Abstract
Inter-individual variability in weight gain and loss under energy surfeit and deficit conditions, respectively, are well recognized but poorly understood phenomena. We documented weight loss variability in an intensively supervised clinical weight loss program and assessed skeletal muscle gene expression and phenotypic characteristics related to variable response to a 900 kcal regimen. Matched pairs of healthy, diet-compliant, obese diet-sensitive (ODS) and diet-resistant (ODR) subjects were defined as those in the highest and lowest quintiles for weight loss rate. Physical activity energy expenditure was minimal and comparable. Following program completion and weight stabilization, skeletal muscle biopsies were obtained. Gene expression analysis of rectus femoris and vastus lateralis indicated upregulation of genes and gene sets involved in oxidative phosphorylation and glucose and fatty acid metabolism in ODS compared with ODR. In vastus lateralis, there was a higher proportion of oxidative (type I) fibers in ODS compared with ODR women and lean controls, fiber hypertrophy in ODS compared with ODR women and lean controls, and lower succinate dehydrogenase in oxidative and oxidative-glycolytic fibers in all obese compared with lean subjects. Intramuscular lipid content was generally higher in obese versus lean, and specifically higher in ODS vs. lean women. Altogether, our findings demonstrate differences in muscle gene expression and fiber composition related to clinical weight loss success.
Keywords: mitochondria, oxidative phosphorylation, fiber type, thermogenesis
Obesity is reaching epidemic proportions in developed countries and represents a significant risk factor for cardiovascular disease, diabetes, and cancer (1). Enrolment in clinical and nonclinical obesity treatment programs is unprecedented. Success in obesity treatment programs is highly variable, related in part to compliance and program characteristics (e.g., type and duration of hypocaloric diets, educational components, and/or exercise-associated energy expenditure). While it is generally well accepted that there is substantial inter-individual variability in the susceptibility to weight gain in response to overfeeding (2, 3), less well understood is the impact of biological factors on weight loss success. However, studies of monozygotic twins have shown greater inter-pair than intra-pair variation in weight loss (4), consistent with the idea that there are important genetic determinants of weight loss success. We have studied the molecular and cellular determinants of variable weight loss in highly compliant subjects in an intensively supervised and interactive hypocaloric clinical obesity treatment program at the Ottawa Hospital. We previously reported differences in muscle mitochondrial energy inefficiencies between program participants exhibiting high versus low weight loss success (5). Here, we extend these findings by demonstrating distinct differences in skeletal muscle gene expression profiles and in structural and metabolic characteristics between individuals who exhibit markedly different degrees of weight loss success in a clinical obesity treatment program.
Factors affecting skeletal muscle energy metabolism are relevant, because skeletal muscle is a major determinant of resting energy expenditure (∼20%), largely due to the fact that it accounts for ∼40% of adult body weight (6). Yet the idea that differences in skeletal energy expenditure characteristics can explain variability in weight loss success has not been well explored. However, available evidence is consistent with this idea. Briefly, physiological and gene expression studies demonstrate that low oxidative capacity in muscle is characteristic of obesity and type 2 diabetes mellitus and predisposition to the latter (7–11). In a long-term follow-up study of obese men, lower proportions of oxidative (type I) fibers were associated with a higher prevalence of obesity (12). Notably, Tanner et al. (13) showed that weight loss following gastric bypass surgery was correlated with proportions of type I fibers in rectus abdominus muscle. Others have demonstrated that whole body resting energy expenditure is correlated with forearm muscle oxygen uptake, a phenomenon hypothesized to explain obesity susceptibility (6). Finally, in rodent obesity studies, a lower proportion of type I fibers was associated with increased risk for high fat diet-induced obesity compared with obesity-resistant rats (14).
We have investigated phenotypic and gene expression differences in rectus femoris and vastus lateralis muscle biopsies from obese diet-sensitive (ODS) and obese diet- resistant (ODR) subjects in an intensively supervised, multidisciplinary clinical weight loss program. Our findings reveal marked increases in the proportion of oxidative fibers and fiber size in vastus lateralis muscle of ODS compared with ODR and lean subjects. Obesity (ODS and ODR) was associated with decreased muscle mitochondrial content. Moreover, exploratory gene expression analysis of rectus femoris and vastus lateralis muscles from ODR and ODS subjects indicate upregulation of gene sets involved in oxidative phosphorylation and glucose and fatty acid metabolism in ODS compared with ODR. These findings provide new insights into factors contributing to weight loss success in obesity.
METHODS
Clinical protocol
The Human Research Ethics Committees of the Ottawa Hospital and the University of Ottawa Heart Institute approved the study. Signed consent was obtained from all participants of our study. A total of 2,878 patients with a body mass index (BMI) of 30–50 kg/m2 entered the Ottawa Hospital Weight Management Program from September 1992 to the end of December 2006. This intensively supervised, multidisciplinary program uses the Optifast 900® total meal replacement for the first 6 or 12 weeks to accomplish safe weight loss in a timely fashion (2–4)]. Percent weight loss was evaluated in the first 6 weeks of the 900 kcal meal replacement. We excluded 316 patients who had insufficient baseline or 6 week data or who failed to complete the initial 6 weeks of study. Males were excluded from study, because total numbers were relatively small. This left 1,868 evaluable women who were ranked according to percent weight loss.
Selection of patients
As in our previous report (5), we studied healthy highly compliant women enrolled in the Ottawa Hospital Weight Management Program. Because adherence to protocol is a major reason for poor response to dietary intervention, every effort was made to remove noncompliant patients. Selection of compliant patients was maximized by studying subjects during the first 6 weeks of an 8 or 12 month weight program with substantial cost to the patient ($1700–$2800). In addition, patients were excluded if they completed <75% of the 26 weekly visits, were absent for more than two visits during the initial 6 weeks on meal replacement, there were physician notes expressing reservations about self-reported compliance, or there was inadequate completion of the laboratory testing protocol.
Patients were also excluded on the basis of medical conditions possibly affecting rate of weight loss, including thyroid indices (TSH, free T3) out of normal range at week 1 or week 13, diabetes mellitus treated with insulin or oral hypoglycemic agents, cigarette smoking, congestive heart failure, obstructive sleep apnea, active malignancy, immobility, or previous bariatric surgery. Patients treated with weight-altering medications, including tricyclic antidepressants, paroxetine, mirtazepine, lithium, valproate, gabapentin, typical and atypical antipsychotics, fluoxetine in doses > 20 mg, bupropion, topiramate, systemic glucocorticoids, and weight management drugs were also excluded. Women meeting these criteria who were in the top quintile of percent weight loss in the first 6 weeks of meal replacement were matched according to initial BMI and age with women in the bottom quintile for the rate of weight loss.
