Abstract
Lipid rafts are cholesterol-rich microdomains of cell membranes that have a variety of roles in cellular processes including receptor-mediated signal transduction. Lipid rafts also occur in embryonic stem (ES) cells, but their role in ES cells is largely unknown. Therefore, we investigated the role of lipid rafts in the maintenance of ES cell self-renewal. In the present study, we observed that the presence of lipid rafts/caveolae. The results from sucrose gradient fractionation showed that the expression of glycoprotein 130 (gp130) and leukemia inhibitory factor receptor β (LIFRβ) was decreased by treatment with methyl-β-cyclodextrin (Mβ-CD) but, interestingly, was not affected by caveolin-1 small interfering RNA (siRNA). In addition, LIF increased phosphorylation of signal transducer and activator of transcription 3 (STAT3) and Akt, and the expression level of c-Myc, which were attenuated by the pretreatment with Mβ-CD. However, caveolin-1 siRNA did not influence LIF-induced phosphorylation of STAT3 and Akt, and expression of c-Myc. Treatment with Mβ-CD and caveolin-1 siRNA decreased expression levels of Oct4 protein and Oct4, Sox2, FoxD3, and Rex1 mRNAs in normal culture conditions. Additionally, Mβ-CD and caveolin-1 siRNA decreased the expression levels of cyclin D1 and cyclin E, and the proliferation index [(S + G2/M)/(G0/G1 + S + G2/M)] of ES cells.
Keywords: cell signaling, ES cell, signal transduction
One of the most important issues in stem cell biology is the mechanism(s) that regulate self-renewal, a crucial aspect of stem cell function (1). To understand the underlying molecular mechanisms of embryonic stem (ES) cell pluripotency, it would be useful to identify the cellular components that permit precise regulation of ES cell self-renewal. To date, a number of pathways have been identified that contribute to the regulation of ES cell self-renewal, and the roles and mechanisms of various factors, such as extrinsic stimulus, intracellular signal molecules, nuclear receptors, transcription factors, and chromatin remodeling, in ES cells have been elucidated (2–5). In the present study, we focused on the role of lipid rafts in the maintenance of ES cell self-renewal. Lipid rafts are small (10–200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes. Bulk lipid rafts are distinguished from caveolae, a subclass of rafts characterized by flask-like invaginations of the plasma membrane and the presence of caveolin-1 (6, 7). To our knowledge, the existence of lipid raft/caveolae in the ES cell membrane has not been explored, and its potential role in ES cells remains unclear.
Lipid rafts/caveolae are involved in various cellular events, such as signal transduction (8, 9), membrane trafficking, immune responses, and cellular growth (10, 11). Signal transduction in mammalian cells is initiated by complex protein-protein interactions between ligands, receptors, and kinases at the level of the plasma membrane. In addition, according to a ligand-receptor signaling threshold model, a critical growth factor concentration and interaction with the appropriate receptors are required to maintain the ability to self-renew (12). It is becoming clear that lipid rafts have a pivotal role as platforms to concentrate receptors and properly assemble the signal transduction (6, 7, 13, 14). For example, receptors in lipid rafts/caveolae may exert their functional roles following induction by insulin (15), transforming growth factor-β (TGF-β) (16), bone morphogenetic protein (BMP) (17), platelet-derived growth factor (PDGF) (18), and receptors in nonraft regions, resulting in cellular physiological responses (19). Therefore, we hypothesized that lipid rafts/caveolae are associated with ES cell self-renewal because of the presence of receptor initiating signals and localization of signal molecules in these specific plasma membrane microdomains.
It is well-known that the self-renewal capacity of ES cells is maintained by various extracellular cues, such as cytokines, growth factors, hormones, fetal animal serum, and serum extract (20–26). The propagation of pluripotent mouse ES cells especially depends upon a cytokine known as leukemia inhibitory factor (LIF) (27). Presently, we attempted to determine the role of lipid rafts in ES cell self-renewal through the examination of involvement of lipid rafts in LIF-induced signaling as one of the factors to maintain ES cell self- renewal. We demonstrate that lipid rafts are present in mouse ES cells and suggest a functional model in which lipid rafts play an important role in maintenance of ES cell self-renewal as a mediator of extracellular cues.
MATERIALS AND METHODS
Materials
Mouse ES cells (ES-E14TG2a) were obtained from the American Type Culture Collection (Manassas, VA). FBS was purchased from Biowhittaker (Walkersville, MD). Fluorescein (FITC)-conjugated goat-anti rabbit IgM anti β-actin antibodies were acquired from Sigma-Aldrich (St. Louis, MO). Anti-cyclin D1, cyclin E, c-myc, caveolin-1, gp130, LIFR and phospho-signal transducer and activator of transcription 3 (STAT3), horseradish peroxidase (HRP)-conjugated goat anti-rabbit and rabbit anti-mouse antibodies, and LIF were acquired from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-Akt308 and phospho-Akt473 antibodies were supplied by New England Biolabs (Ipswich, MA). Methyl-β-cyclodextrin (Mβ-CD) was purchased from Sigma-Aldrich (St. Louis, MO). To exclude the possibility of nonspecific cytotoxicity of Mβ-CD, we prepared the cholesterol-loaded Mβ-CD (CLM) as described by Purdy and Graham (28). The treatment of CLM has no effect on the stem cell markers and cell proliferation markers examined in present study (supplementary Fig. I). In addition, we confirmed that Mβ-CD itself did not affect plasma membrane functions that do not depend on lipid raft microdomains through 2-DG uptake (supplementary Fig. II). All reagents were purchased commercially and were of the highest available purity.
ES cell culture
Mouse ES cells were cultured for 5 days in DMEM (Gibco-BRL, Gaithersburg, MD) supplemented with 3.7 g/l of sodium bicarbonate, 1% penicillin and streptomycin, 1.7 mM L-glutamine, 0.1 mM β-mercaptoethanol, 5 ng/ml mouse LIF, and 15% FBS without a feeder layer. Cells were grown on gelatinized 12-well plates or 60 mm-diameter culture dishes in a 37°C incubator in an atmosphere of 5% CO2. The medium was replaced by serum-free DMEM for 12 h before the experiments to investigate the effect of LIF.
