Abstract
Microsomal triglyceride transfer protein (MTP) is required for the assembly and secretion of apolipoprotein (apo) B-containing lipoproteins. Previously, we demonstrated that the N-terminal 1,000 residues of apoB (apoB:1000) are necessary for the initiation of apoB-containing lipoprotein assembly in rat hepatoma McA-RH7777 cells and that these particles are phospholipid (PL) rich. To determine if the PL transfer activity of MTP is sufficient for the assembly and secretion of primordial apoB:1000-containing lipoproteins, we employed microRNA-based short hairpin RNAs (miR-shRNAs) to silence Mttp gene expression in parental and apoB:1000-expressing McA-RH7777 cells. This approach led to 98% reduction in MTP protein levels in both cell types. Metabolic labeling studies demonstrated a drastic 90–95% decrease in the secretion of rat endogenous apoB100-containing lipoproteins in MTP-deficient McA-RH7777 cells compared with cells transfected with negative control miR-shRNA. A similar reduction was observed in the secretion of rat endogenous apoB48 under the experimental conditions employed. In contrast, MTP absence had no significant effect on the synthesis, lipidation, and secretion of human apoB:1000-containing particles. These results provide strong evidence in support of the concept that in McA-RH7777 cells, acquisition of PL by apoB:1000 and initiation of apoB-containing lipoprotein assembly, a process distinct from the conventional first-step assembly of HDL-sized apoB-containing particles, do not require MTP. This study indicates that, in hepatocytes, a factor(s) other than MTP mediates the formation of the PL-rich primordial apoB:1000-containing initiation complex.
Keywords: VLDL secretion, triglyceride-rich lipoprotein biosynthesis, hepatic lipoproteins, liver, atherosclerosis
Apolipoprotein (apo) B has a fundamental role in the transport and metabolism of plasma triacylglycerols (TAGs) and cholesterol and is synthesized primarily in hepatocytes and enterocytes (1–3). ApoB is present as a single molecule per lipoprotein particle (4) and exists in two forms, apoB100 (the full-length protein) and apoB48 (the N-terminal 48% of apoB100). The two forms derive from the same gene by a posttranscriptional modification of the apoB mRNA at codon 2,153 that converts a glutamine codon to a stop codon (5). Studies by Xie et al. (6) provided the first biologically plausible role for a mammalian intestinal apoB mRNA editing mechanism. ApoB100 is an essential structural component for the formation and secretion of VLDL and is essentially the only apoprotein component of LDL (3, 7, 8). ApoB48 is required for the formation and secretion of chylomicrons and is expressed in mammalian intestine and in the liver of some nonhuman mammals (3, 9).
The processes involved in the assembly of apoB-containing lipoproteins in the liver are complex and are regulated at multiple levels throughout the secretory pathway. The addition of lipids to apoB is widely thought to occur in two steps (3, 10, 11). The first step involves the addition of small amounts of lipids to apoB as it is translated and translocated into the lumen of the endoplasmic reticulum, preventing its degradation and allowing for the formation of a partially lipidated small preVLDL particle in the HDL density range (3, 11, 12). In the second step, this preVLDL particle acquires the bulk of its core lipids and is converted to bona fide VLDL (3, 11, 13), presumably by fusing with a large, VLDL-sized, apoB-free TAG particle (13, 14). Microsomal TAG transfer protein (MTP) plays a pivotal role in the assembly and secretion of apoB-containing lipoproteins (15–18). MTP is widely thought to play a critical role in the first-step assembly of apoB (19–24), but it is not required for the second-step core expansion during VLDL assembly (20, 22, 24, 25). On the other hand, several studies support the concept that MTP is essential for bulk TAG transfer and subsequent conversion of small apoB100-containing lipoproteins to large VLDL-sized particles (26–29) and chylomicrons (30). The intent of this study was not to scrutinize the relative requirement for MTP in the first-step apoB particle assembly or the second-step particle core expansion. Rather, the objective here was to assess the role of MTP in the initial addition of phospholipids (PLs) to the N-terminal 1,000 residues of apoB100 and nucleation of the primordial prenascent apoB-containing initiation complex. We consider this early stage in apoB particle assembly to be distinct from the first-step assembly of HDL-sized apoB lipoproteins.
Previous experimentally derived results in lipoprotein-producing rat hepatoma McA-RH7777 cells (31) and molecular modeling of the βα1 domain (residues 1–1,000) of apoB100 (32) indicated that the N-terminal 1,000 residues of apoB (referred to as apoB:1000, corresponding to apoB22.05) are required for the initiation of apoB particle assembly without a structural requirement for MTP. These results supported the earlier proposal by Segrest et al. (33, 34). This primordial apoB:1000-containing lipoprotein particle was shown to be PL rich with a fixed lipid capacity on the order of 50 PL for a total stoichiometry of 70 lipid molecules/particle (31). Subsequent studies (35) were carried out to determine if MTP lipid transfer activity is required for the formation of the PL-rich primordial apoB:1000-containing lipoprotein particle. Results demonstrated that MTP inhibitors, BMS-200150 and BMS-197636 (36, 37), and small interfering RNA (siRNA)-mediated suppression of Mttp gene expression by 60% in apoB:1000-expressing McA-RH7777 cells had no detectable effect on the synthesis, lipidation, and secretion of apoB:1000-containing particles (35). However, it has been argued that it is possible that the remaining 40% of MTP activity is sufficient to mediate the initiation of apoB lipoprotein assembly (38). This valid point is based on the results of a study demonstrating that the PL transfer activity of MTP is sufficient for the assembly and secretion of primordial apoB lipoproteins in transformed African green monkey kidney fibroblast COS cells (39). To determine if this mechanism is active in hepatocytes, and as a logical and necessary continuation of our studies, we used micro (mi) RNA-based short hairpin RNAs (miR-shRNAs) and silenced the Mttp gene expression in parental and apoB:1000-expressing McA-RH7777 cells by 98%. Results of metabolic labeling studies strongly indicate that PL transfer activity of MTP is not required for the assembly and secretion of primordial prenascent apoB:1000-containing particles in hepatocytes. This study indicates that, in hepatocytes, factor(s) other than MTP are involved in the formation of the PL-rich apoB:1000-containing initiation complex.
MATERIALS AND METHODS
Materials
FBS, sodium deoxycholate, Triton X-100, pheneylmethylsulfonyl fluoride, benzamidine, leupeptin, aprotinin, pepstatin A, fatty acid-free BSA, and rabbit antibody to human albumin were from Sigma Chemical Co. (St. Louis, MO). Horse serum (HS) and antibiotic-antimycotic were obtained from GIBCO BRL Biological Co. (Grand Island, NY). Tris-Glycine gels were obtained from Invitrogen-Novex (Carlsbad, CA). DMEM, MEM, trypsin, and G418 were purchased from Mediatech, Inc. (Herndon, VA). Protein G-Sepharose CL-4B, [3H]glycerol, [14C]oleic acid, and Amplify were from Amersham Pharmacia Biotech (Piscataway, NJ). TRAN35S-LABLE [35S]methionine/cysteine was from MP Biomedicals, Inc. (Irvine, CA). Immobilin polyvinylidene difluoride transfer membrane and Centriprep Centrifugal Filter Devices YM-30 were purchased from Millipore Corp. (Bedford, MA). Affinity purified polyclonal antibody to human apoB100 was prepared in our laboratory and biotinylated as previously described (40). Monospecific polyclonal antibody to rat apoB was prepared in our laboratory as previously described (41). ApoB100 cDNA was a gift from Dr. Zemin Yao (University of Ottawa Heart Institute, Ottawa, Ontario, Canada). Polyclonal antibody to bovine MTP 97 kDa large subunit (15) was kindly provided by Dr. David Gordon (Bristol-Myers Squibb Co).
Construction of truncated ApoB expression plasmid
Truncated apoB cDNA spanning nucleotides 1-3081 of the full-length apoB100 cDNA was prepared from pB100L-L (42) as a PCR template and appropriate primers as previously described (40). Standard cloning procedures were used to identify clones with 100% correct sequence (40). The apoB fragment 3,081 bp (apoB:1000) was ligated into the mammalian expression vector, the Molony murine leukemia virus-based retrovirus LNCX (43), and expression vectors were used for transformation. Clones harboring plasmids containing apoB gene with the correct orientation were identified by restriction enzyme digestion and confirmed by nucleotide sequencing and used to transfect McA-RH7777 cells as previously described in detail (40).