Data management
Clinical information, including medical history, medication, weekly compliance assessments, anthropometric measurements, physicians’ notes, and laboratory values were collected as reported in detail elsewhere (5). Exclusions and rate of weight loss percentiles were described using software developed in this clinic (2) and by individual chart reviews on all patients to verify data.
Physical activity energy expenditure
Activity at work and planned physical activity of obese subjects were assessed by questionnaire prior to weight loss and were maintained at prestudy levels over the first 6 weeks of meal replacement. Activity at work was assessed using a 5-point Likkert scale with a value of 1 defined as an “inactive” (e.g., seated almost exclusively) and a value of 5 defined as a ”very active“ (e.g., walking nearly continuously) work environment. Subjects also recorded the frequency, duration, and type of planned weekly exercise activities. Daily physical activity energy expenditure (PAE in kcal · d−1) was calculated using total body mass and specific energy expenditure coefficients (kcal · kg−1 min−1) of each planned exercise (6) (see also “Results”).
Muscle biopsies
A subset of well-matched women meeting the above criteria who were in the top (ODS) or bottom (ODR) quintile of percent weight loss in the first 6 weeks of meal replacement underwent rectus femoris open muscle biopsy. Biopsies were carried out between 7 and 9 AM after a 12 h fast and several months after completion of the meal replacement program and after a period of at least 4 weeks of weight stabilization. For gene expression analyses, RNA was extracted from biopsied rectus femoris muscle of six matched pairs of ODS and ODR women. For replication studies, needle biopsies of the vastus lateralis muscle were collected from seven pairs of similarly matched ODS and ODR women. For analyses of muscle fiber size, type and for mitochondrial analyses, a 100–150 mg needle biopsy of the vastus lateralis was collected using a Bergstrom needle from an additional group consisting of six matched pairs of ODS and ODR women.
Gene expression analyses
mRNA extraction.
Frozen tissue was homogenized in Trizol and mRNA isolated using Qiagen oligo-dT latex beads. RNA samples were treated with amplification grade DNase1 and RNA concentration determined spectrophotometrically using OD260/280.
Whole genome expression analyses using GeneChip® microarrays.
Hybridization samples were prepared as recommended (www.affymetrix.com) and hybridized to the Affymetrix GeneChip U133A 2.0 Array consisting of 18,400 transcripts and variants including 14,500 well-characterized human genes. Sufficient RNA was obtained from six ODS and 6 ODR subjects for microarray analyses. Gene expression signals were scaled to a target intensity of 150 for all chips. Replicates were compared against each other to spot outliers, and none were detected. Genes with a maximum present call in >50% replicates in either group were kept for further analysis. Multiple statistical tests were performed to increase confidence in the set of genes that differed significantly between the ODS and ODR groups. These tests included the classical t-test, a modified t-test incorporating technology-specific error models (14), class distinction based on signal to noise (15), and partial least squares discriminant analysis (PLS-DA). Multivariate analysis PLS-DA was performed in SIMCA P+ software (Umetrics, Uppsala, Sweden) to identify candidate genes that could distinguish ODS from ODR subjects. Models were validated for fit (R2) and predictability (Q2) by randomly permuting the samples 200 times and recalculating R2 and Q2.
Global pathway analyses
Affymetrix gene expression data from ODS and ODR subjects was subjected to global pathway analysis by use of the gene set enrichment analysis (GSEA) algorithm (15, 16). Because the number of samples is relatively small (six of each class), GSEA was conducted by permuting gene-sets instead of phenotypes (samples). GSEA was conducted on data from the full chip with no prior filtering. One thousand permutations were used to obtain the nominal P-value and gene sets with a false discovery rate <0.05 were considered to be significantly regulated.
Data sharing
All microarray data were deposited in the Gene Expression Omnibus repository (http://www.ncbi.nlm.nih.gov/geo/) and is publicly available (accession no. GSE 17371).
Quantitative real-time RT-PCR
Determination of transcript quantity in key candidate genes was carried out using TaqMan quantitative RT-PCR on cDNA from individual vastus lateralis biopsy samples. Real-time PCR results were generated using a 5′ nuclease assay (TaqMan) and the ABI 7900 Sequence Detection System (Applied Biosystems, Foster City, CA). Following outlier identification and removal, all data were subjected to normalization using β actin and cyclophilin as endogenous reference transcripts. Following normalization, all data were subjected to statistical analysis using ANOVA, with significance criteria of P-value < 0.05 and fold change > 1.5.
Histological analyses
Muscle was immediately chilled in ice-cold PBS and transferred to a 1:2 mixture of ice-cold 20% sucrose in PBS: optimal cutting temperature compound over 2 h. It was then frozen in optimal cutting temperature compound using a liquid-nitrogen cooled isopentane slurry (−140°C) and stored at −80°C. Muscle was sectioned (10 μm) by cryostat and mounted onto slides at –20°C.
Intramuscular neutral lipid content was assessed by Oil Red O staining followed by densitometric analysis of gray-scale images. Briefly, frozen sections were fixed with paraformaldehyde (4% in PBS) for 10 min, then exposed to Oil Red O (Sigma) for 1 h followed by two or three washes with distilled water. Oil Red O staining was quantified using ImageJ software (NIH). Images were converted to 8-bit gray-scale, and the integrated pixel density and area were obtained per muscle fiber. Two images from two different sections were analyzed per subject. For each group, the total numbers of fibers/subject analyzed were (mean ± SEM): 106 ± 6 (ODS), 118 ± 8 (ORD), 136 ± 23 (L).
Mitochondrial content was assessed using the total succinate dehydrogenase (SDH) activity assay in frozen muscle sections (described above). Sections were thawed and activity was assayed over a period of 1 h at 25°C. It was confirmed that the assay was linear over this period of time. Activity at the individual fiber level was visualized colorimetrically and quantified densitometrically from images captured with a Zeiss Axiovert microscope and a LCD camera. All fibers/section were included in the analysis of a section. See below for details of image analysis protocol.