RNA isolation and real-time RT-PCR
Mouse ES cells were harvested after treatment with 10 mM Mβ-CD for 1 h, and then the culture media was changed with Mβ-CD free media every day for 5 days. Mouse ES cells were collected after transfection with 50 nM caveolin-1 siRNA or control siRNA for 24 h and then cultured with normal culture media for 4 days. Total RNA was extracted from mouse ES cells using a RNA extraction kit (Qiagen, Valencia, CA). Reverse transcription was performed on 3 μg of RNA using an AccuPower RT PreMix reverse-transcription system kit (Bioneer, Daejeon, Korea) with oligo (dT)18 primers. The real-time quantification of RNA targets was performed using a Rotor-Gene6000 real-time thermal cycling system (Corbett Research, New South Wales, Australia) using a QuantiTect SYBR Green RT-PCR kit (Qiagen). The primers used are described in Table 1. The 20 μl reaction mixture contained 200 ng cDNA, 0.5 μM of each primer, enzymes, and fluorescent dyes. The data was collected during the extension step and was analyzed using the manufacturer's software. To verify the specificity and identity of the PCR products, the amplification cycles were followed by a high-resolution melting cycle from 65°C to 99°C at a rate of 0.1°C/2 s. When the melting temperature (Tm) is reached, double stranded DNA is denatured and the SYBR is released, which causes a dramatic decrease in fluorescence intensity. The rate of this change was determined by plotting the derivative of the fluorescence relative to the temperature (dF/dT) versus temperature by data analysis software of the real-time PCR instrument. The temperature at which a peak occurs on the plot corresponds to the Tm of the DNA duplex. β-actin of control group was the endogenous control used for calibration and normalization.
TABLE 1.
Primers used for polymerase chain reaction
| Gene | Identification | Primer Sequence | Annealing Temperature |
|---|---|---|---|
| Oct4 | Sense | 5′-CGTGAGACTTTGCAGCCTGA-3′ | 60°C |
| Antisense | 5′-GGGATGTAAGTGATCTGCTG-3′ | ||
| Sox2 | Sense | 5′-GCATGTCCTACTCGCAGCAG-3′ | 53°C |
| Antisense | 5′-GCTGATCATGTCCCGGAGGT-3′ | ||
| FoxD3 | Sense | 5′-TCTCTGGGGCAATCACACTC-3′ | 50°C |
| Antisense | 5′-GTACATTTGTTGATAAAGGG-3′ | ||
| Rex1 | Sense | 5′-GTCTTATCGATGCTGGAGTG-3′ | 55°C |
| Antisense | 5′-AAAGCTCTTCTCGCAGCCAT-3′ | ||
| β-actin | Sense | 5′-AACCGCGAGAAGATGACCCAGATCATGTTT-3′ | 55°C |
| Antisense | 5′-AGCAGCCGTGGCCATCTCTTGCTCGAAGTC-3′ |
siRNA transfection
ES cells were grown until 75% confluence. The cells were transfected for 24 h with either a SMARTpool of caveolin-1 specific small interfering RNAs (siRNA) (Dharmacon, Lafayette, CO) or nontargeting siRNA (negative control; Dharmacon) using Dharmafect transfection reagent (Dharmacon). The construct targeting caveolin-1 was composed of the following 3′ (sense) and 5′ (antisense) primer pairs: 3′-GCUAUUGGCAAGAUAUUCAUU and 5′-UGAAUAUCUUGCCAAUAGCUU; 3′-GCACAUCUGGGCGGUUGUAUU and 5′-UACAACCGCCCAGAUGUGCUU; 3′-GCAAAUACGUGGACUCCGAUU and 5′-UCGGAGUCCACGUAUUUGCUU; 3′-GUCCAUACCUUCUGCGAUCUU and 5′-GAUCGCAGAAGGUAUGGACUU. The nontargeting siRNA was 5′-UGGUUUACAUGUCGACUAA-3′. After 24 h, transfection mixtures were replaced with regular medium, and cells were maintained in normal culture condition (DMEM supplemented with LIF and FBS) before the experiments.
Detergent-free purification of caveolin-rich membrane fraction
Caveolin-enriched membrane fractions were prepared as described previously (29). Cells were washed twice with ice-cold PBS, scraped into 2 ml of 500 mM sodium carbonate (pH 11.0), transferred to a plastic tube, and homogenized with a Sonicator 250 apparatus (Branson Ultrasonic, Danbury, CT, USA) using three 20 s bursts. The homogenate was adjusted to 45% sucrose by the addition of 2 ml 90% sucrose prepared in MES-buffered solution consisting of 25 mM MES (pH 6.5) and 0.15 M NaCl, and placed at the bottom of an ultracentrifuge tube. A 5–35% discontinuous sucrose gradient was formed above (4 ml each of 5% and 35% sucrose, both in MBS containing 250 mM sodium carbonate) and centrifuged at 40,000 rpm for 20 h in a SW 41 rotor (Beckman Coulter, Fullerton, CA). Twelve 1 ml fractions were collected and analyzed by 8–12% SDS-PAGE.
Immunofluorescence staining
Cells were fixed with 3.5% paraformaldehyde in PBS and permeabilized for 10 min with 0.1% (v/v) Triton X-100 and washed three times with PBS, for 10 min each wash. Cells were preincubated with 10% BSA (Sigma-Aldrich) in PBS for 20 min to decrease nonspecific antibody binding. Cells were then incubated for 60 min with primary antibody in a solution containing 1% (v/v) BSA in PBS, and washed with PBS as above. Cells were incubated with 1% (v/v) BSA for 5 min and then incubated for 60 min with FITC-conjugated secondary antibody in PBS containing 1% (v/v) BSA, and washed with PBS as above. Samples were mounted on slides and visualized with a FluoView 300 confocal microscope (Olympus, Tokyo, Japan) equipped with a 400× objective lens.