Development of vectors containing miRNA-based shRNA expression cassettes to silence Mttp gene expression
The pre-miRNA sequences were designed using Invitrogen's RNAi Designer online tool. Seven different double-stranded (ds) oligo duplexes encoding desired miRNA sequences were selected and cloned, either as single or multiple copies, into pcDNA™6.2-GW/miR, a BLOCK-iT™ Pol II miR RNAi expression vector (Invitrogen, Carlsbad, CA). The BLOCK-iT™ Pol II miR RNAi expression vectors are specifically designed to allow expression of engineered miRNA sequences and contain specific miR flanking sequences that allow proper processing of the miRNA. The vectors also contain spectinomycin resistance gene for selection in bacteria and blasticidin resistance gene for selection in mammalian cells. The sequences of two of the most efficient oligo duplexes are as follows. The sequences of first MTP miRNA, targeted to a sequence in exon 10 of MTP (AGTCAGGAAGCTGTGTCAGAA), are: top sequence 5′-TGCTGTTCTGACACAGCTTCCTGACTGTTTTG-GCCACTGACTGACAGTCAGGACTGTGTCAGAA-3′ and bottom sequence 5′-CCTGTTCTGACACAGTCCTGACTGTCAGTCAGTGGCCAAAACAGTCAGGAAGCTGTGTCAGAAC-3′. The sequences of the second MTP miRNA, targeted to the 3′ UTR of MTP (AAATGCTTTGACGTGCCTAA) are: top sequence 5′-TGCTGTTAGGCACGTCAAAGCATTTCGTTTTGGCCACTGACTGACGAAATGCTGACGTGCCTAA-3′ and bottom sequence 5′-CCTGTTAGGCACGTCAGCATTTCGTCAGTCAGTGGCCAAAACGAAATGCTTTGACGTGCCTAAC-3′. The sequences of the negative control oligo duplex are as follows: top sequence 5′-GCTGAAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCACGCAGTACATTT-3′ and bottom sequence 5′-CCTGAAATGTACTGCGTGGAGACGTCA-GTCAGTGGCCAAAACGTCTCCACGCGCAGTACATTTC-3′. Vectors harboring the above oligo duplexes, as single or multiple copies (for maximizing efficiency of gene silencing), were constructed according to the manufacturer's instructions. Escherichia coli DH5α cells were transformed using the vectors harboring MTP miRNA or negative control miRNA. Plasmids were purified using standard techniques, analyzed to confirm correct sequence, and used to transfect parental and apoB:1000-expressing McA-RH7777 cells as previously described (31, 35, 40, 44).
Cell culture and transfection
Rat hepatoma McA-RH7777 cells (referred to as McA-RH hereon) were obtained from American Type Culture Collection (Rockville, MD). Generation of clonal stable transformants of McA-RH cells expressing apoB:1000 (denoting amino acid residues 1–1,000 of the mature protein lacking the signal peptide) has previously been described in detail (40). Cells were grown in DMEM containing 20% HS, 5% FBS, and 0.2 mg/ml G418, and medium was changed every 48 h. All experiments were conducted with 4- to 5-day-old cells as previously described (40). To transfect negative control miRNA or MTP miRNA into parental McA-RH, cells were seeded onto 60-mm dishes and grown in DMEM containing 20% HS and 5% FBS as described above. After 24 h and at approximately 50–60% confluency, cells were transfected using TransIT® –LT1 Transfection Reagent (Mirus, Madison, WI) according to the manufacturer's instructions. Cells were trypsinized 48 h posttransfection and were grown in DMEM containing serum and 10 μg/ml blasticidin (Invitrogen, Carlsbad, CA) to select for clonal stable cells. Approximately 12–20 blasticidin-resistant colonies from each transfection were selected and were then maintained in DMEM containing serum and 5 μg/ml blasticidin for 18–20 days. Medium was changed twice weekly, and cells were expanded every 4 days as described previously (31). This approach was necessary to reduce the level of preexisting MTP which has a long half-life, i.e., 4.4 days in HepG2 cells (45). Transfection of apoB:1000-expressing McA-RH cells was carried out as described for parental McA-RH cells. The efficiency of transfection was established by determining MTP mRNA level by RT-PCR and MTP protein level by Western blot analysis (46) using polyclonal antibody to MTP 97 kDa large subunit (a gift from Dr. David Gordon, Bristol-Myers Squib Pharmaceutical Research Institute, Princeton, NJ).
mRNA analysis
Total RNA from cells was isolated with Trizol Reagent (Invitrogen, Carlsbad, CA). MTP mRNA was determined by one-step semiquantitative RT-PCR using sense strand primer: AGGCTGGGGAAGGGCCCGTC and anti-sense strand primer: AATGTTCTTCACATCCATGT producing a 200 bp fragment of rat MTP mRNA. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level was used as internal control using sense strand primer: GACAAGATGGTGAAGGTCGGT and antisense strand primer: TTGGCCCCACCCTTCAGGTG. MTP and apoB mRNA levels were also determined by quantitative real-time PCR using BIO-RAD MyiQ Single Color Real Time PCR Detection System. ApoB mRNA level was determined using sense stand primer: ATGGGCCCACAGAGGCCTGCCCT and antisense strand primer: AGGTCAGGGTAAAGAGCAAC.
De novo synthesis and secretion of ApoB
Clonal stable transformants of McA-RH cells were grown for 4 days in 6-well or 60 mm dishes. At the start of experiments, maintenance medium was removed, monolayers were washed twice with PBS, and fresh serum-, methionine- and cysteine-free DMEM was added and the incorporation of [35S]methionine/cysteine (100 µCi/ml of medium) into newly synthesized rat endogenous apoB100 and apoB48 (in parental McA-RH cells) and human apoB:1000 (in stable transformants of McA-RH cells) was determined after 4 h or overnight incubation. The [35S]labeled conditioned medium was collected and preservative mixture described previously (35) was added to prevent oxidative and proteolytic damage. Lysis buffer containing preservative cocktail (35) plus leupeptin (50 μg/ml) and pepstatin A (50 μg/ml) was added to PBS-washed cells, and cell lysate was processed as previously described (40). The [35S]labeled human apoB:1000 in cell lysate and secreted into the medium was isolated by immunoprecipitation using monospecific polyclonal antibody to human apoB100 (44). Rat [35S]labeled endogenous apoB100 and apoB48 in the conditioned medium were isolated by immunoprecipitation using monospecific polyclonal antibody to rat apoB (developed in our laboratory) as described (35).
Metabolic labeling of the lipid moiety of ApoB-containing lipoprotein particles
At the start of experiments, maintenance medium was removed; cells were washed twice with PBS and were incubated for 17 h in serum-free DMEM containing [3H]glycerol (7 µCi/ml of medium). In experiments where the radiolabeled lipids associated with apoB-containing particles were determined by autoradiography, cells were labeled with both [3H]glycerol and [14C]oleic acid (0.4 mM) bound to 0.75% BSA to enhance the signal. The labeled conditioned medium was processed and supplemented with preservatives as described above. Cell monolayers were washed with PBS, scraped off the plate in 1.0 ml PBS, and sonicated. The incorporation of [3H]glycerol into various lipid moieties of apoB-containing lipoproteins secreted into the medium was determined by nondenaturing gradient gel electrophoresis (NDGGE) and lipid extraction described below. Aliquots of sonicated cell suspension were analyzed for various lipids described below; protein content was measured by the method of Lowry et al. (47).
NDGGE
The [3H]glycerol-labeled conditioned medium was subjected to 4−20% NDGGE as previously described (31, 40). Gels were stained and the bands corresponding to rat endogenous apoB100 or human apoB:1000, identified by immunoblotting of a duplicate gel, were excised and analyzed for lipids as described below.
Lipid analysis of isolated full-length and truncated ApoB-containing particles
The [3H]labeled lipids associated with the gel-isolated rat endogenous apoB100- or human apoB:1000-containing particles were extracted with chloroform/methanol (2:1) as previously described in detail (31). The extracted lipids were applied to TLC plate and bands corresponding to PL, diacylglycerols (DAGs), and TAG, identified by comparison to known standards, were scraped off the plate and quantified by liquid scintillation (31).
Immunoblot analysis
The human apoB:1000-containing particles were separated by 4–12% SDS-PAGE (48) or 4–20% NDGGE. After electrophoresis, proteins were detected by Western blot analysis (46) using biotinylated antibody to human apoB100 as previously described (40). The level of MTP protein in the cells was determined by Western blot analysis using polyclonal antibody to bovine MTP 97 kDa large subunit (15).
Microsomal triglyceride transfer protein activity assay
The activity of MTP in cell homogenate (100 μg protein) was determined by fluorescence assay (37, 49) using Roar MTP Activity Assay Kit, Roar Biochemical (New York, NY) according to the manufacturer's protocol.
RESULTS
RNAi-mediated silencing of Mttp gene expression in parental McA-RH cells
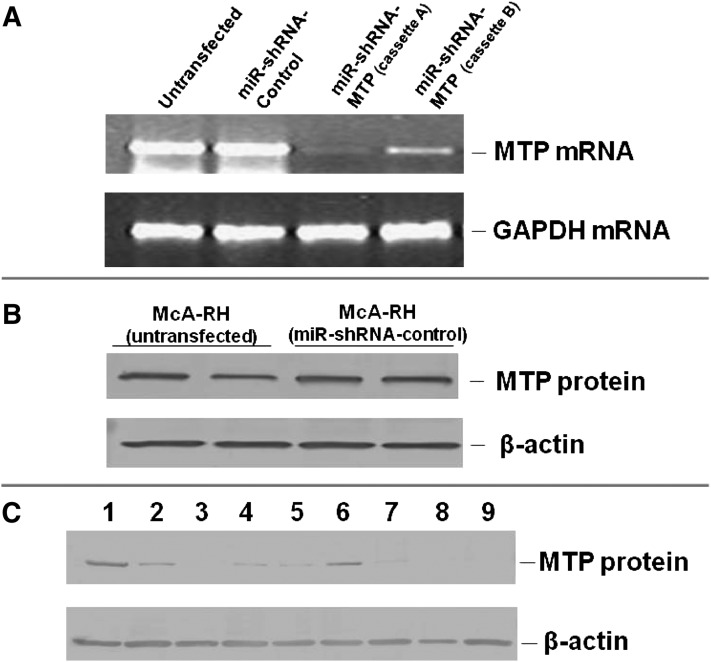
Seven different miR-shRNA-MTP sequences were tested for their capacity to silence Mttp gene expression in McA-RH cells. The sequences of the two most effective oligo duplexes are provided in “Materials and Methods.” To maximize the efficiency of gene silencing, we developed several vectors containing either a single or multiple copies of each or a combination of the two above-mentioned most efficient miR-shRNA-MTP expression cassettes. These experiments led to the identification of two miR-shRNA-MTP expression cassettes (referred to as A and B) that were the most efficient in silencing Mttp gene expression. As shown in Fig. 1, we found equivalent levels of MTP mRNA (Fig. 1A) and protein (Fig. 1B) in both the parental untransfected McA-RH cells and cells that were transfected with miR-shRNA-negative control. Both cassettes A and B markedly decreased MTP mRNA level as compared with parental untransfected McA-RH cells or cells transfected with miR-shRNA-negative control (Fig. 1A). The GAPDH mRNA level was the same in all four cell lines (Fig. 1A) supporting the specificity of miR-shRNA-MTP. The ability of cassette A to silence Mttp gene expression in McA-RH cells was greater than that of cassette B (Fig. 1A).
