Abstract
Moderately elevated levels of plasma plant sterols have been suspected to be causally involved in atherosclerosis. The aim of this study was to investigate whether plant sterols and other markers of sterol metabolism predicted all-cause and cardiovascular mortality in participants of the Ludwigshafen Risk and Cardiovascular health (LURIC) study. A total of 1,257 individuals who did not use statins and at baseline had a mean (± SD) age of 62.8 (± 11.0) years were included in the present analysis. Lathosterol, cholestanol, campesterol, and sitosterol were measured to estimate cholesterol synthesis and absorption. The mean (± SD) time of the follow-up for all-cause and cardiovascular mortality was 7.32 (± 2.3) years. All-cause (P = 0.001) and cardiovascular (P = 0.006) mortality were decreased in the highest versus the lowest lathosterol to cholesterol tertile. In contrast, subjects in the third cholestanol to cholesterol tertile had increased all-cause (P < 0.001) and cardiovascular mortality (P = 0.010) compared with individuals in the first tertile. The third campesterol to cholesterol tertile was associated with increased all-cause mortality (P = 0.025). Sitosterol to cholesterol tertiles were not significantly related to all-cause or cardiovascular mortality. The data suggest that high absorption and low synthesis of cholesterol predict increased all-cause and cardiovascular mortality in LURIC participants.
Keywords: cholesterol absorption, cholesterol synthesis, campesterol, sitosterol, cholestanol, lathosterol
High total and LDL cholesterol represent well-established cardiovascular risk factors (1). Plant sterols are structurally highly similar to cholesterol (2), but their physiological plasma concentration is less than 1 mg/dl (3).
Sitosterolaemia, a rare genetic disorder, is characterized by up to 100-fold higher levels of plasma plant sterols (4). Patients suffering from sitosterolaemia often develop severe premature atherosclerosis (4). Thus, it has been speculated that moderately increased plasma plant sterols might be atherogenic in the general population as well. Epidemiological studies concerning this issue have been controversial (3, 5–18).
Research analyzing a possible link between plant sterols and coronary artery disease (CAD) has to take into account that plasma plant sterols reflect cholesterol absorption (19–21). Interestingly, high absorption and low synthesis of cholesterol have also been speculated to be related to CAD, particularly in subjects taking statins, as was presented for the first time by Miettinen et al. (22).
Recently, we examined the associations of plasma plant sterols and cholesterol metabolism with the severity of CAD in participants of the Ludwigshafen Risk and Cardiovascular Health (LURIC) study (23). LURIC is a large prospective clinical study of individuals who have undergone coronary angiography, and it was designed to investigate biochemical and genetic cardiovascular risk factors (24). High absorption and low synthesis of cholesterol were related to more severe CAD. However, the data did not conclusively support an active role of plant sterols in the progression of vascular disease (23).
Only two previous studies, the Drugs and Evidence-Based Medicine in the Elderly (DEBATE) study and the Helsinki Businessmen Study, have investigated whether cholesterol homeostasis and plasma plant sterols are associated with all-cause mortality (5, 16). However, both studies were very small in the number of participants and events. In the DEBATE cohort, 376 subjects were studied and 64 deaths occurred (5). The Helsinki Businessmen Study reported on 232 subjects and 101 fatal events (16). Moreover, DEBATE enrolled subjects aged 75 years or older and the Helsinki Businessmen Study was limited, because only males (mean age 60 years) were included (5, 16). The relationship of cholesterol metabolism and plasma plant sterols with cardiovascular mortality has been studied once only (16). Furthermore, there is a lack of studies addressing the predictive value of cholesterol metabolism and plasma plant sterols in subjects at intermediate to high cardiovascular risk.
Thus, the objective of the present analysis was to study whether high absorption and low synthesis of cholesterol predict increased all-cause and cardiovascular mortality in LURIC participants. Moreover, we aimed to investigate if particularly high plasma plant sterols are a harbinger of an adverse prognosis.
For that purpose, we measured the plasma concentrations of lathosterol, cholestanol, campesterol, and sitosterol. Lathosterol is a cholesterol precursor and has been shown to be a marker of cholesterol synthesis (20). Cholestanol represents the 5α-saturated derivative of cholesterol and indicates intestinal cholesterol uptake (19). It is poorly absorbed in the gut, endogenously synthesized by enzymatic conversion of cholesterol, and rapidly eliminated via bile (19). The exact physiological mechanisms accounting for the association of the plasma cholestanol level with fractional cholesterol absorption are not fully understood yet. Campesterol and sitosterol are the two most abundant plant sterols, and they are not synthesized in the human body. Their plasma concentrations are tightly correlated, and they are surrogate markers for cholesterol absorption (20).
METHODS
Study design and participants
LURIC is a prospective cohort study with a total of 3,316 participants who were recruited between July 1997 and January 2000 at the Ludwigshafen Heart Center in South-West Germany (24). Inclusion criteria were: German ancestry, clinical stability except for acute coronary syndromes, and the availability of a coronary angiogram. The indications for angiography in individuals in clinically stable condition were chest pain and/or noninvasive test results consistent with myocardial ischemia. Individuals suffering from any acute illness other than acute coronary syndromes, chronic noncardiac diseases, or malignancy within the past 5 years, and those unable to understand the purpose of the study were excluded. The study was approved by the ethics committee at the Ärztekammer Rheinland-Pfalz and was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from all participants. Diabetes mellitus was categorized according to the criteria of the American Diabetes Association (25). Furthermore, individuals with a history of diabetes, or treatment with oral antidiabetics or insulin were considered diabetic. Hypertension was diagnosed if the systolic and/or diastolic blood pressure exceeded 140 and/or 90 mm Hg or if there was a history of hypertension, also evident through the use of antihypertensive drugs.
Measurement of lathosterol, cholestanol, campesterol, and sitosterol was complete in 2,440 LURIC participants with and without statin treatment that were randomly selected and did not have type 1 diabetes (exclusion of seven subjects). A total of 1,257 individuals who did not use lipid lowering drugs were included in the present analysis.
Follow-up
There was a follow-up for all-cause and cardiovascular mortality. The mean (± SD) duration of the follow-up in the entire cohort was 7.32 (± 2.3) years. Information on whether a participant had died or not was obtained from local person registries. Death certificates were used for the classification of the causes of death. The deceased were classified into those who died from cardiovascular and noncardiovascular causes. Cardiovascular death included the following categories: sudden death, fatal myocardial infarction, death due to congestive heart failure, death immediately after intervention to treat CAD, fatal stroke, and other causes of death due to CAD. Two experienced clinicians who were blinded to any data of the study participants independently classified the causes of death. In cases of a disagreement or uncertainty concerning the coding of a specific cause of death, classification was made by a principal investigator of the LURIC study (W.M.) (24).
