Abstract
The endocannabinoid system has recently been attracted interest for its anti-inflammatory and anti-oxidative properties. In this study, we investigated the role of the endocannabinoid system in regulating the oxidized low-density lipoprotein (oxLDL)-induced inflammatory response in macrophages. RAW264.7 mouse macrophages and peritoneal macrophages isolated from Sprague-Dawley (SD) rats were exposed to oxLDL with or without the synthetic cannabinoid WIN55,212-2. To assess the inflammatory response, reactive oxygen species (ROS) and tumor necrosis factor alpha (TNF- α) levels were determined, and activation of the mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-kappa B signaling pathways were assessed. We observed that: i) oxLDL strongly induced ROS generation and TNF- α secretion in murine macrophages; ii) oxLDL-induced TNF- α and ROS levels could be lowered considerably by WIN55,212-2 via inhibition of MAPK (ERK1/2) signaling and NF-kappa B activity; and iii) the effects of WIN55212-2 were attenuated by the selective CB2 receptor antagonist AM630. These results demonstrate the involvement of the endocannabinoid system in regulating the oxLDL-induced inflammatory response in macrophages, and indicate that the CB2 receptor may offer a novel pharmaceutical target for treating atherosclerosis.
Keywords: atherosclerosis, TNF-α, ROS, inflammatory response, CB2 receptor, oxLDL
Atherosclerosis is no longer considered simply a lipid metabolism disorder, but rather, a subacute inflammatory condition of the vascular system. Although altered lipids levels play a role, they probably only represent part of the overall problem.
Oxidation of low-density lipoproteins increases their atherogenicity (1). Accordingly, elevated plasma levels of oxidized low-density lipoprotein (oxLDL) are associated with coronary artery disease (2). There is accumulating evidence that oxLDL binds the lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), thereby stimulating endothelial cells to produce adhesion molecules and recruit leukocytes into the subintimal space. Concomitant pathological effects include an imbalance between NO generation and oxidative stress, endothelial dysfunction, proinflammatory changes to the vessel wall, foam cell formation, apoptosis of smooth muscle cells, and unstable and rupture-prone lesions (3).
The endocannabinoid system has recently attracted interest for its anti-inflammatory and anti-oxidative effects in a number of chronic inflammatory diseases and disorders linked with organ ischemia/reperfusion (I/R) injury. Oxidative stimuli that appear to activate the endocannabinoid system include oxLDL (4), lipopolysaccharide (LPS) (5), hydrogen peroxide, and tumor necrosis factor-α (TNF-α) (6). Further, studies have reported that activation of the CB2 cannabinoid receptor protects against myocardial (7), hepatic (6), and cerebral (8) I/R injury due to its anti-inflammatory and anti-oxidative properties. Specifically, Defer et al. (9) reported that CB2 activation promotes cardiac myocyte and fibroblast survival and protects against I/R injury-induced cardiomyopathy. In contrast, CB1 cannabinoid receptor activation activates p38 mitogen-activated protein kinase (MAPK) signaling, thereby mediating reactive oxygen species (ROS) production and the synthesis of TNF-α and monocyte chemoattractant protein-1 (MCP-1), which are negatively regulated by CB2 via Rap1 (10).
Important cross talk has been revealed between inflammation, generation of reactive oxygen and nitrogen species, and lipid metabolism in the pathogenesis of atherosclerosis. A recent study by Bátkai (11) demonstrated that the endocannabinoid neurotransmitter anandamide (AEA) dose-dependently attenuated TNF-α-induced intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 expression, nuclear factor (NF)-kappa B activation in human coronary artery endothelial cells (HCAEC), and the adhesion of monocytes to HCAECs in a CB1- and CB2-dependent manner. It was also shown that the inhibition of the endocannabinoid AEA metabolizing enzyme, the fatty acid amide hydrolase (FAAH) by gene knock-out method, decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice. In our most recent study, we reported activation of the endocannabinoid system by oxLDL in macrophages; the activated endocannabinoid system promotes cellular cholesterol accumulation in macrophages by upregulating CD36 receptor expression and downregulating ATP-binding cassette transporter (ABCA1) expression (4). In the present study, we hypothesized that the endocannabinoid system is directly involved in regulating oxLDL-induced inflammation and oxidative stress in macrophages.
MATERIALS AND METHODS
Materials
The protocol involved animals was approved by the Experimental Animal Ethics Committee in Shanghai Jiaotong University School of Medicine. Sprague-Dawley (SD) rats were purchased from the Chinese Academy of Sciences, Shanghai Laboratory Animal Center. All cell culture reagents, including FBS, were purchased from Gibco Life Technologies (USA), and culture materials were purchased from Corning Life Science (USA). MEK1 inhibitor PD98059 and MEK 1/2 signaling pathway inhibitor U0126 were obtained from Merck (USA), and WIN55,212-2 (synthetic nonselective CB1/CB2 agonist), AM251 (selective CB1 antagonist), and AM630 (selective CB2 antagonist) were purchased from Tocris (USA). The RevertaidTM First Strand cDNA Synthesis Kit was from Fermentas International Inc. (Canada), and the SYBR® Premix Ex TaqTM (Perfect Real Time) kit from Takara Inc. (Japan). The mouse and rat TNF-α /TNFSF1A kits were purchased from R and D Systems Inc. (USA). The NE-PER Nuclear and Cytoplasmic Extraction Reagents and the LightShift EMSA kit were obtained from Pierce (USA). The ECL Western Blotting Detection Kit was purchased from Amersham Pharmacia Biotech (Germany). The following antibodies were used: rabbit anti-phospho-p44/42 MAPK (ERK1/2) and rabbit anti total-p44/42 MAPK (ERK1/2) (Cell Signaling Technology; MA); rabbit polyclonal anti-GAPDH antibody (Proteinech Group; USA); and donkey anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The ROS assay kit was purchased from Sigma (St. Louis, MO), and the BCA Protein Assay Kit was from Beyotime Institute of Biotechnology (China).
Isolation and oxidative modification of LDL
LDL was isolated from human endotoxin-free heparin plasma by sequential ultracentrifugation (density range, 1.019–1.063 g × ml−1), and dialyzed against PBS at 4°C. LDL protein concentration was determined by a modification of the Lowry method using bovine albumin as the standard. LDL was oxidized with CuSO4 and stored at 4°C as described previously (12).