Fig. 1.
RNAi-mediated silencing of Mttp gene expression in McA-RH cells. A: McA-RH cells were transfected with miR-shRNA-MTP (cassette A or B) or with miR-shRNA-negative control. The mRNA levels of MTP and GAPDH were measured by RT-PCR. B: The levels of MTP protein and β-actin internal control in parental untransfected McA-RH cells and McA-RH cells transfected with miR-shRNA-negative control were determined by Western blot analysis. C: The levels of MTP protein and β-actin internal control in McA-RH cells transfected with miR-shRNA-negative control (lane 1) and several clonal stable transformants of McA-RH cell lines transfected with either miR-shRNA-MTP, cassette A (lanes 2–5) or cassette B (lanes 6–9) were determined by Western blot analysis using antibody to bovine MTP 97 kDa large subunit and antibody to β-actin, respectively.
Several clonal stable transformants of McA-RH cell lines transfected with either expression cassette A (Fig. 1C, lanes 2–5) or cassette B (Fig. 1C, lanes 6–9) were tested for MTP protein levels after 18–20 days in the maintenance medium containing serum and 5 μg/ml blasticidin. This extended time was necessary to allow for degradation of any preexisting MTP protein that has been reported to have a long half-life of 4.4 days in HepG2 cells (45). All clones (Fig. 1C, lanes 2–9) contained significantly lower MTP protein compared with control McA-RH cells (Fig. 1C, lane 1). The β-actin level was the same in all cell lines (Fig. 1C), supporting the specificity of miR-shRNA-MTP cassettes. These studies led to the identification of two stable McA-RH cell lines that did not contain any detectable MTP protein (approximately 2% of that found in the control cells) after normalization for the β-actin level. These cell lines are referred to as: miR-shRNA-MTP cassette A clone #6 (Fig. 1C, lane 3) and miR-shRNA-MTP cassette B, clone #2 (Fig. 1C, lane 9). A plausible reason for the very low level of MTP mRNA in the setting of near absence of MTP protein, observed in this study (Fig. 1), might be a specific feature of miRNA-based shRNA-mediated gene silencing technique. This approach could result in mRNA cleavage and repression of translation as well as inhibition of protein synthesis (50). Therefore, even in the presence of very low levels of MTP mRNA, there could be total absence of MTP protein, shown in Fig. 1.
RNAi-mediated silencing of Mttp gene expression in parental McA-RH cells drastically diminishes the secretion of rat endogenous apoB100 and apoB48
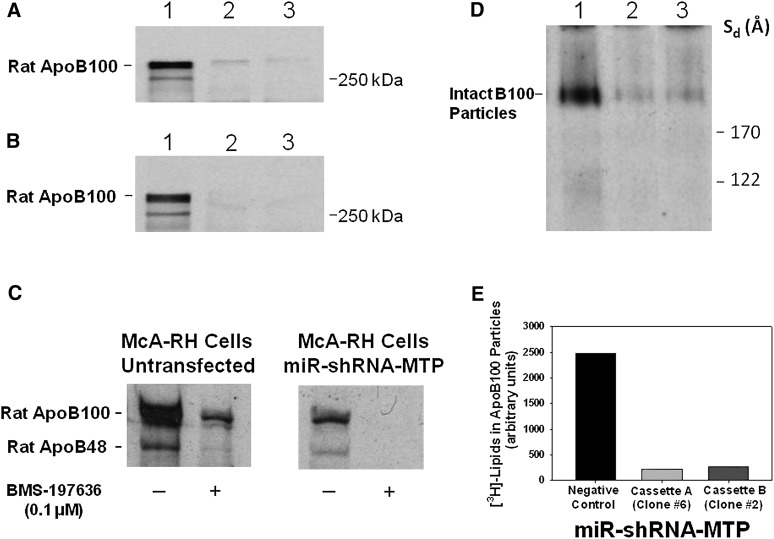
Cells were metabolically labeled with [35S]methionine/cysteine, and the secreted apoB was immunoprecipitated using polyclonal antibody to rat apoB. Labeled proteins were resolved by 4–12% SDS-PAGE and visualized by autoradiography. As expected, the secretion of rat endogenous [35S]labeled apoB100 was drastically decreased (Fig. 2A, lanes 2 and 3) or nearly abolished (Fig. 2B, lanes 2 and 3) with miR-shRNA-MTP cassettes A and B compared with cells transfected with miR-shRNA-negative control (Fig. 2A, B, lane 1). The secretion of the protein with apparent molecular mass of 250 kDa was completely abolished in MTP-deficient McA-RH cells (Fig. 1A, B). Analysis of the intensities of [35S]labeled apoB100 bands by computer- assisted image processing demonstrated >95% inhibition in the secretion of newly synthesized rat apoB100 in MTP-deficient McA-RH cells compared with cells transfected with miR-shRNA-negative control (data not shown).
Fig. 2.
Silencing of Mttp gene expression in parental McA-RH cells drastically diminishes the secretion of rat endogenous apoB100 and apoB48 and lipidation of LDL-sized apoB100-containing particles. A and B: McA-RH cells transfected with miR-shRNA-negative control (lane 1), miR-shRNA-MTP cassette A, clone #6 (lane 2), or miR-shRNA-MTP cassette B, clone #2 (lane 3) were labeled with [35S]methionine/cysteine (100 μCi/ml) for 4 h. The [35S]labeled apoB secreted into the conditioned medium was isolated by immunoprecipitation using monospecific polyclonal antibody to rat apoB. Proteins were separated by 4–12% SDS-PAGE and subjected to autoradiography. C: McA-RH cells with 70% MTP deficiency were metabolically labeled with [35S]methionine/cysteine (100 μCi/ml) for 4 h in the presence or absence of BMS-197636 (0.1 μM). The [35S]labeled apoB proteins in the conditioned medium were immunoprecipitated using monospecific polyclonal antibody to rat apoB; proteins were separated on 6% SDS-PAGE and visualized by autoradiography. D: McA-RH cells transfected with miR-shRNA-negative control (lane 1), miR-shRNA-MTP cassette A, clone #6 (lane 2), or miR-shRNA-MTP cassette B, clone #2 (lane 3) were metabolically labeled with [3H]glycerol (7 μCi/ml) for 17 h. The [3H]labeled conditioned medium was concentrated 10-fold and subjected to 4–20% NDGGE for 48 h. Gels were stained and dried and intact particles were visualized by autoradiography. E: A bar graph illustrating the levels of [3H]labeled lipids associated with intact LDL-sized apoB100-containing particles, shown in Fig. 2D, determined by computer-assisted image processing and normalized to cell protein. Values are representative of six samples from three separate experiments.
Although McA-RH cells are known to secrete predominantly apoB100-containing lipoproteins (51–53), they also secrete apoB48. Therefore, the role of MTP in the secretion of apoB48 was examined by metabolic labeling of parental and MTP-deficient McA-RH cells with [35S] methionine/cysteine in the presence or absence of MTP inhibitors. For a better separation of apoB100 and apoB48, the immunoprecipitated [35S]labeled proteins were separated by 6% SDS-PAGE and visualized by autoradiography. In parental untransfected McA-RH cells, MTP inhibitor BMS-197636 (0.1 µM) caused a 77% decrease in the secretion of [35S]labeled apoB100 as well as the 250 kDa protein, presumed to be apoB48, when compared with cells incubated with Me2SO vehicle control (Fig. 2C). As shown in Fig. 2A, B, secretion of apoB48 could not be detected in McA-RH cells with 98% MTP deficiency. Therefore, to further evaluate the relative importance of MTP in the synthesis and secretion of apoB100 and apoB48, we used McA-RH cells with approximately 70% MTP deficiency. Results showed that the secretion of both [35S]labeled apoB100 and [35S]labeled apoB48 was decreased by 73% in McA-RH cells with 70% MTP deficiency when compared with control untransfected McA-RH cells (Fig. 2C). Furthermore, addition of BMS-197636 (0.1 µM) abolished the secretion of both apoB100 and apoB48 (Fig. 2C). These results suggest that, under the experimental conditions employed, MTP is required for the secretion of both apoB100 and apoB48 in McA-RH cells.