Myofibrillar size analyses were conducted using laminin/meromycin staining. Briefly, the sections analyzed for SDH activity were fixed for 30 min on ice in 3.7% paraformaldehyde. After rinsing, immunostaining was performed for laminin (LAM-89; Sigma; L8271) at 1/125 of stock and subsequently for sarcomeric myosin (MF-20c; DSHB) at 1/5. Primary antibodies were detected using standard ABC amplification techniques (Vector; PK-7100). See below for details of image analysis protocol.
Fiber typing.
Sections immediately adjacent to the SDH/Laminin/MF-20 material were air dried (60 min at room temperature) and stained for type IIa (N2.26, IgG1; DSHB) and type IIx fibers (212-F, IgG1; P. Merrifield). Although 212F reacts with both type IIx and IIb proteins, fibers were categorized as IIx, because human muscle does not translate MyHC-IIb mRNA (7). Staining was enhanced with a biotin-avidin amplification step (M.O.M Basic Kit; Vector BMK 2202, Avidin/Biotin Blocking kit; Vector SP-2001). IIa fibers were detected with Avidin-488 (Invitrogen; A-21370). IIx fibers were detected using alkaline phosphatase (Vector Blue AP Kit III; SK-5300).
Sections from tissue also adjacent to the SDH/Laminin/MF-20 sections (opposing direction) were double labeled for type I (A4.840 IgM; DSHB) and type IIa (N2.26, IgG1; DSHB). IIa staining was repeated to detect hybrid fibers. Antibodies for type I fibers were diluted to 5% of stock and illuminated with Avidin-TXR (Invitrogen; A-820) diluted to 4%. Slides were mounted in anti-fade medium and stored at −20°C.
Image analysis.
Images were taken at equal exposure settings between patients (SDH at 0.13S, 212F at 0.16S, N.2.261 at 5.0S, A4.840 at 5.0S, LAM89 at 6.5S, MF-20 at 5.0S). Laminin and meromycin image sets were used to create analyzable region of interest (ROI) maps using Image-J software, as follows. Contrast was enhanced by eye in the laminin and meromycin photographs. Images were then merged and converted to binary images using Image-J software (http://reb.info.nih.gov/ij/). Each binary image was then completely watershed using the white-pen erasing tool, and damaged/incomplete fibers were removed. A 25 × 25 unit background control ROI was added to each ROI map to assess background intensity. Each fiber was identified as type I, type IIa, type IIx, or hybrid. Hybrid fibers comprised <1% of total fibers and were excluded from analysis. SDH images were analyzed for size and pixilation using the ROI maps and the “Analyze-Measure” function (Image J protocol). Differences in lighting and assay conditions were normalized using a background subtraction calculation. On average, 297 fibers (SD: 29) were examined from each patient.
Statistical analyses of muscle histology.
Means were compared using ANOVA with Tukey's post hoc tests using GraphPad Prism software (Version 4). Data are presented as group means ± SEM unless otherwise indicated.
RESULTS
Whole genome expression analyses
Subject characteristics for the entire cohort of 24 subjects were previously reported (5). For analyses of gene expression, we examined RNA from rectus femoris biopsies of six matched pairs of subjects (Table 1). The percent weight loss in the first 6 weeks of meal replacement was 49% greater (P < 0.0001) for the ODS subjects versus the ODR subjects.
TABLE 1.
Characteristics of study subjects for muscle whole genome expression analysis
| ODS | ODR | |
|---|---|---|
| Age (years) | 46.7 (6.1) | 45.0 (11.6) |
| N | 6 | 6 |
| Baseline at program entry | ||
| Weight (kg) | 98.1 (10.4) | 104.7 (16.9) |
| BMI (kg/m2) | 35.8 (3.8) | 40.7 (6.4) |
| Six weeks of 900 kcal diet | ||
| Weight (kg) | 87.9 (9.5) | 97.5 (16.1) |
| Weight lost (kg) | 10.1 (1.1) | 7.2 (1.1)* |
| % weight loss | 10.4 (0.6) | 7.0 (0.8)** |
| At biopsy | ||
| Weight (kg) | 89.0 (13.2) | 98.8 (21.5) |
| BMI (kg/m2) | 32.5 (5.2) | 35.6 (4.1) |
Mean (SD). *, P < 0.001; **, P < 0.0001. Study subjects with microarray data. The entire study cohort of 12 ODS and 12 ODR subjects were described previously (5).
Upregulation of gene sets involved in oxidative phosphorylation and glucose and fatty acid metabolism in ODS compared with ODR women
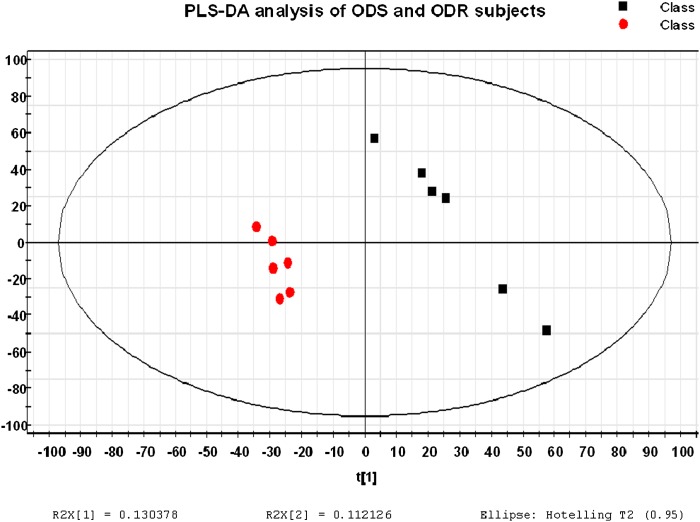
PLS-DA of whole-genome expression data displayed significant separation of the ODS and ODR subjects (Fig. 1), suggestive of significant differences in gene expression between the two groups. The top differentially expressed genes (Table 2) included FRZB (frizzled-related protein B), a negative regulator of canonical Wnt signaling, which, based on two probe sets, had 2.5-fold higher expression in diet-sensitive subjects. Expression of NR1D2 (REV-ERBβ), a transcriptional silencer that regulates expression of lipid uptake genes in skeletal muscle (17), was significantly decreased in skeletal muscle of diet-sensitive subjects, as was expression of ATP1B1 (Na/K-transporting ATPase β-1 chain) and SLC24A3 (sodium/potassium/calcium exchange member 3). GSEA revealed upregulation of gene sets in ODS versus ODR subjects relevant to oxidative phosphorylation, cell cycle, and protein turnover (Table 3).