Laboratory analyses
Quantification of noncholesterol sterols and all other analyses were performed in fasting blood samples collected before coronary angiography. The standard laboratory methods have been described (24). Total cholesterol was measured enzymatically with reagents from Roche, Mannheim, Germany on a Hitachi 717 analyzer (24). Noncholesterol sterols were measured in plasma kept frozen at −80°C from the date of blood withdrawal until the day of analysis applying GC and MS (single ion monitoring mode). Lathosterol, cholestanol, and campesterol were obtained from Steraloids (Newport, US). Sitosterol was from Sigma (Steinheim, Germany). Epicoprostanol (Sigma) was used as internal standard. N-methyl-N-(trimethylsilyl)-trifluoroacetamide (MSTFA) was obtained from ABCR (Karsruhe, Germany), trimethylchlorosilane and pyridine were purchased from Pierce (Rockford, IL). Silica gel and all solvents and reagents of analytical grade were obtained from Merck (Darmstadt, Germany). A Thermo Trace 2000 gas chromatograph coupled to a Fisons MD 800 quadrupole mass spectrometer was used. The column was directly connected to the ion source of the MS. The GC was equipped with a HT5 fused silica capillary column (25 m × 0.22 mm i.d., 0.1 µm film thickness) from SGE (SGE Analytical Science, Griesheim, Germany). The splitless Grob-injector was kept at 220°C. Helium was used as carrier gas with a constant flow of 1 ml/min. The initial column temperature of 200°C was held for 1 min and followed by an increase of 15°C/min to 300°C and a final isothermal hold of 7 min. The transfer line between GC and MS was kept at 310°C. Ion source temperature was 150°C. Electron impact spectra were recorded with electron energy of 70 eV and an emission current of 150 µA. The significant ions were m/z 445.4 for cholestanol, m/z 382.4 for campesterol, m/z 357.3 for sitosterol, m/z 458.5 for lathosterol, and m/z 370.4 for epicoprostanol. A 100 µl portion of the methanolic internal standard solution containing 2 nmol of epicoprostanol and 20 µg/100 µl of butylated hydroxytoluene (Sigma) as antioxidant was admixed to 100 µl of plasma. An amount of 250 µl KOH solution (50% in water) and 800 µl of ethanol absolute were added to the vial. The sealed vial was kept at 75°C for 1 h, cooled to room temperature and 1 ml of water was added. After the addition of 2 ml of hexane, the samples were extracted (10 min, 360° shaker) and centrifuged (3 min, 3,000 rpm). The upper organic layer was decanted and applied on a silica gel column that had been prewashed with 4 ml of hexane. After further elution of 2 ml of hexane, the samples were eluted with 4 ml of hexane/i-propanol (70/30) and the solvent was evaporated under a stream of nitrogen. Then 50 µl of an MSTFA solution [MSTFA containing 1% trimethylchlorosilane, in pyridine (2/1, v/v)] was added. Subsequently, the mixture was shaken and incubated at room temperature for 30 min. Then the samples were dried under nitrogen, resuspended in 100 µl of hexane, transferred to autosampler vials, sealed, and an aliquot of 2 µl was injected into the GC. Linearity of calibration (linear regression with weighting of 1/x2) was examined for all target compounds in the range of 0.012–3.125 nmol/sample by comparing peak areas of standard samples with the internal standard (epicoprostanol). The coefficients of regression were >0.99. Analyses of five control sets (low, medium, and high) on one day and five control sets on five different days as well as measurements of samples after three freeze-thaw cycles and of samples kept 3 h at room temperature before extraction revealed consistent results. Each set of patient samples was accompanied by a set of calibration samples and two sets of control samples. No tendency in any direction could be seen by comparing control values over the whole period of experiments. The investigators and laboratory personnel were blinded to all clinical and biochemical data of the study participants and the samples were analyzed in random order.
Genotyping
Apolipoprotein E (APOE) alleles were determined as described earlier (23). Three groups comprising carriers of at least one APOE 2 allele (APOE 2/2, APOE 2/3, APOE 2/4), APOE 3/3 homozygotes, and the remaining individuals (APOE 3/4 or APOE 4/4) were formed. Data on the APOE genotype were available in 1,255 of 1,257 subjects.
Statistical analysis
We calculated the ratios of the noncholesterol sterols to cholesterol to standardize for variation in cholesterol. Moreover, the ratios of the absorption markers to lathosterol were computed. Tertiles of the noncholesterol sterol to cholesterol ratios and of the absorption marker to lathosterol ratios were established. Baseline clinical and biochemical characteristics are presented according to the noncholesterol sterol to cholesterol tertiles. In the case of continuous variables, we report means and SD or medians and inter-quartile ranges. Categorical data are expressed as numbers and percentages of subjects. Comparisons between the three groups according to the noncholesterol to cholesterol tertiles were made with logistic regression for categorical data and with the general linear model for continuous variables. The Cox proportional hazards model was used to test the relationships of the noncholesterol sterol to cholesterol and the absorption marker to lathosterol tertiles with all-cause and cardiovascular mortality. Two models of adjustment were applied, model 1 with the covariates sex and age and model 2 with the covariates sex, age, body mass index (BMI), type 2 diabetes, hypertension, smoking, and C-reactive protein (CRP). Backward stepwise logistic regression selection was used and the results of the final step are shown. In addition, Kaplan-Meier curves were calculated. The Kaplan Meier curves for cardiovascular mortality in the subgroup of individuals without type 2 diabetes (model 2) are depicted in Fig. 1. All statistical tests were 2-sided and P < 0.05 was considered significant. The SPSS 15.0 statistical package (SPSS Inc.) was used.
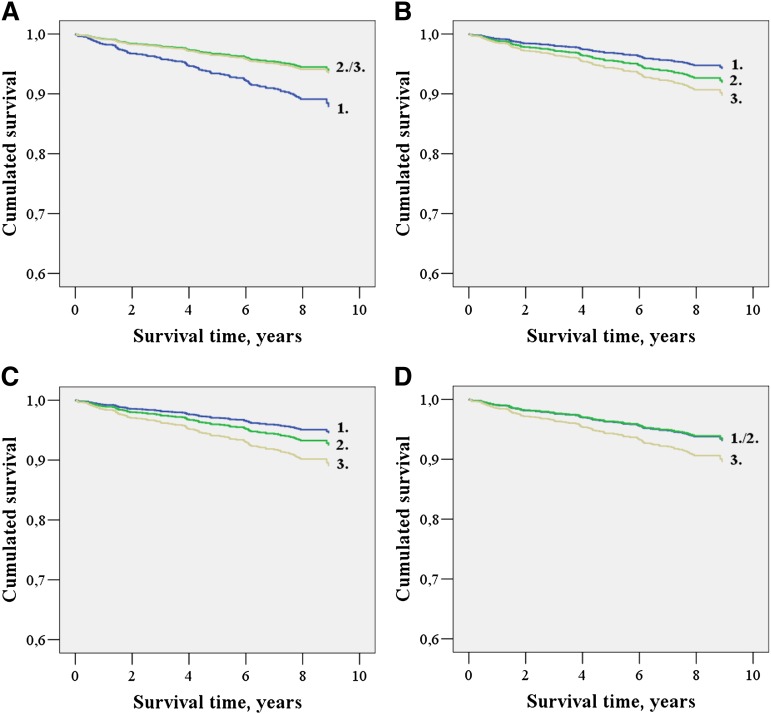
Fig. 1.
Cumulated survival functions for cardiovascular mortality in subjects without diabetes according to lathosterol to cholesterol (A), cholestanol to cholesterol (B), campesterol to cholesterol (C), and sitosterol to cholesterol (D) tertiles (1.-3.).
Statement of responsibility
All authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Noncholesterol sterol concentrations
The absolute mean plasma concentrations (± SD) of lathosterol, cholestanol, campesterol, and sitosterol were 8.94 (± 5.15) µmol/l, 7.52 (± 3.21) µmol/l, 7.46 (± 4.68) µmol/l, and 3.84 (± 2.44) µmol/l, respectively. All noncholesterol sterols were positively correlated with total cholesterol (r = 0.348, P < 0.001; r = 0.494, P < 0.001; r = 0.412, P < 0.001; and r = 0.393, P < 0.001 for lathosterol, cholestanol, campesterol, and sitosterol, respectively). The relationships among the noncholesterol sterol ratios and their correlations with age and metabolic parameters are shown in supplementary Table I.
