldehyde-3-phosphate dehydrogenase, hypoxanthine phosphoribosyltransferase 1, and ribosomal phosphoprotein P0) found not to change with age and/or treatment in adipose tissue.
Immunophenotyping and Fluorescence-Activated Cell Sorting (FACS)
To determine the percentage of macrophages in 1 g of adipose tissue, another group of YAL rat (3 months old), OAL rat (∼20 months old), and OCR rats (∼20 months old) were studied. At necropsy, adipose tissue was immediately excised for the isolation of macrophages and stromal vascular cells as described before with slight modification (25). Briefly, stromal vascular cells isolated from adipose tissue samples were cooled on ice and counted using a hemocytometer. Following counting, cells were centrifuged at 500g for 5 minutes and resuspended in fluorescence-activated cell sorting (FACS) buffer (phosphate buffered saline containing 5 mM ethylenediaminetetracetic acid and 0.2% [wt/vol] bovine serum albumin) at a concentration of 7 × 106 cells/mL. Cells were incubated in the dark at 4°C on a bidirectional shaker for 30 minutes in Fc Block (14 μg/mL; BD Pharmingen, San Jose, CA), followed by additional incubation of 30 minutes with fluorophore-conjugated primary antibodies (CD11b–fluorescein isothiocyanate, 2 μg/mL; Serotec, Raleigh, NC) and HIS-36–phycoerythrin (5 μg/mL; eBioscience, San Diego, CA), and 1 mL of FACS buffer was added to the cell conjugate. Cells were centrifuged, washed twice, and fixed with 1% paraformaldehyde. The acquisition of cells was done using Facscan and analysis was performed using FlowJo software (Tree Star Inc., Ashland, AZ).
Analytical Procedures
Plasma glucose was measured by the glucose oxidase method (Glucose Analyzer II; Beckman Instruments, Inc., Palo Alto, CA). Plasma insulin was measured by radioimmunoassay, using rat and porcine insulin standards. FFA were measured using WACO NEFA C Kit (Waco Chemicals USA, Richmond, VA). Plasma [3-3H]-glucose radioactivity was measured in duplicate in the supernatants of Ba(OH)2 and ZnSO4 precipitates of plasma samples (20 μL) after evaporation to dryness to eliminate tritiated water. Under steady-state conditions for plasma glucose concentrations, the rate of glucose disappearance (Rd) equals the rate of glucose appearance (Ra). The latter was calculated as the ratio of the rate of infusion of [3-3H]-glucose (decay per minutes per minute) and the steady-state plasma [3-3H]-glucose–specific activity (decay per minutes per milligrams). A time course for developing insulin resistance was then established by calculated slopes and determining when primary outputs of the glucose clamp including glucose infusion rate, glucose uptake, and endogenous glucose production were significantly impaired as compared with saline controls.
Statistical Analysis
All values are presented as means ± SE. Statistical analyses for cross-sectional measures were performed using either a one-way (group) or two-way Analysis of variance (Group × Treatment). When a significant effect was observed, planned contrasts with Tukey’s adjustment or t tests, when appropriate, were applied to determine individual differences across groups. For glucose clamp data, a hierarchical linear model was fit incorporating terms for group (YAL, OCR, and OAL), treatment (saline and IL), and time (0–300 minutes) with random effects for animals. An omnibus test for differences across groups was based upon the three-way interaction term (Group × Treatment × Time) and specific planned contrasts were carried out when appropriate. Likewise, estimates of the slopes over time and 95% confidence intervals were calculated from the appropriate linear combinations of regression coefficients, and group differences were determined from those estimates. A p value ≤ .05 was considered to be statistically significant. All analyses were performed using either SPSS v15 (SPSS, Chicago, IL) or Stata v11 (Stata Corp., College Station, TX).
RESULTS
Phenotypic and Basal Metabolic Characteristics of YAL, OCR, and OAL rats
Comparison of YAL and OCR rats revealed no significant differences in body weight or visceral fat (Table 1). As compared with YAL and OCR, OAL rats weighed more and had a greater amount of visceral fat (p < .05, Table 1). Furthermore, prior to the initiation of saline or IL, there were no significant differences among groups for glucose (not shown). OAL had greater insulin than YAL and OCR and greater FFA levels than YAL (p = .05) but not OCR (Table 1, p = .11).