Fig. 1.
PLSDA scoreplot. PLSDA demonstrates a clear separation between the ODS (red) and ODR (black) subjects based on global gene expression profiles.
TABLE 2.
Whole genome expression analyses of rectus femoris muscle from ODS versus ODR subjects
| Probe ID Symbol | Gene Name | Gene | Mean ODR | Mean ODS | ODS vs. ODR | Log Ratio ODS/ODR | P-Value t-test | Resolver |
|---|---|---|---|---|---|---|---|---|
| 203697_at | FRZB | frizzled related protein | 69.3 | 174.7 | up | 1.33 | 0.001 | 0.000 |
| 202814_s_at | HIS1 | HEXIM1 (HMBA inducible) | 323.0 | 197.7 | down | −0.71 | 0.002 | 0.020 |
| 202814_s_at | NRID2 | REV_ERBβ | 867.67 | 493.23 | down | −0.81 | 0.002 | 0.010 |
| 208029_s_at | LC27 | putative integral membrane transporter | 1819.6 | 1316.1 | down | −0.47 | 0.014 | 0.005 |
| 201242_S_AT | ATP1B1 | ATPase Na+ /K+ transporting β1 polypeptide | 4802.6 | 3076.0 | down | −0.64 | 0.021 | 0.002 |
| 57588_at | SLC24A3 | solute carrier family Na+/K+/Ca++ exchanger 3 | 24 486.0 | 295.7 | down | −0.72 | 0.034 | 0.050 |
The top differentially expressed genes identified through microarray analysis are shown. Columns 1–3 contain the Affymetrix reported probeset ID, the gene symbol, and the gene name, respectively. Columns 4 and 5 contain the average gene expression signals recorded for the ODR and ODS groups, respectively. Columns 6 and 7 show the direction of change and the magnitude of change (log2) in gene expression in the ODS group compared with the ODR group. Columns 8 and 9 show the statistical significance of differential expression for the genes as ascertained by a classical t-test or by a modified t-test based on the Resolver error model for gene expression analysis (Rosetta Inpharmatics, Seattle, WA).
TABLE 3.
GSEA
| Name | Size | ES | NES | Nominal P-value | FDR Q-value |
|---|---|---|---|---|---|
| RIBOSOMAL_PROTEINS | 92 | 0.72 | 3.16 | 0 | 0 |
| PROTEASOME | 17 | 0.65 | 1.91 | 0.002 | 0.04 |
| CELL_CYCLE_REGULATOR | 24 | 0.57 | 1.86 | 0 | 0.06 |
| MOOTHA_VOXPHOS | 77 | 0.41 | 1.74 | 0 | 0.18 |
| ELECTRON_TRANSPORT_CHAIN | 90 | 0.38 | 1.64 | 0.008 | 0.379 |
All genes on the chip were ranked by differences in expression between ODR and ODS subjects using the t-test. An enrichment score (ES) was calculated for each gene set. NES: enrichment score normalized for differences in gene set size; FDR q-value: false discovery rate.
We further explored these relationships in a second group of similar subjects, where we obtained vastus lateralis muscle biopsies from matched ODS and ODR subjects (n = 7 in each group). By quantitative-RT-PCR, the top differentially expressed genes with higher expression in ODS versus ODR included PPARD, relevant to mitochondrial biogenesis (P = 0.01), and SLC25A3 (P = 0.0009), which functions to catalyze the cotransport of phosphate and a proton into the mitochondrial matrix (Table 4). Expression of IRS2 was also higher (P = 0.02) in diet-sensitive versus diet-resistant subjects. Although to a lesser extent compared with the above studies of rectus femoris biopsies, vastus lateralis FRZB expression was increased by 11% in ODS subjects compared with ODR subjects (P = 0.02).
TABLE 4.
Top differentially expressed genes in vastus lateralis muscle biopsies from ODS versus ODR subjects
| Gene ID | Name | ODS | ODR | Fold Difference ODS vs. ODR | P |
|---|---|---|---|---|---|
| SLC25A3 | mitochondrial phosphate carrier protein | 244215 ± 11324 | 155457 ± 14401 | 1.6 | 0.0009 |
| IRS2 | insulin receptor substrate-2 | 9900 ± 1502 | 5653 ± 744 | 1.8 | 0.0390 |
| GPAM | mitochondrial glycerol-3-PO4 acyltransferase | 66168 ± 13975 | 35912 ± 4452 | 1.8 | 0.0367 |
| PPARD | PPAR delta | 11269 ± 870 | 7527 ± 850 | 1.5 | 0.0132 |
N = 7 in each group; P-values <0.05 and fold change > 1.5.
Characterization of subjects involved in muscle fiber phenotype analyses
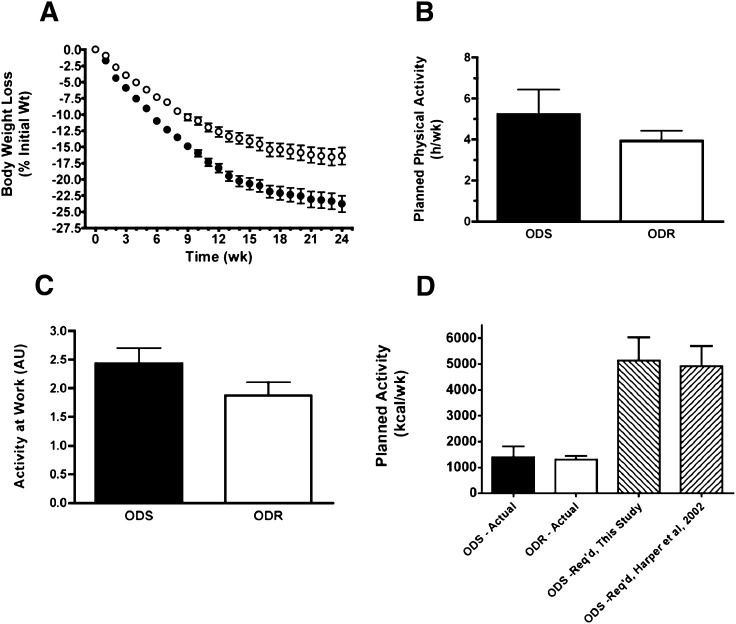
Fifteen additional pairs of ODS and ODR subjects were recruited for these analyses. Age, height, body weight, and BMI were similar for all ODS and ODR subjects at the onset of calorie restriction (Table 5). Similarly, age and height of the lean control subjects were comparable to those of obese subjects at the time of muscle biopsy. After 6 weeks of calorie restriction, ODS and ODR groups had lost 11.2 ± 0.34% and 7.3 ± 0.33% (P < 0.001) of initial body weight, respectively (Fig. 2A). The rate of weight loss was 45% greater for ODS than ODR subjects (1.52 ± 0.10 and 1.05 ± 0.08 kg/wk, respectively) at 6 weeks of calorie restriction (P < 0.001; Table 5).