Baseline characteristics according to lathosterol to cholesterol tertiles
The cholestanol, campesterol, and sitosterol to cholesterol ratios and all absorption marker to lathosterol ratios were inversely related to the lathosterol to cholesterol tertiles (all P < 0.001) (Table 1). There were no differences in sex across the tertiles of the lathosterol to cholesterol ratio. Subjects in the high lathosterol to cholesterol tertile versus individuals in the low lathosterol to cholesterol tertile were significantly younger (P < 0.001) (Table 1). A high lathosterol to cholesterol ratio was associated with increased BMI, waist circumference, and prevalence of type 2 diabetes (no insulin) (all P < 0.001). In addition, a high lathosterol to cholesterol ratio was correlated with increased fasting glucose (P = 0.004), fasting insulin (P < 0.001), systolic blood pressure (P = 0.013), and systemic hypertension (P < 0.001) (Table 1). Diastolic blood pressure and the HbA1c were not significantly related to the lathosterol to cholesterol tertiles (Table 1). Decreased LDL and HDL cholesterol and elevated triglycerides were associated with high lathosterol to cholesterol ratio (P = 0.003, P < 0.001, and P < 0.001, respectively) (Table 1). The total cholesterol concentration, CRP, and smoking were not significantly associated with the lathosterol to cholesterol tertiles. The APOE 3/4 and APOE 4/4 genotypes were more frequent in individuals with a high lathosterol to cholesterol ratio (P = 0.035) (Table 1).
TABLE 1.
Baseline characteristics of the study population by lathosterol to cholesterol tertiles
| Lathosterol to Cholesterol Tertiles |
||||
|---|---|---|---|---|
| 1st tertile | 2nd tertile | 3rd tertile | ||
| N | 419 | 419 | 419 | Pa |
| Lathosterol: cholesterol (mmol/mol) | 0.90 (0.71–1.06) | 1.53 (1.38–1.69) | 2.50 (2.16–3.00) | |
| Cholestanol: cholesterol (mmol/mol) | 1.60 (1.33–1.90) | 1.33 (1.08–1.60) | 1.21 (0.97–1.42) | <0.001d |
| Campesterol: cholesterol (mmol/mol) | 1.55 (1.17–2.21) | 1.21 (0.88–1.71) | 1.02 (0.75–137) | <0.001d |
| Sitosterol: cholesterol (mmol/mol) | 0.76 (0.56–1.10) | 0.63 (0.46–0.85) | 0.54 (0.41–0.72) | <0.001d |
| Cholestanol: lathosterol | 1.78 (1.38–2.61) | 0.88 (0.69–1.08) | 0.47 (0.35–0.58) | <0.001d |
| Campesterol: lathosterol | 1.78 (1.21–2.81) | 0.77 (0.57–1.11) | 0.39 (0.28–0.56) | <0.001d |
| Sitosterol: lathosterol | 0.88 (0.60–1.43) | 0.40 (0.29–0.56) | 0.21 (0.15–0.30) | <0.001d |
| Male gender | 268 (64.0) | 282 (67.3) | 281 (67.1) | 0.681c |
| Age (years) | 64.5 ± 10.9 | 62.3 ± 11.2 | 61.5 ± 10.6 | <0.001b |
| BMI (kg/m ) | 26.0 (3.9) | 27.3 (4.1) | 28.1 (4.1) | <0.001 |
| Waist circumference (cm) | 95 ± 13 | 99 ± 11 | 101 ± 12 | <0.001 |
| Type 2 diabetes | ||||
| No | 304 (72.6) | 307 (73.3) | 276 (65.9) | |
| Yes, no insulin treatment | 87 (20.8) | 94 (22.4) | 127 (30.3) | <0.001 |
| Yes, insulin treatment | 28 (6.7) | 18 (4.3) | 16 (3.8) | 0.296 |
| HbA1c (%) | 6.3 ± 1.2 | 6.3 ± 1.3 | 6.4 ± 1.3 | 0.172 |
| Fasting glucose (mg/dl) | 108 ± 32 | 113 ± 37 | 115 ± 37 | 0.004 |
| Fasting insulin (mU/l) | 7.0 (5.0–11.0) | 8.0 (5.0–12.0) | 10.0 (7.0–15.0) | <0.001d |
| Systemic hypertension | 293 (69.9) | 291 (69.5) | 320 (76.4) | <0.001 |
| Systolic blood pressure (mmHg) | 141 ± 24 | 143 ± 23 | 144 ± 23 | 0.013§ |
| Diastolic blood pressure (mmHg) | 81 ± 11 | 82 ± 11 | 82 ± 11 | 0.506§ |
| Total cholesterol (mg/dl) | 199 ± 37 | 203 ± 37 | 202 ± 34 | 0.327 |
| LDL cholesterol (mg/dl) | 124 ± 32 | 126 ± 33 | 119 ± 30 | 0.003 |
| HDL cholesterol (mg/dl) | 42 ± 12 | 40 ± 11 | 39 ± 10 | <0.001 |
| Triglycerides (mg/dl) | 116 (90–157) | 138 (106–183) | 171 (128–236) | <0.001d |
| Smoking | ||||
| Never | 173 (41.3) | 169 (40.3) | 171 (40.8) | |
| Past | 176 (42.0) | 168 (40.1) | 177 (42.2) | 0.890 |
| Current | 70 (16.7) | 82 (19.6) | 71 (16.9) | 0.240 |
| CRP (mg/l) | 3.78 (1.24–9.80) | 2.94 (1.24–7.54) | 3.02 (1.31–6.95) | 0.097d |
| APOE genotype (419/417/419) | ||||
| E3/3 | 276 (65.9) | 269 (64.5) | 250 (59.7) | |
| E2/2, E2/3, E2/4 | 70 (16.7) | 67 (16.1) | 74 (17.7) | 0.390 |
| E3/4, E4/4 | 73 (17.4) | 81 (19.4) | 95 (22.7) | 0.035 |
Values are means ± SD or medians with inter-quartile ranges in the cases of continuous variables. Values represent numbers (percentages) of subjects in the cases of categorical variables.
General linear model or logistic regression, adjusted for age and gender.
General linear model, adjusted for gender only.
Logistic regression, adjusted for age only. § Additionally adjusted for the use of antihypertensive drugs.
General linear model of logarithmically transformed values.
Baseline characteristics according to cholestanol, campesterol, and sitosterol to cholesterol tertiles
All absorption sterol to cholesterol ratios were positively correlated with each other and with the absorption marker to lathosterol ratios (all P < 0.001). There was a significant inverse relationship between all absorption sterol to cholesterol tertiles and the lathosterol to cholesterol ratio (all P < 0.001) (supplementary Tables II–IV). Only the campesterol to cholesterol tertiles were negatively correlated with age (P < 0.001). None of the absorption marker to cholesterol tertiles was significantly associated with gender. All absorption marker to cholesterol tertiles were inversely related to BMI, waist circumference, type 2 diabetes (no insulin), HbA1c, and fasting glucose and insulin (all P ≤ 0.002) (supplementary Tables II–IV). Systemic hypertension was not significantly associated with the absorption marker to cholesterol tertiles. However, the cholestanol to cholesterol tertiles showed a significant association with systolic and diastolic blood pressure (P = 0.027 and P = 0.031, respectively) (supplementary Tables II–IV). In contrast to the cholestanol to cholesterol tertiles, the campesterol and sitosterol to cholesterol tertiles were positively correlated with total cholesterol (P = 0.001 and P = 0.011, respectively), LDL cholesterol (both P ≤ 0.001), and HDL cholesterol (both P < 0.001) (supplementary Tables II–IV). Neither past or current smoking nor the APOE genotypes were significantly associated with any of the absorption sterol to cholesterol tertiles. CRP was inversely related to the tertiles of the campesterol and sitosterol to cholesterol ratios (both P < 0.001), whereas the cholestanol to cholesterol tertiles were not significantly associated with CRP (supplementary Tables II–IV).