RNAi-mediated silencing of MTP gene expression in parental McA-RH cells markedly reduces the lipid content and alters the lipid composition of rat endogenous apoB100-containing particles
The effect of MTP silencing on the lipidation of rat endogenous apoB100-containing particles is shown in Fig. 2D. As expected, compared with McA-RH cells transfected with miR-shRNA-negative control (Fig. 2 D, lane 1), there was a marked reduction in [3H]labeled lipid content of rat endogenous LDL-sized apoB100-containing particles secreted by McA-RH cells transfected with either miR-shRNA-MTP, cassette A (Fig. 2D, lane 2) or cassette B (Fig. 2D, lane 3). The plot of the relative intensities of [3H]labeled lipids associated with the LDL-sized bands in Fig. 2D, demonstrated greater than 90% decrease in [3H]labeled lipid content of secreted LDL-sized particles in MTP-deficient McA-RH cells when compared with cells transfected with miR-shRNA-negative control (Fig. 2E). Quantitative determination of lipids associated with intact particles showed a reduction of 78% (with cassette A) and 74% (with cassette B) in the [3H]labeled content of LDL-sized apoB100-containing lipoproteins secreted by MTP-deficient McA-RH cells (data not shown). This decrease was more pronounced in TAG than in PL and DAG, resulting in the secretion of LDL-sized apoB100-containing particles that contained a lower percent content of TAG and higher percent contents of PL (data not shown).
Effect of MTP deficiency on the synthesis and secretion of human apoB:1000 in stable transformants of McA-RH cells
We previously showed that inactivation of MTP lipid transfer activity by inhibitors BMS-197636 and BMS-200150 had no detectable effect on the secretion of human apoB:1000 in stable transformants of McA-RH cells (35). Similar results were obtained in apoB:1000-expressing McA-RH cells with siRNA-mediated 60% reduction in Mttp gene expression (35). However, we could not rule out the possibility that the remaining 40% of MTP would be sufficient to support the initiation of apoB particle assembly. Achieving the challenging task of near complete silencing of MTP expression in parental McA-RH cells in the present study provided us with a unique opportunity to determine if MTP is in fact required for the formation of prenascent PL-rich apoB:1000-lipoprotein particle. To this end, stable transformants of apoB:1000-expressing McA-RH cells were transfected with the miR-shRNA-MTP targeted to the 3′ UTR of MTP, which was determined to be the most efficient in silencing Mttp gene expression in parental McA-RH cells (Figs. 1 and 2).
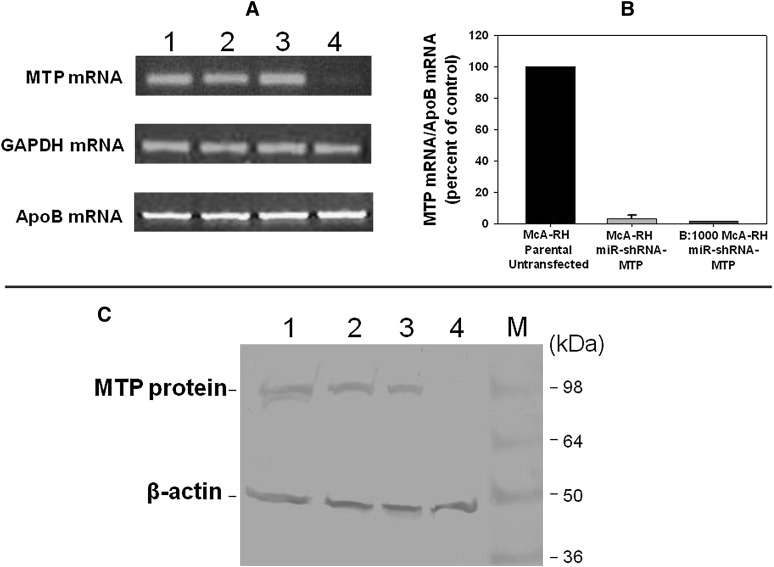
As shown in Fig. 3A (representative of three individual dishes), we found equivalent levels of MTP mRNA in parental untransfected McA-RH cells (Fig. 3A, lane 1), control apoB:1000-expressing McA-RH cells (Fig. 3A, lane 2), and apoB:1000-expressing McA-RH cells transfected with miR-shRNA-negative control (Fig. 3A, lane 3). Transfection of apoB:1000-expressing McA-RH cells with miR-shRNA-MTP (Fig. 3A, lane 4) decreased the levels of MTP mRNA by 90% compared with negative control apoB:1000-expressing McA-RH cells (Fig. 3A, lane 3). The GAPDH mRNA level was the same in all four cell lines supporting the specificity of miR-shRNA-MTP (Fig. 3A). The apoB mRNA levels were the same in all four cell lines (Fig. 3A), indicating that MTP deficiency does not alter ApoB gene expression in McA-RH cells. The MTP mRNA levels were also determined by real-time PCR (Fig. 3B) and confirmed the results obtained by semiquantitative RT-PCR (Fig. 3A). The MTP mRNA levels in MTP-deficient parental and apoB:1000-expressing McA-RH cells were decreased by approximately 98% when compared with control parental untransfected McA-RH cells (Fig. 3B).
Fig. 3.
RNAi-mediated silencing of Mttp gene expression in stable transformants of apoB:1000-expressing McA-RH cells. A: The mRNA levels of MTP, apoB, and GAPDH in parental untransfected McA-RH cells (lane 1), control apoB:1000-expressing McA-RH cells (lane 2), and apoB:1000-expressing McA-RH cells transfected with either miR-shRNA-negative control (lane 3) or miR-shRNA-MTP targeted to 3′UTR of MTP (lane 4) were measured by RT-PCR. B: The mRNA levels of parental untransfected McA-RH cells, MTP-deficient McA-RH cells, and MTP-deficient apoB:1000-expressing McA-RH cells were determined by real-time PCR. C: The levels of MTP protein and β-actin internal control in parental untransfected McA-RH cells (lane 1), control apoB:1000-expressing McA-RH cells (lane 2), and apoB:1000-expressing McA-RH cells transfected with either miR-shRNA-negative control (lane 3) or miR-shRNA-MTP targeted to 3′UTR of MTP (lane 4) were determined by Western blot analysis using antibody to bovine MTP 97 kDa large subunit and antibody to β-actin, respectively.
Western blot analysis, using polyclonal antibody to bovine MTP 97 kDa large subunit (15), of cell lysate demonstrated equal MTP protein levels in parental untransfected McA-RH cells (Fig. 3C, lane 1), control apoB:1000-expressing McA-RH cells (Fig. 3C, lane 2), and apoB:1000-expressing McA-RH cells transfected with miR-shRNA-negative control (Fig. 3C, lane 3). After 6 weeks in culture, the levels of MTP protein in apoB:1000-expressing McA-RH cells transfected with miR-shRNA-MTP (Fig. 3C, lane 4) was decreased by 98% compared with cells transfected with miR-shRNA-negative control (Fig. 3C, lane 3). The β-actin level was the same in all cell lines (Fig. 3C), establishing the specificity of miR-shRNA-MTP. Thus, we were able to achieve near complete silencing of Mttp gene expression, at both mRNA and protein levels, in stable transformants of apoB:1000-expressing McA-RH cells using miR-shRNA-MTP targeted to the 3′UTR of MTP. The activity of residual MTP in MTP-deficient cells was determined using a Roar MTP Activity Assay Kit (Roar Biochemical, New York, NY). Under the assay condition suggested by the manufacturer (Roar Biochemical), MTP activity in MTP-knockdown apoB:1000-expressing McA-RH cells was approximately 8% of that of wild-type cells.
RNAi-mediated total silencing of Mttp gene expression has no significant effect on the synthesis and secretion of apoB:1000-containing particle expressed in stable transformants of McA-RH cells
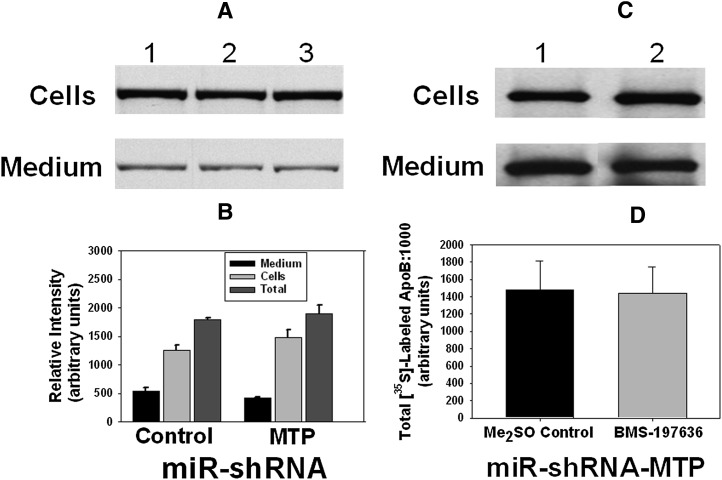
As shown in Fig. 4A, there were no significant differences in either the secretion or cellular accumulation of [35S]labeled apoB:1000 between the control apoB:1000-expressing McA-RH cells (Fig. 4A, lane 1) and cells that were transfected with either miR-shRNA-negative control (Fig. 4A, lane 2) or miR-shRNA-MTP (Fig. 4A, lane 3). The relative intensities of [35S]labeld apoB:1000 bands in medium and cells from triplicate dishes were determined by computer-assisted image processing, normalized for cell protein, and plotted (Fig. 4B). Results demonstrated that the near absence of MTP did not impair the synthesis and secretion of apoB:1000 in stable transformants of McA-RH cells. The modest decrease in the secretion of apoB:1000 was not statistically significant.