TABLE 5.
Characteristics of subjects for vastus lateralis muscle phenotype analyses
| ODS | ODR | L | |
|---|---|---|---|
| N | 15 | 15 | 15 |
| Baseline at program entry | |||
| Age (years) | 45.4 ± 2.3 | 46.7 ± 2.7 | — |
| Body mass (kg) | 102.8 ± 2.9 | 101.9 ± 3.1 | — |
| BMI (kg/m2) | 38.5 ± 0.9 | 38.5 ± 1.3 | — |
| Six weeks of CR | |||
| Body mass (kg) | 91.4 ± 2.5 | 94.4 ± 2.9 | — |
| BMI (kg/m2) | 34.2 ± 0.7 | 35.7 ± 1.3** | — |
| Mass lost (kg) | 11.4 ± 0.6 | 7.5 ± 0.4** | — |
| Rate of mass loss (g/day) | 217.2 ± 13.0 | 149.4 ± 11.6** | — |
| Six months weight management | |||
| Body mass (kg) | 78.0 ± 1.9 | 85.0 ± 2.6 | — |
| BMI (kg/m2) | 29.2 ± 0.4 | 32.2 ± 1.2** | — |
| Mass lost (kg) | 13.3 ± 1.3 | 9.4 ± 1.2** | — |
| Rate of mass loss (g/day) | 45.6 ± 7.3 | 37.3 ± 8.0 | — |
| At biopsy | |||
| Age (years) | 48.5 ± 2.1 | 50.7 ± 2.6 | 51.1 ± 2.4 |
| Height (cm) | 163.4 ± 1.6 | 162.9 ± 1.6 | 163.0 ± 2.0 |
| Body mass (kg) | 91.1 ± 3.2 | 93.5 ± 3.1 | 51.9 ± 1.5** |
| BMI (kg/m2) | 34.1 ± 1.1 | 35.3 ± 1.3 | 19.5 ± 0.4** |
| Adipose mass (kg) | 40.4 ± 1.9 | 43.9 ± 2.2 | 12.3 ± 1.0** |
| Fat-free mass (kg) | 50.6 ± 1.4 | 49.7 ± 1.1 | 39.3 ± 0.6** |
CR, calorie restriction. *, P < 0.05; **, P < 0.01. Data are mean ± SEM.
Fig. 2.
Weight loss and physical activity characteristics of obese subjects involved in vastus lateralis muscle phenotype analyses. A: Rate of weight loss, expressed as a percentage of initial body weight in ODS (filled symbols) and ODR (open symbols) subjects. (Mean ± SEM; ODS: N = 15; ODR: N = 15). B: Work-related physical activity in arbitrary units (1 = inactive; 5 = very active). C: Planned physical activity (hours/week). D: Planned PAE (kcal/wk): actual and the calculated amount required for the additional weight loss of ODS compared with ODR subjects. The energy required for the additional weight loss of ODS compared with ODR subjects was calculated for subjects involved in this study and in our previous study (5).
Physical activity level does not account for weight loss differences
To exclude the possibility that differences in weight loss were affected by PAE, we assessed both activity at work and planned activity. As part of the program, a physical activity questionnaire was administered prior to the onset of calorie restriction. Results were used to ensure that activity was maintained at preprogram levels over the first 6 weeks of the program. Activity at work was assessed using a 5-point Likkert scale with a value of 1 defined as an “inactive” work environment (e.g., seated almost exclusively) and a value of 5 defined as a “very active” work environment (e.g., walking nearly continuously). ODS and ODR subjects had similar levels of work-related activity (Fig. 2B). Similarly, subjects were asked to record the frequency, duration, and type of planned weekly exercise activities. There were no differences in planned activity between ODS and ODR cohorts (h/wk; Fig. 2C). We then calculated the PAE (in kcal · wk−1) using the specific energy expenditure coefficients (kcal · kg−1. min−1) of each planned exercise (18). There were no significant differences between the ODS and ODR groups (Fig. 2D). To explore the theoretical energy cost associated with the difference in weight loss at the 6 week time point (3.9 kg) between ODS and ODR groups, we then calculated the energy expenditure that would be required for ODS subjects to lose this additional weight. We used the estimated energy densities of lean body mass (7.6 MJ/kg) and fat body mass (39.5 KJ/kg) from Hall (19) and the MRI whole body results from Ross et al. (20). The latter reported body weight loss (5.2 kg), fat mass loss (4.1 kg), and skeletal muscle mass loss (0.5 kg) with calorie restriction in a nonexercised obese group. We then assumed that the remainder of the weight, not accounted for by either fat mass loss or skeletal muscle mass, also fell into the lean mass category (i.e., total lean = 1.1 kg), equivalent to an 80/20 split for fat/lean mass percentages. This conservatively underestimates the ratio for our study subjects (5), because they were slightly more obese than the group studied by Ross et al. (20). This theoretical energy content (lost as body weight) is almost 4-fold greater than the calculated PAE (Fig. 2D), arguing against a role for differences in activity underlying the weight loss variability.
