Abstract
Bile acids are important regulatory molecules that can activate specific nuclear receptors and cell signaling pathways in the liver and gastrointestinal tract. In the current study, the chronic bile fistula (CBF) rat model and primary rat hepatocytes (PRH) were used to study the regulation of gluconeogenic genes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) and the gene encoding short heterodimeric partner (SHP) by taurocholate (TCA). The intestinal infusion of TCA into the CBF rat rapidly (1 h) activated the AKT (∼9-fold) and ERK1/2 (3- to 5-fold) signaling pathways, downregulated (∼50%, 30 min) the mRNA levels of PEPCK and G-6-Pase, and induced (14-fold in 3 h) SHP mRNA. TCA rapidly (∼50%, 1–2 h) downregulated PEPCK and G-6-Pase mRNA levels in PRH. The downregulation of these genes by TCA was blocked by pretreatment of PRH with pertussis toxin (PTX). In PRH, TCA plus insulin showed a significantly stronger inhibition of glucose secretion/synthesis from lactate and pyruvate than either alone. The induction of SHP mRNA in PRH was strongly blocked by inhibition of PI3 kinase or PKCζ by specific chemical inhibitors or knockdown of PKCζ by siRNA encoded by a recombinant lentivirus. Activation of the insulin signaling pathway appears to be linked to the upregulation of farnesoid X receptor functional activity and SHP induction.
Keywords: protein kinase C zeta, GW4064, chronic bile fistula rat, primary rat hepatocyte, serine/threonine kinase AKT (protein kinase B)
Bile acids are detergent molecules that are synthesized from cholesterol in the liver hepatocytes. Bile acid synthesis represents one of two main output pathways of cholesterol from the body, the other being biliary cholesterol secretion. Bile acids are conjugated to either glycine or taurine before they are actively transported across the hepatocyte canalicular membrane into the biliary ductule system along with cholesterol and phospholipids. Bile salts carry out important physical-chemical functions in the body by promoting lipid solubilization and absorption in the small intestines (1).
Bile acids also function as important regulatory molecules in the liver and intestines. Bile acids are capable of activating specific nuclear receptors [i.e., farnesoid X receptor (FXR), pregnane X receptor (PXR), Vitamin D], a Gαs-protein-coupled surface receptor (TGR-5), and cell signaling pathways (i.e., JNK1/2, ERK1/2, AKT) (2–6). Activation of FXR in the liver results in the increased expression of a number of target genes, including the bile salt export pump ABCB11 and the phospholipid transporter ABCB4. These transporters decrease intracellular bile salt and lipid levels by increasing transport rates into bile. In addition, activation of FXR downregulates the genes encoding hepatic cholesterol 7α-hydroxylase (CYP7A1) and sterol 12α-hydroxylase (CYP8B1), which control the rate of bile acid synthesis and the ratio of cholic acid to chenodeoxycholic acid, respectively. However, repression of CYP7A1 gene by bile acids appears to be primarily through the activation of the gene encoding FGF15/19 in the ileum (7–10). FGF15/19 has been reported to bind and activate the tyrosine kinase FGFR4 receptor in hepatocytes, which then activates the JNK1/2 pathway, downregulating the gene encoding CYP7A1. Activation of the JNK1/2 pathway in primary hepatocytes has been shown to repress CYP7A1 mRNA (11) possibly by altering the amount and/or phosphorylation state of hepatocyte nuclear factor 4α (HNF4α), a positive acting transcription factor that regulates CYP7A1 and CYP8B1 gene expression in the liver (12).
Our laboratories (13–20) and others (3–6, 21, 22) have reported that bile acids can activate the JNK1/2, ERK1/2, and AKT cell signaling pathways in primary hepatocytes. We have reported that conjugated and free bile acids activate the ERK1/2 and AKT signaling pathways by distinctly different mechanisms. Unconjugated hydrophobic bile acids appear to activate the ERK1/2 and AKT signaling pathways via induction of mitochondria-generated reactive oxygen species (ROS) (18). In contrast, conjugated bile acids activate the ERK1/2 and AKT pathways in primary hepatocytes through pertussis toxin (PTX)-sensitive Gαi-protein-coupled receptor(s) (GPCR) (19, 20).
There is growing evidence that bile acids may play a role in the regulation of glucose metabolism in the liver. We have shown that bile acids enhance the activity of the insulin receptor and AKT signaling cascade in primary hepatocytes (17). Activation of this signaling cascade resulted in a marked stimulation of glycogen synthase (GS) activity in primary hepatocytes via a GPCR-dependent mechanism. We have recently shown in the chronic bile fistula rat model that intestinal infusion of taurocholate (TCA) rapidly activates the AKT signaling pathway and GS activity (20). A number of recent studies indicate that bile acids, FXR, and SHP may play a role in the regulation of glucose metabolism in the liver (23–28). However, it is unclear how bile acids coordinately regulate cell signaling pathways and nuclear receptors that control glucose metabolism. In the present study, we provide evidence that TCA, through activation of GPCR(s) and the PI3K/PDK-1/AKT pathway (insulin signaling pathway), can regulate gluconeogenic genes. Moreover, it appears that activation of the PI3K/PDK-1/PKCζ pathway is required for the optimal activation of FXR by taurocholate, which induces the gene encoding the small heterodimeric partner (SHP). SHP is a nuclear receptor protein without a DNA binding domain that has been reported to interact with other nuclear receptors and transcription factors (i.e., HNF4α, FOX01, CEBPα), inhibiting their function.
MATERIALS AND METHODS
Materials
Wortmannin, pertussis toxin, AKTi-1/2, PKCζ pseudosubstrate, and Gö6976 were purchased from Calbiochem (San Diego, CA). GW4064 was purchased from Tocris (Ellisville, MO). Anti phospho-FOX01/FOX03a antibodies (thr24, ser254, ser319) were from Cell Signaling Technology (Danvers, MA). Anti actin was from Cayman Chemicals (Ann Arbor, MI). Anti PKCζ was purchased from US Biological (Swampscott, MA). Taurocholate was purchased from Sigma. All other antibodies were from Santa Cruz (Santa Cruz, CA).
Bile fistula rat
Biliary fistulas and intraduodenal cannulas were placed in male Sprague-Dawley rats under brief anesthesia as previously described (20, 29). After surgery, they were placed in individual metabolic cages with water and normal chow ad libitum. All animals received continuous infusion of glucose-electrolyte replacement solution. After 48 h of chronic biliary diversion, taurocholate was infused at a rate of 1.05 ml per 100 g rat per h and at a concentration of 36 μmoles per 100 g rat per h for the time indicated. At the end of the experiment, 0.1g of liver was harvested to isolate RNA (see below), and the rest of the liver was flash-frozen in liquid nitrogen in several pieces. One piece was used to make total cell lysates (see Western blot analysis below). Animal research was conducted in conformity with PHS policy and with approval of the Institutional Animal Care and Use Committee.
Primary rat hepatocytes
Primary rat hepatocyte monolayer cultures were prepared from male Sprague-Dawley rats by the collagenase-perfusion technique of Bissell and Guzelian as described previously (30). Cells were handled as described in figure legends. They were plated in serum-free Williams E medium containing penicillin, dexamethasone (0.1 μM), and thyroxine (1 µM).
Measurement of rates of glucose secretion/synthesis in primary rat hepatocytes
Primary rat hepatocytes were prepared as described above, washed without insulin, and plated in a 12-well plate at a concentration of 5 × 105 cells in 1 ml of DMEM (no added glucose) containing 5% FBS. Cells were incubated overnight at 37°C in 5% CO2, washed 3 times with warm PBS, and the various treatments added with or without 20 mM sodium lactate + 2 mM sodium pyruvate in a volume of 400 μl per well. Cells were incubated 1 h at 37°C followed by the addition of 100 μl of DMEM (without glucose) + 20 mM sodium lactate + 2 mM sodium pyruvate with or without 100μM dibutyryl-cAMP + 50 nM dexamethasone and incubated for 2.5 h. Culture supernatants were harvested and centrifuged for 75 s at maximum speed in a microcentrifuge. Supernatants (100 μl) were then assayed for glucose levels using a WAKO Autokit. Each assay contained a blank with media only and a standard glucose solution as a positive control.
Western blot analysis
Total cell lysates were prepared as described previously (20). Thirty µg of protein were resolved on 10% Bis-Tris or 7% Tris-acetate NuPage gels and transferred to Nitrocellulose membranes. Immunoblots were blocked 1 h at RT with 5% nonfat milk in TBS buffer and then incubated with primary antibodies in 1% nonfat milk for 40 h at 4°C. Immunoreactive bands were detected using horseradish peroxidase-conjugated secondary antibodies and the Western Lightning Chemiluminescence Reagent Plus (PerkinElmer). The densities of immunoblot bands were analyzed using ImageJ computer software (National Institutes of Health).