Fig. 4.
RNAi-mediated total silencing of Mttp gene expression has no effect on the secretion or cellular accumulation of apoB:1000 in stable transformants of McA-RH cells. A: Control apoB:1000-expressing McA-RH cells (lane 1) and cells transfected with either miR-shRNA-negative control (lane 2) or miR-shRNA-MTP targeted to 3′UTR of MTP were labeled with [35S]methionine/cysteine (100 μCi/ml) for 4 h. The [35S]labeled apoB:1000 secreted into the conditioned medium and accumulated in the cells were isolated by immunoprecipitation using monospecific polyclonal antibody to human apoB100. Proteins were separated by 4-12% SDS-PAGE and subjected to autoradiography. B: A bar graph illustrating the levels of [35S]labeled apoB:1000 in cells and medium, shown in Fig. 4A, determined by computer-assisted image processing and normalized to cell protein. Values are mean ± SE of triplicate dishes. C: MTP-deficient apoB:1000-expressing McA-RH cells were metabolically labeled with [35S]methionine/cysteine (100 μCi/ml) for 4 h in the absence (lane 1) or presence (lane 2) of BMS-197636 (0.1 μM). The [35S]labeled apoB:1000 secreted into the conditioned medium and accumulated in the cells were isolated by immunoprecipitation using monospecific polyclonal antibody to human apoB100 and analyzed by SDS-PAGE and autoradiography. D: A bar graph illustrating the total [35S]labeled apoB:1000 in cells and medium, shown in Fig. 4C, determined by computer-assisted image processing and normalized to cell protein. Values are mean ± SE of triplicate dishes.
To assess if the remaining 2% MTP protein detected in MTP-deficient cells was sufficient to mediate the transfer of PL to apoB:1000 and particle assembly, we employed BMS-197636, a potent inhibitor of MTP lipid transfer activity (37). Cells were labeled with [35S]methionine/cysteine in the presence or absence of 0.1 μM BMS-197636 and labeled apoB:1000 in the conditioned medium, and cell lysate was immunoprecipitated with polyclonal antibody to human apoB100 and analyzed by SDS-PAGE and autoradiography. Results demonstrated that inhibition of the residual PL transfer activity of MTP had no further effect on the net synthesis and secretion of apoB:1000 (Fig. 4C). Thus, total elimination of MTP activity did not significantly affect the total synthesis and secretion of apoB:1000 (Fig. 4D).
MTP deficiency has no detectable effect on the content or composition of lipids associated with intact ApoB:1000-containing particles secreted by stable transformants of McA-RH cells
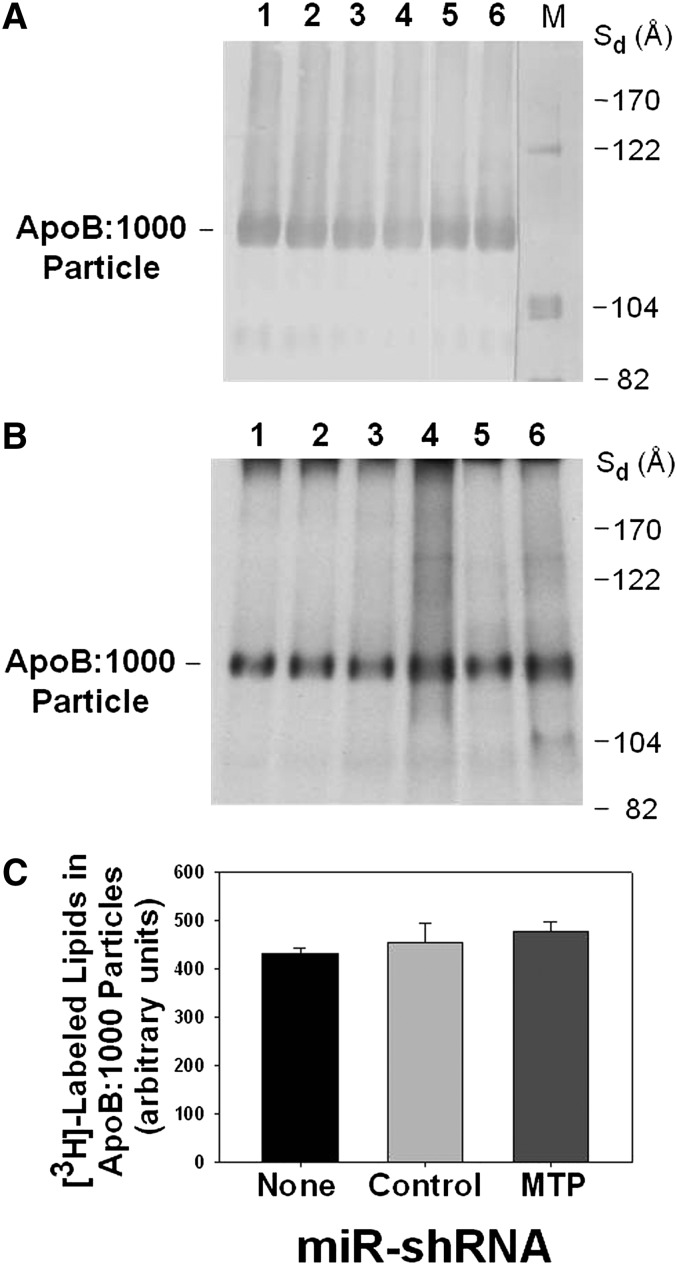
Cells were metabolically labeled with [3H]glycerol for 17 h, and labeled conditioned medium was subjected to NDGGE. Gels were run in duplicates; one gel was analyzed by Western blotting using polyclonal antibody to human apoB100 (Fig. 5A), and the second gel was stained and subjected to autoradiography (Fig. 5B). Western blot analysis demonstrated equal levels of apoB:1000 in intact particles secreted by control apoB:1000-expressing McA-RH cells (Fig. 5A, lanes 1 and 2) and cells transfected with either miR-shRNA-negative control (Fig. 5A, lanes 3 and 4) or miR-shRNA-MTP (Fig. 5A, lanes 5 and 6). Similarly, there were no detectable differences between the [3H]labeled lipid content of intact apoB:1000-containing particles secreted by control apoB:1000-expressing McA-RH cells (Fig. 5B, lanes 1 and 2) and cells transfected with either miR-shRNA-negative control (Fig. 5A, lanes 3 and 4) or miR-shRNA-MTP (Fig. 5B, lanes 5 and 6). A plot of the relative intensities of [3H]labeled lipids associated with the apoB:1000-containing bands and normalized for cell protein showed that the absence of MTP had no effect on the lipid content of the secreted apoB:1000-containing particles (Fig. 5C).
Fig. 5.
RNAi-mediated total silencing of Mttp gene expression has no effect on the lipid content of secreted intact apoB:1000-containing particles in stable transformants of McA-RH cells. Cells were metabolically labeled with [3H]labeled glycerol (7 μCi/ml) for 17 h. The labeled conditioned medium was concentrated 10-fold and subjected to 4-20% NDGGE for 48 h; gels were run in duplicates. A: Western blot analysis of apoB:1000-containing particles secreted by control apoB:1000-expressing McA-RH cells (lanes 1 and 2), cells transfected with miR-shRNA-negative control (lanes 3 and 4) and cells transfected with miR-shRNA-MTP targeted to 3′ UTR of MTP (lanes 5 and 6). B: Gel was dried and the lipid contents of apoB:1000-containing particles secreted by control apoB:1000-expressing McA-RH cells (lanes 1 and 2), cells transfected with miR-shRNA-negative control (lanes 3 and 4), and cells transfected with miR-shRNA-MTP targeted to 3′ UTR of MTP (lanes 5 and 6) were visualized by autoradiography. C: A bar graph illustrating the levels of [3H]labeled lipid content of the secreted apoB:1000, shown in Fig. 5B, determined by computer-assisted image processing and normalized to cell protein. Values are mean ± SE of triplicate dishes.
In a separate series of four experiments, the [3H]labeled apoB:1000-containing particles were excised from the native gels, and lipids were extracted from the particles as previously described (31). Quantitative determination of [3H]labeled lipids likewise demonstrated identical levels of lipids associated with apoB:1000-containing particles secreted by stable transformants of McA-RH cells in the presence or absence of MTP (Table 1). Furthermore, no changes were observed in the lipid composition of the apoB:1000-containing particles secreted by MTP-deficient McA-RH cells compared with control cells (Table 1). Complete silencing of MTP in apoB:1000-expressing McA-RH cells had no major effect on the accumulation of [3H]labeled total lipids in the cells compared with the control apoB:1000-expressing cells (Table 1). There was a modest increase of approximately 15% in the lipids accumulated in cells transfected with miR-shRNA-negative control compared with control apoB:1000-expressing cells (Table 1). The exact reason for this increase is currently not clear but could potentially be due to the nonspecific effects of miR-shRNA. With the exception of a small increase in TAG, the composition of newly synthesized lipids that accumulated in MTP-deficient apoB:1000-expressing McA-RH cells were not different from those in control cells (Table 1).