The relationships of the noncholesterol sterol to cholesterol and absorption sterol to lathosterol tertiles with mortality in the whole group
During the follow-up, 304 (24.2%) of 1,257 subjects had died. Of 304 deaths, 192 (63.2%) were accounted for by cardiovascular events and 112 (36.8%) deaths were due to noncardiovascular reasons (Table 2).
TABLE 2.
Noncholesterol sterol to cholesterol ratios, absorption sterol to lathosterol ratios, and mortality in the whole group
| All-Cause Mortality (events: 304) |
Cardiovascular Mortality (events: 192) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | HRa | Pa | HRb | Pb | HRa | Pa | HRb | Pb | |
| Lathosterol: cholesterol | |||||||||
| 1st tertile | 419 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 419 | 0.71 (0.54–0.92) | 0.009 | 0.76 (0.59–1.00) | 0.047 | 0.61 (0.43–0.86) | 0.004 | 0.64 (0.45–0.90) | 0.011 |
| 3rd tertile | 419 | 0.54 (0.41–0.73) | <0.001 | 0.61 (0.45–0.82) | 0.001 | 0.57 (0.40–0.80) | 0.002 | 0.60 (0.41–0.87) | 0.006 |
| Cholestanol: cholesterol | |||||||||
| 1st tertile | 419 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 419 | 1.46 (1.09–1.97) | 0.012 | 1.33 (0.98–1.80) | 0.066 | 1.28 (0.88–1.86) | 0.193 | 1.20 (0.82–1.76) | 0.342 |
| 3rd tertile | 419 | 1.88 (1.41–2.50) | <0.001 | 1.71 (1.28–2.29) | <0.001 | 1.72 (1.21–2.44) | 0.003 | 1.60 (1.12–2.30) | 0.010 |
| Campesterol: cholesterol | |||||||||
| 1st tertile | 419 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 419 | 0.92 (0.69–1.22) | 0.561 | 0.95 (0.71–1.26) | 0.710 | 0.86 (0.60–1.23) | 0.408 | 0.91 (0.64–1.31) | 0.623 |
| 3rd tertile | 419 | 1.27 (0.97–1.66) | 0.080 | 1.38 (1.04–1.82) | 0.025 | 1.14 (0.81–1.59) | 0.462 | 1.29 (0.91–1.84) | 0.157 |
| Sitosterol: cholesterol | |||||||||
| 1st tertile | 419 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 419 | 0.85 (0.64–1.11) | 0.236 | 0.92 (0.70–1.21) | 0.533 | 0.82 (0.58–1.16) | 0.253 | 0.91 (0.64–1.28) | 0.575 |
| 3rd tertile | 419 | 0.96 (0.73–1.26) | 0.770 | 1.08 (0.81–1.42) | 0.610 | 0.94 (0.67–1.32) | 0.728 | 1.11 (0.78–1.57) | 0.577 |
| Cholestanol: lathosterol | |||||||||
| 1st tertile | 419 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 419 | 1.21 (1.09–1.64) | 0.222 | 1.21 (0.88–1.66) | 0.228 | 1.08 (0.74–1.59) | 0.684 | 1.14 (0.77–1.68) | 0.519 |
| 3rd tertile | 419 | 1.82 (1.37–2.41) | <0.001 | 1.65 (1.23–2.22) | 0.001 | 1.71 (1.21–2.42) | 0.003 | 1.64 (1.13–2.37) | 0.008 |
| Campesterol: lathosterol | |||||||||
| 1st tertile | 419 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 419 | 1.03 (0.77–1.38) | 0.845 | 1.04 (0.77–1.39) | 0.816 | 0.97 (0.67–1.41) | 0.883 | 1.02 (0.70–1.48) | 0.920 |
| 3rd tertile | 419 | 1.43 (1.09–1.88) | 0.010 | 1.37 (1.03–1.81) | 0.032 | 1.38 (0.98–1.95) | 0.063 | 1.43 (1.00–2.04) | 0.052 |
| Sitosterol: lathosterol | |||||||||
| 1st tertile | 419 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 419 | 0.93 (0.69–1.24) | 0.607 | 0.90 (0.67–1.21) | 0.496 | 0.87 (0.60–1.26) | 0.462 | 0.87 (0.60–1.26) | 0.459 |
| 3rd tertile | 419 | 1.31 (1.00–1.72) | 0.052 | 1.24 (0.94–1.64) | 0.132 | 1.29 (0.92–1.81) | 0.138 | 1.30 (0.92–1.85) | 0.139 |
HR, hazard ratio calculated by Cox regression.
Model 1: adjusted for gender and age.
Model 2: adjusted for gender, age, BMI, hypertension, type 2 diabetes, smoking, and CRP.
All-cause mortality.
All-cause mortality was increased in subjects belonging to the low lathosterol to cholesterol tertile (P < 0.001) and those allocated to the high cholestanol to cholesterol tertile (P < 0.001) adjusting for sex and age. After additional adjustment for cardiovascular risk factors (BMI, type 2 diabetes, hypertension, smoking, and CRP), the relationships of the lathosterol and cholestanol to cholesterol tertiles with all-cause mortality remained significant (P = 0.001 and P < 0.001, respectively) (Table 2). The association of the campesterol to cholesterol tertiles with all-cause mortality reached significance applying the multivariate model (P = 0.025) (Table 2). The sitosterol to cholesterol ratio was not significantly related to all-cause mortality in the entire cohort (Table 2). High cholestanol and campesterol to lathosterol ratios were significantly associated with increased all-cause mortality in the whole cohort adjusting for sex and age (P < 0.001 and P = 0.010, respectively) and using the multivariate model (P = 0.001 and P = 0.032, respectively). In agreement, the association of the sitosterol to lathosterol ratio with all-cause mortality reached borderline significance controlling for sex and age (P = 0.052).
Cardiovascular mortality.
Subjects in the low lathosterol to cholesterol tertile or high cholestanol to cholesterol tertile were at increased risk of death from cardiovascular causes adjusting for sex and age (P = 0.002 and P = 0.003, respectively) and using the multivariate model (P = 0.006 and P = 0.010, respectively) (Table 2). The campesterol and sitosterol to cholesterol tertiles were not significantly related to cardiovascular mortality controlling for sex and age and after multivariate adjustment (Table 2). Cardiovascular mortality was positively correlated with increased cholestanol to lathosterol ratio (P ≤ 0.008 for both models of adjustment). There was a tendency toward an association of elevated campesterol to lathosterol ratio with increased cardiovascular mortality in the entire group (P ≤ 0.063 for both models of adjustment). The sitosterol to lathosterol tertiles were not significantly related to cardiovascular mortality in the entire cohort (Table 2).
Addition of the APOE genotype as a covariate did not change the results (data not shown).