Table 1.
Phenotypic Measures and Basal Metabolic Parameters of YAL, OCR, and OAL Rats
| YAL (n = 13) | OCR (n = 10) | OAL (n = 11) | |
| Body weight (g) | 296.6 ± 5.2a | 334.4 ± 11.9a | 521.2 ± 17.3b |
| Visceral fat (g) | 4.98 ± 0.43a | 4.29 ± 0.58a | 19.4 ± 2.19b |
| Food intake (g/day) | 19.2 ± 2.45b | 12a* | 21.3 ± 3.76b |
| Insulin (ng/mL) | 1.0 ± 0.2a | 1.4 ± 0.3a | 2.8 ± 0.2b |
| FFA (meq/L) | 0.64 ± 0.08a | 0.71 ± 0.06ab | 1.03 ± 0.17b |
Notes: Basic phenotypic characteristics including body weight, visceral fat, food intake, and plasma insulin and FFA levels in YAL, OCR, and OAL rats. Data are means ± SE. Different letters denote a significant difference between groups, p < .05. FFA = free fatty acids.
Food intake was clamped at 12 g/day.
Metabolic Parameters During the Clamp Studies in YAL, OCR, and OAL Rats
During the hyperinsulinemic clamps, there was no effect of group (p = .79) or Group × Treatment interaction for FFA (p = .43), but a significant effect of treatment was detected, which was observed within each group (Table 2, p < .001). Comparing glucose levels during the clamp (Table 2), there was a significant main effect for group with OAL being greater than YAL or OCR (p < .001) but neither treatment (p = .20) nor its interaction (p = .56) was significant. Likewise, a group effect was observed for clamp insulin (p = .003) with OAL as a group being significantly greater than YAL or OCR but neither treatment (p = .75) nor its interaction (p = .97) was significant (Table 2).
Table 2.
Metabolic Parameters During the Hyperinsulinemic–Euglycemic Clamp Study
| YAL Sal (n = 7) | YAL IL (n = 6) | OCR Sal (n = 5) | OCR IL (n = 5) | OAL Sal (n = 5) | OAL IL (n = 6) | |
| Clamp FFA (meq/L) | 0.11 ± 0.04 | 2.06 ± 0.26* | 0.33 ± 0.05 | 1.94 ± 0.47* | 0.86 ± 0.25 | 1.86 ± 0.12* |
| Clamp glucose (mmol/L) | 6.71 ± 0.10 | 6.38 ± 0.11 | 6.61 ± 0.22 | 6.61 ± 0.05 | 8.13 ± 0.06 | 7.52 ± 0.22 |
| Clamp insulin (ng/mL) | 3.96 ± 0.33 | 4.18 ± 0.11 | 4.67 ± 0.44 | 4.64 ± 0.08 | 6.23 ± 0.96 | 6.49 ± 0.82 |
Notes: YAL, OAL, and OCR rats underwent 5 hours of saline (Sal) or IL infusion during a hyperinsulinemic–euglycemic clamp. The average plasma FFA, glucose, and insulin levels (clamp) over the last hour are shown. Data are means ± SE. FFA = free fatty acids; IL = intralipid.
Significant effect of treatment within group, p < .05.
Glucose Infusion Rate During the Clamp Studies in YAL, OCR, and OAL Rats
The glucose infusion rate required to maintain plasma glucose levels in the three groups is shown in Figure 1A–C. Because the trajectory of glucose infusion rate over time was determined to be biphasic, the effect of time is represented by a piecewise linear model with a breakpoint at time = 120 minutes for this outcome. Using a two-variable hierarchical linear model, a significant Group × Treatment × Time interaction was observed up to time = 120 minutes (early phase; p = .007) and more than 120 minutes (late phase; p = .01). Based upon the calculated slopes during the early phase (0–120 minutes), glucose infusion rate was significantly attenuated with IL in YAL (p < .001) and OCR (p < .001) but not OAL (p = .25). During the late phase (>120 minutes), there was no effect of treatment on glucose infusion rate in YAL (p = .91) or OCR (p = .14) nor was there a significant difference in treatment effect between YAL and OCR (p = 0.64), but glucose infusion rate was significantly decreased by treatment in OAL (p < .01).