RNA isolation and RT-PCR
RNA was isolated using the SV Total RNA Isolation Kit (Promega) and quantified using a Victor3V plate reader. The reverse transcription was accomplished using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The real-time quantitative RT-PCR reactions were done using gene-specific primers and a Bio-Rad iQ5 Multicolor Real-Time PCR Detection System with iQTM SYBR Green Supermix as a fluorescent dye to detect the presence of double-stranded DNA. The mRNA levels were normalized to internal β-actin and GAPDH mRNA. The primer sequences were the following: rSHP: forward, CCAAGGAGTACGCATACC, reverse: AGCCATGAGGAGGATTCG; rPEPCK1: forward: GTGTCATCCGCAAGCTGAAG, reverse: CTTTCGATCCTGGCCACATC; rG6Pase: forward: CCCAGACTAGAGATCCTGACAGAAT, reverse: GCACAACGCTCTTTTCTTTTACC; rABCB11: forward TTCTGTTCTCCACCACTATCG, reverse: TTGCTTCTGACCACCACTC; rβ-actin: forward: TATCGGCAATGAGCGGTTCC, reverse: AGCACTGTGTTGGCATAGAGG; rGAPDH: forward: GACATCAAGAAGGTGGTG, reverse: CAGCATCAAAGGTGGAAG.
Lentiviral siRNA for downregulating PKCζ
The sense sequence of the siRNA cassettes specifically targeting the nucleotides (549-568) of PKCζ was designed through siRNA Target Finder (Ambion, Austin, TX). A two-step PCR strategy was performed using two separate reverse primers to generate a siRNA expression cassette (SEC) consisting of human U6 promoter and a hairpin siRNA cassette plus terminator and subcloned into pLL3.7 vector, which encodes the CMV-promoted EGFP (enhanced green fluorescent protein) marker as an internal control. The resulting lentiviral siRNA vector was confirmed by restriction enzyme digestion. The sequence of PKCζ siRNA is 5′-AGCCAAGCGCTTTAACAGGA-3′. The recombinant lentiviruses were produced by transient transfection of HEK293FT cells (from Invitrogen) using Effectene Transfection Reagent (Qiagen). Briefly, HEK293FT cells were cultured in high-glucose DMEM, supplemented with 10% FBS, penicillin/streptomycin (100 U/ml), and 500 µg/ml of G418. The subconfluent cells in a 10 cm culture dish were cotransfected with lentiviral vector (10 μg), and the lentiviral packaging vectors pRSV-REV (2 μg), pMDLg/pRRE (5 μg), and the vesicular stomatitis virus G glycoprotein (VSVG) expression vector pMD2G (3 μg). The viruses were collected from the culture supernatants on day 3 posttransfection, freeze-thawed, and centrifuged at 4000 rpm for 10 min. Titers were determined by infecting HEK293FT with serial dilutions of concentrated lentivirus, counting EGFP-expressing cells after 48 h under fluorescent microscopy. The knockdown efficiency was determined after infecting primary rat hepatocytes by Western blot analysis. Primary hepatocytes were infected with the appropriate amount of lentivirus for 40 h and then treated with TCA for 3 h. RNA was harvested, and SHP mRNA levels were determined by QRT-PCR.
Statistical analysis
Data were analyzed by Student's t-test. Level of significance was set at P < 0.05, N≥ 3.
RESULTS
Activation of cell signaling pathways by TCA in the chronic bile fistula rat
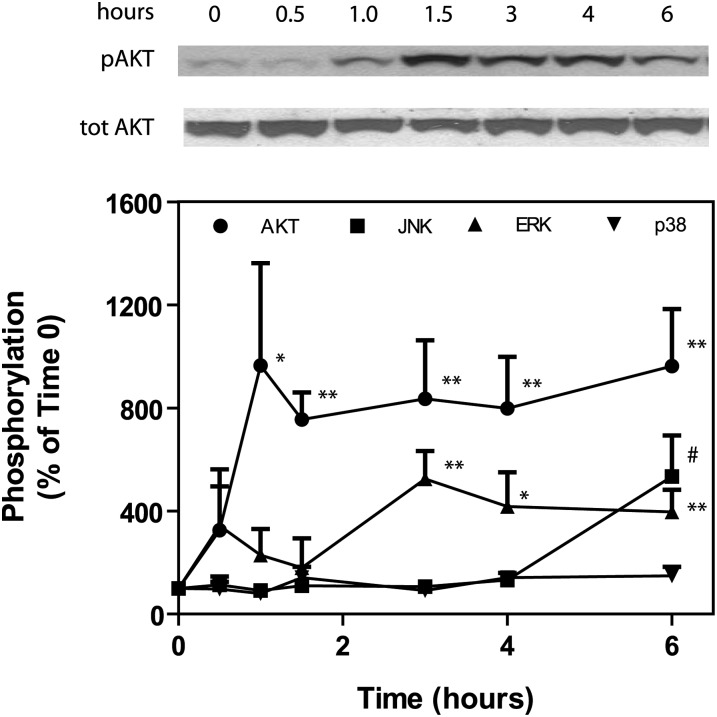
The chronic bile fistula rat model has been used in the past to study the regulation of bile acid and cholesterol synthesis in the liver (10, 29, 31). In this model, bile is diverted for 48 h allowing for equilibration of bile acid, cholesterol, and phospholipid synthesis to a higher steady-state in the liver. A single bile acid is then infused into the small intestine at a rate that is not toxic to the liver. In the current experiments, we infused taurocholate (TCA) at a rate of 36 µmol per 100 g rat per h, which has been previously shown not to be hepatotoxic (29). In contrast to other bile acids, TCA is not metabolized in the liver. Hence, activation of cell signaling pathways and nuclear receptors in the liver is due solely to TCA and not toxicity or bile acid metabolites. Infusion of TCA resulted in the rapid (1 h) and dramatic activation of AKT (∼9-fold) and ERK1/2 (3- to 5-fold) pathways (Fig. 1). We have previously reported that activation of the AKT and ERK1/2 pathways by conjugated bile acids in primary hepatocytes is through a PTX/dominant negative Gαi-dependent pathway (18–20). These results suggest the involvement of Gαi-protein-coupled receptors in hepatocytes that are activated by TCA. The infusion of TCA also activated the JNK1/2 signaling cascade, but only after 6 h of TCA infusion. These results may indicate that TCA activation of JNK1/2 in the liver is primarily through stimulation of the synthesis of FGF15/19 in the intestine (ileum) (7). The promoter of the gene encoding FGF15/19 has a functional FXR element that is activated by bile acids (7). This hormone has been reported to activate the FGFR-4 receptor in the liver, which turns on the JNK1/2 pathway, resulting in the downregulation of the gene encoding CYP7A1. There was no significant activation of p38 MAPK pathway by TCA in the chronic bile fistula model over a 6 h period (Fig. 1).
Fig. 1.
Taurocholate rapidly activates AKT and ERK1/2 in the chronic bile fistula rat. Bile fistulas were placed in rats and bile drained for 48 h after surgery as previously described (20, 29). Vehicle (PBS) or taurocholate was infused intraduodenally at a rate of 1.05 ml/h/100 g rat and a concentration of 36 μmol/h/100 g rat. Animals were harvested at times indicated, and liver pieces from each animal isolated and snap-frozen. A piece of liver from each animal was thawed and homogenized in lysis buffer and ATK, ERK1/2, JNK1/2, and p38 MAPK activity was determined by Western blot as previously described (16) (± SEM, N = 4). *P < 0.05, **P < 0.01 compared with time 0. #Includes 6 h (N = 4), 7 h, and 8 h (N = 1 each), P < 0.05 compared with time 0. All other points are not significant compared with time 0.