The relationships of the noncholesterol sterol to cholesterol and absorption sterol to lathosterol tertiles with mortality in subjects without diabetes
In the subgroup of 887 subjects without diabetes, 162 (18.3%) had died during a mean (± SD) follow-up of 7.62 (± 2.05) years. Of the deceased, 94 (58.0%) and 68 (42.0%) individuals had died from cardiovascular diseases and noncardiovascular reasons, respectively (Table 3). The mortality associations of the noncholesterol sterol to cholesterol ratios and the absorption sterol to lathosterol ratios were more pronounced in persons without type 2 diabetes.
TABLE 3.
Noncholesterol sterol to cholesterol ratios, absorption sterol to lathosterol ratios, and mortality in subjects without diabetes
| All-Cause Mortality (events: 162) |
Cardiovascular Mortality (events: 94) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | HRa | Pa | HRb | Pb | HRa | Pa | HRb | Pb | |
| Lathosterol:cholesterol | |||||||||
| 1st tertile | 304 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 307 | 0.62 (0.43–0.89) | 0.010 | 0.69 (0.48–1.00) | 0.048 | 0.46 (0.28–0.75) | 0.002 | 0.49 (0.30–0.81) | 0.005 |
| 3rd tertile | 276 | 0.54 (0.36–0.82) | 0.003 | 0.66 (0.43-0.99) | 0.047 | 0.47 (0.28–-0.80) | 0.005 | 0.53 (0.31–0.91) | 0.022 |
| Cholestanol:cholesterol | |||||||||
| 1st tertile | 273 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 309 | 1.71 (1.11–2.63) | 0.015 | 1.59 (1.03–2.46) | 0.036 | 1.47 (0.83–2.57) | 0.184 | 1.42 (0.80–2.50) | 0.228 |
| 3rd tertile | 305 | 2.16 (1.43–3.28) | <0.001 | 1.86 (1.22–2.86) | 0.004 | 2.02 (1.19–3.43) | 0.010 | 1.81 (1.05–3.12) | 0.032 |
| Campesterol:cholesterol | |||||||||
| 1st tertile | 272 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 295 | 1.14 (0.75–1.72) | 0.539 | 1.25 (0.82–1.89) | 0.303 | 1.23 (0.71–2.13) | 0.454 | 1.39 (0.79–2.42) | 0.252 |
| 3rd tertile | 320 | 1.64 (1.12–2.39) | 0.011 | 1.76 (1.19–2.61) | 0.005 | 1.79 (1.08–2.96) | 0.024 | 2.06 (1.21–3.48) | 0.007 |
| Sitosterol:cholesterol | |||||||||
| 1st tertile | 272 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 299 | 0.99 (0.67–1.47) | 0.961 | 1.10 (0.74–1.64) | 0.625 | 0.88 (0.52–1.51) | 0.646 | 0.98 (0.57–1.68) | 0.939 |
| 3rd tertile | 316 | 1.25 (0.86–1.82) | 0.244 | 1.33 (0.90–1.96) | 0.148 | 1.36 (0.84–2.21) | 0.211 | 1.54 (0.93–2.53) | 0.091 |
| Cholestanol:lathosterol | |||||||||
| 1st tertile | 245 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 314 | 1.37 (1.11–2.13) | 0.169 | 1.14 (0.73–1.79) | 0.570 | 1.11 (0.62–2.00) | 0.723 | 0.97 (0.53–1.76) | 0.918 |
| 3rd tertile | 308 | 1.90 (1.25–2.89) | 0.003 | 1.49 (0.96–2.30) | 0.076 | 1.89 (1.10–3.22) | 0.021 | 1.57 (0.89–2.74) | 0.118 |
| Campesterol: lathosterol | |||||||||
| 1st tertile | 260 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 313 | 1.32 (0.75–1.72) | 0.211 | 1.22 (0.79–1.88) | 0.368 | 1.50 (0.84–2.68) | 0.173 | 1.45 (0.81–2.61) | 0.214 |
| 3rd tertile | 314 | 1.73 (1.16–2.59) | 0.007 | 1.52 (1.00–2.29) | 0.048 | 1.98 (1.14–3.42) | 0.015 | 1.84 (1.05–3.23) | 0.033 |
| Sitosterol: lathosterol | |||||||||
| 1st tertile | 266 | 1.0 reference | 1.0 reference | 1.0 reference | 1.0 reference | ||||
| 2nd tertile | 309 | 1.24 (0.81-1.90) | 0.329 | 1.23 (0.80-1.89) | 0.357 | 1.39 (0.77-2.50) | 0.270 | 1.40 (0.78-2.54) | 0.262 |
| 3rd tertile | 312 | 1.59 (1.07-2.38) | 0.022 | 1.47 (0.98-2.22) | 0.064 | 1.94 (1.12-3.36) | 0.018 | 1.92 (1.10-3.36) | 0.023 |
HR, hazard ratio calculated by Cox regression.
Model 1: adjusted for gender and age.
Model 2: adjusted for gender, age, BMI, hypertension, smoking, and CRP.
All-cause mortality.
In agreement with the results in the entire cohort, all-cause mortality was higher at a low lathosterol to cholesterol ratio and at high cholestanol and campesterol to cholesterol ratios adjusting for sex and age (P = 0.003, P < 0.001, and P = 0.011, respectively) and using the multivariate model (P = 0.047, P = 0.004, and P = 0.005, respectively) (Table 3). The sitosterol to cholesterol tertiles were not significantly related to all-cause mortality (Table 3). All absorption marker to lathosterol ratios were significantly and positively associated with all-cause mortality in the subgroup without diabetes adjusting for sex and age (all P ≤ 0.022) (Table 3). After multivariate adjustment, the association of the campesterol to lathosterol ratio with all-cause mortality remained statistically significant (P = 0.048) (Table 3).
Cardiovascular mortality.
Low lathosterol to cholesterol and high cholestanol and campesterol to cholesterol ratios were predictive of increased cardiovascular mortality in subjects without diabetes controlling for sex and age (P = 0.005, P = 0.010, and P = 0.024, respectively) and after additional adjustment for cardiovascular risk factors (P = 0.022, P = 0.032, and P = 0.007, respectively) (Table 3) (Fig. 1). The sitosterol to cholesterol ratio did not correlate with cardiovascular mortality (Table 3) (Fig. 1). All absorption marker to lathosterol ratios were positively correlated with cardiovascular mortality adjusting for sex and age (all P ≤ 0.021) (Table 3). Using the multivariate model, the associations of the campesterol and sitosterol to lathosterol ratios with cardiovascular mortality (P = 0.033 and P = 0.023, respectively) remained statistically significant (Table 3).
Additional adjustment for the APOE genotype did not markedly alter the results (data not shown).
DISCUSSION
This prospective analysis indicates that high absorption and low synthesis of cholesterol estimated by measurement of plasma noncholesterol sterols are predictive of increased all-cause and cardiovascular mortality in subjects referred to coronary angiography.
Our study on plant sterols, cholesterol homeostasis and mortality is until now the largest and most comprehensive of its kind. We examined a cohort of 1,257 subjects at intermediate to high cardiovascular risk and the analysis relies on a total of 304 fatal events. The plasma concentrations of the noncholesterol sterols were similar to the data published previously (3, 14). Furthermore, the results confirm earlier studies reporting associations of cholesterol metabolism with BMI (26), glucose (27), insulin (28), and type 2 diabetes (29). Confounding due to functional food consumption can be ruled out, because blood samples had been taken before products enriched with plant sterols or stanols were brought onto the market in Germany.