Figure 1.
Effect of saline (Sal) and intralipid (IL) infusion on glucose infusion rate (GIR) in (A) YAL, (B), OCR and (C) OAL rats. All rats underwent 5 hours of Sal infusion (control) or IL at a rate of 1.5 mL/h during the hyperinsulinemic clamp (3 mU/kg/min). Symbols and error bars are means ± SE (*p ≤ .05 vs Sal).
In addition to modifying the slope of the outcome trajectory, a treatment may shift the curve upward or downward. We quantified treatment shift by the mean difference in modeled (not observed) outcome at time = 30 minutes (the earliest time of observations). In none of the groups was a statistically significant shift observed at time = 30 minutes in response to treatment. However, OAL rats began the glucose clamps in a near maximal insulin-resistant state as demonstrated by a significant downward shift in their overall curves as compared with other groups (p < .001). In response to IL infusion at specific time points within groups, the glucose infusion rate in YAL animals was significantly lower than saline controls at 70 and 80 minutes, but animals did not demonstrate a level similar to old insulin-resistant rats (glucose infusion rate = ∼10 mg/kg/min) until 210 minutes (Figure 1A). OCR animals receiving IL infusion rapidly reached a glucose infusion rate significantly lower than saline (Figure 1B, p < .05) and similar to that of OAL by 100 minutes. During the last hour, IL-infused OCR rats demonstrated an even further decline in glucose infusion rate approaching 4 mg/kg/min.
Glucose Uptake During the Clamp Studies in YAL, OCR, and OAL Rats
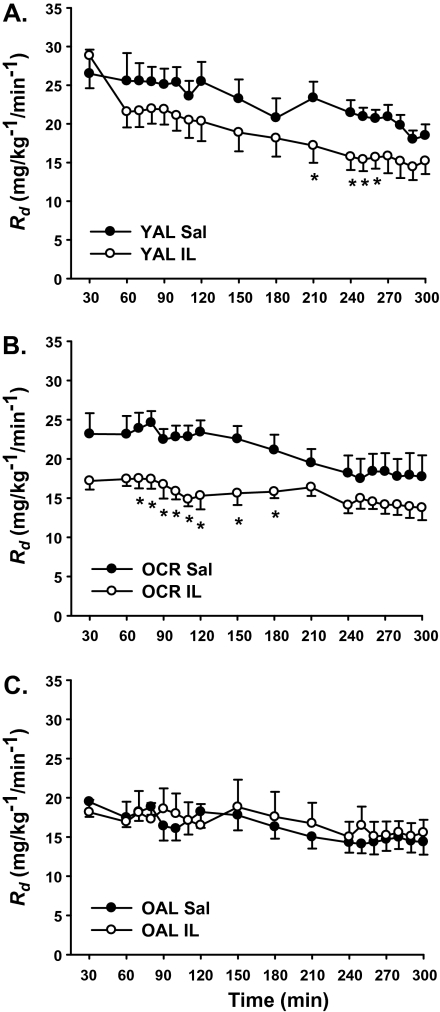
A significant Group × Treatment × Time interaction was observed for glucose uptake (p < .001), and the panels are shown in Figure 2. Based upon the slopes for each group, IL led to an attenuation in glucose uptake in YAL (p = .01) and OCR (p = .002) but not OAL (p = 0.22). In response to IL, the calculated downward shift for OCR was 7.4 mg/kg/min, (p = .002) but not in YAL (2.3 mg/kg/min, p = .26) or OAL (0.3 mg/kg/min, p = .89). Indeed, insulin-stimulated glucose uptake in IL-infused YAL rats at 30 minutes was ∼29 mg/kg/min (Figure 2A), but this was followed by a slow and steady decline in the rate of glucose uptake that was not significantly less than saline controls until 210 minutes and only remained significant until 270 minutes of the clamp (p < .05). In contrast, OCR rats more rapidly reached significance as compared with their saline controls (Figure 2B, p < .05) than YAL (70 vs 210 minutes). Thus, there was no significant effect of treatment in OAL, whereas a significant effect of treatment was observed in YAL and OCR. In addition, there was a significant difference in the effect of IL treatment between YAL and OCR (p < .001). Similar to glucose infusion rate, the overall curve for glucose uptake in OAL was substantially shifted downward relative to other groups (Figure 2C, p < .01) and IL infusion failed to lower glucose uptake, suggesting that glucose uptake was already maximally impaired in OAL.