Downregulation of PEPCK and G-6-Pase mRNA by TCA in the chronic bile fistula rat and primary rat hepatocytes
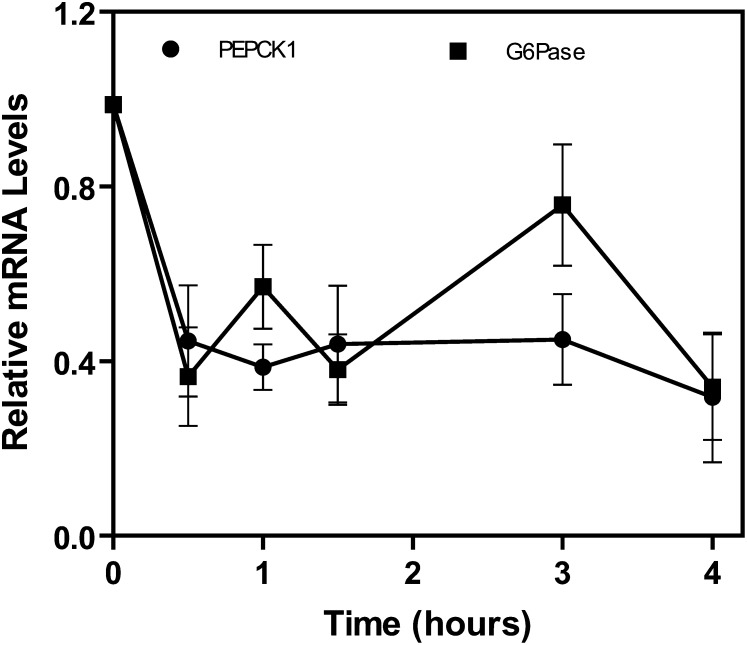
Bile acids have been reported to regulate the rate-limiting gluconeogenic genes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) in various cell culture and animal models (4, 23–28). Most, but not all (32), studies report that the rate-limiting gluconeogenic genes are downregulated by bile acids or FXR agonists. It has been hypothesized that the downregulation of these genes is through induction of SHP, which has been reported to physically interact with HNF-4α and FOX01 to inhibit PEPCK and G-6-Pase gene expression (24, 28). In the chronic bile fistula rat, intestinal infusion of TCA rapidly (∼50%, 30 min) downregulated mRNA levels of PEPCK and G-6-Pase (Fig. 2).
Fig. 2.
Taurocholate rapidly downregulates PEPCK and G-6-Pase mRNA in the chronic bile fistula rat. Pieces of frozen liver (100 mg) from the chronic bile fistula rats, harvested at various time points (0–4 h, see Fig. 1), were used to extract total mRNA. This mRNA was used for reverse transcription and then for real-time quantitative RT-PCR to quantitate PEPCK and G-6-Pase mRNA as described in “Materials and Methods.“ N = 3 for all points. G-6-Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate carboxykinase.
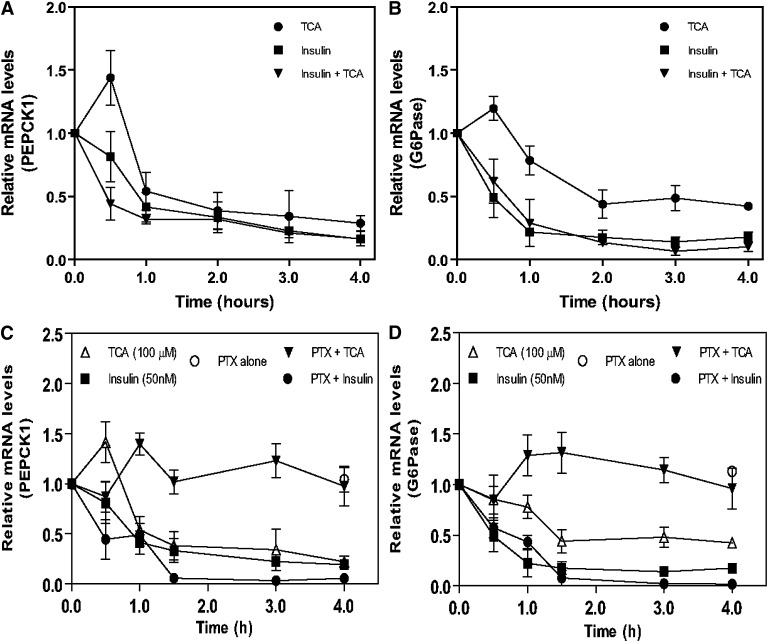
To further investigate the mechanism of regulation of PEPCK and G-6-Pase mRNA by TCA, we used primary rat hepatocytes as a model. The addition of TCA alone to primary rat hepatocytes in culture rapidly (1–2 h) downregulated both PEPCK (Fig. 3A) and G-6-Pase mRNA levels (Fig. 3B). As expected, the addition of insulin alone to primary rat hepatocytes rapidly downregulated PEPCK and G-6-Pase mRNA levels. Interestingly, the downregulation of G-6-Pase and PEPCK mRNA by TCA, in the absence of insulin, was completely blocked by pretreating primary hepatocytes with PTX (Fig. 3C, D). PTX alone does not affect PEPCK or G6Pase mRNA levels. As we also know that PTX does not affect the ability of TCA to enter the cell (19), these results suggest that TCA can regulate G-6-Pase and PEPCK-1 mRNA through GPCR(s), which we have shown to activate the PI3K/PDK-1/AKT signaling cascade (17–20).
Fig. 3.
Downregulation of PEPCK and G-6-Pase mRNA in primary rat hepatocytes by taurocholate is inhibited by pertussis toxin. Primary rat hepatocytes were prepared as described in “Materials and Methods.” After 20 h, cells were treated with either TCA (50 µM), insulin (50 nM), or both for a 4 h period, harvested at the same time, mRNA isolated and subjected to RT-PCR analysis to quantitate PEPCK and G-6-Pase mRNA as described in “Materials and Methods” (panels A and B). In some experiments, cells were treated 4 h after plating with pertussis toxin (300 ng/ml) for 16 h prior to treatment with TCA or insulin (panels C and D). N = 3 for all points. G-6-Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate carboxykinase; PTX, pertussis toxin; TCA, taurocholate.
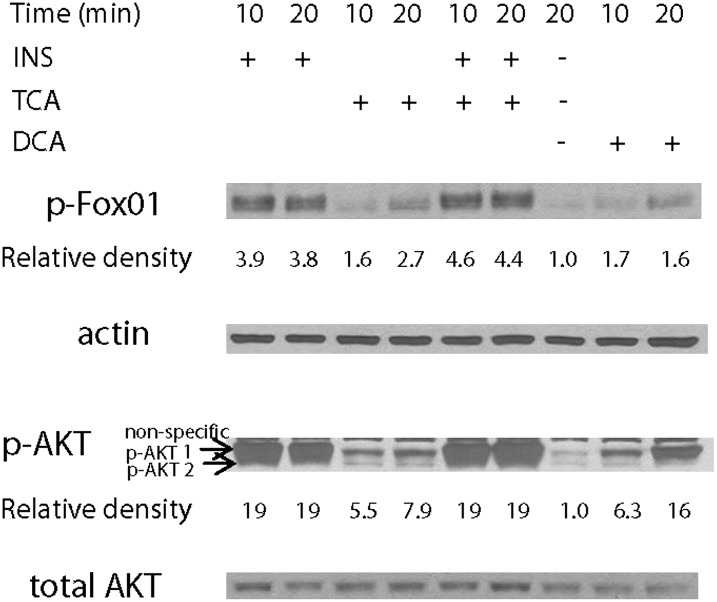
We next determined if the addition of TCA would result in the activation of AKT and phosphorylation of FOX01, a transcription factor known to regulate gluconeogenic genes. Insulin, TCA, and DCA all significantly activated the AKT-1 isoform. In primary hepatocytes, insulin, TCA, and DCA induced an increase in FOX01 phosphorylation on thr24 (Fig. 4). Surprisingly, in these same experiments there was no detectable phosphorylation of FOX01 at ser256 or ser319 following the addition of either insulin or TCA (data not shown). A similar pattern of FOX01 phosphorylation was seen using liver extracts prepared from bile fistula rats (see supplementary Fig I).
Fig. 4.
Taurocholate and insulin increase the phosphorylation of FOX01 on thr24 in primary rat hepatocytes (representative blot). Primary rat hepatocytes were prepared without added insulin as described in “Materials and Methods.” After 24 h, insulin (50 nM), TCA (50 µM), both, or deoxycholate (50 µM) were added and incubated for 10 or 20 min and harvested as cell lysates for Western blot analysis. Relative increases in p-FOX01-thr24 (compared with actin) and p-AKT1/2 (compared with total AKT) are indicated. N = 3. DCA, deoxycholate; FOX01, forkhead box O1; INS, insulin; TCA, taurocholate.