The plasma lathosterol to cholesterol ratio, indicating cholesterol synthesis, was inversely related to all-cause and cardiovascular mortality. In contrast, the plasma cholestanol to cholesterol ratio, which is a surrogate marker for cholesterol absorption, was associated with increased all-cause and cardiovascular mortality. Whereas the plasma campesterol to cholesterol ratio was correlated with increased all-cause mortality, the plasma sitosterol to cholesterol ratio did not significantly predict mortality during the follow-up. There was a more pronounced association of cholesterol metabolism with all-cause and cardiovascular mortality when diabetics were excluded from the analysis. Hence, the prospective data extend the findings of our recent cross-sectional analysis on the relationships of cholesterol metabolism and plasma plant sterols with the severity of CAD (23). Increased ratios of cholestanol and campesterol to cholesterol and decreased lathosterol to cholesterol ratio were correlated with high Friesinger Score. However, the sitosterol ratio was not significantly related to the angiographic data (23).
Why are there discrepancies among the relationships of the three absorption marker to cholesterol ratios with the severity of CAD (23) and mortality?
A possible reason for this observation is confounding due to nutritional factors. Elevated plasma plant sterols not only reflect increased cholesterol absorption but also a higher amount of plant-based food in the diet (15, 20, 30, 31). Such a diet rich in fruits, seeds, vegetable protein, and polyunsaturated fatty acids is considered healthy and may reduce cardiovascular risk. In some studies, the plasma sitosterol concentration was more dependent on the composition of the diet than plasma campesterol (15, 31, 32). Plasma cholestanol is not known to increase in response to high consumption of plant-based foods. Interestingly, it has even been shown that the plasma concentration of cholestanol was inversely related to the dietary intake of plant sterols and the polyunsaturated versus saturated fat ratio (19).
The inflammatory state, a predictor of increased mortality (33), potentially causes divergent results, too. We found that plant sterols unlike cholestanol were significantly negatively associated with inflammation estimated by CRP. This finding is in accordance with an earlier study (34) and further supported by a modest increase of CRP across the tertiles of the cholestanol to cholesterol ratio in the DEBATE collective (5). Importantly, our results obtained from model 2 (multivariate model including adjustment for CRP) were more similar versus those derived from model 1 (covariates sex and age only).
In addition, the associations of the absorption markers with CAD and mortality could be discrepant due to differences in the individual metabolic pathways and the genetic regulation of the various sterols. For example, the relationships of campesterol and sitosterol with single nucleotide polymorphisms in the ATP binding cassette transporter G5 and G8 genes were not congruent (35).
Finally, the impact of cholesterol metabolism on CAD and mortality is not large in participants of the LURIC study, which also represents a possible explanation for some inconsistency.
It has been found that the ratios of the absorption markers to lathosterol are better estimates of cholesterol absorption and synthesis than the ratios of the absorption and synthesis markers to cholesterol (20). Of note, compared with the absorption and synthesis marker to cholesterol ratios the absorption marker to lathosterol ratios were more consistently associated with the severity of CAD (23) and mortality. High sitosterol to lathosterol ratio was significantly related to increased all-cause and cardiovascular mortality in the subgroup of individuals without diabetes, too.
How do our findings correspond with previous work? In agreement with the LURIC follow-up, high absorption and low synthesis of cholesterol were associated with increased all-cause mortality in the DEBATE cohort (5). On the contrary, high absorption marker to cholesterol ratios were not associated with increased mortality in the Helsinki Businessmen Study (16). Hence, data on the relationships of cholesterol metabolism and plasma plant sterols with CAD and mortality remain inconsistent (3, 5–18, 23).
The controversial results might in part be caused by the interrelations between cholesterol homeostasis and type 2 diabetes (29). Type 2 diabetes increases mortality from vascular complications (36) and is characterized by high synthesis and low absorption of cholesterol (29). Insulin treatment also modulates cholesterol metabolism (37). Another possible explanation for divergent findings could be selection bias with regard to age. Individuals with high synthesis and low absorption of cholesterol representing a characteristic of the metabolic syndrome may die before reaching old age (26). On the other hand, low synthesis and high absorption of cholesterol particularly in old age is possible to reflect a poor nutritional state indicating frailty (26). In our cohort, however, the associations of cholesterol metabolism with mortality were independent of the BMI ruling out malnutrition as a confounder.
It has been speculated that the repeatedly observed association of high cholesterol absorption with CAD and increased mortality is accounted for by a higher cholesterol lifetime burden (5). In line with this consideration and confirmed by an animal study (38), inhibition of cholesterol absorption has been suggested to be favorable in the prevention and treatment of CAD (5). Plant sterol and stanol margarines and ezetimibe are available to block intestinal cholesterol uptake. Both plant sterols and stanols and ezetimibe are effective in lowering LDL cholesterol (39–43). However, there is a lack of prospective clinical studies to demonstrate that cardiovascular risk can be reduced by the use of cholesterol absorption inhibitors. In the Simvastatin and Ezetimibe in Aortic Stenosis study, a combination therapy of simvastatin and ezetimibe did not reduce all-cause mortality and only modestly decreased ischemic events compared with placebo (42). Moreover, the effects of lipid lowering with ezetimibe on carotid intima media thickness have been contradictory (43, 44).
To the contrary, there is broad evidence that with the use of statins a reduction in coronary events can be achieved in subjects with hypercholesterolemia (45, 46). Yet, the well-established effectiveness of statin treatment in lowering plasma total and LDL cholesterol and reducing cardiovascular risk has been suggested to depend on baseline cholesterol metabolism. Importantly, several reports have shown individuals with high cholesterol absorption not to profit from statins (22, 47). It is of interest if those patients with high cholesterol absorption will profit from drugs interfering with intestinal cholesterol uptake. They may be screened by measuring plasma noncholesterol sterols before the start of a lipid-lowering therapy.
We do not think that a moderate plasma plant sterol elevation also seen in individuals taking plant sterol margarines (48) is atherogenic. In agreement, the administration of plant sterols and stanols has been shown to retard lesion formation in LDL receptor- or APOE-deficient mice (49, 50). However, the use of plant sterols cannot be definitely recommended before prospective clinical trials with hard cardiovascular end points have shown their effectiveness to reduce cardiovascular risk (51).
Previous studies addressing the effects of the APOE genotype on the noncholesterol sterol to cholesterol ratios have been conflicting (52, 53). In our cohort, the combined APOE 3/4 and 4/4 genotypes were associated with a high lathosterol to cholesterol ratio, whereas the cholestanol, campesterol, and sitosterol to cholesterol ratios were not related to the APOE genotype.
Several limitations pertain to the LURIC study. First, systematic information for the dietary habits of the participants has not been available, and potential confounding due to nutritional factors can therefore not be controlled for. Yet, to estimate cholesterol absorption, we have also quantified the plasma concentration of cholestanol, which is less dependent on vegetable food intake than the plasma levels of campesterol and sitosterol (19). Second, sample storage for up to 10 y is possible to have affected plasma sterol concentrations. However, this is improbable, because sterols stored at −80 degrees were proven stable in a set of samples over a period of 10 y in a previously published study (17). Third, the LURIC cohort consists of patients with an indication for coronary angiography. Therefore, our findings may not be generalizable to subjects at lower cardiovascular risk. The fact that in Germany in 1997–2000 many coronary patients with total and LDL cholesterol levels higher than the international recommendations were without statin treatment reflects problems with the implementation of total and LDL cholesterol goals (54, 55).
In conclusion, our findings suggest that high absorption and low synthesis of cholesterol predict increased all-cause and cardiovascular mortality in participants of the LURIC cohort who did not take statins. A specific pathophysiological role of plant sterols in atherogenesis, however, seems unlikely in subjects without sitosterolaemia.