Figure 2.
Effect of saline (Sal) and intralipid (IL) infusion on glucose uptake (Rd) in (A) YAL, (B) OCR, and (C) OAL rats. Symbols and error bars are means ± SE (*p ≤ .05 vs Sal).
Endogenous Glucose Production During the Clamp Studies in YAL, OCR, and OAL Rats
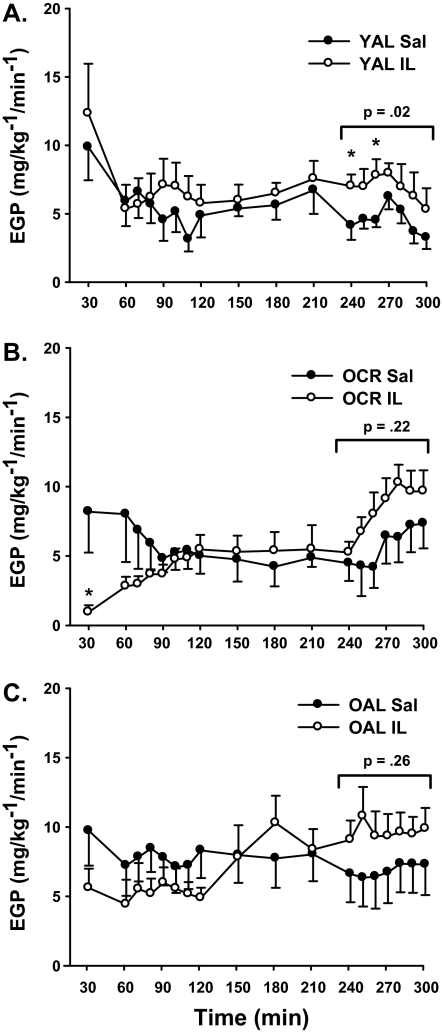
A three-way (Group × Treatment × Time) interaction was observed for endogenous glucose production (p = .001). Although glucose production rates showed no significant change in slope or shift with IL infusion in YAL rats (p = .60), there was a significant and similar downward shift and upward slope over time in both OCR (p = .01) and OAL rats (p < .05). Furthermore, OCR and OAL differed significantly in their response to IL infusion as compared with YAL (p < .001). Interestingly, within-group comparisons revealed that endogenous glucose production rates were significantly higher only in YAL rats by 240 and 260 minutes (Figure 3A, p < .05). Furthermore, when averaging glucose production rates over the last hour of the clamp, IL infusion raised endogenous glucose production levels in all three groups, but this was only statistically significant for YAL rats (4.53 ± 0.56 vs 7.20 ± 0.78 mg/kg/min, p = .02) and not for OCR (5.75 ± 1.55 vs 8.39 ± 1.12 mg/kg/min, p = .22) or OAL (6.87 ± 1.72 vs 9.64 ± 1.44 mg/kg/min; Figure 3, p = .26).
Figure 3.
Effect of saline (Sal) and intralipid (IL) infusion on endogenous glucose production (EGP) in (A) YAL, (B) OCR, and (C) OAL rats. Symbols and error bars are means ± SE (*p ≤ .05 vs Sal).