Cell culture
Peritoneal macrophages were isolated from SD rats (250–350g) and cultured with DMEM with 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FBS under standard conditions as previously described (13). The mouse RAW264.7 macrophage line (ATCC; Manassas, VA) was cultured in the same culture medium. Before stimulation, nonadherent cells were removed by washing twice with DMEM, and then incubated with oxLDL, WIN55,212-2, AM251, AM630, PD98059, or U0126 alone or in combination. The treated cells were subsequently harvested for the following procedures.
Flow cytometry for measurement of ROS
ROS generation in RAW264.7 macrophages and peritoneal macrophages isolated from SD rats was determined with the ROS assay kit and by flow cytometry monitoring the oxidation of 2′,7′-dichlorodihydrofluorescein (DCFH). After a 30-min pretreatment with WIN55,212-2, PD98059, U0126, AM251, and/or AM630, cells were treated with oxLDL for 1 h. Cells were then loaded with 5 μM DCFH-diacetate for 30 min in the dark, and then resuspended in PBS for flow cytometry. DCF fluorescence was read at 488-nm excitation and 525-nm emission.
ELISA analysis of TNF-α protein levels
TNF-α levels in the cell culture media were determined by mouse and rat TNF-α/TNFSF1A kits according to the manufacturer's instructions. Concentrations were expressed as pg/ml. Three separate experiments were conducted with duplicate samples. Sensitivity for the TNF-α enzyme immunoassays was 5.1 pg/ml, and intra-assay and inter-assay coefficients of variation were less than 10%.
Determination of TNF-α by quantitative real-time PCR
Macrophage TNF-α and glyceraldehydes phosphate dehydrogenase (GAPDH) mRNA levels were determined using the SYBR® Premix Ex TaqTM (Perfect Real Time) kit and an ABI7900 analyzer (Applied Biosystems Inc., CA). The following primers were used: mouse TNF-α sense: 5′-CCT CCC TCT CAT CAG TTC TA-3′ and antisense: 5′-ACT TGG TGG TTT GCT ACG AC-3′; rat TNF-α sense: 5′-TTG CTT CTT CCC TGT TCC-3′ and antisense: 5′-CTG GGC AGC GTT TAT TCT-3′; GAPDH sense: 5′-GTG AAG GTC GGA GTC AAC G-3′ and antisense: 5′-TGA GGT CAA TGA AGG GGT C-3′. Total RNA (2 μg) was first reverse-transcribed to cDNA with the RevertaidTM First Stand cDNA Synthesis Kit. Real-time polymerase chain reaction (PCR) was performed as follows: preliminary denaturation at 95°C for 10 s, followed by 40 cycles of 95°C for 5 s, and 60°C for 30 s. Relative expression was determined with the ΔΔCt method using GAPDH as the internal control.
Western blot analysis
RAW264.7 macrophages and peritoneal macrophages isolated from SD rats were lysed in 100 μl RIPA buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 10 mM EDTA, 1% Tergitol-type NP-40, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethanesulphonylfluoride (PMSF), 10 μg/ml aprotinin, and 2 mM NaF. Insoluble materials were removed by centrifugation at 12,000 rpm at 4°C for 20 min, and then protein concentration was determined using the Enhanced BCA Protein Assay Kit. After boiling for 5 min with 5× Laemmli sample buffer, equal protein amounts (20 μg) were resolved by 10% SDS-polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose membranes at 4°C. After blocking for 1 h with 5% nonfat dry milk in TBS containing 0.1% Tween 20, membranes were incubated overnight at 4°C with primary antibodies (dilution, 1:2000 for p-ERK1/2, t-ERK1/2, and GAPDH), and then incubated with secondary antibodies and visualized by chemiluminescence using an ECL Western Blotting Detection Kit. Finally, densitometric analysis of the immunoblots was performed with Quantity One 4.4.0 software (Bio-Rad, Hercules, CA).
EMSA to detect NF-kappa B activity
Nuclear extract preparation and conditions for the electrophoretic mobility shift assay (EMSA) reactions were described previously (14). The 22-mer synthetic double-stranded oligonucleotides used as NF-kappa B probes in the gel shift assay were 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ and 3′-TCA ACT CCC CTG AAA GGG TCC G - 5′.
Statistical methods
One-way ANOVA was followed by the Student-Newman-Keuls posthoc test to compare means between different groups. Continuous data were expressed as mean ± standard error of the mean (SEM). P < 0.05 (two-tailed) was accepted as statistically significant.
RESULTS
WIN55,212-2 decreases oxLDL-induced ROS generation in macrophages
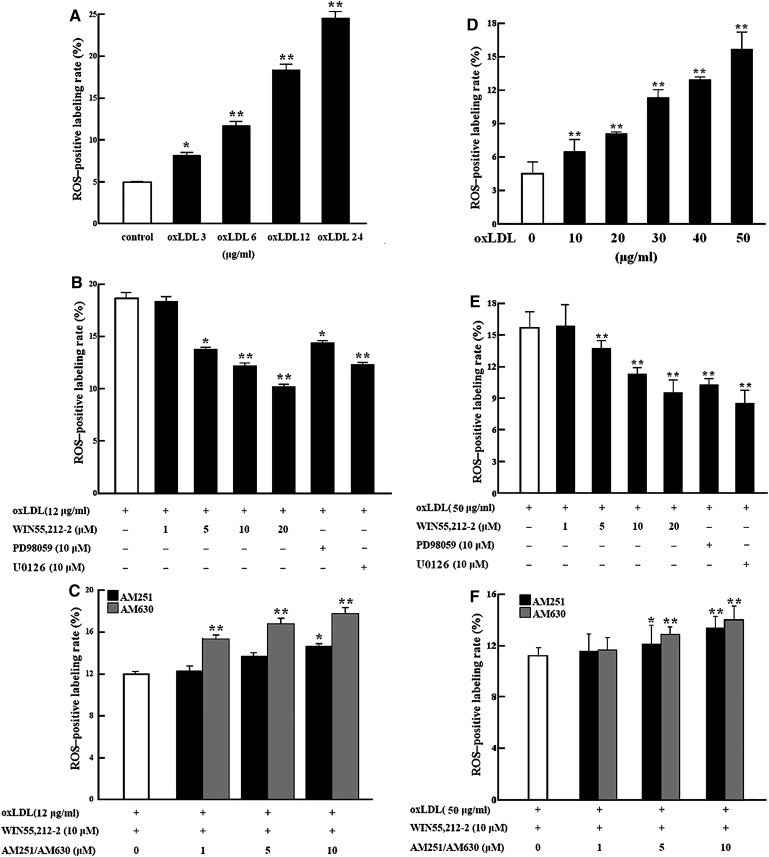
After oxLDL treatment with or without receptor agonist/antagonists or signaling pathway inhibitors, intracellular ROS was determined in macrophages by flow cytometry using the nonfluorescent probe DCFH-diacetate, which is converted to highly fluorescent dichlorofluorescein (DCF) by ROS. Exposure of RAW264.7 macrophages to oxLDL for 1 h led to a dose-dependent increase in ROS production, with mean increase in DCF-positive cells of 2.9%, 6.4%, 13.3%, and 19.8% at concentrations of 3, 6, 12, and 24 μg/ml oxLDL, respectively (Fig. 1A). Pretreatment with WIN55,212-2 (1–20 μM) attenuated the ROS generation induced by 12 μg/ml of oxLDL in a dose- dependent manner (Fig. 1B). However, the effect of WIN55,212-2 (10 μM) was blocked by AM251 or AM630 pretreatment, with AM630 demonstrating a stronger effect than AM251 (Fig. 1C). Interestingly, increased intracellular ROS by oxLDL treatment (12 μg/ml) was also significantly decreased by coincubation with PD98059 or U0126, two inhibitors of the MEK signaling pathway (Fig. 1B). A similar phenomenon was also observed in peritoneal macrophages isolated from SD rats (Fig. 1D–F), although higher doses of oxLDL (10–50 μg/ml) were required to achieve similar intracellular ROS generation in these cells.