TABLE 1.
Effect of RNAi-mediated silencing of MTP on the [3H]glycerol-labeled lipids accumulated in the cells and associated with secreted human apoB:1000-containing particles in stable transformants of McA-RH cells
|
3H-Labeled Lipids Associated with Secreted ApoB:1000-Containing Particles |
3H-Labeled Lipids Accumulated in the Cells |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| McA-RH cell line | miR-shRNA | Total lipids | PL | DAG | TAG | Total lipids | PL | DAG | TAG |
| cpm/mg cell protein | % composition | cpm/mg cell protein | % composition | ||||||
| ApoB:1000-Expressing | None | 2,691 ± 189 | 68.3 ± 1.0 | 8.4 ± 0.8 | 23.3 ± 0.9 | 75,707 ± 5,965 | 93.0 ± 0.4 | 4.5 ± 0.3 | 2.5 ± 0.2 |
| ApoB:1000-Expressing | Negative control | 2,864 ± 198 | 70.4 ± 1.3 | 6.7 ± 0.5 | 22.9 ± 1.5 | 88,621 ± 5,634 | 93.4 ± 0.6 | 4.1 ± 0.3 | 2.6 ± 0.4 |
| ApoB:1000-Expressing | MTP 3′UTR | 2,651 ± 221 | 69.0 ± 0.8 | 8.6 ± 0.6 | 22.6 ± 0.8 | 79,785 ± 7,891 | 89.4 ± 0.7 | 5.6 ± 0.1 | 5.1 ± 0.7 |
Control stable transformants of apoB:1000-expressing McA-RH cells and cells that were transfected with either miR-shRNA-negative control or miR-shRNA-MTP targeted to 3′ UTR of MTP were incubated with serum-free DMEM containing [3H]labeled glycerol (7 µCi/ml of medium) for 17–20 h. Secreted apoB1000-containing particles were isolated by NDGGE and analyzed for lipids as described in “Experimental Procedures.” Values are mean ± SE of nine samples from four separate experiments.
DISCUSSION
Despite myriad studies on the role of MTP in VLDL secretion, its requirement for the initial step of apoB particle assembly remains controversial. The mechanisms involved in the assembly and secretion of apoB-containing lipoproteins have be en under intense investigation in our laboratory. Specifically, we have focused on structural elements in apoB that are required for the initiation of particle assembly. Based on our experimentally derived results (31) and molecular modeling of the βα1 domain (residues 1–1,000) of apoB100, referred to as apoB:1000 (32), we previously proposed a model for the initiation of apoB particle assembly. In this model, the first 1,000 residues of apoB are required for the formation of a stable initiation complex that is PL rich and has a maximum capacity on the order of 50 molecules of PLs (31). We proposed that this primordial apoB particle can be formed without a structural requirement for MTP via four salt bridges between a tandem series of four charged residues (Arg997, Glu998, Asp999, and Arg1000) located at the C-terminal end of the model, with a complementary tandem series of four charges (Glu720, His719, Lys718, and Asp717) in the unmodeled loop region (residues 670–745) (32). In a recent publication (44), our site-directed mutation studies provided experimentally derived evidence in support of this model.
The above results did not exclude the possible functional role of MTP in the formation of the PL-rich apoB:1000-containing particles. To address this issue, we initially tested the effects of two inhibitors of lipid transfer activity of MTP, BMS-l97636, and BMS-200150 (36, 37) on the synthesis and secretion of apoB:1000 in McA-RH cells (35). Results demonstrated that inhibition of MTP activity had no effect on the synthesis, lipidation, and secretion of apoB:1000-containing particles in stable transformants of McA-RH cells (35). Considering the possibility that these inhibitors may not be totally specific, we were compelled to examine the secretion of apoB:1000 in siRNA-mediated MTP-knockdown McA-RH cells. Results showed that a 60% reduction in Mttp gene expression had no detectable effect on the assembly and secretion of apoB:1000-containing particles (35). However, it has been argued that the remaining 40% of MTP might be sufficient to mediate the initiation of apoB particle assembly (38). One of the most convincing means of precluding this possibility was to achieve complete silencing of Mttp gene expression in McA-RH cells and demonstrate unimpaired secretion of intact lipidated apoB:1000-containing particles. To this end, we used miR-shRNAs and succeeded in decreasing the MTP protein level in parental McA-RH cells by 98%. This magnitude of reduction in MTP protein level is similar to that reported in liver-specific Mttp conditional knockout mice (28, 54). Consistent with the study by Raabe et al. (28) in liver-specific Mttp conditional knockout mice, we did not detect any difference in apoB mRNA levels between the wild-type and MTP-deficient McA-RH cells, indicating that MTP deficiency does not alter Apob gene expression.
The marked reduction in the synthesis, lipidation, and secretion of rat endogenous apoB100 in MTP-deficient McA-RH cells corresponded to the decrease in MTP mRNA and protein levels in these cells. MTP deficiency also diminished the secretion of a 250 kDa protein to the same extent as that of apoB100. Because this protein was immunoprecipitated by a monospecific polyclonal antibody to rat apoB, it is presumed to be rat endogenous apoB48. Thus, under the experimental conditions employed in this study, we conclude that MTP is required for the secretion of both apoB100 and apoB48 in rat hepatoma McA-RH cells. Although studies in mice with liver-specific inactivation of Mttp gene have consistently demonstrated a marked reduction (>95%) in the plasma levels and hepatic secretion of apoB100 (28, 29, 55), results on apoB48 are more variable. Using liver-specific Mttp knockout mice, one study found a marked reduction in plasma apoB48 (54), while another study found moderate decreases in both the plasma level and hepatic secretion of apoB48 (28). Yet, a third study observed variable changes in plasma apoB48 ranging from no change to ∼50% reduction and a modest decrease in hepatic secretion of apoB48 (28). The variability in the plasma levels of apoB48 in the above studies could be explained by potential differences in the intestinal secretion of apoB48 in liver-specific Mttp knockout mice under different dietary conditions. However, the observed reduction in hepatic apoB48 would imply that the secretion of both apoB100 and apoB48 from hepatocytes requires the presence of functional MTP. MTP is thought to facilitate the stabilization of newly synthesized apoB48 (20) and apoB100 (23) shortly after completion of translation but before the addition of any major amount of lipids to the particle. In the absence of MTP, the majority of apoB100 and apoB48 undergo intracellular degradation and only small amounts are secreted (56, 57). Our results on the role of MTP in the secretion of apoB100 and apoB48 in McA-RH cells are not in conflict with the above published studies.
In striking contrast to the marked reduction in the secretion of rat endogenous apoB100 and apoB48, the near absence of MTP had no significant effect on the synthesis and secretion of apoB:1000 in stable transformants of McA-RH cells. Likewise, identical levels of labeled lipids were found associated with apoB:1000-containing particles secreted by cells that did not express any detectable MTP and cells that expressed the full complement of functional MTP. Importantly, the lipid composition of the secreted apoB:1000-containing particles was the same regardless of the presence or absence of MTP expression. These results do not support the notion that PL transfer activity of MTP is sufficient to initiate apoB particle assembly in hepatocytes, a process that is apparently active in nonhepatic COS cells (39). The detection of trace amounts of MTP protein (2%) and activity (∼8%) in MTP-deficient apoB:1000-expressing McA-RH cells evoked the possibility that this residual MTP could be sufficient to support the assembly and secretion of apoB:1000-containing particles. However, addition of BMS-197636, a potent MTP inhibitor, did not have any significant effect on the synthesis and secretion of apoB:1000 in MTP-deficient McA-RH cells. MTP has a distinct preference for the transfer of nonpolar lipids, and its lipid transfer rate decreases in the order of TAG (100%) > cholesteryl esters (66%) > DAG (10.2%) > cholesterol (8.5%) > squalene (6.9%) > PL (4.1%) (58). Considering that PL transfer activity of MTP is only 6.2% of its cholesteryl ester transfer activity (measured by MTP Activity Assay Kit), the calculated PL transfer activity of residual MTP in MTP-deficient apoB:1000-expressing McA-RH cells would be 0.50%. Because BMS-197636 at 0.1 μM inhibits the PL transfer activity of MTP by 60% (37), a significant decrease in the secretion of PL-rich apoB:1000-containing particles would have been expected in BMS-197636-treated cells if this process were dependent on MTP activity. Clearly, this was not the case, further supporting our concept that a factor(s) other than MTP mediates the assembly of PL-rich apoB:1000-containing initiation complex.
An inevitable question is how our results can be reconciled with the well-established role of MTP in the assembly and secretion of apoB-containing lipoproteins (16, 17). A prudent answer is that our data neither dispute the role of MTP in the first-step assembly of small, HDL-sized apoB100- or apoB48-containing nascent particles nor are sufficient in scope to allow any speculation of the role of MTP in the second-step particle core expansion. The present study was designed to resolve the fundamental question of requirement of MTP in the earliest stage of apoB particle assembly. Results strongly support our concept that the initial addition of fixed numbers of PL molecules to the apoB “lipid pocket” (31) and nucleation of apoB:1000-containing initiation complex occurs independently of MTP. There are ample published studies that support our conclusion. In McA-RH cells, the secretion of human apoB29 and apoB18 was not affected by MTP inhibitors (59). In HepG2 cells, synthesis of apoB polypeptides the size of 100–200 kDa was insensitive to MTP inhibitors, suggesting that functional MTP was not required for the initiation of apoB100 translation (21). Murine mammary- derived cells, which lack MTP, secrete human apoB41on small HDL-sized particles (60). Sequences in the C terminus of apoB29 bind PL and are secreted by cells lacking MTP (61), whereas segments equal to or greater than apoB48 containing the N-terminal 17% of the protein respond to MTP activity (62). Our results are in accord with the above published studies.