Western blot analysis
Cell homogenates containing 20 μg protein were separated by 10%–12% SDS-PAGE and transferred to polyvinylidene fluoride transfer membranes (Pall, Gelman Laboratory, Ann Arbor, MI). Each membrane was washed with TBST [10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.05% Tween-20] and blocked with 5% skimmed milk for 1 h prior to incubation with a 1:1,000 dilution of the appropriate primary antibody. Each membrane was washed, and primary antibodies were detected with a 1:10,000 dilution of HRP-conjugated goat anti-rabbit IgG or goat anti-mouse IgG. The reactive bands were visualized with enhanced chemiluminescence (Amersham Pharmacia Biotech, Franklin Lakes, NJ) on KODAK chemiluminescence BioMax film (Carestream Health Inc., Rochester, NY).
FACS analysis
Cells were dissociated in trypsin/EDTA, pelleted by centrifugation, and resuspended at approximately 106 cells/ml in PBS containing 0.1% BSA. The cells were then fixed with 70% ice-cold ethanol for 30 min at 4°C, followed by incubation in a freshly prepared nuclei staining buffer consisting of 250 μg/ml propidium iodide (PI) and 100 μg/ml RNase for 30 min at 37°C. Cell cycle histograms were generated after analyzing the PI-stained cells by fluorescence-activated cell sorting (FACS) (Beckman Coulter). The samples were analyzed using CXP software (Beckman Coulter) and the proliferation indices [(S + G2/M)/(G0/G1 + S + G2/M)] were calculated.
Statistical analysis
The results are reported as the mean ± SE, and all experiments were analyzed by ANOVA. Analysis was followed by a comparison of the treatment means with the control using the Bonferroni-Dunn test. Statistical significance was defined as P < 0.05.
RESULTS
Presence of lipid raft/caveolae and localization of LIF receptor in lipid rafts
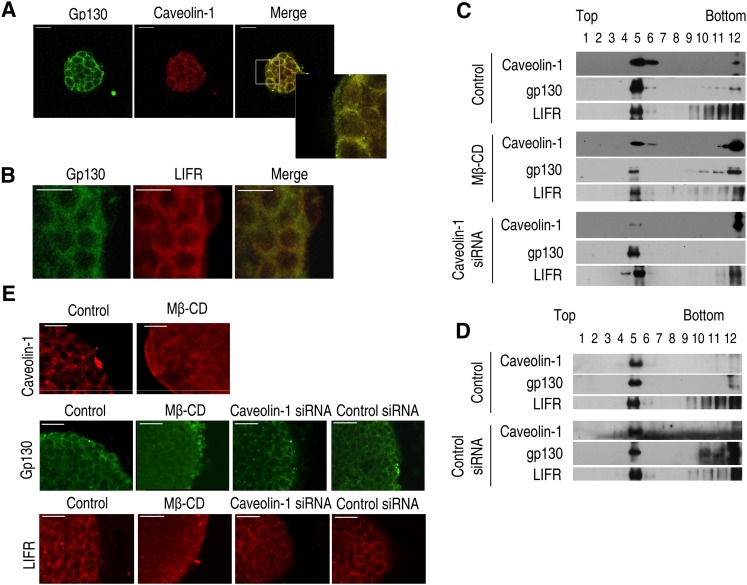
Mouse ES cells can self-renew with the addition of recombinant LIF protein to the culture medium (30, 31). The receptor for LIF is a heterodimeric complex consisting of glycoprotein 130 (gp130) and the LIF receptor (also referred as to LIFRβ) (32). To visualize gp130, caveolin-1, and LIFRβ expression in the plasma membrane of mouse ES cells, immunofluorescence staining was carried out. The staining revealed expression of gp130, caveolin-1, and LIFRβ mainly in the plasma membrane fraction (Fig. 1A). In addition, it was observed that gp130 is colocalized with LIFRβ (Fig. 1B). To assess whether LIFRβ and gp130 colocalized with the lipid raft fraction, the latter fractions were prepared by detergent-free purification using discontinuous sucrose density gradient centrifugation. The plasma membrane lipid raft fraction was found to reside mainly in the light buoyant membranes (Fig. 1C, fractions 4, 5, 6). Western blot analysis for LIFRβ, gp130, and caveolin-1 demonstrated localization of gp130 and LIFRβ in the lipid raft fraction (Fig. 1C). Control siRNA did not effect caveolin-1, gp130, or LIFRβ in lipid raft fraction in control condition (Fig. 1D). Importantly, gp130 and LIFRβ expression in the lipid raft fraction was significantly decreased by methyl-β-cyclodextrin (Mβ-CD) but was not affected by caveolin-1 siRNA or control siRNA. The results of immunofluorescent staining showed that distribution of gp130 and LIFRβ in the plasma membrane was altered by Mβ-CD but not by caveolin-1siRNA (Fig. 1E).
Fig. 1.
Localization of gp130 and LIFRβ in lipid raft structure in mouse ES cells. (A) Gp130 and caveolin-1 were detected by immunostaining with anti-gp130 and anti-caveolin-1 antibodies. Insets are higher magnifications of the portions of the image in the white boxes. (B) Gp130 and LIFRβ were detected by immunostaining. (C, D) Lysates of control, 10 mM Mβ-CD-treated or transfected cells with caveolin-1 siRNA or control siRNA were subjected to discontinuous sucrose density gradient fractionation, and then gp130, LIFRβ, and caveolin-1 were detected. Each fraction was assessed by Western blot. (E) Cells were incubated with or without 10 mM Mβ-CD for 1 h and immunostained with anti-caveolin-1 antibody. In addition, mouse ES cells were incubated with or without 10 mM Mβ-CD, transfected with caveolin-1 siRNA or control siRNA, and immunostained with anti-gp130 and LIFRβ antibody. Each example is representative of three experiments. The size bar represents 50 μm. ES cell, embryonic stem cell; LIFRβ, leukemia inhibitory factor receptor β; Mβ-CD, methyl-β-cyclodextrin; siRNA, small interfering RNA.