Expression of Fat-Derived Peptide Genes at the End of the Clamp Period
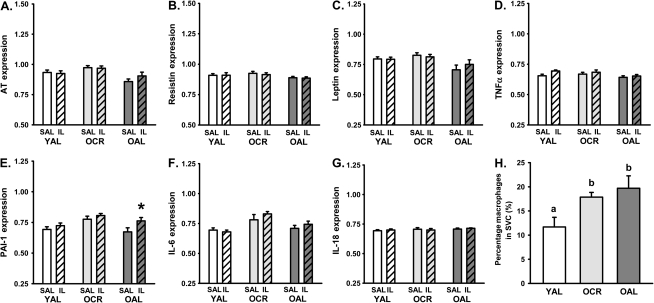
At the end of the clamps with saline or IL, we studied gene expression of several cytokines (AT, resistin, leptin, TNF-α, PAI-1, IL-6, and IL-18) that are associated with a proinflammatory state and insulin resistance in visceral (Figure 4) and subcutaneous fat (Figure 5) depots. In visceral fat, a significant main effect of group and treatment were observed only for PAI-1 (Figure 4E, p < .05) and IL-6 (Figure 4F, p < .01), but no significant interaction was observed. In response to IL, PAI-1 expression was significantly increased in OCR (p < .05) but not in OAL (p = .07) or YAL (p = .43), whereas IL-6 expression was increased in OCR and OAL (p < .05) but not YAL (p = .63). In subcutaneous fat, a significant main effect of group and treatment was observed for PAI-1 (p < .05) but gene expression was only increased by IL in OAL (p = .05).
Figure 4.
Effect of saline (Sal) and intralipid (IL) infusion on gene expression of adipokines from visceral fat in YAL, OCR, and OAL rats during a hyperinsulinemic clamp including (A) angiotensinogen (AT), (B) resistin, (C) leptin, (D) tumor necrosis factor-α (TNFα), (E) plasminogen-activating inhibitor-1 (PAI-1), (F) interleukin (IL)-6, and (G) IL-18. All values were normalized to the expression of the housekeeping gene beta-2 microglobulin. Macrophage content as a percentage of stromal vascular cells in 1 g of visceral fat was assessed by flow cytometry and is shown in panel (H). Data are means ± SE. Different letters denote a significant difference between groups in panel H (p ≤ .05). *Denotes a significant difference between IL and Sal within groups (p ≤ .05).
Figure 5.
Effect of saline (Sal) and intralipid (IL) infusion on gene expression of adipokines from subcutaneous fat in YAL, OCR, and OAL rats during a hyperinsulinemic clamp, including (A) angiotensinogen (AT), (B) resistin, (C) leptin, (D) tumor necrosis factor-α (TNFα), (E) plasminogen-activating inhibitor-1 (PAI-1), (F) interleukin (IL)-6, and (G) IL-18. All values were normalized to the expression of the housekeeping gene beta-2 microglobulin Macrophage content as a percentage of stromal vascular cells in 1 g of SC fat was assessed by FACS is shown in panel (H). Data are means ± SE. Different letters denote a significant difference between groups (p < .05). *Denotes a significant difference between IL and Sal within groups (p ≤ .05).
Adipose Tissue Macrophages in YAL, OCR, and OAL Rats
Macrophage content as a percentage of the stromal vascular fraction was assessed in 1 g of visceral or subcutaneous adipose tissue with FACS analysis. YAL rats had the lowest amount of macrophages in both visceral (Figure 4H) and subcutaneous adipose tissue (Figure 5H), as expressed as a percentage of the stromal vascular cell fraction. However, both old groups of rats, regardless of adiposity status, had a significantly greater percentage of macrophages compared with that of YAL in both subcutaneous and visceral fat (p < .05), but no significant difference was found between OCR and OAL rats (Figures 4H and 5H).
DISCUSSION
The goal of this study was to determine if aging per se increases the susceptibility of rats to a lipid provocation as demonstrated by the development of more rapid and severe insulin resistance during a hyperinsulinemic–euglycemic clamp and whether this might be linked to greater gene expression of proinflammatory cytokines from adipose tissue. To test this hypothesis, we utilized an experimental paradigm of YAL, OAL, and a separate group of old rats, which were caloric restricted since early adulthood (OCR), thus allowing for the discrimination of aging per se from unfavorable changes in body composition with aging. As our data show, despite their chronological age, OCR rats were very similar to YAL animals in many respects including body weight, visceral fat mass, FFA, insulin levels and demonstrated a similar response to insulin during a control (infused with saline) hyperinsulinemic–euglycemic clamp.