Fig. 1.
Regulation of ROS generation in cultured murine macrophages. To detect ROS generation, 106 cells were treated for 1 h in 6-well plates, loaded with the DCFH probe, and incubated for 30 min; then flow cytometry analysis was performed by monitoring DCF fluorescence. Mean increase in ROS-positive cells is shown for three separate experiments with duplicate samples. A–C: RAW264.7 macrophages. D–F: Primary rat peritoneal macrophages. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01. DCF, dichlorofluorescein; DCFH, 2′,7′-dichlorodihydrofluorescein; ROS, reactive oxygen species.
WIN55,212-2 regulation of TNF-α in macrophages
Regulation of TNF-alpha protein release.
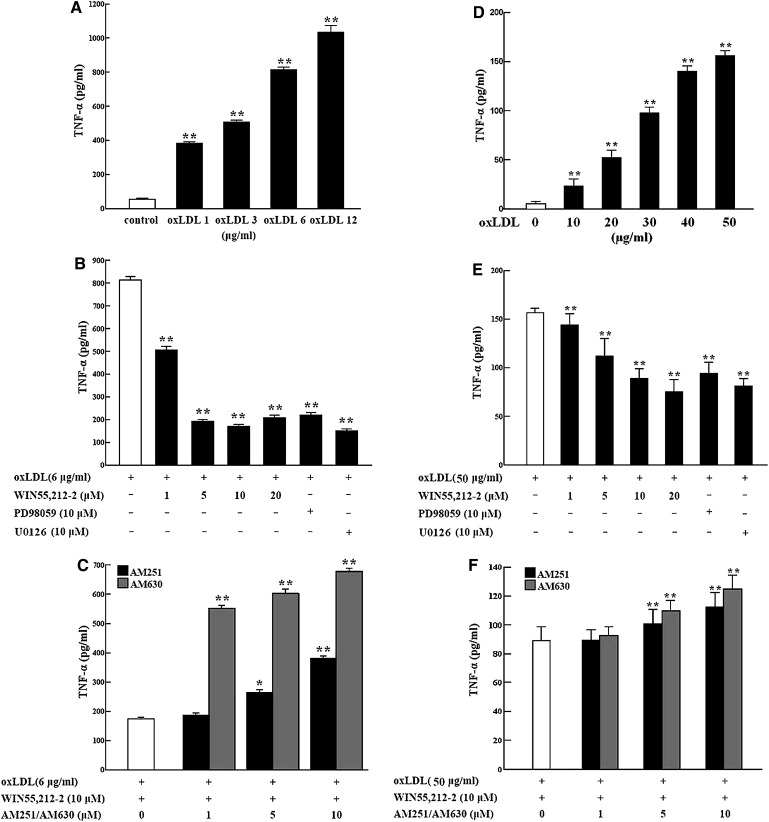
After 24-h oxLDL treatment (1–12 μg/ml) of RAW264.7 macrophages, ELISA analysis showed that TNF-α protein levels were dose-dependently increased in the cell culture media (Fig. 2A). However, the increased TNF-α secretion induced by 6 μg/ml of oxLDL was markedly decreased by cotreatment with WIN55,212-2 (1–20 μM); a sharp decrease was attained above 5 μM WIN55,212-2, with a maximum effect at 10 μM (Fig. 2B). In cells treated with 6 μg/ml oxLDL, the effect of WIN55,212-2 (10 μM) in decreasing TNF-α protein secretion was partially blocked by AM251 or AM630 pretreatment, with AM630 demonstrating a stronger effect than AM251 (Fig. 2C). As observed in the ROS assay, the increased TNF-α protein levels produced by 6 μg/ml of oxLDL were also significantly attenuated by coincubation with PD98059 and U0126 (Fig. 2B). A similar phenomenon was observed in SD rat peritoneal macrophages incubated with oxLDL (10–50 μg/ml) (Fig. 2D–F).
Fig. 2.
Regulation of TNF-α release from murine macrophages. A total of 106 cells in 6-well plates were treated for 24 h with oxLDL alone or combined with WIN55,212-2, PD98059, U0126, AM251, and/or AM630. TNF-α levels (pg/ml) in the cell culture media were determined by ELISA from three separate experiments with duplicate samples A–C: RAW264.7 macrophages. D–F: primary rat peritoneal macrophages). Data are shown as mean ± SEM. *P < 0.05; **P < 0.01. AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; AM630, 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl) methanone; WIN55,212-2, (R)-(+)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmeth anone mesylate; oxLDL, oxidized low-density lipoprotein; PD98059, 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one; TNF-α, tumor necrosis factor-α; U0126, 1,4-diamino-2,3-dicyano-1,4-bis (o-aminophenylmercapto) butadiene monoethanolate.
Regulation of TNF-alpha mRNA expression.