Increased proportion of oxidative fibers in ODS subjects
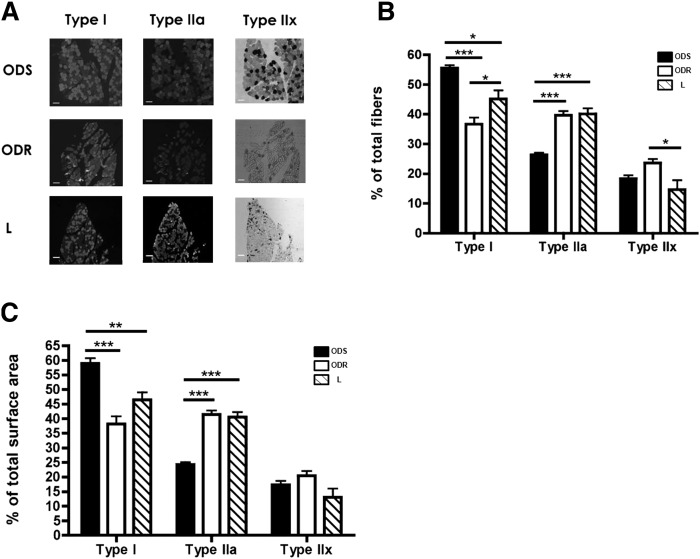
Six of the latter 15 pairs of ODS and ODR subjects consented to vastus lateralis biopsies. Fiber typing analyses revealed that the proportion of type I oxidative fibers was 52% higher in the ODS compared with the ODR group (P < 0.001). It was also significantly greater in the ODS than in the lean subjects (P < 0.05). Of additional importance is the significantly decreased proportion of type I fibers in the ODR compared with the lean subjects (P < 0.05). Type I fibers comprised only 36.7 ± 2.5% of all fibers in ODR subjects, whereas type I fibers in ODS and lean subjects accounted for 55.6 ± 1.1% and 45.2 ± 3.0% of fibers, respectively (Fig. 3 A, B).
Fig. 3.
Increased proportion of oxidative (type I) fibers in ODS than in ODR and lean subjects. A: Representative images of vastus lateralis sections from ODS, ODR, and lean subjects. Scale bar represents 250 μm. B: Fiber type proportions, expressed as a proportion of total fibers in ODS, ODR, and lean groups (mean ± SEM; N = 6 matched pairs of subjects). C: Fiber type proportions, expressed as a percentage of muscle surface area in ODS, ODR, and lean groups (mean ± SEM; N = 6 matched pairs of subjects).
While there were no fiber-specific differences in fiber size, the proportional contributions of the fiber types to muscle cross-sectional area differed between subject groups. The proportion of muscle area as type I oxidative fibers were 55% greater in ODS than in ODR muscle (P < 0.001; Fig. 3C). Interestingly, the contribution of type I fibers to muscle cross-sectional area was 28% greater in ODS than in muscle from lean subjects. There were no proportional differences by area in the contribution of type I fibers between the ODR and lean groups. The proportion of muscle area as type IIa fibers was 41% and 40% lower in ODS compared with ODR and lean subjects, respectively (P < 0.001). There were no significant differences in the proportion of area as type IIx fibers between groups.
Myofibrillar hypertrophy in ODS subjects
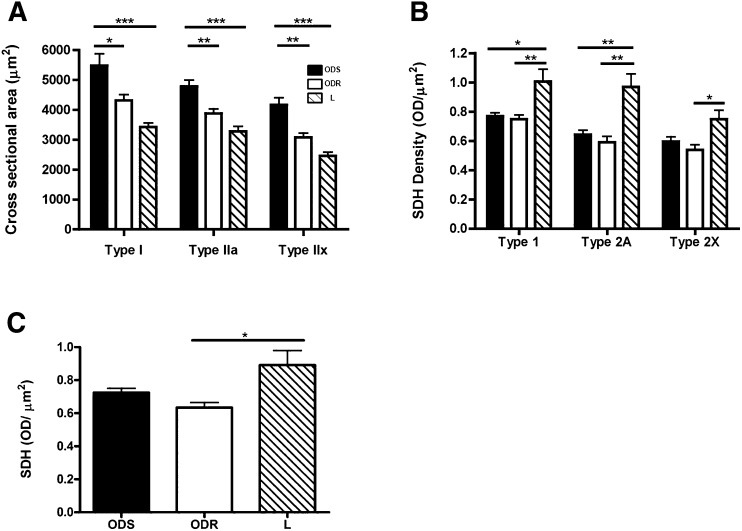
We then sought to characterize differences in fiber size. The cross-sectional area of type I fibers was 27% and 60% greater in ODS than in ODR and lean subjects (P < 0.05; P < 0.001), respectively (Fig. 4A). The type IIa cross-sectional area was 23% and 46% greater in ODS muscle compared with ODR and lean muscle (P < 0.01; P < 0.001), respectively. Finally, the IIx area was 35% and 69% greater in ODS than in ODR and lean muscle (P < 0.01; P < 0.001), respectively. Thus, regardless of fiber type, the cross-sectional fiber area was greater in muscle of ODS subjects.
Fig. 4.
Fiber surface area and SDH activity analyses. A: Hypertrophy of types I, IIa, and IIx in vastus lateralis of ODS compared with ODR and lean subjects. Average fiber cross-sectional area in ODS, ODR, and lean groups (mean ± SEM; N = 6 matched pairs of subjects). Mitochondrial content: B: SDH activity per unit area of the three major fiber types in vastus lateralis of ODS, ODR, and lean subjects (mean ± SEM; N = 6 matched pairs of subjects). C: Overall SDH activity per unit area of vastus lateralis of ODS, ODR, and lean subjects (Mean ± SEM; N = 6 matched pairs of subjects).
Lower muscle fiber-specific and mass-specific mitochondrial content in obesity
To determine possible differences in mitochondrial content between groups, we assessed total activity of SDH. When SDH activity was analyzed on a fiber-specific basis (Fig. 4B), activity was lower in types I and IIa in ODS and ODR compared with lean subjects. In type IIx fibers, activity was lower in ODR than in lean subjects (P < 0.05). When analyzed on a mass-specific basis (Fig. 4C), activity was lower in muscle of ODR compared with lean subjects (P < 0.05). Interestingly, there were no differences in SDH activity between ODS and ODR muscle (Fig. 4B, C; see “Discussion”).