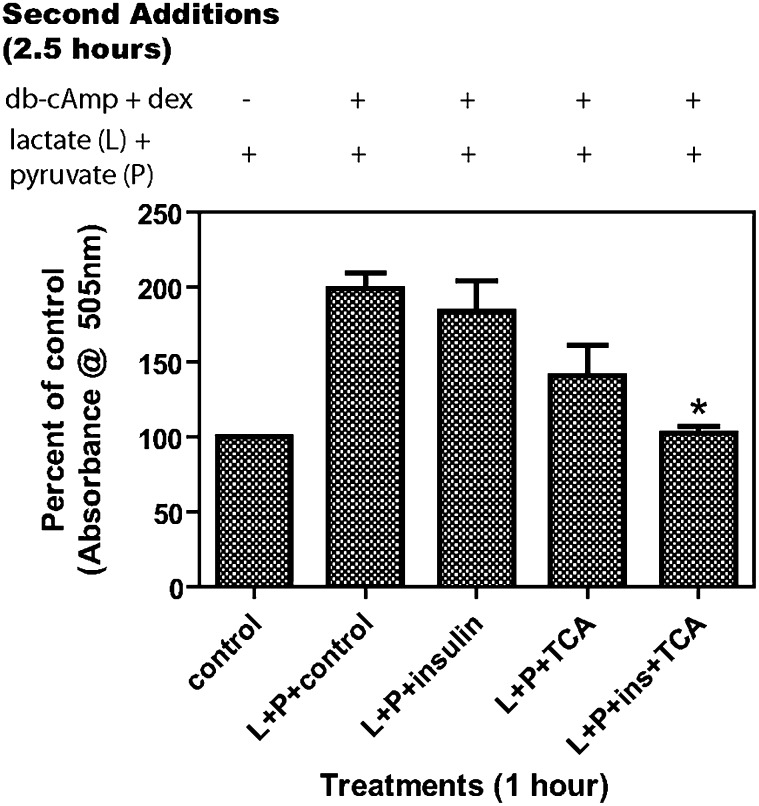
The effects of TCA and insulin on the rates of glucose secretion/synthesis were next determined in primary rat hepatocyte cultures. The individual addition of either TCA or insulin alone only modestly inhibited rates of glucose secretion/synthesis under our experimental conditions. In contrast, the addition of both TCA and insulin significantly inhibited rates of glucose secretion/synthesis (Fig. 5).
Fig. 5.
Insulin and TCA together have a stronger inhibitory effect on glucose synthesis by rat hepatocytes. Primary rat hepatocytes were prepared without insulin as described in “Materials and Methods” and plated at 5 × 105 cells per 1ml in a 12-well plate in glucose-free DMEM with 5% FBS. After 20 h, the cells were washed three times with warm PBS and suspended in 400 µl of glucose-free DMEM with appropriate treatments (100 μM TCA and/or 50 nM insulin) with or without L+P (20 mM lactate + 2 mM pyruvate). After 1 h incubation at 37°C, 100 µl of glucose-free DMEM with 500 μM dibutyryl-cAMP and 250 nM dexamethasone and/or L+P were added. Cells were incubated 2.5 h at 37°C, and supernatants harvested and 100 µl assayed for glucose with 1.5 ml of WAKO reagent. N = 3, *P < 0.001 compared with L+P+control. Results are expressed as % of control.
Induction of SHP mRNA by TCA in the chronic bile fistula rat
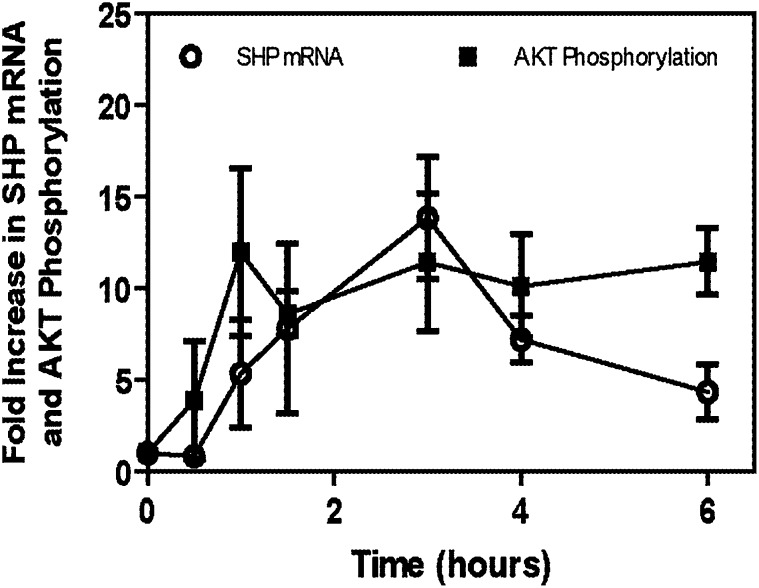
The small heterodimeric partner (SHP) is a nuclear receptor without a DNA binding domain. SHP can physically interact with other nuclear receptors and transcription factors to inhibit their function (24, 28). SHP has been reported to be involved in the regulation of hepatic glucose metabolism (23–28). Specifically, SHP has been reported to interact with HNF4α, FOX01 (24), and C/EBPα (28), resulting in the downregulation of PEPCK and G-6-Pase. HNF4α, C/EBPα, and FOX01 have been reported to be important in regulating gluconeogenic genes (24, 28, 33–35). The intraduodenal infusion of TCA into the chronic bile fistula rat resulted in a dramatic increase (∼14-fold at 3 h) in the steady-state mRNA levels of SHP (Fig. 6). It was also observed that there was about a 30 min lag between the activation of the AKT signaling pathway and induction of SHP.
Fig. 6.
Taurocholate rapidly induces SHP mRNA in the chronic bile fistula rat. Bile fistulas were placed in rats and bile drained for 48 h after surgery as previously described (20, 29). Vehicle (PBS) or taurocholate was intraduodenally infused at a rate of 1.05 ml/h/100 g rat and a concentration of 36 μmol/h/100 g rat as described in Fig. 1. Animals were harvested at times indicated, and the liver of each animal was isolated and snap-frozen. A piece of frozen liver (100 mg) from the chronic bile fistula rats, harvested at various time points (0–6 h, see Fig. 1), was used to extract total mRNA. This mRNA was used for reverse transcription and then for real-time quantitative RT-PCR to quantitate SHP mRNA as described in “Materials and Methods.” N = 3 for all points. SHP, small heterodimeric partner; TCA, taurocholate.
Role of the insulin signaling pathway in TCA induction of SHP in primary hepatocytes
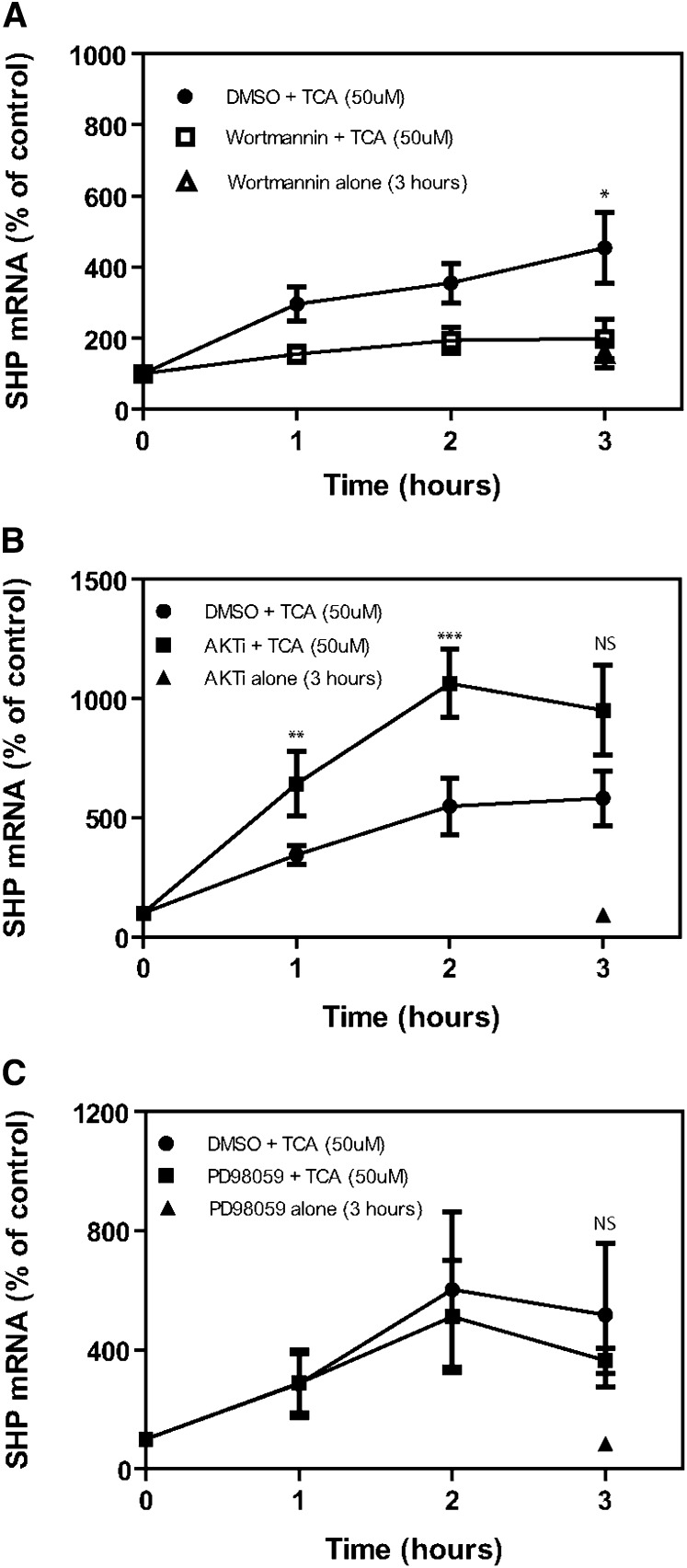
Because of the time lag between the activation of AKT and induction of SHP mRNA in the chronic bile fistula rat model (Fig. 6), we hypothesized that the activation of the insulin signaling pathway by TCA might be required for maximal activation of FXR and induction of SHP mRNA. We used the primary rat hepatocyte model to test this hypothesis. First, we treated freshly prepared primary rat hepatocytes with either Wortmannin (PI3K inhibitor), PD98059 (an ERK1/2 inhibitor), or AKTi-1/2 (an AKT kinase inhibitor). TCA (50 μM) was then added to the culture medium, and mRNA levels of SHP, an FXR target gene, were determined over a 3 h period. The data (Fig. 7A) show that Wortmannin significantly blocked the ability of TCA to induce SHP mRNA in primary hepatocytes in the absence of insulin. In contrast, ERK1/2 inhibitor (Fig. 7C) had no significant effect on the ability of TCA to induce SHP mRNA. In contrast, the AKT inhibitor enhanced the induction of SHP by TCA (Fig. 7B). Finally, a p38 MAPK and a JNK1/2 inhibitor were also used but failed to inhibit SHP mRNA induction by TCA (data not shown). These results indicated that kinases upstream of AKT signaling pathway may be involved in the activation of FXR by TCA.