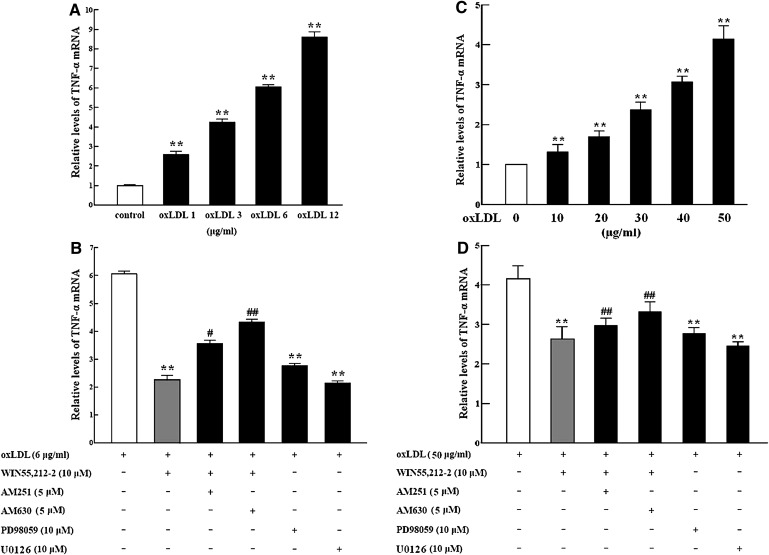
TNF-α mRNA expression showed the same trend as protein expression, as determined by real-time RT-PCR analysis, (Fig. 3A–D). TNF-α mRNA levels increased with oxLDL exposure in cultured RAW264.7 murine macrophages and primary rat peritoneal macrophages (Fig. 3A, C), but the oxLDL-induced increase was significantly attenuated by WIN55,212-2 (10 μM). The effect of WIN55,212-2 was blocked by AM251 or AM630, with AM630 demonstrating a stronger effect than AM251 (Fig. 3B, D). Similarly, oxLDL-induced TNF-α mRNA expression was also decreased by PD98059 and U0126 (Fig. 3B, D).
Fig. 3.
Regulation of TNF-α mRNA expression in murine macrophages. Cells were incubated for 24 h with oxLDL alone or combined with WIN55,212-2, AM251, AM630, PD98059, and/or U0126. TNF-α mRNA levels were determined by real-time PCR analysis. Data are expressed as relative expression compared with control from three separate experiments with duplicate samples. A and B: RAW264.7 macrophages. C and D: primary rat peritoneal macrophages. **P < 0.01 compared with vehicle control (represented by a white bar); #P < 0.05 and ##P < 0.01 compared with cells cotreated with WIN55,212-2 and oxLDL (represented by a gray bar). AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; AM630, 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol- 3-yl](4-methoxyphenyl) methanone; WIN55,212-2, (R)-(+)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo[1,2,3-de]-1,4-benzoxazin- 6-yl]-1-naphthalenylmeth anone mesylate; oxLDL, oxidized low-density lipoprotein; PD98059, 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran- 4-one; TNF-α, tumor necrosis factor-α; U0126, 1,4-diamino-2,3-dicyano-1,4-bis (o-aminophenylmercapto) butadiene monoethanolate.
Mechanism of WIN55,212-2 regulation of TNF-alpha and ROS in murine macrophages
WIN55,212-2 inhibits NF-kappa B DNA-binding activity.
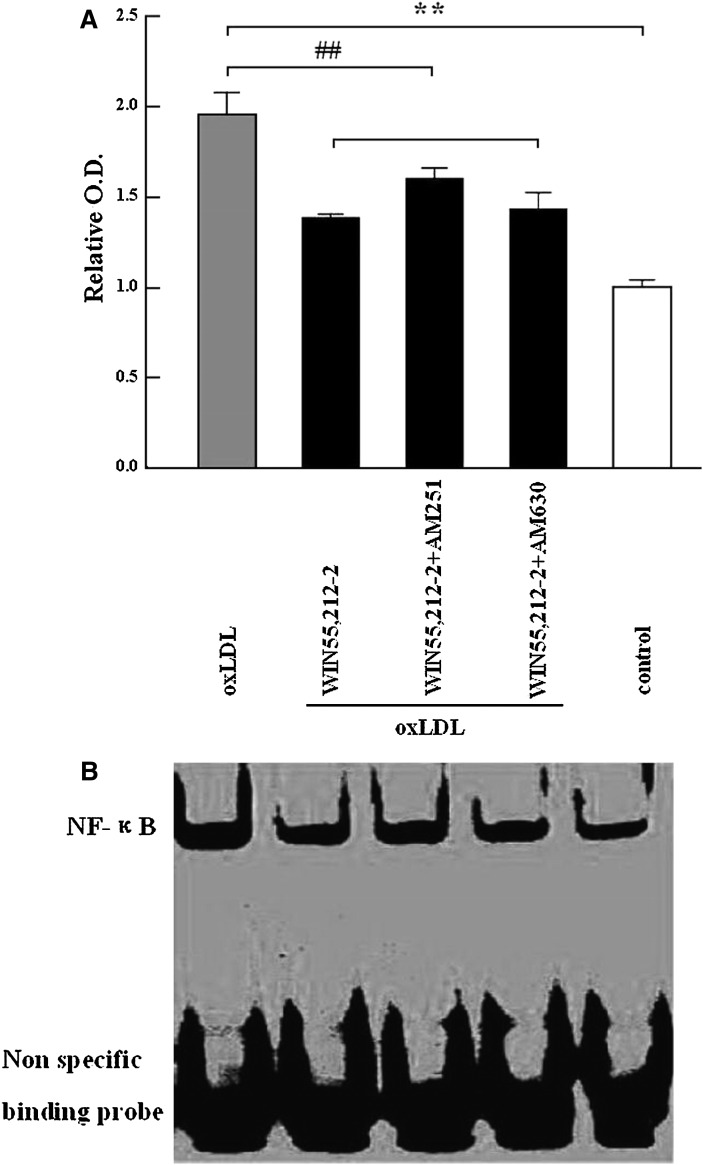
NF-kappa B DNA-binding activity in RAW264.7 macrophages was determined by EMSA. The results showed that 24-h incubation with oxLDL (6 μg) increased NF-kappa B DNA-binding activity approximately 1.9-fold (P < 0.01) compared with cells cultured in DMEM alone. However, coincubation with 5 μM WIN55,212-2 resulted in a 30% decrease in NF-kappa B activity (P < 0.01). AM251 (5 μM) or AM630 (5 μM) blocked the effects of WIN55,212-2; however, this was not statistically significant (Fig. 4A, B).
Fig. 4.