Higher intramuscular neutral lipid content in obesity
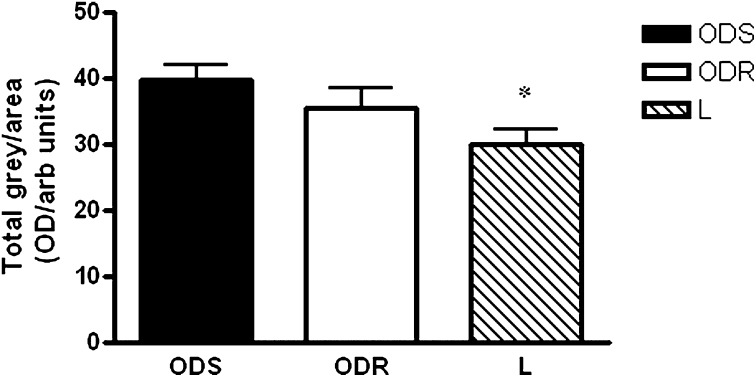
Greater levels of intramuscular lipid have been reported in obese compared with lean subjects (21–23), as well as in type I versus type II fibers from vastus lateralis (21, 22, 24, 25). It was therefore of interest to determine intramuscular lipid content in ODS, ODR, and L muscle ex vivo. Images of Oil Red O-stained muscle sections were converted to gray-scale, quantified densitometrically, and values expressed per total area of muscle analyzed. Lipid content/muscle area was ∼26% higher in muscle from obese subjects (ODR and ODS combined) compared with L subjects (P = 0.02, unpaired t-test; not shown). When the obese subjects were separated into ODS and ODR, only lipid content of ODS muscle was significantly higher, by ∼33%, than that of L muscle (P < 0.05, one-way ANOVA, Bonferroni post test, ODS vs. L; Fig. 5).
Fig. 5.
Intramuscular lipid content. Intramuscular lipid content was determined by Oil Red O staining followed by densitometric analyses of muscle fibers. Optical densities (OD) were expressed per total area of muscle analyzed. Lipid content of muscle from L subjects was significantly lower than that from ODS subjects (*: P < 0.05; one-way ANOVA, Bonferroni post hoc test, L vs. ODS). Values are means ± SEM, N = 6/group.
DISCUSSION
Distinct gene expression signatures in ODS compared with ODR and lean control women
Skeletal muscle capacity for oxidative metabolism is an important determinant of whole body energy metabolism, relevant to obesity and weight loss success. Previous reports have revealed that gene sets involved in oxidative phosphorylation (OXPHOS) and mitochondrial function are downregulated in subjects with type 2 diabetes as compared with controls (8, 9). In support of the hypothesis that differences in weight loss in response to calorie restriction are in part determined by innate differences in skeletal muscle characteristics, global gene expression profiles allowed separation of the ODS and ODR subjects (Fig. 1). Due to the phenotypic similarities between ODS and ODR subjects (both groups being obese), the differences in gene expression were often subtle. Limiting sample sizes, high variability in human samples, and small differences in gene expression reduced the statistical power of the study, and consequently, too much reliance on statistical significance, especially multiple testing adjustments, would have resulted in false negatives. We therefore searched for biological relevance in addition to statistical significance with greater emphasis on the former. In this regard, we have essentially followed the procedures outlined in Mootha et al. (8) and have used the permutation-based P-value and the magnitude of the normalized enrichment scores as the criterion for pathway evaluation without further consideration for multiple-testing corrections. By GSEA of microarray data from rectus femoris biopsies, we have demonstrated upregulation of gene sets relevant to oxidative phosphorylation, cell cycle, and protein turnover in ODS compared with ODR subjects.
Pertinent to the role of Wnt signaling in the determination of muscle fiber type, we have demonstrated increased rectus femoris expression of FRZB in ODS compared with ODR women. Wnts are expressed in skeletal muscle and modulate myocyte differentiation and the expression of oxidative versus glycolytic fibers (26). FRZB is a negative regulator of Wnt/Frizzled-1 signaling via the β catenin pathway but does not impair Wnt-5a signaling via the Ca/PKC pathway and thus may promote oxidative fiber formation (27). Further studies are required to address this hypothesis.
Differential regulation of genes relevant to mitochondrial biogenesis and function were confirmed by quantitative-RT-PCR in the second study. In particular, expression of PPARD was increased by 1.5-fold in ODS versus ODR women. In concert with PGC1α, PPARδ promotes a switch to type I fibers and markedly enhances the transcription of oxidative phosphorylation and uncoupling protein genes. Consistent with enhanced oxidative capacity, the expression of mitochondrial phosphate carrier protein (SLC25A3) was increased 1.6-fold and that of mitochondrial glycerol-3-phosphate acyltransferase (GPAM) by 1.8-fold in vastus lateralis of ODS versus ODR subjects.
Muscle fiber phenotypic characteristics
In human muscles, unlike those of small mammals (e.g., rodents), there is substantial variability in fiber type proportions (28, 29). Despite wide acceptance of inter- individual fiber type heterogeneity and its importance in exercise performance variability among athletes (30), the role of fiber type heterogeneity in weight loss is very poorly understood. Our results indicate that a greater proportion of type I fibers in ODS is a predominant feature distinguishing the ODS from ODR and lean subjects. Comprehensive investigations of fiber type composition of human vastus lateralis muscle have shown that type I fibers typically account for 51–55% of all fibers, regardless of age (31). Our results indicate that, among obese individuals, fiber composition does vary and may affect propensity for weight loss.
It appears likely that intrinsic energetic efficiency is lower in type I compared with type II fibers. The latter is supported by in vivo spectroscopy results of higher in vivo uncoupling in muscles that are largely oxidative than in those that are largely glycolytic (32). Indeed, this is consistent with our findings of increased mitochondrial proton leak in rectus femoris of ODS compared with ODR subjects (5).
Potential mechanisms contributing to greater oxidative fiber content
Fiber type is determined predominantly by genetic and developmental factors. Importantly, weight loss in obese subjects does not change muscle fiber type proportions (33). However, hypoxia, endurance exercise training, and motor neuron activity promote conversion of type IIx to type IIa fibers by increasing intracellular Ca2+ and activating transcription of myoglobin, MEF2, IGF, PPARGC1A, and gene sets important in mitochondrial biogenesis and oxidative metabolism (34–42). In our study, there were no differences in physical activity in ODS and ODR subjects. Moreover, the amount of activity required for fiber type conversion (i.e., from the highly glycolytic type IIx to the oxidative glycolytic type IIa fibers) is much greater than the low intensity day-to-day activity of our subjects [e.g., (43)]. Indeed recent findings (11) demonstrate in overweight/obese adults that 16 weeks of diet, or diet and exercise (3–5 d/wk of 30–40 min/d of moderate intensity treadmill walking), resulted in no change in vastus lateralis fiber types. Thus, the observed differences in fiber composition between ODS and ODR subjects may have genetic or epigenetic origins.