Fig. 7.
Effects of kinase inhibitors on induction of SHP mRNA in primary rat hepatocytes. Primary rat hepatocytes were prepared without added insulin as described in “Materials and Methods.” After 2 h plating, cells were treated with vehicle, Wortmannin (100 nM), AKTi (1 µM), or PD98059 (50 µM) for 30 min and then TCA (50 μM) for 1, 2, or 3 h. Cells were harvested at 3 h, and mRNA isolated and used for reverse transcription and then for real-time quantitative RT-PCR to quantitate SHP mRNA as described in “Materials and Methods.” *P < 0.03, **P < 0.079, ***P < 0.018, NS = not significant. There was no significant difference when mRNA levels were normalized to rGAPDH. Results expressed as % of time 0 control. N = 4. SHP, small heterodimeric partner; TCA, taurocholate.
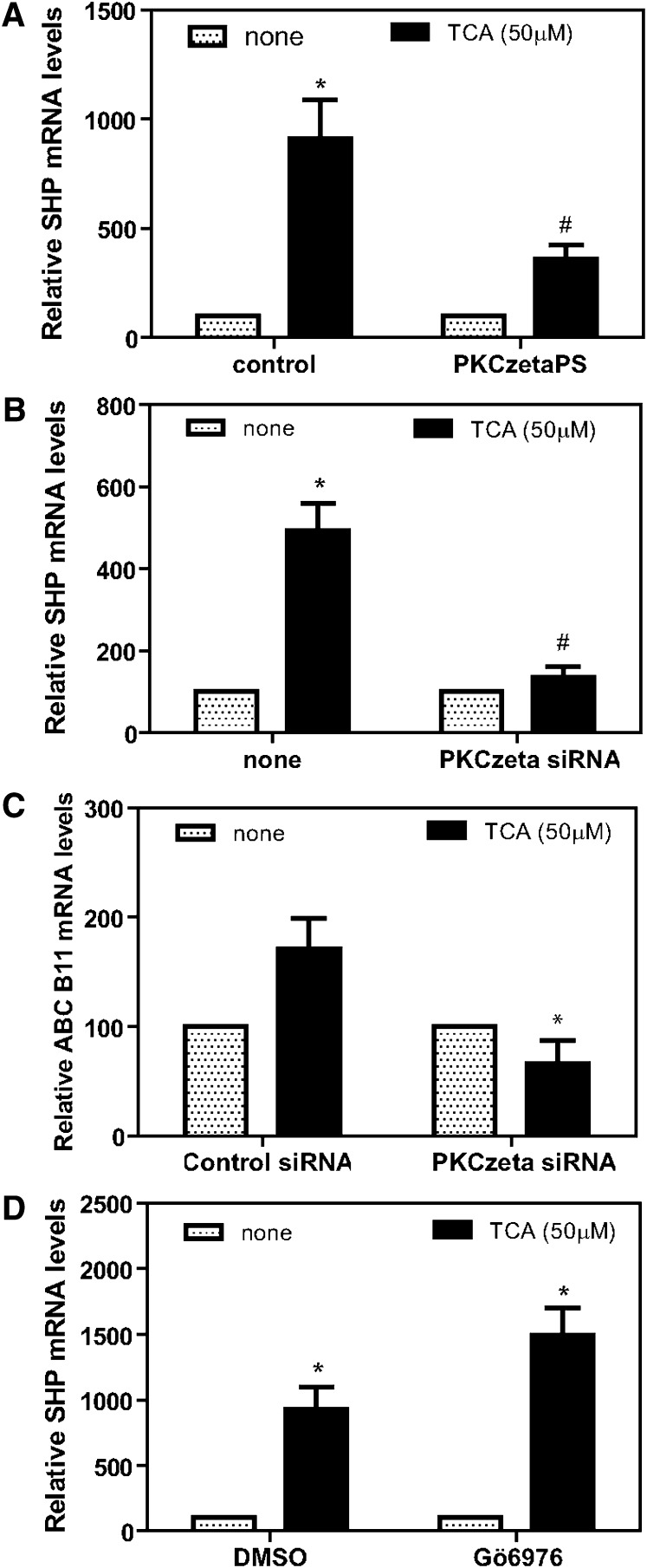
We next tested the hypothesis that PKCζ, which is activated by PDK-1, regulates FXR and alters its ability to induce SHP. In this regard, Frankenberg et al. (36) has reported that PKCζ can phosphorylate FXR, altering its cellular location. A specific inhibitor of PKCζ (PKCζPS) was used to initially test this hypothesis. The addition of PKCζPS significantly blocked the ability of TCA to induce SHP in primary rat hepatocytes in culture (Fig. 8A). Moreover, siRNA knockdown of PKCζ mRNA and protein (see supplementary Fig II) using a recombinant lentivirus also significantly blocked the ability of TCA to induce SHP mRNA (Fig. 8B) and a second FXR target gene ABCB11 (Fig. 8C). In contrast, the Ca2+-dependent PKC isozyme inhibitor Gö6976 failed to block the induction of SHP by TCA (Fig. 8D).
Fig. 8.
Effect of protein kinase C inhibitors on induction of SHP mRNA by TCA in primary rat hepatocytes. Primary rat hepatocytes were prepared without insulin as described in “Materials and Methods.” After 2 h plating, cells were treated with either vehicle (control) or PKCζ pseudosubstrate (80 µM) for 1.5 h and then TCA (50 µM) for 3 h (panel A: *P < 0.005, #P < 0.005). Lentivirus encoding siRNA for PKCζ was added to primary hepatocytes in a minimal volume for 3 h, media were brought to normal volume, and cells incubated for 20 h. Cells were then treated with TCA for 3 h, mRNA was isolated and used for reverse transcription, and the DNA was used for real-time quantitative RT-PCR to quantitate SHP or ABCB11 mRNA as described in “Materials and Methods” (panel B: *P < 0.03, #P < 0.012; panel C: *P < 0.04 compared with control+TCA). In other experiments, after 2 h plating, cells were treated with either vehicle (DMSO) or Gö6976 (1 µM) for 1.5 h and then TCA (50 µM) for 3 h (panel D: *P < 0.009 compared with none added). DMSO+TCA versus Gö6976+TCA = not significant. N = 3 for all experiments. SHP, small heterodimeric partner; TCA, taurocholate.