However, based upon several outcomes of the hyperinsulinemic–euglycemic clamps during IL infusion, OCR rats appeared to demonstrate a more rapid development of insulin resistance than YAL. Indeed, OCR demonstrated a significantly greater upward slope for glucose production and downward slope for glucose uptake than YAL, and glucose infusion rate and glucose uptake were also impaired sooner in OCR than YAL (saline as reference). This suggests a greater baseline susceptibility to FFA-induced insulin resistance with aging. Importantly, this observation could not have been obtained in OAL rats because these animals were already insulin resistant in regard to glucose uptake at the outset of the clamp and IL infusion failed to further impair glucose disposal. Interestingly, endogenous glucose production rates behaved similarly in OCR and OAL rats in response to IL with a significant downward shift and increase over time throughout the clamp, whereas no significant change in slope was observed in YAL. However, during the last hour, glucose production was only significantly elevated during IL infusion in YAL animals (∼6 to 7 mg/kg/min), though their rates remained less than those observed in old (∼9 to 11 kg/min) during the last hour. Taken together, this novel paradigm has shown for the first time that greater susceptibility of insulin action to FFA may be an important feature of aging.
The exact cause(s) of increased susceptibility to FFA with aging are unknown. Randle initially hypothesized that elevated FFA levels directly switch glucose oxidation to FFA oxidation, thereby reducing glucose uptake (26). However, insulin resistance is not induced immediately by infusion of FFA as would be expected by this hypothesis. In fact, infusion of an IL emulsion during an insulin clamp does not begin to significantly impair glucose uptake until 90 minutes later and progressively decreases until a nadir is reached 4–7 hours later (27), which is similar to our observation here. This phenomenon suggests that augmentation of FFA fluxes induce insulin resistance in a time-dependent manner, which requires the activation of several biologic processes, such as glycosylation, expression of various transcription factors, and inflammatory pathways (19). It should also be noted that while others have demonstrated impaired glucose uptake with prolonged lipid infusion (14,28), there are contradictory findings as to whether intracellular glucose-6-phosphate increases following prolonged lipid infusion supporting Randle's hypothesis (14,26), or decreases, suggesting reduced insulin-induced glucose transport into muscle as the primary defect (28).
Because aging has been characterized as a proinflammatory state (29,30) and nutrients have been shown to induce adipose tissue–derived inflammation and impair insulin action (7–9), we hypothesized that similar to what we observed with glucosamine infusion (22) that increased vulnerability to FFA-induced insulin resistance with aging was in part the result of a heightened sensitivity of nutrient-sensing pathways linked to inflammation. Consistent with our hypothesis, IL infusion failed to significantly increase fat-derived cytokine expression from visceral fat in young rats but resulted in a marked increase in PAI-1 and IL-6 expression from visceral fat in both OAL and OCR rats. This is in line with our prior studies (21,22), and the general contention that visceral fat is an important contributor to the proinflammatory state associated with obesity (4,31). Likewise, we also observed an increase in PAI-1 expression in subcutaneous fat from OAL receiving IL. These observations seem relevant considering both PAI-1 and IL-6 have been shown to increase with aging and obesity (6,32) and are linked to important age-related diseases, such as type 2 diabetes (33,34) and cardiovascular disease (35,36).
It is worth pointing out that while nutrients provoked expression of proinflammatory cytokines in both OAL and OCR rats, OAL rats also had five times more visceral fat mass than their old lean counterparts (Table 1). As a consequence, the absolute amount of cytokines produced is likely far greater in OAL than OCR animals. Indeed, human studies have shown that increasing amounts of visceral adiposity are independently associated with greater systemic inflammation (6). Furthermore, other biologic changes may also be responsible for heightened inflammation with aging such as declines in sex steroids (37,38) and increased prevalence of senescent cells, which have been shown to have a proinflammatory secretory phenotype (39) and can accumulate in metabolically relevant tissues with aging, such as liver (40). Thus, these results do not rule out a role for inflammation in this process but instead have identified a novel distinction between aging per se and the aging–obesity interaction on the relative contribution of inflammation from adipose tissue. Furthermore, other mechanisms may also contribute to chronic or sustained insulin resistance with aging and obesity, including lipotoxicity (41) and mitochondrial dysfunction, although the latter is somewhat controversial (42–44).