Relationship between lipid rafts and LIF-induced signaling
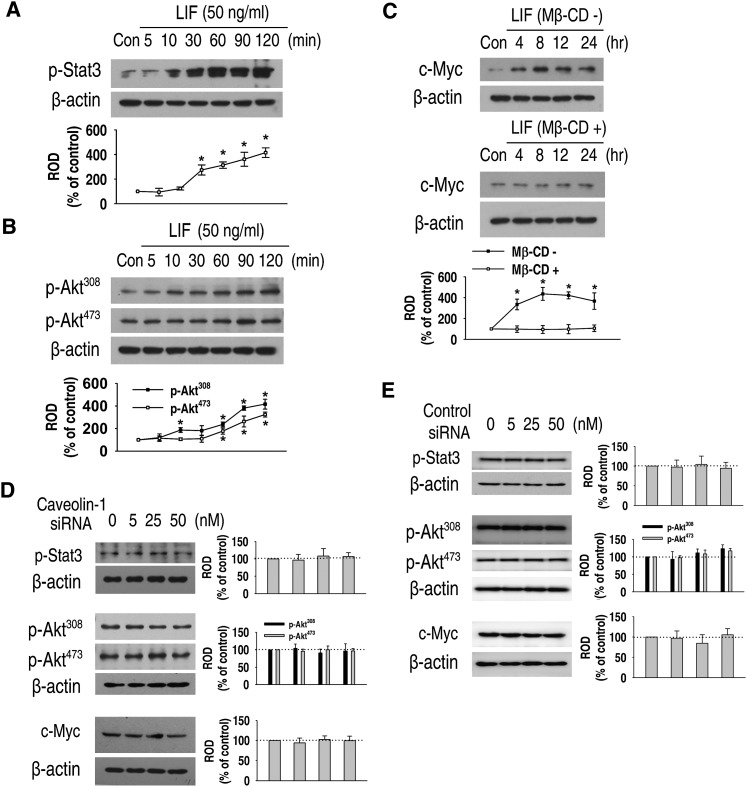
It is well-known that LIF induces the activation of STAT3 and PI3K/Akt signaling, which converge in c-Myc expression through the gp130 protein in mouse ES cells (4, 33, 34). We investigated the effect of Mβ-CD and caveolin-1 siRNA in LIF-induced signals. LIF significantly increased the phosphorylation of STAT3 (Fig. 2A) and Akt (Fig. 2B). c-Myc protein expression was also increased by LIF treatment. However, the increase of c-Myc expression level was attenuated by a 1 h pretreatment with 10 mM Mβ-CD (Fig. 2C).
Fig. 2.
Relationship between lipid rafts and LIF-induced signaling. (A, B) Mouse ES cells were treated with LIF for 0–120 min, and the phosphorylation of STAT3 and Akts (Ser473, Thr308) was detected by Western blot. (C) The cells were incubated for 0–24 h with or without a 1 h preincubation with 10 mM Mβ-CD. c-Myc protein expression was detected by Western blot. (D, E) Cells were transfected for 24 h with a SMARTpool of caveolin-1 siRNA or nontarget control siRNA (0–50 nM) and the phosphorylation of STAT3, Akts, and the expression of c-Myc protein was detected by Western blot. Zero represents no siRNA. Each example is representative of three experiments. The graph denotes the mean ± SE of three experiments for each condition determined from densitometry relative to β-actin. P < 0.05 versus control. ES cell, embryonic stem cell; LIF, leukemia inhibitory factor; Mβ-CD, methyl-β-cyclodextrin; ROD, relative optical density; siRNA, small interfering RNA; STAT3, signal transducer and activator of transcription 3.
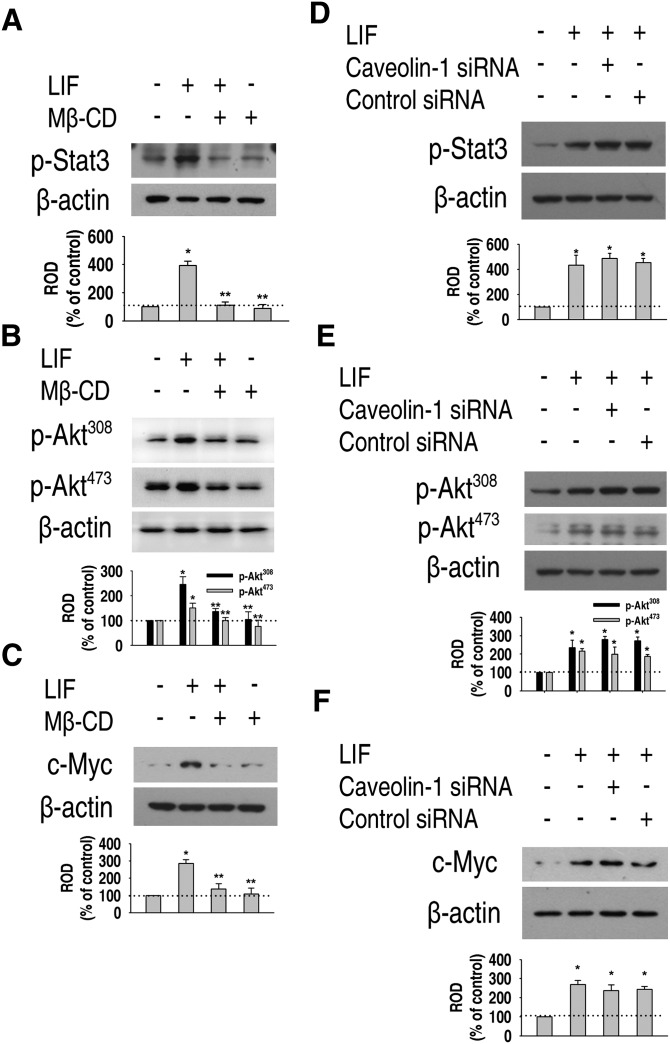
Next, we investigated the involvement of caveolin-1 in LIF-induced signaling. Caveolin-1 siRNA or control siRNA did not affect phosphorylation of STAT3 and Akt, or protein expression of c-Myc in normal culture conditions (Fig. 2D, E). We investigated the effect of Mβ-CD and caveolin-1 siRNA in LIF-induced downstream signals. LIF-induced phosphorylation of STAT3 (Fig. 3A) and Akt (Fig. 3B), as well as c-Myc expression (Fig. 3C), were significantly attenuated by a 1 h pretreatment with 10 mM Mβ-CD. However, intriguingly, caveolin-1 siRNA did not abrogate LIF-induced phosphorylation of STAT3 (Fig. 3D), Akt (Fig. 3E), or c-Myc expression (Fig. 3F). These results suggest that LIF-induced downstream signals are not mediated by caveolae or caveolin-1.