Effects of the cannabinoid WIN55,212-2 on oxLDL-induced NF-kappa B DNA-binding activity in RAW264.7 macrophages. After treatment with oxLDL (6 μg/ml) alone or in combination with WIN55,212-2 (10 μM) and AM251(5 μM) or AM630 (5 μM) for 24 h, 8 μg of nuclear extract was prepared and subjected to EMSA analysis. Data in Fig. 4A are expressed as mean ± SEM of the relative optical density from three separate experiments with duplicate samples. In Fig. 4B, the upper bands reflect NF-kappa B bound to DNA and the lower bands show nonspecific probe. **P < 0.01 compared with vehicle (DMEM) control (represented by a white bar); ##P < 0.01 compared with oxLDL treatment (represented by a gray bar). AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; AM630, 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl) methanone; oxLDL, oxidized low-density lipoprotein; WIN55,212-2, (R)-(+)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmeth anone mesylate.
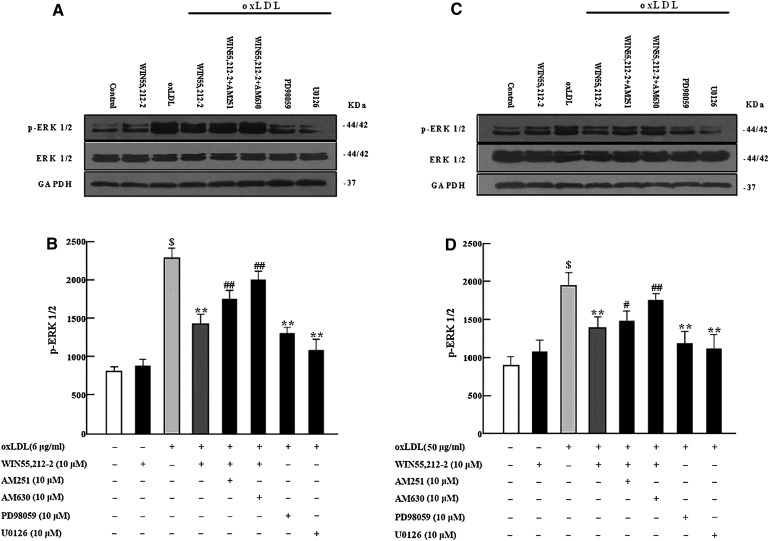
WIN55,212-2 decreased oxLDL-induced phosphorylation of ERK1/2.
Western blot analysis was performed to determine the role of the MAPK (ERK1/2) pathway in WIN55,212-2 regulation of TNF-α and ROS in murine macrophages (Fig. 5A–D). A 10-min incubation with oxLDL led to a high level of ERK1/2 phosphorylation compared with the control cultured in DMEM alone; however, pretreatment with WIN55,212-2 (10 μM) significantly decreased oxLDL-induced ERK1/2 activation (P < 0.01). The WIN55,212-2-mediated decrease in ERK1/2 activation was partly blocked by AM251 (10 μM) or AM630 (10 μM), with AM630 demonstrating a greater effect than AM251. A 30-min pretreatment with PD98059 (10 μM) or U0126 (10 μM) appeared to inhibit oxLDL-induced ERK1/2 phosphorylation.
Fig. 5.
Effects of the cannabinoid WIN55,212-2 on oxLDL-induced ERK1/2 activation in cultured murine macrophages. After treatment with oxLDL alone or combined with WIN55,212-2 (10 μM), AM251 (10 μM), AM630 (10 μM), PD98059 (10 μM), or U0126 (10 μM) for 10 min, 20 μg of protein was prepared and p-ERK1/2 was detected by Western blot analysis with anti-phospho-ERK1/2. Incubation with oxLDL significantly increased ERK1/2 activation compared with vehicle control ($P < 0.01). ERK1/2 activation was inhibited by coincubation with WIN55,212-2, PD98059, or U0126 compared with vehicle control (**P < 0.01); effects of WIN55,212-2 were markedly attenuated by AM251 or AM630 (#P < 0.05##P < 0.01). Data are expressed as relative optical density compared with GAPDH from three separate experiments with duplicate samples. A and B: RAW264.7 macrophages. C and D: primary peritoneal macrophages. AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; AM630, 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H- indol-3-yl](4-methoxyphenyl) methanone; GAPDH, glyceraldehydes phosphate dehydrogenase; oxLDL, oxidized low-density lipoprotein; PD98059, 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one; ROS, reactive oxygen species; U0126, 1,4-diamino-2,3-dicyano-1,4-bis (o-aminophenylmercapto) butadiene monoethanolate; WIN55,212-2, (R)-(+)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmeth anone mesylate.
DISCUSSION
There were several major findings in this study: (i) oxLDL-induced TNF-α expression and ROS generation in RAW264.7 mouse macrophages and in rat peritoneal macrophages were considerably reduced by the synthetic cannabinoid WIN55,212-2; (ii) WIN55,212-2 reduced TNF-α expression and ROS production, mainly via the CB2 receptor, and partially linked with the CB1 receptor; (iii) MAPK and NF-kappa B were inhibited by WIN55,212-2-activated CB1/CB2 receptor signaling, suggesting their involvement in the attenuation of TNF-α expression and ROS generation.
Atherosclerosis is no longer considered simply a lipid metabolism disorder, but rather a subacute inflammatory condition of the vascular system. The initiating event in atherosclerosis remains unclear, but it is known that activation of the acute phase response can result in a shift to a more oxidizing/inflammatory lipid profile, which may play a crucial role in atherosclerosis initiation and progression. The binding of oxLDL to the LOX-1 receptor leads to endothelial injury and monocyte recruitment as well as other pathological effects (3). Macrophages in the subintimal space engulf oxLDL through the scavenger receptor pathways and form foam cells, which are prone to apoptosis. Foam cells also produce excessive ROS (15), and a variety of proinflammatory cytokines, including TNF-α and interleukin (IL)-1β (12, 16), accelerate the vicious circle of oxidation and inflammation (17, 18). Eventually, the oxidizing/inflammatory process promotes lesion progression and results in unstable and rupture-prone lesions.