Macrophages are considered to be a significant source for many fat-derived proinflammatory cytokines, and the percentage of macrophages in fat has been shown to increase in obesity (25). Interestingly, we observed that the percentage of macrophages in the stromal vascular cell fraction from both visceral and subcutaneous fat increased with age regardless of obesity status. To our knowledge, such an increase in adipose tissue macrophage content with aging has not been reported. Indeed, the dogma has been that greater levels of macrophage infiltration occurs only with expansion of adipose tissue mass, yet this finding challenges that notion by demonstrating an effect of aging per se. However, as previously mentioned, OAL were substantially more obese than OCR rats such that these animals would still have a predictably greater absolute number of macrophages.
In summary, we demonstrate that aging per se confers a unique susceptibility to FFA-induced insulin resistance as demonstrated by a more rapid impairment in glucose uptake and glucose infusion rate in OCR rats as compared with YAL, when challenged with nutrients. Furthermore, aging per se is associated with a heightened proinflammatory response to nutrients in adipose tissue, including increased PAI-1 and IL-6 expression in OAL and OCR animals. Thus, although lifelong calorie restriction may prevent age-related changes in body composition and maintain a “youthful” phenotype, it does not prevent the accumulation of adipose tissue macrophages, the inflammatory response to excess nutrients, or the increased vulnerability to FFA-induced insulin resistance with aging.
FUNDING
This work was supported by grants from the National Institutes of Health (AG21654 and AG18381 to N.B. and K08 AG027462 to R.H.M.), the Core laboratories of the Albert Einstein Diabetes Research and Training Center (DK20541), and the American Association of Obstetricians and Gynecologists Foundation with the Society of Maternal-Fetal Medicine (to F.H.E). D.M.H. is supported by a T32 Training Grant (T32AG23475).
Acknowledgments
The authors would like to acknowledge Temuri Budagov, Lingguang Cui, and Hongquian Liang for technical assistance.
References
- 1.Barzilai N, Rossetti L. Age-related changes in body composition are associated with hepatic insulin resistance in conscious rats. Am J Physiol. 1996;270(6 Pt 1):E930–E936. doi: 10.1152/ajpendo.1996.270.6.E930. [DOI] [PubMed] [Google Scholar]
- 2.Koh-Banerjee P, Wang Y, Hu FB, et al. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159(12):1150–1159. doi: 10.1093/aje/kwh167. [DOI] [PubMed] [Google Scholar]
- 3.Bjorntorp P. Abdominal fat distribution and the metabolic syndrome. J Cardiovasc Pharmacol. 1992;20(Suppl 8):S26–S28. [PubMed] [Google Scholar]
- 4.Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790(10):1117–1123. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 6.Cartier A, Cote M, Lemieux I, et al. Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metabolism. 2009;58(10):1452–1458. doi: 10.1016/j.metabol.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Hutley L, Prins JB. Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci. 2005;330(6):280–289. doi: 10.1097/00000441-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;22:82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 9.Gabriely I, Barzilai N. The role of fat cell derived peptides in age-related metabolic alterations. Mech Ageing Dev. 2001;122(14):1565–1576. doi: 10.1016/s0047-6374(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 10.Bonadonna RC, Groop LC, Simonson DC, DeFronzo RA. Free fatty acid and glucose metabolism in human aging: evidence for operation of the Randle cycle. Am J Physiol. 1994;266(3 Pt 1):E501–E509. doi: 10.1152/ajpendo.1994.266.3.E501. [DOI] [PubMed] [Google Scholar]
- 11.Tessari P. Changes in protein, carbohydrate, and fat metabolism with aging: possible role of insulin. Nutr Rev. 2000;581:11–19. doi: 10.1111/j.1753-4887.2000.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 12.Gao Z, Zhang X, Zuberi A, et al. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18(8):2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 13.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46(1):3–10. [PubMed] [Google Scholar]
- 14.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93(6):2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Galaz C, Fernandez-Agullo T, Perez C, et al. Long-term food restriction prevents ageing-associated central leptin resistance in wistar rats. Diabetologia. 2002;45(7):997–1003. doi: 10.1007/s00125-002-0851-4. [DOI] [PubMed] [Google Scholar]