Fig. 3.
Effect of Mβ-CD and caveolin-1 siRNA in LIF-induced signaling. (A, B) Mouse ES cells were preincubated for 1 h with 10 mM Mβ-CD prior to a 1 h LIF treatment, and phosphorylation of STAT3 and Akt was detected by Western blot. (C) The cells were preincubated for 1 h with 10 mM Mβ-CD prior to a 24 h LIF treatment, and c-Myc protein expression was detected. (D, E) Cells were transfected with either a SMARTpool of caveolin-1 siRNA or nontargeting control siRNA (0–50 nM) for 24 h, and phosphorylation of STAT3 and Akt was detected. (F) Cells were transfected with either a SMARTpool of caveolin-1 siRNA or nontargeting control siRNA (0–50 nM) for 24 h, and c-Myc protein expression was detected. Each example is representative of three experiments. The graph denotes the mean ± SE of three experiments for each condition determined from densitometry relative to β-actin. P < 0.05 versus control. ES cell, embryonic stem cell; LIF, leukemia inhibitory factor; Mβ-CD, methyl-β-cyclodextrin; ROD, relative optical density; siRNA, small interfering RNA; STAT3, signal transducer and activator of transcription 3.
Role of lipid rafts/caveolae in ES cell self-renewal
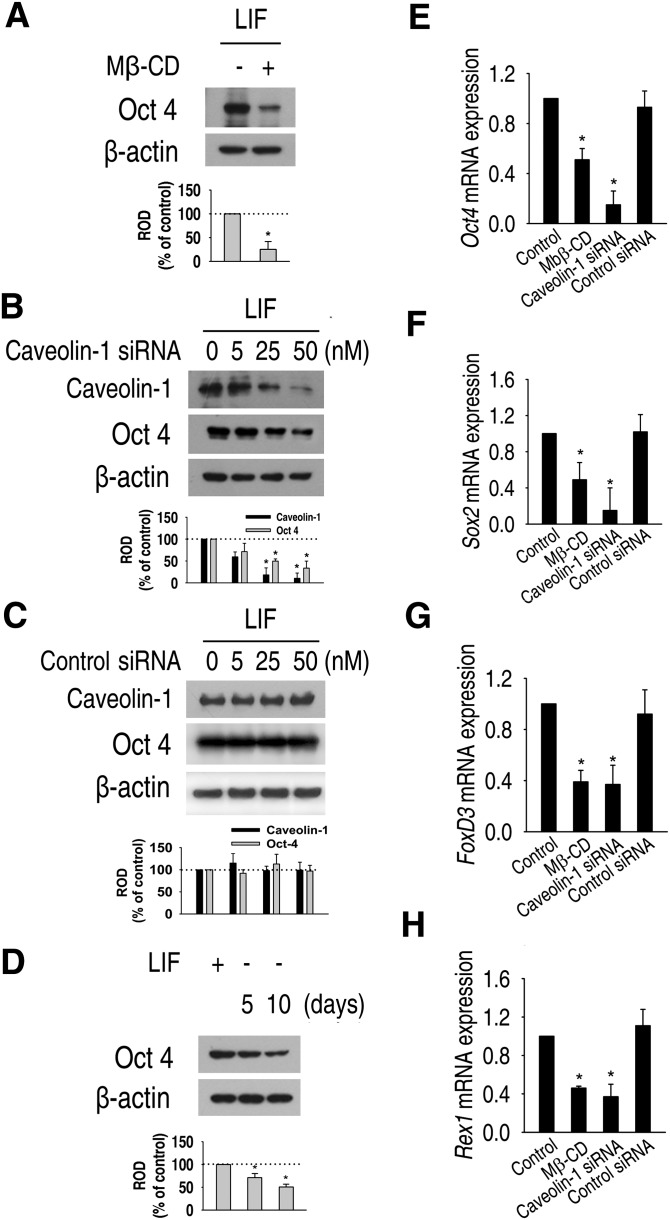
To examine the role of lipid rafts/caveolae in maintenance of pluripotency and proliferation of mouse ES cells, mouse ES cells were incubated with or without 10 mM Mβ-CD for 1 h everyday for 5 days, followed by replacement with media lacking Mβ-CD, and then Oct4 protein expression was examined. As shown in Fig. 4A, Mβ-CD significantly decreased Oct4 protein expression level. Next, the effect of caveolin-1 knockdown on Oct4 expression was assessed. Oct4 protein expression was decreased by caveolin-1 siRNA in a dose-dependent manner (Fig. 4B), but Oct4 protein expression was not influenced by control siRNA (Fig. 4C). In addition, the level of Oct4 protein expression was decreased by withdrawal of LIF in a time- dependent manner (Fig. 4D). To confirm these results, the expression of pluripotency marker mRNAs was examined using real-time RT-PCR. As shown in Fig. 4–H, mRNA expression levels of Oct4, Sox2, FoxD3, and Rex1 were significantly decreased by Mβ-CD and caveolin-1 siRNA. These results suggest that lipid rafts/caveolae play an important role in the maintenance of ES cell pluripotency.
Fig. 4.
Role of lipid rafts in the maintenance of pluripotency of mouse ES cells. (A) Mouse ES cells were cultured with or without 10 mM Mβ-CD for 1 h everyday for 4 days. (B, C) Cells were transfected for 24 h with different doses of SMARTpool of caveolin-1 siRNA or nontarget control siRNA (0–50 nM), and expressions of caveolin-1 and Oct4 protein were detected. Zero represents no siRNA. (D) Cells were cultured with or without LIF for 5 and 10 days, and Oct4 protein expression levels were detected by Western blot. Each example is representative of three experiments. The graphs denote the mean ± SE of three experiments for each condition determined from densitometry relative to β-actin. (E–H) Mouse ES cells were harvested after culture in normal culture condition for 5 days, cultured with 10 mM Mβ-CD for 1 h every day for 5 days in normal culture condition, and cultured in normal culture condition for 4 days after transfection with 50 nM caveolin-1 or nontarget control siRNA for 24 h, respectively. Real-time RT-PCR quantification of Oct4, Sox2, FoxD3, and Rex1 was carried out. The data is reported as the mean ± SE of three independent experiments, each conducted in triplicate. *P < 0.05 versus control. ES cell, embryonic stem cell; LIF, leukemia inhibitory factor; Mβ-CD, methyl-β-cyclodextrin; ROD, relative optical density; siRNA, small interfering RNA; STAT3, signal transducer and activator of transcription 3.