Cannabinoids have been used therapeutically for the past several millennia (19), but it is only in the last few decades that the biological basis for their effects has begun to be studied. Owing to enormous differences in outcomes according to dosage, type of endocannabinoid, and cell type, only some of the mechanisms have been elucidated. Based on the affirmative evidence, endocannabinoids are ubiquitous lipid signaling molecules that exert a number of central and peripheral actions mediated primarily by the specific receptors CB1 and CB2. CB1 receptors are expressed mainly in the brain but also in some peripheral tissues (20), and CB2 receptors have been detected mainly in immune cells (21). Studies have reported that increased endocannabinoids and activated CB1 receptors in peripheral tissues are closely linked with multiple cardiometabolic risk factors, including obesity and increased serum lipid production in rodents and humans (22, 23), and as well as cellular cholesterol accumulation in macrophages in vitro (4). Selective CB1 receptor antagonists, such as rimonabant, have been implicated to reduce body weight and waist circumference, reduce triglyceride, and increase high density lipoprotein cholesterol (24, 25). Controversially, they have been withdrawn from the market because of the increased risk of depression by acting on the cerebral CB1 receptor. The CB2 receptor, which, unlike the CB1 receptor, does not induce major side effects, has been shown to be closely linked with immunoregulation and therefore shows potential for treating chronic inflammatory diseases, such as multiple sclerosis (26), colonic inflammation (27), and atherosclerosis (28).
The endocannabinoid system has recently attracted wide interest for its anti-inflammatory and anti-oxidative properties. Accumulating evidence indicates that synthetic or endogenous cannabinoids protect against central nervous system injury by factors including ischemic/reperfusion (29, 30) and hyperglycemia (31). Although the exact mechanisms of these neuroprotective effects are not completely understood, numerous CB receptor-dependent as well as receptor-independent processes have been suggested, of which the CB2 receptor-mediated anti-inflammatory and anti-oxidative activities may be the most important (32).
In a hepatic I/R injury animal model, the endocannabinoids AEA and 2-arachidonoylglycerol (2-AG) increased several-fold in the liver and positively correlated with the degree of hepatic damage and the serum levels of TNF-α, macrophage inflammatory protein (MIP)-1α, and MIP-2. Furthermore, a brief exposure of hepatocytes to various oxidants (hydrogen peroxide, peroxynitrite) or inflammatory stimuli (endotoxin, TNF-α) also increases endocannabinoid levels. Interestingly, the selective CB2 cannabinoid receptor agonist JWH133 protects against I/R damage by attenuating inflammatory cell infiltration, tissue and serum TNF-α, MIP-1α and MIP-2 levels, tissue lipid peroxidation, and expression of adhesion molecule ICAM-1 in vivo. Accordingly, I/R-induced hepatic damage and inflammation are increased in CB2−/− mice (6). In addition, low doses of tetrahydrocannabinol (THC), the major psychoactive cannabinoid compound of marijuana, appear to inhibit the progression of established atherosclerotic lesions. This therapeutic effect is associated with reduced proliferation and interferon (IFN)-γ secretion by lymphoid cells as well as reduced macrophage infiltration into atherosclerotic lesions (28). These antiatherosclerotic effects of THC appear to be concentration-dependent.
Di Filippo et al. (7) demonstrated that treatment with the synthetic nonselective CB1/CB2 receptor agonist WIN55,212-2 significantly reduced the extent of myocardial infarct in mice suffering from myocardial I/R injury; cardioprotection was accompanied by lower myeloperoxidase activity and IL-1β and CXC chemokine ligand levels in the injured tissue. This cardioprotective effect was almost abolished by the selective CB2 antagonist AM630, but it was not affected by the selective CB1 antagonist AM251. In addition, JWH133 reduced myocardial infarct size in mice with I/R injury, and the cardioprotective effect was linked with reduced oxidative stress and neutrophil infiltration in the infarcted myocardium (33). Taken as a whole, these findings indicate that endocannabinoids exert cardioprotective effects via the CB2 receptor (34).
In our previous study, we demonstrated that the endocannabinoid system is activated by oxLDL in macrophages. In addition, we observed that WIN55,212-2 promoted cellular cholesterol accumulation in macrophages, which was associated with increased CD36 expression and decreased ABCA1 expression via peroxisome proliferator-activated receptor γ (PPARγ) (4). To test the hypothesis that the endocannabinoid system is involved in regulating oxLDL-induced inflammation and oxidative stress, we determined the effect of WIN55,212-2 on oxLDL-induced TNF-α expression and ROS generation in RAW264.7 macrophages and in peritoneal macrophages isolated from rats. We observed that elevated TNF-α and ROS were dose-dependently attenuated by WIN55,212-2. Our results were consistent with previous studies: WIN55,212-2 decreased IL-1 β-induced TNF-α release in human astrocytes (35) and lipopolysaccharide (LPS)-stimulated TNF-α production in human mononuclear cells (36) in vitro. Additionally, there is increasing evidence that WIN55,212-2 reduces ROS formation in cortical neuron cultures treated with FeCl2 (37) and protects against I/R damage by decreasing inflammation and oxidative stress (6, 7, 32–34). Thus, the endocannabinoid system appears to play a role in the pathophysiology of atherosclerosis by attenuating oxLDL-induced inflammation and oxidative stress in macrophages.
Previous studies have reported that oxLDL activates MAPK signaling to stimulate inflammation and oxidative stress in macrophages via LOX-1 (3, 38). We therefore hypothesized that MAPK (ERK1/2) signaling may be involved in the attenuation of oxLDL-induced ROS generation and TNF-α expression by cannabinoids. We found that WIN55,212-2 inhibited oxLDL-mediated ERK1/2 phosphorylation in RAW264.7 macrophages and in peritoneal macrophages isolated from rats. Further, the MEK inhibitors PD98059 and U0126 suppressed oxLDL-induced TNF-α expression and ROS formation in cultured macrophages, which supports the requirement of ERK1/2 in oxLDL-induced inflammation and oxidative stress. Thus, the MAPK signaling pathway may be involved the WIN55,212-2 regulation of TNF-α expression and ROS formation.
Nuclear factor NF-kappa B also participates in the regulation of inflammatory response, apoptosis, and oxidative stress (39, 40). In cultured cells, NF-kappa B is activated by a variety of proatherogenic factors, such as inflammatory cytokines, oxLDL, and oxidative stress (40–42). In the present study, we found that WIN55,212-2 significantly reduced oxLDL-induced NF-kappa B activation. Consistent with our findings, recent studies report that β-caryophyllene, a natural CB2 agonist, inhibits LPS-induced proinflammatory cytokine expression in peripheral blood and attenuates LPS-stimulated ERK1/2 and JNK1/2 phosphorylation in monocytes (43). Also, β-caryophyllene induces a switch from a TH1 to a TH2 immune response by inhibiting the pathway triggered by activation of the toll-like receptor complex CD14/TLR4/MD2 (44). Many studies have focused on the role of the CB2 receptor in the anti-inflammatory and anti-oxidative effects of WIN55,212-2 (6, 7, 32–34). In the present study, we observed that the selective CB2 receptor antagonist AM630 blocked the effects of WIN55,212-2 on oxLDL-induced TNF-α expression, ROS generation, and ERK1/2 phosphorylation. These results support an important role for the CB2 receptor in the anti-inflammation and anti-oxidation actions of WIN55,212-2. However, AM251 or AM630 did not exert strong effects on the ability of WIN55,212-2 to reduce NF-kappa B activity, which suggests a mechanism other than CB1/CB2 receptors in the regulation of inflammation and oxidative stress by endocannabinoids.