Activation of the insulin signaling pathway inhibits induction of SHP by the FXR agonist GW4064
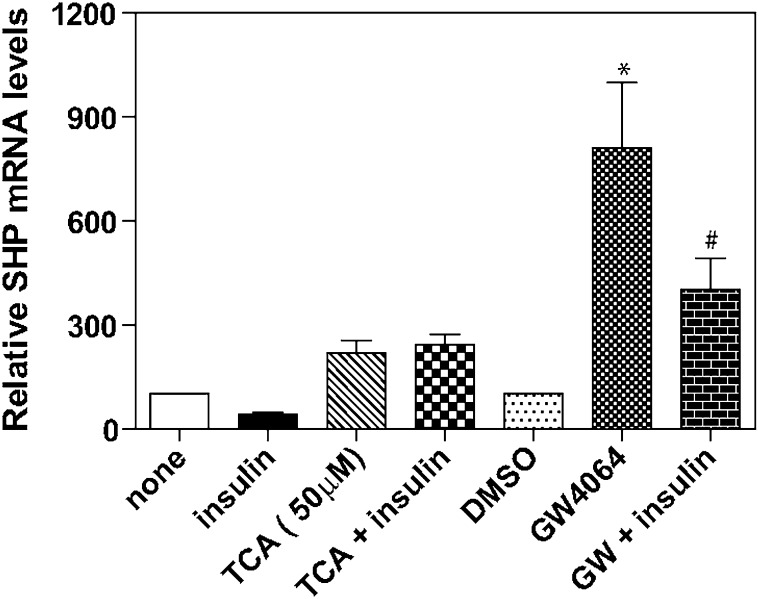
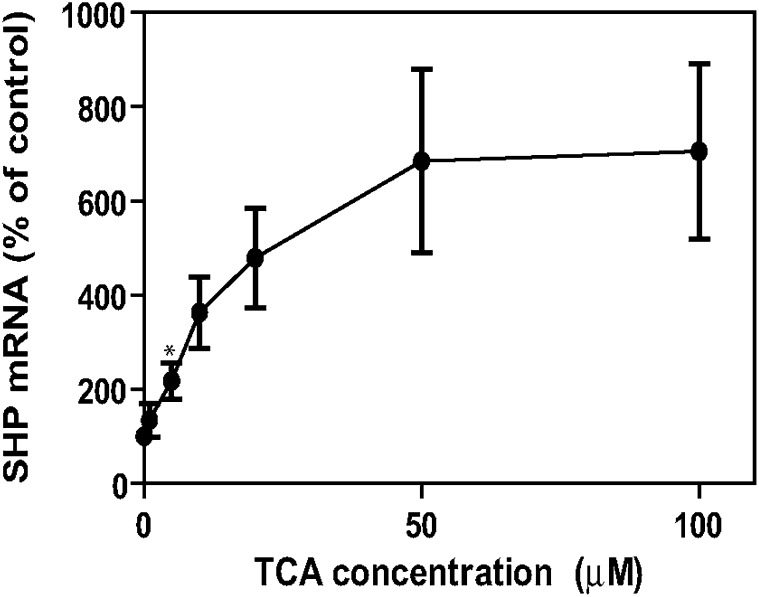
When we tested the induction of SHP mRNA by GW4064 in the presence and absence of added insulin, we discovered that there was a significant decrease in the level of SHP mRNA induced by this agonist in the presence of insulin (Fig. 9). In this regard, note that GW4064 was discovered using a cell-free in vitro screening technique and recombinant protein (37). However, the effect of cell signaling pathways on the ability of GW4064 to activate FXR has not been investigated. The addition of insulin had no effect on induction of SHP by TCA (Fig. 9). Finally, in primary rat hepatocytes, TCA induced SHP mRNA in primary hepatocytes at concentrations as low as 5 µM (Fig. 10), which is in marked contrast to the in vitro studies that required >1 mM TCA for activation of FXR (37). Therefore, in primary rat hepatocytes, TCA appears to be a very good activator of FXR.
Fig. 9.
Effect of insulin on the induction of SHP mRNA by TCA and GW4064 in primary rat hepatocytes. Primary rat hepatocytes were prepared without insulin as described in “Materials and Methods.” After 2 h plating, cells were treated with TCA (50 μM) or vehicle or GW4064 (5 µM) with or without insulin (50 nM) for 3 h, mRNA isolated, and the DNA used for reverse transcription and then for real-time quantitative RT-PCR to quantitate SHP mRNA as described in “Materials and Methods.” *P < 0.001, #P < 0.006 compared with DMSO control. N = 3 for all experiments. SHP, small heterodimeric partner; TCA, taurocholate.
Fig. 10.
Effect of taurocholate concentration on the induction of SHP mRNA in primary rat hepatocytes. Primary rat hepatocytes were prepared without insulin as described in “Materials and Methods.” After 2 h plating, cells were treated with varying concentrations (1, 5, 10, 20, 50, or 100 μM) of TCA for 3 h. mRNA was isolated and used for reverse transcription, and the DNA was used for real-time quantitative RT-PCR to quantitate SHP mRNA as described in “Materials and Methods.” *P < 0.01, N = 4. SHP, small heterodimeric partner; TCA, taurocholate.
DISCUSSION
In recent years it has become clear that bile acids are important regulatory molecules capable of activating specific nuclear receptors, G-protein coupled receptors (TGR5), and various cell signaling pathways in the liver and gastrointestinal tract (2–6). Bile acids have been reported to activate the AKT pathway (insulin signaling pathway) and the nuclear receptor FXR in hepatocytes (18–21, 38–40). We have previously reported that conjugated (but not unconjugated) bile acids activate the AKT and ERK1/2 signaling pathways via unidentified Gαi-protein-coupled receptor(s) in primary rat hepatocytes (18, 19) and in vivo (20). The activation of ERK1/2 and AKT signaling pathways by bile acids in hepatocytes appears not to involve the GPCR TGR5, because its gene is not highly expressed in hepatocytes, is Gαs coupled, and is activated by both conjugated and unconjugated bile acids (3, 41, 42). In the current study, we used the chronic bile fistula rat model and primary rat hepatocytes to study the regulation of the rate-limiting gluconeogenic genes PEPCK and G-6-Pase and the induction of SHP by TCA. We wanted to determine if there is a link between bile acid-activated cell signaling pathways and FXR activation. The SHP promoter has a functional FXR binding site, which allows bile acids to induce this gene. In the chronic bile fistula rat, we observed rapid (1 h) activation of the AKT (∼9-fold) and ERK1/2 (3- to 5-fold) signaling pathways following the intraduodenal infusion of TCA at a concentration that has been shown not to be hepatotoxic (29).
We measured the effect of TCA on the regulation of PEPCK and G-6-Pase in the chronic bile fistula rat and in primary hepatocytes. The data show a very rapid downregulation of both PEPCK and G-6-Pase mRNA following the infusion of TCA in the chronic bile fistula rat (Fig. 2). Consistent with the in vivo data there was also a rapid (1–2 h) downregulation of PEPCK and G-6-Pase mRNA in primary rat hepatocytes by either TCA or insulin (Fig. 3). Moreover, the downregulation of G-6-Pase and PEPCK mRNA by TCA was inhibited by pretreatment of hepatocytes with PTX (Fig. 3C, D). These data are consistent with our previous results showing that TCA activates the AKT pathway via Gαi-protein-coupled receptor(s) and suggest that bile acids may function much like insulin in helping to coordinately regulate glucose metabolism in the liver. In this regard, we have previously reported that the addition of insulin or bile acids to primary hepatocytes results in the activation of GS activity (17, 20). Interestingly, the addition of both a bile acid and insulin resulted in an additive effect on GS activity (20) and a stronger repression of glucose synthesis (Fig. 5). The addition of TCA or insulin to primary hepatocytes resulted in the rapid phosphorylation of FOX01, a key transcription factor controlling PEPCK and G-6-Pase gene expression (Fig. 4). The phosphorylated form of FOX01 moves from the nucleus to the cytosol and becomes inactive as a transcriptional activator of gluconeogenic genes (43). Finally, SHP has been reported to physically interact with FOX01, CEBPα, and HNF4α, inhibiting their function. All three of these transcription factors have been reported to be involved in the upregulation of gluconeogenic genes (24, 28). Hence, the combined effect of activation of the insulin signaling pathway and induction of SHP by TCA may be a rapid and long-lasting repression of the rate-limiting gluconeogenic genes (Fig. 5).