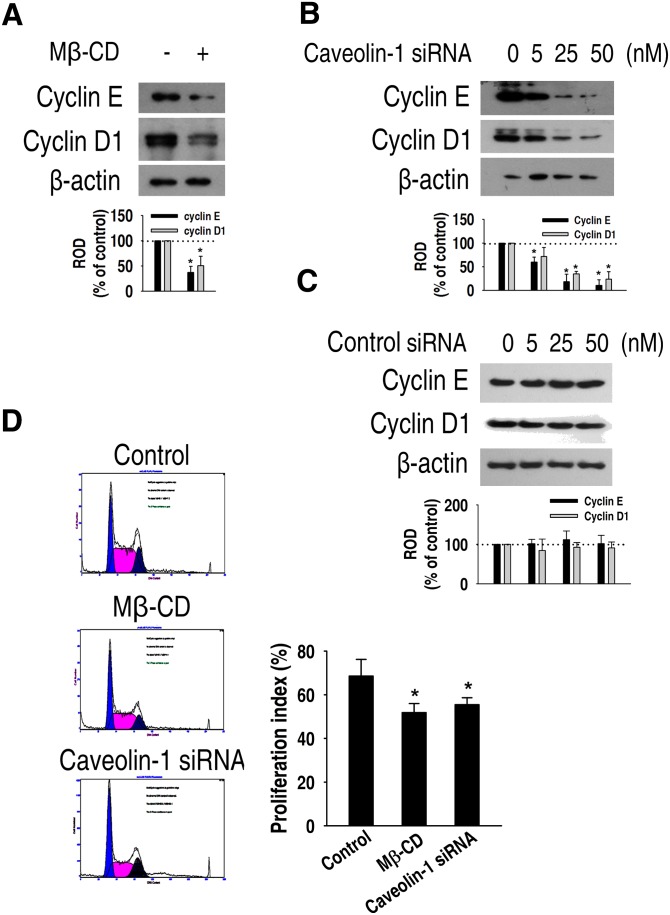
Next, we examined the relationship between the proliferative capacity of mouse ES cells and lipid rafts/caveolae. As shown in Fig. 5A, Mβ-CD significantly decreased the cyclin E and cyclin D1 expression levels in normal culture conditions. In addition, the expression levels of these proteins were decreased by caveolin-1 siRNA in a dose- dependent manner (Fig. 5B, C). Furthermore, Mβ-CD and caveolin-1 siRNA significantly decreased the proliferation index compared with control (control: 68.56 ± 7.63%; MβCD: 51.86 ± 4.1%; caveolin-1 siRNA: 55.50 ± 3.15%) (Fig. 5D). Taken together, these results suggest that lipid rafts/caveolae have a functional role in ES cell self-renewal.
Fig. 5.
Role of lipid rafts on the proliferation of mouse ES cells. (A) Mouse ES cells were incubated with or without 5 mM Mβ-CD for 24 h and then cyclin E and cyclin D1 protein expression was detected by Western blot. (B, C) Cells were transfected for 24 h with different doses (0–50 nM) of SMARTpool of caveolin-1 siRNA or nontarget control siRNA, and cyclin E and cyclin D1 expression was detected by Western blot. Zero represents no siRNA. Each example shown is representative of three experiments. The graphs denote the mean ± SE of three experiments for each condition determined from densitometry relative to β-actin. (D) Mouse ES cells were incubated with 5 mM Mβ-CD and 50 nM caveolin-1 siRNA, harvested, and subjected to PI staining for cell cycle analysis by flow cytometry. The gates were configured manually to determine the percentage of cells in S phase based on the DNA content. The data is calculated using proliferation index [(S + G2/M)/(G0/G1 + S + G2/M)] and reported as the mean ± SE of three independent experiments, each conducted in triplicate. *P < 0.05 versus control. ES cell, embryonic stem cell; LIF, leukemia inhibitory factor; Mβ-CD, methyl-β-cyclodextrin; ROD, relative optical density; siRNA, small interfering RNA; STAT3, signal transducer and activator of transcription 3.
DISCUSSION
In the present study, we demonstrated the presence of lipid rafts/caveolae and its role in self-renewal of mouse ES cells. Of the various functions of lipid rafts, evidence has accumulated for their role as a cell membrane signaling platform where multiple signals are initiated (35, 36). For example, various cytokine and growth factor receptors are constitutively localized in lipid rafts or are translocated into lipid rafts upon ligand binding, and lipid raft localization is essential for downstream signaling (37, 38). Indeed, localization of BMP receptors in lipid raft microdomains and this membrane region influences BMP signaling (17), and TGF-β receptors can form a complex in lipid rafts as well as in nonraft regions (16). Moreover, the PDGF receptor may be sequestered in a raft compartment at the developmental stage, with the rafts functioning in defining the downstream signaling response to PDGF in oligodendrocytes (39). Therefore, we hypothesized that signaling by a variety of extacellular cues for self-renewal of ES cells might be mediated by lipid rafts/caveolae. The present results are, to our knowledge, the first report of the presence and potential function of lipid rafts/caveolae in mouse ES cells.