CONCLUSION
We demonstrated for the first time that the cannabinoid WIN55,212-2 protects against oxLDL-induced inflammation and oxidative stress in murine macrophages via the CB2 receptor, which suggests that the CB2 receptor might be a promising pharmacological target for atherosclerosis therapy. However, the involvement of other receptors and mechanisms cannot be ruled out at this stage.
The present study should be interpreted within the context of its limitation. WIN55,212-2 is a nonselective cannabinoid receptor agonist; therefore, further studies are needed using a more specific CB2 receptor agonist, such as HU308 or JWH-133, to verify our results.
Supplementary Material
Acknowledgments
The authors thank Li-ming Wang from the central laboratory in Renji Hospital for his excellent technical assistance.
Footnotes
Abbreviations:
- ABCA1
- ATP-binding cassette transporter
- AEA
- anandamide
- 2-AG
- 2-arachidonoylglycerol
- AM251
- N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- AM630
- 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl) methanone
- DCF
- dichlorofluorescein
- DCFH
- 2′,7′-dichlorodihydrofluorescein
- ERK
- extracellular signal-regulated kinase
- FAAH
- fatty acid amide hydrolase
- GAPDH
- glyceraldehydes phosphate dehydrogenase
- I/R
- ischemia/reperfusion
- ICAM
- intercellular adhesion molecule
- LDL
- low-density lipoprotein
- LOX-1
- lectin-like oxidized low-density lipoprotein receptor-1
- LPS
- lipopolysaccharide
- MAPK
- mitogen-activated protein kinase
- MCP-1
- monocyte chemoattractant protein-1
- NF
- nuclear factor
- oxLDL
- oxidized low-density lipoprotein
- PD98059
- 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one
- ROS
- reactive oxygen species
- SD
- rats, Sprague-Dawley rats
- THC
- Δ9-tetrahydrocannabinol
- TNF-α
- tumor necrosis factor-α
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis (o-aminophenylmercapto) butadiene monoethanolate
- WIN55
- 212-2, (R)-(+)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmeth anone mesylate
This work was supported by Grant 09ZR1418100 from the Shanghai Municipal Natural Science Foundation, Grant 08XD14026 from the Program of Shanghai Subject Chief Scientist, Grant 30600242 from the National Natural Science Foundation of China, and Grant YG08PETZD03 from Shanghai Jiao Tong University.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
REFERENCES
- 1.Steinberg D., Parthasarathy S., Carew T., Khoo J., Witztum J. 1989. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 320: 915–924. [DOI] [PubMed] [Google Scholar]
- 2.Holvoet P., Vanhaecke J., Janssens S., Van de Werf F., Collen D. 1998. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 98: 1487–1494. [DOI] [PubMed] [Google Scholar]
- 3.Ogura S., Kakino A., Sato Y., Fujita Y., Iwamoto S., Otsui K., Yoshimoto R., Sawamura T. 2009. LOX-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ. J. 73: 1993–1999. [DOI] [PubMed] [Google Scholar]
- 4.Jiang L. S., Pu J., Han Z. H., Hu L. H., He B. 2009. Role of activated endocannabinoid system in regulation of cellular cholesterol metabolism in macrophages. Cardiovasc. Res. 81: 805–813. [DOI] [PubMed] [Google Scholar]
- 5.Liu J., Wang L., Harvey-White J., Osei-Hyiaman D., Razdan R., Gong Q., Chan A. C., Zhou Z., Huang B. X., Kim H. Y., et al. 2006. A biosynthetic pathway for anandamide. Proc. Natl. Acad. Sci. USA. 103: 13345–13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bátkai S., Osei-Hyiaman D., Pan H., El-Assal O., Rajesh M., Mukhopadhyay P., Hong F., Harvey-White J., Jafri A., Haskó G., et al. 2007. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 21: 1788–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Filippo C., Rossi F., Rossi S., D'Amico M. 2004. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia- reperfusion injury: involvement of cytokine/chemokines and PMN. J. Leukoc. Biol. 75: 453–459. [DOI] [PubMed] [Google Scholar]
- 8.Ashton J. C., Rahman R. M., Nair S. M., Sutherland B. A., Glass M., Appleton I. 2007. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci. Lett. 412: 114–117. [DOI] [PubMed] [Google Scholar]
- 9.Defer N., Wan J., Souktani R., Escoubet B., Perier M., Caramelle P., Manin S., Deveaux V., Bourin M. C., Zimmer A., et al. 2009. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J. 23: 2120–2130. [DOI] [PubMed] [Google Scholar]
- 10.Han K. H., Lim S., Ryu J., Lee C. W., Kim Y., Kang J. H., Kang S. S., Ahn Y. K., Park C. S., Kim J. J. 2009. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 84: 378–386. [DOI] [PubMed] [Google Scholar]
- 11.Bátkai S., Rajesh M., Mukhopadhyay P., Haskó G., Liaudet L., Cravatt B. F., Csiszár A., Ungvári Z., Pacher P. 2007. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am. J. Physiol. Heart Circ. Physiol. 293: H909–H918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen L. H., Zhou L., Wang B. Y., Pu J., Hu L. H., Chai D. J., Wang L., Zeng J. Z., He B. 2008. Oxidized low-density lipoprotein induces differentiation of RAW264.7 murine macrophage cell line into dendritic-like cells. Atherosclerosis. 199: 257–264. [DOI] [PubMed] [Google Scholar]
- 13.Jones B. G., Dickinson P. A., Gumbleton M., Kellaway I. W. 2002. The inhibition of phagocytosis of respirable microspheres by alveolar and peritoneal macrophages. Int. J. Pharm. 236: 65–79. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y. H., Lin S. J., Chen J. W., Ku H. H., Chen Y. L. 2002. Magnolol attenuates VCAM-1 expression in vitro in TNFalpha-treated human aortic endothelial cells and in vivo in the aorta of cholesterol-fed rabbits. Br. J. Pharmacol. 135: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkel T., Holbrook N. J. 2000. Oxidants, oxidative stress and the biology of ageing. Nature. 408: 239–247. [DOI] [PubMed] [Google Scholar]