In the chronic bile fistula rat model, we observed a rapid induction of SHP mRNA following the intestinal infusion of TCA (Fig. 6). The kinetics of induction showed that SHP mRNA levels followed closely (∼30 min) the activation of the insulin signaling pathway. In primary rat hepatocytes, Wortmannin significantly inhibited the induction of SHP mRNA by TCA (Fig. 7A). These results suggest that the insulin signaling pathway must be activated to get optimal induction of SHP mRNA by TCA. There was no significant inhibition of SHP induction when specific chemical inhibitors of the ERK1/2 (Fig. 7C) or p38 MAPK (data not shown) were added to primary hepatocytes in culture. Moreover, the inhibition of AKT by a specific chemical inhibitor failed to inhibit SHP induction by TCA (Fig. 7B). These results suggest that PDK-1, which is upstream of AKT, may be involved in regulating SHP induction by TCA, as PKCζ is known to be activated by PDK-1 (44). Inhibition of PKCζ by a specific chemical inhibitor or siRNA significantly blocked the induction of SHP by TCA (Fig. 8A, B). In this regard, it has recently been reported that FXR can be phosphorylated by PKCζ, and this may allow the translocation of FXR to the nucleus (36). Moreover, PKCα and PKCβI have been recently reported to phosphorylate FXR, which promotes transcriptional activity of FXR target genes in plasmids in HepG2 cells, possibly by recruiting the coactivator PGC1α (45). Our laboratories have previously reported that TCA rapidly activates PKCα and PKCβI in primary rat hepatocytes at relatively low concentrations (13, 14). However, in primary rat hepatocytes, we did not observe any significant inhibition of SHP mRNA induction by TCA when the Ca2+-dependent PKC isozyme inhibitor Gö6976 was used at concentrations reported to inhibit FXR-dependent induction in other cells (45)(Fig. 8D). The explanation for these results is not clear, but they could be due to use of different cell types (i.e., primary hepatocytes versus HepG2; FXR promoter constructs versus FXR genes in chromosome; and chenodeoxycholic acid versus TCA as activators of FXR). Additional studies will be required to resolve this issue.
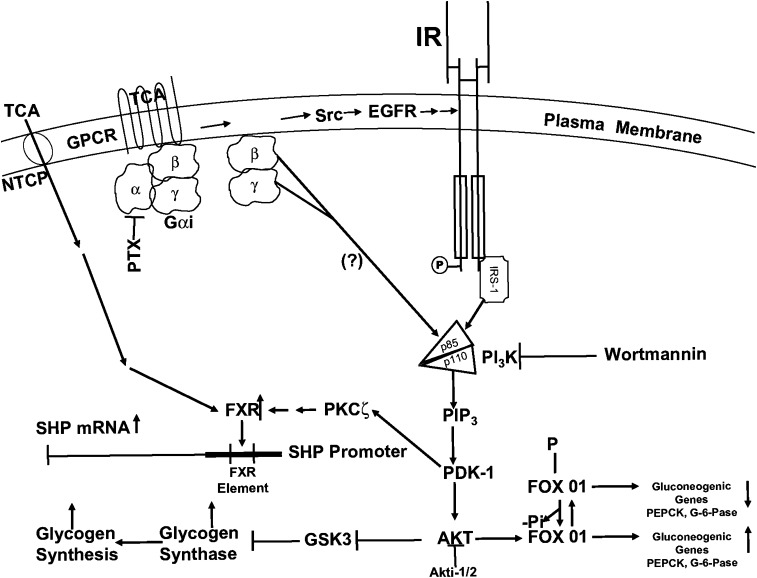
In vitro studies using recombinant FXR show that TCA (EC50 >1 mM) is a very poor activator of FXR, whereas chenodeoxycholic acid (EC50 10 μM) and GW4064 (EC50 70 nM) are excellent activators (37–40). In the current study in primary rat hepatocytes, TCA is a very good inducer of SHP at concentrations as low as 5 μM (Fig. 10). Interestingly, inhibition of PI3K or PKCζ markedly reduced the ability of TCA to induce SHP (Figs. 7A and 8A, B). In contrast, activation of the insulin signaling pathway significantly reduces the ability of GW4064 to induce SHP in primary hepatocytes (Fig. 9). In total, our current interpretation of these data can be summarized by the model shown in Fig. 11. In this model, TCA activates the insulin signaling pathway via Gαi-protein-coupled receptor(s). The activation of PI3K and downstream kinases help regulate glucose metabolism in a manner similar to insulin by repressing gluconeogenic genes and activating GS activity (18–20). The activation of the insulin signaling pathway and PKCζ appears to be crucial for the optimal induction of SHP by TCA. Currently, it is unclear how PKCζ regulates FXR and SHP induction. It has been reported that FXR might be phosphorylated by different isoforms of PKC (36, 45); however, we have not been able to confirm this observation by overexpression of the gene encoding FXR in primary rat hepatocytes (unpublished observations). Finally, it has recently been reported that activation of the ERK1/2 plays an important role in regulating the turnover rate of SHP protein (46). Therefore, the activation of ERK1/2 (Fig. 1) by TCA may help stabilize SHP protein. The induction of SHP protein may be involved in the long term (>3 h) repression of gluconeogenic genes.
Fig. 11.
Cell signaling model for the regulation of glucose metabolism in hepatocytes by taurocholate. TCA can rapidly activate Gαi-dependent G-protein coupled receptor(s) that activate the AKT (insulin signaling pathway). Activated AKT is known to downregulate gluconeogenic genes by phosphorylation of FOX01 and to activate glycogen synthase by phosphorylation of GSK3. PDK-1 in the insulin signaling pathway activates PKCζ, which increases the ability of TCA to activate the farnesoid X receptor (FXR) and increases the transcription of the gene encoding SHP. The induction of SHP protein may play a role in the long term (>3 h) repression of gluconeogenic genes. EGFR, epidermal growth factor receptor; GPCR, G-protein-coupled receptor; GSK3, glycogen synthase kinase-3; IR, insulin receptor; NTCP, Na+/taurocholate cotransporting polypeptide; PDK-1, phosphoinositide-dependent protein kinase-1; PI3K, phosphoinositide-3-kinase; PTX, pertussis toxin; Src, Src kinase; SHP, small heterodimeric partner; TCA, taurocholate.
There are many examples of cell signaling pathways and nuclear receptors interacting to produce a highly regulated physiological response (47). Therefore, the activation of cell signaling pathways and specific nuclear receptors by bile acids may be considered a coordinate physiological response to nutrients. Additional studies are needed to determine if different bile acids produce the same or different physiological responses during their enterohepatic circulation. Such studies may determine if the bile acid pool composition plays an important role in regulating the activation of cell signaling pathways and nuclear receptors in the liver and, hence, metabolism.
Supplementary Material
Footnotes
Abbreviations:
- CBF
- chronic bile fistula
- CYP8B1
- 12α-hydroxylase
- CYP7A1
- cholesterol 7α-hydroxylase
- HNF4α
- hepatocyte nuclear factor 4α
- FXR
- farnesoid X receptor
- G-6-Pase
- glucose-6-phosphatase
- GPCR
- G-protein-coupled receptor
- GS
- glycogen synthase
- PEPCK
- phosphoenolpyruvate carboxykinase
- PRH
- primary rat hepatocyte
- PTX
- pertussis toxin
- SHP
- short heterodimeric partner
- TCA
- taurocholate
This work was supported by National Institutes of Health Grants DK-057543 and DK-078233. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Hofmann A. F. 2007. Biliary secretion and excretion in health and disease: current concepts. Ann. Hepatol. 6: 15–27. [PubMed] [Google Scholar]
- 2.Scotti E., Gilardi F., Godio C., Gers E., Krneta J., Mitro N., De Fabiani E., Caruso D., Crestani M. 2007. Bile acids and their signaling pathways: eclectic regulators of diverse cellular functions. Cell. Mol. Life Sci. 64: 2477–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. 2008. Targeting bile acid signaling for metabolic diseases. Nat. Rev. Drug Discov. 7: 678–693. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen A., Bouscarel B. 2008. Bile acids and signal transduction: role in glucose homeostasis. Cell. Signal. 20: 2180–2197. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. 2009. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 89: 147–191. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y., Edwards P. A. 2008. FXR signaling in metabolic disease. FEBS Lett. 582: 10–18. [DOI] [PubMed] [Google Scholar]
- 7.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C. L., McDonald J. G., Luo G., Jones S. A., Goodwin B., Richardson J. A., et al. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2: 217–225. [DOI] [PubMed] [Google Scholar]
- 8.Rao A., Haywood J., Craddock A. L., Belinsky M. G., Kruh G. D., Dawson P. A. 2008. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc. Natl. Acad. Sci. USA. 105: 3891–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis R. A., Attie D. D. 2008. Deletion of the ileal basolateral bile acid transporter identifies the cellular sentinels that regulate the bile acid pool. Proc. Natl. Acad. Sci. USA. 105: 4965–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandak W. M., Heuman D. M., Hylemon P. B., Chiang J. Y. L., Vlahcevic Z. R. 1995. Failure of intravenous infusion of taurocholate to down-regulate cholesterol 7α-hydroxylase in rats with biliary fistulas. Gastroenterology. 108: 533–544. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S., Stravitz R. T., Dent P., Hylemon P. B. 2001. Down-regulation of cholesterol 7α-hydroxylase (CYP7A1) gene expression by bile acid in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J. Biol. Chem. 276: 15816–15822. [DOI] [PubMed] [Google Scholar]