In general, mouse ES cells can be maintained undifferentiated by LIF and FBS (30, 31). This is due to the regulated expression of transcription factors and the signals for differentiation, at least in part, by LIF supplementation or by several extrinsic factors found in FBS that include BMPs and Wnt. Appropriately, we investigated the involvement of lipid rafts/caveolae in LIF-induced signals as one of the cytokines essential for maintenance of self-renewal of mouse ES cells. At first, we observed that lipid rafts/caveolae reside in the plasma membrane of mouse ES cells along with the coexpression of the gp130 LIF receptor subunit. In addition, gp130 was detected in caveolin-enriched membrane fractions. These observations suggested that gp130 could interact with caveolin-1, with the LIF-induced signal being mediated by caveolin-1. However, gp130 expression and distribution in the lipid raft fraction were changed by 10 mM Mβ-CD, which is known to disrupt lipid raft structure and inhibit lipid raft clustering in response to cytokine stimulation by binding to and sequestering cholesterol from the plasma membrane (11, 40, 41); however, knockdown of caveolin-1 had no effect. In addition, LIF-induced phosphorylation of STAT3, Akt, and c-Myc expression was attenuated by Mβ-CD but not by caveolin-1 siRNA. These results indicate that the LIFR is located in noncaveolae lipid rafts or at least that LIFR-initiated signals are not mediated by caveolin-1.
Next, we determined the effect of Mβ-CD and caveolin-1 siRNA to prove the role of lipid rafts/caveolae in the maintenance of self-renewal of mouse ES cells. Destruction of lipid rafts by Mβ-CD and caveolin-1 siRNA downregulated the expression of Oct4, a pluripotency marker protein of ES cells, as well as the expression of caveolin-1. A similar result was obtained upon withdrawal of LIF. Correspondingly, Mβ-CD and caveolin-1 siRNA significantly decreased mRNA expression of Oct4, Sox2, Rex1, and FoxD3, which are related to ES cell pluripotency. We assume that lipid rafts/caveolae are important for maintenance of efficient self- renewal of mouse ES cells, and when inhibited, mouse ES cells did not keep the self-renewal markers expression level of undifferentiated state. We also show that disruption of lipid rafts and caveolin-1 siRNA resulted in declined expression of the cell cycle regulatory proteins cyclin D1 and cyclin E and decreased ES cell proliferation. The most obvious feature of the pluripotent cell cycle, apart from it being unusually rapid, is its short G1 phase. A contributing factor to this is likely to be the activity of cyclin D1/E and perhaps associated kinases. This is supported by a large body of evidence demonstrating cyclin E to be a rate-limiting factor for the G1-to-S transition (42–45). One important point of these data is that the self-renewal marker was decreased not only by Mβ-CD but also by the knockdown of caveolin-1. In general, a variety of factors are needed for the expression of transcription factors and signals for ES cell self-renewal, except for LIF. In other words, these results indicate the importance of lipid rafts/caveolae for the function of other factors necessary for ES cell self-renewal.
CONCLUSION
The present study demonstrates the importance of lipid rafts/caveolae in maintaining self-renewal of mouse ES cells. We propose that lipid rafts are required for the efficient signal transduction to effectively maintain self- renewal of mouse ES cells. Further investigations should reveal the nature of the participation of lipid rafts/caveolae with other factors implicated in ES cell self-renewal or differentiation. It will also be interesting to evaluate if lipid rafts are important for self-renewal of human ES cells.
Supplementary Material
Footnotes
Abbreviations:
- BMP
- bone morphogenetic protein
- ES cell
- embryonic stem cell
- LIF
- leukemia inhibitory factor
- Mβ-CD
- methyl-β-cyclodextrin
- PDGF
- platelet-derived growth factor
- siRNA
- small interfering RNA
- STAT3
- signal transducer and activator of transcription 3
- TGF-β
- transforming growth factor-β.
This study was supported by Korea Science and Engineering Foundation (KOSEF) Grant MEST 2009-0081395 and a graduate fellowship of the Brain Korea 21 Project from the Ministry of Education, Science and Technology, Republic of Korea.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Niwa H. 2001. Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct. Funct. 26: 137–148. [DOI] [PubMed] [Google Scholar]
- 2.Gao X., Tate P., Hu P., Tjian R., Skarnes W. C., Wang Z. 2008. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc. Natl. Acad. Sci. USA. 105: 6656–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong Y., Mangelsdorf D. J. 2009. Nuclear receptor regulation of stemness and stem cell differentiation. Exp. Mol. Med. 41: 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paling N. R., Wheadon H., Bone H. K., Welham M. J. 2004. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J. Biol. Chem. 279: 48063–48070. [DOI] [PubMed] [Google Scholar]
- 5.Stewart R., Stojkovic M., Lako M. 2006. Mechanisms of self-renewal in human embryonic stem cells. Eur. J. Cancer. 42: 1257–1272. [DOI] [PubMed] [Google Scholar]
- 6.Simons K., Ikonen E. 1997. Functional rafts in cell membranes. Nature. 387: 569–572. [DOI] [PubMed] [Google Scholar]
- 7.Galbiati F., Razani B., Lisanti M. P. 2001. Emerging themes in lipid rafts and caveolae. Cell. 106: 403–411. [DOI] [PubMed] [Google Scholar]
- 8.Alonso M. A., Millán J. 2001. The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. J. Cell Sci. 114: 3957–3965. [DOI] [PubMed] [Google Scholar]
- 9.Pike L. J. 2003. Lipid rafts: bringing order to chaos. J. Lipid Res. 44: 655–667. [DOI] [PubMed] [Google Scholar]
- 10.Bang B., Gniadecki R., Gajkowska B. 2005. Disruption of lipid rafts causes apoptotic cell death in HaCaT keratinocytes. Exp. Dermatol. 14: 266–272. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki S., Iwama A., Morita Y., Eto K., Ema H., Nakauchi H. 2007. Cytokine signaling, lipid raft clustering, and HSC hibernation. Ann. N. Y. Acad. Sci. 1106: 54–63. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan S., Benatar T., Rose-John S., Lauffenburger D. A., Zandstra P. W. 2002. Ligand/receptor signaling threshold (LIST) model accounts for gp130-mediated embryonic stem cell self-renewal responses to LIF and HIL-6. Stem Cells. 20: 119–138. [DOI] [PubMed] [Google Scholar]