- 16.Tedgui A., Mallat Z. 2006. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 86: 515–581. [DOI] [PubMed] [Google Scholar]
- 17.Landmesser U., Hornig B., Drexler H. 2004. Endothelial function: a critical determinant in atherosclerosis? Circulation. 109: II27–II33. [DOI] [PubMed] [Google Scholar]
- 18.McCord J. M. 1987. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed. Proc. 46: 2402–2406. [PubMed] [Google Scholar]
- 19.Adams I. B., Martin B. R. 1996. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 91: 1585–1614. [PubMed] [Google Scholar]
- 20.Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 346: 561–564. [DOI] [PubMed] [Google Scholar]
- 21.Munro S., Thomas K. L., Abu-Shaar M. 1993. Molecular characterizaton of a peripheral receptor for cannabinoids. Nature. 365: 61–65. [DOI] [PubMed] [Google Scholar]
- 22.Osei-Hyiaman D., DePetrillo M., Pacher P., Liu J., Radaeva S., Ba'tkai S., Harvey-White J., Mackie K., Offertáler L., Wang L., et al. 2005. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Invest. 115: 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engeli S., Bohnke J., Feldpausch M., Gorzelniak K., Janke J., Batkai S., Pacher P., Harvey-White J., Luft F. C., Sharma A. M., et al. 2005. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 54: 2838–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Despres J. P., Golay A., Sjostrom L. 2005. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. 353: 2121–2134. [DOI] [PubMed] [Google Scholar]
- 25.Van Gaal L. F., Rissanen A. M., Scheen A. J., Ziegler O., Rossner S. 2005. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 365: 1389–1397. [DOI] [PubMed] [Google Scholar]
- 26.Pryce G., Ahmed Z., Hankey D. J., Jackson S. J., Croxford J. L., Pocock J. M., Ledent C., Petzold A., Thompson A. J., Giovannoni G., et al. 2003. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain. 126: 2191–2202. [DOI] [PubMed] [Google Scholar]
- 27.Massa F., Marsicano G., Hermann H., Cannich A., Monory K., Cravatt B. F., Ferri G. L., Sibaev A., Storr M., Lutz B. 2004. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Invest. 113: 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steffens S., Veillard N. R., Arnaud C., Pelli G., Burger F., Staub C., Karsak M., Zimmer A., Frossard J. L., Mach F. 2005. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 434: 782–786. [DOI] [PubMed] [Google Scholar]
- 29.Su B., Dong H., Ma R., Zhang X., Ding Q., Xiong L. 2009. Cannabinoid 1 receptor mediation of spinal cord ischemic tolerance induced by limb remote ischemia preconditioning in rats. J. Thorac. Cardiovasc. Surg. 138: 1409–1416. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M., Martin B. R., Adler M. W., Razdan R. K., Jallo J. I., Tuma R. F. 2007. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J. Cereb. Blood Flow Metab. 27: 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dagon Y., Avraham Y., Link G., Zolotarev O., Mechoulam R., Berry E. M. 2007. The synthetic cannabinoid HU-210 attenuates neural damage in diabetic mice and hyperglycemic pheochromocytoma PC12 cells. Neurobiol. Dis. 27: 174–181. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P., Haskó G. 2008. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 153: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montecucco F., Lenglet S., Braunersreuther V., Burger F., Pelli G., Bertolotto M., Mach F., Steffens S. 2009. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J. Mol. Cell. Cardiol. 46: 612–620. [DOI] [PubMed] [Google Scholar]
- 34.Hajrasouliha A. R., Tavakoli S., Ghasemi M., Jabehdar-Maralani P., Sadeghipour H., Ebrahimi F., Dehpour A. R. 2008. Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB2 receptors in the rat heart. Eur. J. Pharmacol. 579: 246–252. [DOI] [PubMed] [Google Scholar]
- 35.Sheng W. S., Hu S., Min X., Cabral G. A., Lokensgard J. R., Peterson P. K. 2005. Synthetic cannabinoid WIN55,212–2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 49: 211–219. [DOI] [PubMed] [Google Scholar]
- 36.Isachenko E. G., Vitkina T. I., Berdyshev E. V. 2004. Effect of the cannabinoid receptor ligand WIN-55,212-2 on the TNFalpha production by human mononuclear cells. Eksp. Klin. Farmakol. 67: 49–50. [PubMed] [Google Scholar]
- 37.Kim S. H., Won S. J., Mao X. O., Jin K., Greenberg D. A. 2005. Involvement of protein kinase A in cannabinoid receptor mediated protection from oxidative neuronal injury. J. Pharmacol. Exp. Ther. 313: 88–94. [DOI] [PubMed] [Google Scholar]
- 38.Santini E., Lupi R., Baldi S., Madec S., Chimenti D., Ferrannini E., Solini A. 2008. Effects of different LDL particles on inflammatory molecules in human mesangial cells. Diabetologia. 51: 2117–2125. [DOI] [PubMed] [Google Scholar]
- 39.Collins T., Cybulsky M. I. 2001. NF-kappaB: pivotal mediator or innocent bystanderin atherogenesis? J. Clin. Invest. 107: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen-Heininger Y. M., Poynter M. E., Baeuerle P. A. 2000. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic. Biol. Med. 28: 1317–1327. [DOI] [PubMed] [Google Scholar]
- 41.Flohe L., Brigelius-Flohe R., Saliou C., Traber M. G., Packer L. 1997. Redox regulation of NF-kappa B activation. Free Radic. Biol. Med. 22: 1115–1126. [DOI] [PubMed] [Google Scholar]
- 42.Bourcier T., Sukhova G., Libby P. 1997. The nuclear factor kappa-B signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J. Biol. Chem. 272: 15817–15824. [DOI] [PubMed] [Google Scholar]
- 43.Gertsch J., Leonti M., Raduner S., Racz I., Chen J. Z., Xie X. Q., Altmann K. H., Karsak M., Zimmer A. 2008. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA. 105: 9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gertsch J. 2008. Anti-inflammatory cannabinoids in diet: towards a better understanding of CB(2) receptor action? Commun. Integr. Biol. 1: 26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.