In conclusion, we have produced viable MTP-deficient parental and apoB:1000-expressing McA-RH cells using miR-shRNAs. We found that the near absence of MTP in parental McA-RH cells abolished the secretion of rat endogenous apoB100 and apoB48 and diminished the secretion of lipidated apoB100-containing particles. In striking contrast, MTP deficiency had no significant effect on the synthesis, lipidation, and secretion of human apoB:1000-containing particles. These results provide highly compelling evidence in support of our concept that, in hepatocytes, early acquisition of PLs by apoB:1000 leading to the assembly of the primordial apoB:1000-containing initiation complex occurs independently of MTP. This study underscores the need for considering targets other than MTP when designing strategies aimed at preventing hepatic overproduction of apoB-containing lipoproteins at the early stage of their biogenesis.
Acknowledgments
The authors thank Dr. David Gordon and Dr. J. R. Wetterau (Bristol-Myers Squib Pharmaceutical Research Institute, Princeton, NJ) for providing BMS-197636, BMS-200150, and the antibody to MTP. We wish to thank Dr. Zemin Yao (University of Ottawa Heart Institute, Ottawa, Ontario) for providing apoB100 cDNA and Dr. William Hubbard (University of Alabama at Birmingham) for helpful discussion of this study.
Footnotes
Abbreviations:
- Apo
- apolipoprotein
- ApoB:1000
- amino-terminal 22.05% (residues 1–1,000) of the mature protein
- DAG
- diacylglycerol
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- HS
- horse serum
- miRNA
- micro RNA
- miR-shRNA
- microRNA-based short hairpin RNA
- MTP
- microsomal triglyceride transfer protein
- NDGGE
- nondenaturing gradient gel electrophoresis
- PL
- phospholipid
- siRNA
- small interfering RNA
- TAG
- triacylglycerol
This work was supported by the National Institutes of Health Grant HL-084685. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Blasiole D. A., Davis R. A., Attie A. D. 2007. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol. Biosyst. 3: 608–619. [DOI] [PubMed] [Google Scholar]
- 2.Schumaker V. N., Phillips M. L., Chatterton J. E. 1994. Apolipoprotein B and low-density lipoprotein structure: implications for biosynthesis of triglyceride-rich lipoproteins. Adv. Protein Chem. 45: 205–248. [DOI] [PubMed] [Google Scholar]
- 3.Fisher E. A., Ginsberg H. N. 2002. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 277: 17377–17380. [DOI] [PubMed] [Google Scholar]
- 4.Elovson J., Chatterton J. E., Bell G. T., Schumaker V. N., Reuben M. A., Puppione D. L., Reeve J. R., Jr., Young N. L. 1988. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J. Lipid Res. 29: 1461–1473. [PubMed] [Google Scholar]
- 5.Chan L. 1992. Apolipoprotein B, the major protein component of triglyceride-rich and low density lipoproteins. J. Biol. Chem. 267: 25621–25624. [PubMed] [Google Scholar]
- 6.Xie Y., Luo J., Kennedy S., Davidson N. O. 2007. Conditional intestinal lipotoxicity in Apobec-1−/− Mttp-IKO mice: a survival advantage for mammalian intestinal apolipoprotein B mRNA editing. J. Biol. Chem. 282: 33043–33051. [DOI] [PubMed] [Google Scholar]
- 7.Knott T. J., Pease R. J., Powell L. M., Wallis S. C., Rall S. C., Jr., Innerarity T. L., Blackhart B., Taylor W. H., Marcel Y., Milne R., et al. 1986. Complete protein sequence and identification of structural domains of human apolipoprotein B. Nature. 323: 734–738. [DOI] [PubMed] [Google Scholar]
- 8.Yang C. Y., Chen S. H., Gianturco S. H., Bradley W. A., Sparrow J. T., Tanimura M., Li W. H., Sparrow D. A., DeLoof H., Rosseneu M., et al. 1986. Sequence, structure, receptor-binding domains and internal repeats of human apolipoprotein B-100. Nature. 323: 738–742. [DOI] [PubMed] [Google Scholar]
- 9.Bell-Quint J., Forte T., Graham P. 1981. Synthesis of two forms of apolipoprotein B by cultured rat hepatocytes. Biochem. Biophys. Res. Commun. 99: 700–706. [DOI] [PubMed] [Google Scholar]
- 10.Alexander C. A., Hamilton R. L., Havel R. J. 1976. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J. Cell Biol. 69: 241–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olofsson S. O., Stillemark-Billton P., Asp L. 2000. Intracellular assembly of VLDL: two major steps in separate cell compartments. Trends Cardiovasc. Med. 10: 338–345. [DOI] [PubMed] [Google Scholar]
- 12.Davis R. A. 1999. Cell and molecular biology of the assembly and secretion of apolipoprotein B-containing lipoproteins by the liver. Biochim. Biophys. Acta. 1440: 1–31. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton R. L., Wong J. S., Cham C. M., Nielsen L. B., Young S. G. 1998. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J. Lipid Res. 39: 1543–1557. [PubMed] [Google Scholar]
- 14.Boren J., Rustaeus S., Olofsson S. O. 1994. Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. J. Biol. Chem. 269: 25879–25888. [PubMed] [Google Scholar]
- 15.Wetterau J. R., Aggerbeck L. P., Bouma M. E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., Gregg R. E. 1992. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 258: 999–1001. [DOI] [PubMed] [Google Scholar]
- 16.Gordon D. A. 1997. Recent advances in elucidating the role of the microsomal triglyceride transfer protein in apolipoprotein B lipoprotein assembly. Curr. Opin. Lipidol. 8: 131–137. [DOI] [PubMed] [Google Scholar]
- 17.Hussain M. M., Shi J., Dreizen P. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44: 22–32. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y., Newberry E. P., Young S. G., Robine S., Hamilton R. L., Wong J. S., Luo J., Kennedy S., Davidson N. O. 2006. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281: 4075–4086. [DOI] [PubMed] [Google Scholar]
- 19.Du E. Z., Wang S. L., Kayden H. J., Sokol R., Curtiss L. K., Davis R. A. 1996. Translocation of apolipoprotein B across the endoplasmic reticulum is blocked in abetalipoproteinemia. J. Lipid Res. 37: 1309–1315. [PubMed] [Google Scholar]
- 20.Gordon D. A., Jamil H., Gregg R. E., Olofsson S. O., Boren J. 1996. Inhibition of the microsomal triglyceride transfer protein blocks the first step of apolipoprotein B lipoprotein assembly but not the addition of bulk core lipids in the second step. J. Biol. Chem. 271: 33047–33053. [DOI] [PubMed] [Google Scholar]
- 21.Benoist F., Grand-Perret T. 1997. Co-translational degradation of apolipoprotein B100 by the proteasome is prevented by microsomal triglyceride transfer protein. Synchronized translation studies on HepG2 cells treated with an inhibitor of microsomal triglyceride transfer protein. J. Biol. Chem. 272: 20435–20442. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell D. M., Zhou M., Pariyarath R., Wang H., Aitchison J. D., Ginsberg H. N., Fisher E. A. 1998. Apoprotein B100 has a prolonged interaction with the translocon during which its lipidation and translocation change from dependence on the microsomal triglyceride transfer protein to independence. Proc. Natl. Acad. Sci. USA. 95: 14733–14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rustaeus S., Stillemark P., Lindberg K., Gordon D., Olofsson S. O. 1998. The microsomal triglyceride transfer protein catalyzes the post-translational assembly of apolipoprotein B-100 very low density lipoprotein in McA-RH7777 cells. J. Biol. Chem. 273: 5196–5203. [DOI] [PubMed] [Google Scholar]
- 24.Pan M., Liang Js J. S., Fisher E. A., Ginsberg H. N. 2002. The late addition of core lipids to nascent apolipoprotein B100, resulting in the assembly and secretion of triglyceride-rich lipoproteins, is independent of both microsomal triglyceride transfer protein activity and new triglyceride synthesis. J. Biol. Chem. 277: 4413–4421. [DOI] [PubMed] [Google Scholar]
- 25.Kulinski A., Rustaeus S., Vance J. E. 2002. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J. Biol. Chem. 277: 31516–31525. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., McLeod R. S., Yao Z. 1997. Normal activity of microsomal triglyceride transfer protein is required for the oleate-induced secretion of very low density lipoproteins containing apolipoprotein B from McA-RH7777 cells. J. Biol. Chem. 272: 12272–12278. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Tran K., Yao Z. 1999. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within microsomes in McA-RH7777 cells. A unified model for the assembly of very low density lipoproteins. J. Biol. Chem. 274: 27793–27800. [DOI] [PubMed] [Google Scholar]