- 13.Mañes S., Mira E., Gómez-Moutón C., Lacalle R. A., Keller P., Labrador J. P., Martínez-A C. 1999. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 18: 6211–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roepstorff K., Thomsen P., Sandvig K., van Deurs B. 2002. Sequestration of epidermal growth factor receptors in non-caveolar lipid rafts inhibits ligand binding. J. Biol. Chem. 277: 18954–18960. [DOI] [PubMed] [Google Scholar]
- 15.He H. J., Kole S., Kwon Y. K., Crow M. T., Bernier M. 2003. Interaction of filamin A with the insulin receptor alters insulin-dependent activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 278: 27096–27104. [DOI] [PubMed] [Google Scholar]
- 16.Zuo W., Chen Y. G. 2009. Specific activation of mitogen- activated protein kinase by transforming growth factor-β receptors in lipid rafts is required for epithelial cell plasticity. Mol. Biol. Cell. 20: 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartung A., Bitton-Worms K., Rechtman M. M., Wenzel V., Boergermann J. H., Hassel S., Henis Y. I., Knaus P. 2006. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol. Cell. Biol. 26: 7791–7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stehr M., Adam R. M., Khoury J., Zhuang L., Solomon K. R., Peters C. A., Freeman M. R. 2003. Platelet derived growth factor-BB is a potent mitogen for rat ureteral and human bladder smooth muscle cells: dependence on lipid rafts for cell signaling. J. Urol. 169: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 19.Munro S. 2003. Lipid rafts: elusive or illusive? Cell. 115: 377–388. [DOI] [PubMed] [Google Scholar]
- 20.Martin G. R. 1981. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 78: 7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans M. J., Kaufman M. H. 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature. 292: 154–156. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida K., Chambers I., Nichols J., Smith A., Saito M., Yasukawa K., Shoyab M., Taga T., Kishimoto T. 1994. Maintenance of the pluripotential phenotype of embryonic stem cells through direct activation of gp130 signalling pathways. Mech. Dev. 45: 163–171. [DOI] [PubMed] [Google Scholar]
- 23.Rathjen J., Lake J. A., Bettess M. D., Washington J. M., Chapman G., Rathjen P. D. 1999. Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J. Cell Sci. 112: 601–612. [DOI] [PubMed] [Google Scholar]
- 24.Ying Q. L., Nichols J., Chambers I., Smith A. 2003. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 115: 281–292. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa K., Matsui H., Ohtsuka S., Niwa H. 2004. A novel mechanism for regulating clonal propagation of mouse ES cells. Genes Cells. 9: 471–477. [DOI] [PubMed] [Google Scholar]
- 26.Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A. H. 2004. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10: 55–63. [DOI] [PubMed] [Google Scholar]
- 27.Niwa H., Ogawa K., Shimosato D., Adachi K. 2009. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 460: 118–122. [DOI] [PubMed] [Google Scholar]
- 28.Purdy P. H., Graham J. K. 2004. Effect of cholesterol-loaded cyclodextrin on the cryosurvival of bull sperm. Cryobiology. 48: 36–45. [DOI] [PubMed] [Google Scholar]
- 29.Song K. S., Li S., Okamoto T., Quilliam L. A., Sargiacomo M., Lisanti M. P. 1996. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. J. Biol. Chem. 271: 9690–9697. [DOI] [PubMed] [Google Scholar]
- 30.Smith A. G., Nichols J., Robertson M., Rathjen P. D. 1992. Differentiation inhibiting activity (DIA/LIF) and mouse development. Dev. Biol. 151: 339–351. [DOI] [PubMed] [Google Scholar]
- 31.Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., Wagner E. F., Metcalf D., Nicola N. A., Gough N. M. 1998. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 336: 684–687. [DOI] [PubMed] [Google Scholar]
- 32.Ernst M., Jenkins B. J. 2004. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 20: 23–32. [DOI] [PubMed] [Google Scholar]
- 33.Hocke G. M., Cui M. Z., Fey G. H. 1995. The LIF response element of the α2 macroglobulin gene confers LIF-induced transcriptional activation in embryonal stem cells. Cytokine. 7: 491–502. [DOI] [PubMed] [Google Scholar]
- 34.Cartwright P., McLean C., Sheppard A., Rivett D., Jones K., Dalton S. 2005. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 132: 885–896. [DOI] [PubMed] [Google Scholar]
- 35.Simons K., Toomre D. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39. [DOI] [PubMed] [Google Scholar]
- 36.Patra S. K. 1785. 2008. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim. Biophys. Acta. (2): 182–206. [DOI] [PubMed] [Google Scholar]
- 37.Sehgal P. B., Guo G. G., Shah M., Kumar V., Patel K. 2002. Cytokine signaling: STATs in plasma membrane rafts. J. Biol. Chem. 277: 12067–12074. [DOI] [PubMed] [Google Scholar]
- 38.Limpert A. S., Karlo J. C., Landreth G. E. 2007. Nerve growth factor stimulates the concentration of TrkA within lipid rafts and extracellular signal-regulated kinase activation through c-Cbl-associated protein. Mol. Cell. Biol. 27: 5686–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baron W., Decker L., Colognato H., Ffrench-Constant C. 2003. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr. Biol. 13: 151–155. [DOI] [PubMed] [Google Scholar]
- 40.Gustavsson J., Parpal S., Karlsson M., Ramsing C., Thorn H., Borg M., Lindroth M., Peterson K. H., Magnusson K. E., Strâlfors P. 1999. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J. 13: 1961–1971. [PubMed] [Google Scholar]
- 41.Parpal S., Karlsson M., Thorn H., Strålfors P. 2001. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J. Biol. Chem. 276: 9670–9678. [DOI] [PubMed] [Google Scholar]
- 42.Ohtsubo M., Roberts J. M. 1993. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 259: 1908–1912. [DOI] [PubMed] [Google Scholar]
- 43.Knoblich J. A., Sauer K., Jones L., Richardson H., Saint R., Lehner C. F. 1994. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 77: 107–120. [DOI] [PubMed] [Google Scholar]
- 44.Resnitzky D., Gossen M., Bujard H., Reed S. I. 1994. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol. Cell. Biol. 14: 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wimmel A., Lucibello F. C., Sewing A., Adolph S., Müller R. 1994. Inducible acceleration of G1 progression through tetracycline-regulated expression of human cyclin E. Oncogene. 9: 995–997. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.