Supplementary Material
Acknowledgments
The authors extend appreciation to the participants of the LURIC study; without their collaboration this article would not have been written. We thank Prof. Dr. Wolfgang Erwa and the LURIC study team either temporarily or permanently involved in patient recruitment and sample and data handling, the laboratory staff at the Ludwigshafen General Hospital, and the Universities of Freiburg, Ulm, and Graz. Furthermore, we thank Eva-Maria Matzold and Anna Strallegger for excellent technical assistance, and we are grateful for the support by the GEN-AU project GOLD - Genomics Of Lipid-associated Disorders.
Footnotes
Abbreviations:
- APOE
- apolipoprotein E
- BMI
- body mass index
- CAD
- coronary artery disease
- CRP
- C-reactive protein
- DEBATE
- Drugs and Evidence-Based Medicine in the Elderly study
- LURIC
- Ludwigshafen Risk and Cardiovascular health
- MSTFA
- N-methyl-N-(trimethylsilyl)-trifluoroacetamide
Supported by the GEN-AU project GOLD-Genomics of Lipid-associated Disorders.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four tables.
REFERENCES
- 1.Wilson P. W. F., D'Agostino R. B., Levy D., Belanger A. M., Silbershatz H., Kannel W. B. 1998. Prediction of coronary heart disease using risk factor categories. Circulation. 97: 1837–1847. [DOI] [PubMed] [Google Scholar]
- 2.Ostlund R. E., Jr. 2002. Phytosterols in human nutrition. Annu. Rev. Nutr. 22: 533–549. [DOI] [PubMed] [Google Scholar]
- 3.Wilund K. R., Yu L., Xu F., Vega G., Grundy S., Cohen J. C., Hobbs H. H. 2004. Plant sterol levels are not associated with atherosclerosis in mice and men. Arterioscler. Thromb. Vasc. Biol. 24: 2326–2332. [DOI] [PubMed] [Google Scholar]
- 4.Salen G., Horak I., Rothkopf M., Cohen J. L., Speck J., Tint G. S., Shore V., Dayal B., Chen T., Shefer S. 1985. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolaemia with xanthomatosis. J. Lipid Res. 26: 1126–1133. [PubMed] [Google Scholar]
- 5.Strandberg T. E., Tilvis R. S., Pitkala K. H., Miettinen T. A. 2006. Cholesterol and glucose metabolism and recurrent cardiovascular events among elderly. J. Am. Coll. Cardiol. 48: 708–714. [DOI] [PubMed] [Google Scholar]
- 6.Assmann G., Cullen P., Erbey J., Ramey D. R., Kannenberg F., Schulte H. 2006. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: Results of a nested case-control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr. Metab. Cardiovasc. Dis. 16: 13–21. [DOI] [PubMed] [Google Scholar]
- 7.Matthan N. R., Pencina M., LaRoque J. M., Jacques P. F., D'Agostino R. B., Schaefer E. J., Lichtenstein A. H. 2009. Alterations in markers of cholesterol absorption and synthesis characterize Framingham Offspring study participants with coronary heart disease. J. Lipid Res. 50: 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajaratnam R. A., Gylling H., Miettinen T. A. 2000. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J. Am. Coll. Cardiol. 35: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 9.Glueck C. J., Speirs J., Tracy T., Streicher P., Illig E., Vandegrift J. 1991. Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol and premature coronary heart disease in hyperphytosterolemic probands and their first degree relatives. Metabolism. 40: 842–848. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland W. H. F., Williams M. J. A., Nye E. R., Restieaux N. J., de Jong S. A., Walker H. L. 1998. Associations of plasma noncholesterol sterol levels with severity of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 8: 386–391. [Google Scholar]
- 11.Sudhop T., Gottwald B. M., von Bergmann K. 2002. Serum plant sterols as a potential risk factor for coronary heart disease. Metabolism. 51: 1519–1521. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen T. A., Railo M., Lepäntalo M., Gylling H. 2005. Plant sterols in serum and in atherosclerotic plaques of patients undergoing carotid endarterectomy. J. Am. Coll. Cardiol. 45: 1794–1801. [DOI] [PubMed] [Google Scholar]
- 13.Weingärtner O., Lütjohann D., Ji S., Weisshoff N., List F., Sudhop T., von Bergmann K., Gertz K., König J., Schäfers H. J., et al. 2008. Vascular effects of diet supplementation with plant sterols. J. Am. Coll. Cardiol. 51: 1553–1561. [DOI] [PubMed] [Google Scholar]
- 14.Pinedo S., Vissers M. N., von Bergmann K., Elharchaoui M. D., Lutjohann D., Luben R., Wareham N. J., Kastelein J. J. P., Khaw K. T., Boeckholdt S. M. 2007. Plasma levels of plant sterols and the risk of future coronary artery disease in apparently healthy men and women; The Prospective Epic-Norfolk Population Study. J. Lipid Res. 48: 139–144. [DOI] [PubMed] [Google Scholar]
- 15.Escurriol V., Cofan M., Moreno-Iribas C., Larranaga N., Martinez C., Navarro C., Rodriguez L., Gonzalez C. A., Corella D., Ros E. 2010. Phytosterol plasma concentrations and coronary heart disease in the prospective Spanish EPIC cohort. J. Lipid Res. 51: 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strandberg T. E., Gylling H., Tilvis R. S., Miettinen T. A. 2010. Serum plant and other noncholesterol sterols, cholesterol metabolism and 22-year mortality among middle-aged men. Atherosclerosis. 210: 282–287. [DOI] [PubMed] [Google Scholar]
- 17.Fassbender K., Lutjohann D., Dik M. G., Bremmer M., Koenig J., Walter S., Liu Y., Letiembre M., von Bergmann K., Jonker C. 2008. Moderately elevated plant sterol levels are associated with reduced cardiovascular risk-The LASA study. Atherosclerosis. 196: 283–288. [DOI] [PubMed] [Google Scholar]
- 18.Windler E., Zyriax B. C., Kuipers F., Linseisen J., Boeing H. 2009. Association of plasma phytosterol concentrations with incident coronary heart disease Data from the CORA study, a case-control study of coronary artery disease in women. Atherosclerosis. 203: 284–290. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen T. A., Tilvis R. S., Kesaniemi Y. A. 1989. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism. 38: 136–140. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen T. A., Tilvis R. S., Kesämiemi Y. A. 1990. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am. J. Epidemiol. 131: 20–31. [DOI] [PubMed] [Google Scholar]
- 21.Matthan N. R., Lichtenstein A. H. 2004. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 174: 197–205. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen T. A., Gylling H., Strandberg T., Sarna S. 1998. Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. BMJ. 316: 1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silbernagel G., Fauler G., Renner W., Landl E. M., Hoffmann M. M., Winkelmann B. R., Boehm B. O., März W. 2009. The relationships of cholesterol metabolism and plasma plant sterols with the severity of coronary artery disease. J. Lipid Res. 50: 334–341. [DOI] [PubMed] [Google Scholar]
- 24.Winkelmann B. R., Marz W., Boehm B. O., Zotz R., Hager J., Hellstern P., Senges J.; LURIC Study Group (Ludwigshafen Risk and Cardiovascular Health). 2000. Rationale and design of the LURIC study: a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics. 2(1, Suppl. 1): S1–S73. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. 2006. Diagnosis and classification of diabetes mellitus. Diabetes Care. 29(Suppl. 1): S43–S48. [PubMed] [Google Scholar]