- 17.Xiao C, Giacca A, Lewis GF. The effect of high-dose sodium salicylate on chronically elevated plasma non-esterified fatty acid-induced insulin resistance and {beta}-cell dysfunction. Am J Physiol Endocrinol Metab. 2009;297:E1205–E1211. doi: 10.1152/ajpendo.00313.2009. [DOI] [PubMed] [Google Scholar]
- 18.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkins M, Barzilai N, Liu R, et al. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest. 1997;99(9):2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krogh-Madsen R, Plomgaard P, Akerstrom T, et al. Effect of short-term intralipid infusion on the immune response during low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab. 2008;294(2):E371–E379. doi: 10.1152/ajpendo.00507.2007. [DOI] [PubMed] [Google Scholar]
- 21.Einstein FH, Atzmon G, Yang XM, et al. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes. 2005;54(3):672–678. doi: 10.2337/diabetes.54.3.672. [DOI] [PubMed] [Google Scholar]
- 22.Einstein FH, Fishman S, Bauman J, et al. Enhanced activation of a “nutrient-sensing” pathway with age contributes to insulin resistance. FASEB J. 2008;22(10):3450–3457. doi: 10.1096/fj.08-109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Issad T, Kuo M. O-GlcNAc modification of transcription factors, glucose sensing and glucotoxicity. Trends Endocrinol Metab. 2008;19(10):380–389. doi: 10.1016/j.tem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Atzmon G, Yang XM, Muzumdar R, et al. Differential gene expression between visceral and subcutaneous fat depots. Horm Metab Res. 2002;34(11–12):622–628. doi: 10.1055/s-2002-38250. [DOI] [PubMed] [Google Scholar]
- 25.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 27.Jucker BM, Rennings AJ, Cline GW, Shulman GI. 13C and 31P NMR studies on the effects of increased plasma free fatty acids on intramuscular glucose metabolism in the awake rat. J Biol Chem. 1997;272(16):10464–10473. doi: 10.1074/jbc.272.16.10464. [DOI] [PubMed] [Google Scholar]
- 28.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97(12):2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8(3–4):572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 30.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102(2–3):199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 31.Gabriely I, Ma XH, Yang XM, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51(10):2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- 32.Tofler GH, Massaro J, Levy D, et al. Relation of the prothrombotic state to increasing age (from the Framingham Offspring Study) Am J Cardiol. 2005;96(9):1280–1283. doi: 10.1016/j.amjcard.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 33.Kanaya AM, Harris T, Goodpaster BH, Tylavsky F, Cummings SR. Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care. 2004;27(6):1375–1380. doi: 10.2337/diacare.27.6.1375. [DOI] [PubMed] [Google Scholar]
- 34.Festa A, Williams K, Tracy RP, Wagenknecht LE, Haffner SM. Progression of plasminogen activator inhibitor-1 and fibrinogen levels in relation to incident type 2 diabetes. Circulation. 2006;113(14):1753–1759. doi: 10.1161/CIRCULATIONAHA.106.616177. [DOI] [PubMed] [Google Scholar]
- 35.Rodondi N, Marques-Vidal P, Butler J, et al. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am J Epidemiol. 2010;171(5):540–549. doi: 10.1093/aje/kwp428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morange PE, Saut N, Alessi MC, et al. Association of plasminogen activator inhibitor (PAI)-1 (SERPINE1) SNPs with myocardial infarction, plasma PAI-1, and metabolic parameters: the HIFMECH study. Arterioscler Thromb Vasc Biol. 2007;27(10):2250–2257. doi: 10.1161/ATVBAHA.107.149468. [DOI] [PubMed] [Google Scholar]
- 37.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39(5):687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Nakhai Pour HR, Grobbee DE, Muller M, van der Schouw YT. Association of endogenous sex hormone with C-reactive protein levels in middle-aged and elderly men. Clin Endocrinol (Oxf) 2007;66(3):394–398. doi: 10.1111/j.1365-2265.2007.02745.x. [DOI] [PubMed] [Google Scholar]
- 39.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Jurk D, Maddick M, et al. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8(3):311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 41.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Bhashyam S, Parikh P, Bolukoglu H, et al. Aging is associated with myocardial insulin resistance and mitochondrial dysfunction. Am J Physiol Heart Circ Physiol. 2007;293(5):H3063–H3071. doi: 10.1152/ajpheart.00163.2007. [DOI] [PubMed] [Google Scholar]
- 43.Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59(1):89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]