- 28.Raabe M., Veniant M. M., Sullivan M. A., Zlot C. H., Bjorkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson S. L., Skogsberg J., Bjorkegren J. 2004. The low density lipoprotein receptor prevents secretion of dense apoB100-containing lipoproteins from the liver. J. Biol. Chem. 279: 831–836. [DOI] [PubMed] [Google Scholar]
- 30.Levy E., Stan S., Delvin E., Menard D., Shoulders C., Garofalo C., Slight I., Seidman E., Mayer G., Bendayan M. 2002. Localization of microsomal triglyceride transfer protein in the Golgi: possible role in the assembly of chylomicrons. J. Biol. Chem. 277: 16470–16477. [DOI] [PubMed] [Google Scholar]
- 31.Manchekar M., Richardson P. E., Forte T. M., Datta G., Segrest J. P., Dashti N. 2004. Apolipoprotein B-containing lipoprotein particle assembly: lipid capacity of the nascent lipoprotein particle. J. Biol. Chem. 279: 39757–39766. [DOI] [PubMed] [Google Scholar]
- 32.Richardson P. E., Manchekar M., Dashti N., Jones M. K., Beigneux A., Young S. G., Harvey S. C., Segrest J. P. 2005. Assembly of lipoprotein particles containing apolipoprotein-B: structural model for the nascent lipoprotein particle. Biophys. J. 88: 2789–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segrest J. P., Jones M. K., Dashti N. 1999. N-terminal domain of apolipoprotein B has structural homology to lipovitellin and microsomal triglyceride transfer protein: a “lipid pocket” model for self-assembly of apob-containing lipoprotein particles. J. Lipid Res. 40: 1401–1416. [PubMed] [Google Scholar]
- 34.Segrest J. P., Jones M. K., De Loof H., Dashti N. 2001. Structure of apolipoprotein B-100 in low density lipoproteins. J. Lipid Res. 42: 1346–1367. [PubMed] [Google Scholar]
- 35.Dashti N., Manchekar M., Liu Y., Sun Z., Segrest J. P. 2007. Microsomal triglyceride transfer protein activity is not required for the initiation of apolipoprotein B-containing lipoprotein assembly in McA-RH7777 cells. J. Biol. Chem. 282: 28597–28608. [DOI] [PubMed] [Google Scholar]
- 36.Wetterau J. R., Gregg R. E., Harrity T. W., Arbeeny C., Cap M., Connolly F., Chu C. H., George R. J., Gordon D. A., Jamil H., et al. 1998. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science. 282: 751–754. [DOI] [PubMed] [Google Scholar]
- 37.Rava P., Athar H., Johnson C., Hussain M. M. 2005. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J. Lipid Res. 46: 1779–1785. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Z. G., Liu Y., Hussain M. M., Atkinson D., McKnight C. J. 2008. Reconstituting initial events during the assembly of apolipoprotein B-containing lipoproteins in a cell-free system. J. Mol. Biol. 383: 1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rava P., Ojakian G. K., Shelness G. S., Hussain M. M. 2006. Phospholipid transfer activity of microsomal triacylglycerol transfer protein is sufficient for the assembly and secretion of apolipoprotein B lipoproteins. J. Biol. Chem. 281: 11019–11027. [DOI] [PubMed] [Google Scholar]
- 40.Dashti N., Gandhi M., Liu X., Lin X., Segrest J. P. 2002. The N-terminal 1000 residues of apolipoprotein B associate with microsomal triglyceride transfer protein to create a lipid transfer pocket required for lipoprotein assembly. Biochemistry. 41: 6978–6987. [DOI] [PubMed] [Google Scholar]
- 41.Dashti N., McConathy W. J., Ontko J. A. 1980. Production of apolipoproteins E and A-I by rat hepatocytes in primary culture. Biochim. Biophys. Acta. 618: 347–358. [DOI] [PubMed] [Google Scholar]
- 42.Yao Z. M., Blackhart B. D., Johnson D. F., Taylor S. M., Haubold K. W., McCarthy B. J. 1992. Elimination of apolipoprotein B48 formation in rat hepatoma cell lines transfected with mutant human apolipoprotein B cDNA constructs. J. Biol. Chem. 267: 1175–1182. [PubMed] [Google Scholar]
- 43.Miller A. D., Miller D. G., Garcia J. V., Lynch C. M. 1993. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 217: 581–599. [DOI] [PubMed] [Google Scholar]
- 44.Manchekar M., Richardson P. E., Sun Z., Liu Y., Segrest J. P., Dashti N. 2008. Charged amino acid residues 997–1000 of human apolipoprotein B100 are critical for the initiation of lipoprotein assembly and the formation of a stable lipidated primordial particle in McA-RH7777 cells. J. Biol. Chem. 283: 29251–29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin M. C., Gordon D., Wetterau J. R. 1995. Microsomal triglyceride transfer protein (MTP) regulation in HepG2 cells: insulin negatively regulates MTP gene expression. J. Lipid Res. 36: 1073–1081. [PubMed] [Google Scholar]
- 46.Burnette W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate–polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112: 195–203. [DOI] [PubMed] [Google Scholar]
- 47.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 48.Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 49.Athar H., Iqbal J., Jiang X. C., Hussain M. M. 2004. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45: 764–772. [DOI] [PubMed] [Google Scholar]
- 50.Dykxhoorn D. M., Novina C. D., Sharp P. A. 2003. Killing the messenger: short RNAs that silence gene expression. Nat. Rev. Mol. Cell Biol. 4: 457–467. [DOI] [PubMed] [Google Scholar]
- 51.Yao Z., McLeod R. S. 1994. Synthesis and secretion of hepatic apolipoprotein B-containing lipoproteins. Biochim. Biophys. Acta. 1212: 152–166. [DOI] [PubMed] [Google Scholar]
- 52.Sparks J. D., Collins H. L., Sabio I., Sowden M. P., Smith H. C., Cianci J., Sparks C. E. 1997. Effects of fatty acids on apolipoprotein B secretion by McArdle RH-7777 rat hepatoma cells. Biochim. Biophys. Acta. 1347: 51–61. [DOI] [PubMed] [Google Scholar]
- 53.Cardozo C., Wu X., Pan M., Wang H., Fisher E. A. 2002. The inhibition of microsomal triglyceride transfer protein activity in rat hepatoma cells promotes proteasomal and nonproteasomal degradation of apoprotein b100. Biochemistry. 41: 10105–10114. [DOI] [PubMed] [Google Scholar]
- 54.Chang B. H., Liao W., Li L., Nakamuta M., Mack D., Chan L. 1999. Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein/low density lipoprotein production in a viable conditional knockout mouse. J. Biol. Chem. 274: 6051–6055. [DOI] [PubMed] [Google Scholar]
- 55.Lieu H. D., Withycombe S. K., Walker Q., Rong J. X., Walzem R. L., Wong J. S., Hamilton R. L., Fisher E. A., Young S. G. 2003. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 107: 1315–1321. [DOI] [PubMed] [Google Scholar]
- 56.Leiper J. M., Bayliss J. D., Pease R. J., Brett D. J., Scott J., Shoulders C. C. 1994. Microsomal triglyceride transfer protein, the abetalipoproteinemia gene product, mediates the secretion of apolipoprotein B-containing lipoproteins from heterologous cells. J. Biol. Chem. 269: 21951–21954. [PubMed] [Google Scholar]
- 57.Gordon D. A., Jamil H., Sharp D., Mullaney D., Yao Z., Gregg R. E., Wetterau J. 1994. Secretion of apolipoprotein B-containing lipoproteins from HeLa cells is dependent on expression of the microsomal triglyceride transfer protein and is regulated by lipid availability. Proc. Natl. Acad. Sci. USA. 91: 7628–7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jamil H., Dickson J. K., Jr., Chu C. H., Lago M. W., Rinehart J. K., Biller S. A., Gregg R. E., Wetterau J. R. 1995. Microsomal triglyceride transfer protein. Specificity of lipid binding and transport. J. Biol. Chem. 270: 6549–6554. [DOI] [PubMed] [Google Scholar]
- 59.Nicodeme E., Benoist F., McLeod R., Yao Z., Scott J., Shoulders C. C., Grand-Perret T. 1999. Identification of domains in apolipoprotein B100 that confer a high requirement for the microsomal triglyceride transfer protein. J. Biol. Chem. 274: 1986–1993. [DOI] [PubMed] [Google Scholar]
- 60.Herscovitz H., Kritis A., Talianidis I., Zanni E., Zannis V., Small D. M. 1995. Murine mammary-derived cells secrete the N-terminal 41% of human apolipoprotein B on high density lipoprotein-sized lipoproteins containing a triacylglycerol-rich core. Proc. Natl. Acad. Sci. USA. 92: 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carraway M., Herscovitz H., Zannis V., Small D. M. 2000. Specificity of lipid incorporation is determined by sequences in the N-terminal 37 of apoB. Biochemistry. 39: 9737–9745. [DOI] [PubMed] [Google Scholar]
- 62.Gretch D. G., Sturley S. L., Wang L., Lipton B. A., Dunning A., Grunwald K. A., Wetterau J. R., Yao Z., Talmud P., Attie A. D. 1996. The amino terminus of apolipoprotein B is necessary but not sufficient for microsomal triglyceride transfer protein responsiveness. J. Biol. Chem. 271: 8682–8691. [DOI] [PubMed] [Google Scholar]