- 26.Simonen P., Gylling H., Howard A. N., Miettinen T. A. 2000. Introducing a new component of the metabolic syndrome: low cholesterol absorption. Am. J. Clin. Nutr. 72: 82–88. [DOI] [PubMed] [Google Scholar]
- 27.Gylling H., Hallikainen M., Kolehmainen M., Toppinen L., Pihlajamäki J., Mykkänen H., Agren J. J., Rauramaa R., Laakso M., Miettinen T. A. 2007. Cholesterol synthesis prevails over absorption in metabolic syndrome. Transl. Res. 149: 310–316. [DOI] [PubMed] [Google Scholar]
- 28.Pihlajamäki J., Gylling H., Miettinen T. A., Laakso M. 2004. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J. Lipid Res. 45: 507–512. [DOI] [PubMed] [Google Scholar]
- 29.Simonen P. P., Gylling H. K., Miettinen T. A. 2002. Diabetes contributes to cholesterol metabolism regardless of obesity. Diabetes Care. 25: 1511–1515. [DOI] [PubMed] [Google Scholar]
- 30.Chan Y. M., Varady K. A., Lin Y., Trautwein E., Mensink R. P., Plat J., Jones P. J. 2006. Plasma concentrations of plant sterols: physiology and relationship with coronary heart disease. Nutr. Rev. 64: 385–402. [DOI] [PubMed] [Google Scholar]
- 31.Escurriol V., Cofán M., Serra M., Bulló M., Basora J., Salas-Salvadó J., Corella D., Zazpe I., Martínez-González M. A., Ruiz-Gutiérrez V., et al. 2009. Serum sterol responses to increasing plant sterol intake from natural foods in the Mediterranean diet. Eur. J. Nutr. 48: 373–382. [DOI] [PubMed] [Google Scholar]
- 32.Muti P., Awad A. B., Schünemann H., Fink C. S., Hovey K., Freudenheim J. L., Wu Y. W., Bellati C., Pala V., Berrino F. 2003. A plant food-based diet modifies the serum beta-sitosterol concentration in hyperandrogenic postmenopausal women. J. Nutr. 133: 4252–4255. [DOI] [PubMed] [Google Scholar]
- 33.Sabatine M. S., Morrow D. A., Jablonski K. A., Rice M. M., Warnica J. W., Domanski M. J., Hsia J., Gersh B. J., Rifai N., Ridker P. M., et al. 2007. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 115: 1528–1536. [DOI] [PubMed] [Google Scholar]
- 34.Hallikainen M., Kolehmainen M., Schwab U., Laaksonen D. E., Niskanen L., Rauramaa R., Pihlajamäki J., Uusitupa M., Miettinen T. A., Gylling H. 2007. Serum adipokines are associated with cholesterol metabolism in the metabolic syndrome. Clin. Chim. Acta. 383: 126–132. [DOI] [PubMed] [Google Scholar]
- 35.Berge K. E., von Bergmann K., Lutjohann D., Guerra R., Grundy S. M., Hobbs H. H., Cohen J. C. 2002. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J. Lipid Res. 43: 486–494. [PubMed] [Google Scholar]
- 36.Haffner S. M., Lehto S., Rönnemaa T., Pyörälä K., Laakso M. 1998. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339: 229–234. [DOI] [PubMed] [Google Scholar]
- 37.Scoppola A., Testa G., Frontoni S., Maddaloni E., Gambardella S., Menzinger G., Lala A. 1995. Effects of insulin on cholesterol synthesis in type II diabetes patients. Diabetes Care. 18: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg M. E., Smith J. D., Sehayek E. 2009. Moderately decreased cholesterol absorption rates are associated with a large atheroprotective effect. Arterioscler. Thromb. Vasc. Biol. 29: 1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miettinen T. A., Puska P., Gylling H., Vanhanen H., Vartiainen E. 1995. Serum cholesterol lowering by sitostanol ester margarine in a mildly hypercholesterolemic random population. N. Engl. J. Med. 333: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 40.Law M. 2000. Plant sterol and stanol margarines and health. BMJ. 320: 861–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudhop T., Reber M., Tribble D., Sapre A., Taggart W., Gibbons P., Musliner T., von Bergmann K., Lütjohann D. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J. Lipid Res. Epub ahead of print. April 20, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossebø A. B., Pedersen T. R., Boman K., Brudi P., Chambers J. B., Egstrup K., Gerdts E., Gohlke-Bärwolf C., Holme I., Kesäniemi Y. A., et al. 2008. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 359: 1343–1356. [DOI] [PubMed] [Google Scholar]
- 43.Kastelein J. J., Akdim F., Stroes E. S., Zwinderman A. H., Bots M. L., Stalenhoef A. F., Visseren F. L., Sijbrands E. J., Trip M. D., Stein E. A., et al. 2008. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 358: 1431–1443. [DOI] [PubMed] [Google Scholar]
- 44.Fleg J. L., Mete M., Howard B. V., Umans J. G., Roman M. J., Ratner R. E., Silverman A., Galloway J. M., Henderson J. A., Weir M. R., et al. 2008. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J. Am. Coll. Cardiol. 52: 2198–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shepherd J., Cobbe S. M., Ford I., Isles C. G., Lorimer A. R., MacFarlane P. W., McKillop J. H., Packard C. J. 1995. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 333: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 46.Sacks F. M., Pfeffer M. A., Moye L. A., Rouleau J. L., Rutherford J. D., Cole T. G., Brown L., Warnica J. W., Arnold J. M., Wun C. C., et al. 1996. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 335: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 47.Miettinen T. A., Strandberg T. E., Gylling H. 2000. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients. Arterioscler. Thromb. Vasc. Biol. 20: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 48.Fransen H. P., de Jong N., Wolfs M., Verhagen H., Verschuren W. M., Lütjohann D., von Bergmann K., Plat J., Mensink R. P. 2007. Customary use of plant sterol and plant stanol enriched margarine is associated with changes in serum plant sterol and stanol concentrations in humans. J. Nutr. 137: 1301–1306. [DOI] [PubMed] [Google Scholar]
- 49.Moghadasian M. H., Mc Manus B. M., Godin D. V., Rodrigues B., Frolich J. J. 1999. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apolipoprotein E-deficient mice: Possible mechanisms of action. Circulation. 99: 1733–1739. [DOI] [PubMed] [Google Scholar]
- 50.Plat J., Beugels I., Gijbels M. J., de Winter M. P., Mensink R. P. 2006. Plant sterol or stanol esters retard lesion formation in LDL receptor-deficient mice independent of changes in serum plant sterols. J. Lipid Res. 47: 2762–2771. [DOI] [PubMed] [Google Scholar]
- 51.Weingärtner O., Böhm M., Laufs U. 2009. Controversial role of plant sterol esters in the management of hypercholesterolaemia. Eur. Heart J. 30: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kesäniemi Y. A., Ehnholm C., Miettinen T. A. 1987. Intestinal cholesterol absorption efficiency in man is related to apoprotein E phenotype. J. Clin. Invest. 80: 578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Bergmann K., Lütjohann D., Lindenthal B., Steinmetz A. 2003. Efficiency of intestinal cholesterol absorption in humans is not related to apoE phenotype. J. Lipid Res. 44: 193–197. [DOI] [PubMed] [Google Scholar]
- 54.Silber S., Krischke I., Prohaska M. 2000. Undertreatment in secondary prevention of patients with coronary heart disease after revascularization. Herz. 25: 623–626. [DOI] [PubMed] [Google Scholar]
- 55.van Dam M., van Wissen S., Kastelein J. J. 2002. Declaring war on undertreatment: rationale for an aggressive approach to lowering cholesterol. J. Cardiovasc. Risk. 9: 89–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.