- 12.Jahan A., Chiang J. Y. 2005. Cytokine regulation of human sterol 12-α-hydroxylase (CYP8B1) gene. Am. J. Physiol. Gastrointest. Liver Physiol. 288: G685–G695. [DOI] [PubMed] [Google Scholar]
- 13.Stravitz R. T., Rao Y. P., Vlahcevic Z. R., Gurley E. C., Jarvis W. D., Hylemon P. B. 1996. Hepatocellular protein kinase C activation by bile acids: implications for regulation of cholesterol 7 alpha-hydroxylase. Am. J. Physiol. 271: G293–G303. [DOI] [PubMed] [Google Scholar]
- 14.Rao Y. P., Stravitz R. T., Vlahcevic Z. R., Gurley E. C., Sando J. J., Hylemon P. B. 1997. Activation of protein kinase C alpha and delta by bile acids: correlation with bile acid structure and diacylglycerol formation. J. Lipid Res. 38: 2446–2454. [PubMed] [Google Scholar]
- 15.Qiao L., Studer E., Leach K., McKinstry R., Gupta S., Decker R., Kukreja R., Valerie K., Nagarkatti P., El Deiry W., et al. 2001. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen activated protein kinase signaling module enhances DCA-induced apoptosis. Mol. Biol. Cell. 12: 2629–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao Y. P., Studer E. J., Stravitz R. T., Gupta S., Qiao L., Dent P., Hylemon P. B. 2002. Activation of the Raf-1/MEK/ERK cascade by bile acids occurs via the epidermal growth factor receptor in primary rat hepatocytes. Hepatology. 35: 307–314. [DOI] [PubMed] [Google Scholar]
- 17.Han S. I., Studer E., Gupta S., Fang Y., Qiao L., Li W., Grant S., Hylemon P. B., Dent P. 2004. Bile acids enhance the activity of the insulin receptor and glycogen synthase in primary rodent hepatocytes. Hepatology. 39: 456–463. [DOI] [PubMed] [Google Scholar]
- 18.Fang Y., Han S. I., Mithcell C., Gupta S., Studer E., Grant S., Hylemon P. B., Dent P. 2004. Bile acids induce mitochondrial ROS which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology. 40: 961–971. [DOI] [PubMed] [Google Scholar]
- 19.Dent P., Fang Y., Gupta S., Studer E., Mitchell C., Spiegel S., Hylemon P. B. 2005. Conjugated bile acids promote ERK 1/2 and AKT activation via a pertussis toxin-sensitive mechanism in murine and human hepatocytes. Hepatology. 42: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 20.Fang Y., Studer E., Mitchell C., Grant S., Pandak W. M., Hylemon P. B., Dent P. 2007. Conjugated bile acids regulate hepatocyte glycogen synthase in vitro and in vivo via Gαi signaling. Mol. Pharmacol. 71: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 21.Rust C., Karnitz L. M., Paya C. V., Moscat J., Simari R. D., Gores G. J. 2000. The bile acid taurochenodeoxycholate activates phosphatidylinositol 3-kinase-dependent survival signaling cascade. J. Biol. Chem. 275: 20210–20216. [DOI] [PubMed] [Google Scholar]
- 22.Raufman J. P., Shant J., Guo C. Y., Roy S., Cheng K. 2008. Deoxycholyltaurine rescues human colon cancer cells from apoptosis by activating EGFR-dependent PI3K/Akt signaling. J. Cell. Physiol. 215: 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Fabiani E., Mitro N., Gilardi F., Caruso D., Galli G., Crestani M. 2003. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J. Biol. Chem. 278: 39124–39132. [DOI] [PubMed] [Google Scholar]
- 24.Yamagata K., Daitoku H., Shimamoto Y., Matsuzaki H., Hirota K., Ishida J., Fukamizu A. 2004. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J. Biol. Chem. 279: 23158–23165. [DOI] [PubMed] [Google Scholar]
- 25.Duran-Sandoval D., Cariou B., Percevault F., Hennuyer N., Grefhorst A., Van Dijk T. H., Gonzalez F. J., Fruchart J-C., Kuipers F., Staels B. 2005. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J. Biol. Chem. 280: 29971–29979. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., Willson T. M., Edwards P. S. 2006. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA. 103: 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma K., Saha P. K., Chan L., Moore D. D. 2006. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Invest. 116: 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park M. J., Kong H. J., Kim H. Y., Kim H. H., Kim J. H., Cheong J. H. 2007. Transcriptional repression of gluconeogenic gene PEPCK by the orphan nuclear receptor SHP through inhibitory interaction with C/EBP alpha. Biochem. J. 402: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heuman D. M., Hernandez C. R., Hylemon P. B., Kubaska W. M., Hartman C., Vlahcevic Z. R. 1988. Regulation of bile acid synthesis. I. Effects of conjugated ursodeoxycholate and cholate on bile acid synthesis in chronic bile fistula rat. Hepatology. 8: 358–365. [DOI] [PubMed] [Google Scholar]
- 30.Bissell D. M., Guzelian P. S. 1980. Degradation of endogenous hepatic heme by pathways not yielding carbon monoxide: studies in normal liver and primary hepatocyte culture. Ann. N. Y. Acad. Sci. 349: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandak W. M., Li Y. C., Chiang J. Y., Studer E. J., Gurley E. C., Heuman D. M., Vlahcevic Z. R., Hylemon P. B. 1991. Regulation of cholesterol 7α-hydroxylase mRNA and transcriptional activity by taurocholate and cholesterol in the chronic biliary diverted rat. J. Biol. Chem. 266: 3416–3421. [PubMed] [Google Scholar]
- 32.Stayrook K. R., Bramlett K. S., Savkur R. S., Ficorilli J., Cook T., Christe M. E., Michael L. F., Burris T. P. 2005. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 146: 984–991. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Z., Guo S., Copps K., Dong X., Kollipara R., Rodgers J. T., Depinho R. A., Puigserver P., White M. F. 2009. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat. Med. 15: 1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross D. N., Wan M., Birnbaum M. J. 2009. The role of FOXO in the regulation of metabolism. Curr. Diab. Rep. 9: 208–214. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi C. M., Emanuelli B., Kahn C. R. 2006. Critical nodes in signaling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7: 85–96. [DOI] [PubMed] [Google Scholar]
- 36.Frankenberg T., Miloh T., Chen F. Y., Ananthanarayanan M., Sun A. Q., Balasubramaniyan N., Aries I., Setchell K. D., Suchy F. J., Shneider B. L. 2008. The membrane protein ATPase class I type 8B member 1 signals through protein kinase C zeta to activate the farnesoid X receptor. Hepatology. 48: 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maloney P. R., Parks D. J., Haffner C. D., Fivush A. M., Chandra G., Plunket K. D., Creech K. L., Moore L. B., Wilson J. G., Lewis M. C., et al. 2000. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 43: 2971–2974. [DOI] [PubMed] [Google Scholar]
- 38.Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. 1999. Identification of a nuclear receptor for bile acids. Science. 284: 1362–1365. [DOI] [PubMed] [Google Scholar]
- 39.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Chen J., Hollister K., Sowers C., Forman B. M. 1999. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 3: 543–553. [DOI] [PubMed] [Google Scholar]
- 41.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., et al. 2003. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 278: 9435–9440. [DOI] [PubMed] [Google Scholar]
- 42.Keitel V., Donner M., Winandy S., Kubitz R., Häussinger D. 2008. Expression and function of the bile acid receptor TGR5 in Kuffer cells. Biochem. Biophys. Res. Commun. 372: 78–84. [DOI] [PubMed] [Google Scholar]
- 43.Nakae J., Oki M., Cao Y. 2008. The FoxO transcription factors and metabolic regulation. FEBS Lett. 582: 54–67. [DOI] [PubMed] [Google Scholar]
- 44.Hirai T., Chida K. 2003. Protein kinase C zeta (PKCzeta): activation mechanisms and cellular functions. J. Biochem. 133: 1–7. [DOI] [PubMed] [Google Scholar]
- 45.Gineste R., Sirvent A., Paumelle R., Helleboid S., Aquilina A., Darteil R., Hum D. W., Fruchart J-C., Staels B. 2008. Phosphorylation of farnesoid X receptor by protein kinase C promotes transcriptional activity. Mol. Endocrinol. 22: 2433–2447. [DOI] [PubMed] [Google Scholar]
- 46.Miao J., Xiao Z., Kanamaluru D., Min G., Yau P. M., Veenstra T. D., Ellis E., Strom S., Suino-Powell K., Xu H. E., et al. 2009. Bile acid signaling pathways increase stability of small heterodimer partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 23: 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weigel N. L., Moore N. L. 2007. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol. Endocrinol. 21: 2311–2319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.