Hypertrophy of muscle fibers
The greater cross-sectional area of all fiber types in ODS compared with ODR does not appear to be affected by subject age. We studied subjects ranging in age from 36 to 62 years; all but one was younger than 60 years. Our finding is consistent with observations that age-associated sarcopenia, which predominantly reflects a loss of fiber number and to a lesser degree fiber size, which are not substantially manifested until after age 60 (31). Moreover, although loss of muscle mass with aging has been associated with reduced fiber size and number (31, 44), the relative proportion of type I to type II fibers in vastus lateralis (45) and other skeletal muscles (46) is not altered with aging. Thus, age does not appear to be a factor contributing to the observed differences in fiber size in the present investigation.
Increased oxidative fiber content, but not mitochondrial content, is associated with greater rate of weight loss in diet-sensitive subjects
Because the lower mass-specific mitochondrial SDH activity in type I and II fibers is comparable in ODS and ODR groups compared with the lean group (Fig. 4 B, C), the loss of mitochondrial content, per se, does not appear to be an immediate determinant of poor diet responsiveness. However, we found increased type I and decreased type IIa fibers in ODS subjects with reciprocal decrease and increases in types I and IIa fibers in ODR subjects. Because both types I and IIa fibers have high mitochondrial contents compared with the highly glycolytic type IIx fibers (21, 47), the absence of significant differences in total mitochondrial content between ODS and ODR muscle is not surprising. Type IIa fibers are estimated to have approximately 80% of the mitochondrial content as type I fibers (21, 47). Notably, muscle mitochondrial capacity is not altered (e.g., increased) by weight loss (11) despite increased insulin sensitivity and decreased intramyocellular lipid. Thus, although reduced mitochondrial content may reduce overall weight loss responsiveness in obese individuals, it does not contribute to the weight loss differential observed here. While speculative, but consistent with the lower energetic efficiency of type I fibers (32), our findings support the idea that mitochondria from type I fibers are energetically distinct from those in type IIa fibers, and further research is needed.
Intramuscular neutral lipid content
Intramuscular lipid content has been reported to be higher in obese compared with lean subjects (21–23). Our results also demonstrate greater intramuscular lipid in obese (ODS and ODR, combined) compared with L muscle. Higher lipid content has been observed in type I versus type II fibers from vastus lateralis (21, 22, 24, 25). Thus, the greater lipid content of ODS (but not ODR) compared with L muscle is generally consistent with the higher proportion of type I fibers in ODS muscle. That the increase (∼33%) in lipid content of ODS versus L was slightly greater than the increase (∼26%) in the proportion of type I fibers suggests that type I fibers within ODS muscle contain slightly more lipid as compared with L muscle. Exercise training is associated with increased muscle lipid content (48, 49); however, there were no differences in physical activity between ODS and ODR subjects. Weight loss is also associated with a reduction in intramuscular triglyceride levels (23). The lower degree of weight loss exhibited by ODR subjects may have resulted in a reduced lipid loss in ODR muscle, leading to the greater-than- predicted lipid content, based on type I fiber content, of ODR compared with ODS muscle.
Rate of weight loss was not compromised by initial body composition
Initial body composition can influence rate of weight loss at a given level of calorie restriction and total energy expenditure (TEE) (19, 50). Ideally, stored fat is mobilized during energy deficit to forestall excessive catabolism of lean tissue. However, compared with fat mass (39.5 MJ · kg−1), ∼5.2 times more lean mass (7.6 MJ · kg−1) must be lost to satisfy an energy deficit (51). Thus, the greater weight loss ODS versus ODR could potentially reflect a greater proportional mobilization of energy from lean reserves.
In our study, average body fat mass of ODS and ODR subjects prior to calorie restriction was well above 30 kg and comparable between groups. In calorie-restricted obese subjects with an initial body fat mass of greater than 30 kg, fat is the predominant component lost (51). MRI-based estimates of calorie-restricted obese women revealed that muscle and body fat accounted for ∼10% and 80% of weight lost, respectively, when initial body fat mass was 39.2 kg (20). Thus, it seems unlikely that the greater weight loss by the ODS group reflects a greater mobilization of lean body mass, implying that ODS subjects had a greater intrinsic TEE than ODR subjects.
Estimates of energy expenditure
Daily TEE of ODS and ODR subjects during calorie restriction would equal the energy consumed (900 kcal/day) plus the estimated energy content of body mass lost. The energy content of weight loss during the first 6 weeks for ODS and ODR subjects (11.4 and 7.5 kg, respectively) was approximately 40,600 ± 1,960 kcal (170 ± 8.2 MJ) and 25,900 ± 1,190 kcal (108 ± 5.0 MJ), respectively, according to the estimates of Hall (51). The estimated daily TEE for ODS and ODR subjects is 1,867 ± 47 kcal (7,812 ± 197 KJ) and 1,517 ± 28 kcal (6,347 ± 117 KJ), respectively. The TEE of the ODS group is thus 350 kcal/day (or 23%) greater than that of the ODR group (P < 0.001). There were no differences in total body mass, fat mass, or fat-free mass (Table 5), consistent with intrinsic differences in TEE between the groups.
Estimates of PAE revealed no differences in either expenditure related to physical activity at work or planned physical activity. It is noteworthy that the PAE in ODS and ODR (1,397 ± 420 kcal · d−1 and 1,254 ± 129 kcal · d−1) account for only ∼25% of the difference theoretically required to explain the weight loss difference.
In summary, these studies demonstrate altered expression of genes relevant to fuel uptake and oxidation in a comparison of well-matched obese individuals who differ solely in their propensity for weight loss. Our findings of vastus lateralis fiber hypertrophy and a higher proportion of oxidative (type I) and lower oxidative-glycolytic (type IIa) fibers in ODS compared with ODR subjects and to lean controls, in conjunction with no differences in mass-specific mitochondrial content are consistent with the idea that the differences in the bioenergetic demands of types I and IIa fibers (32) are quantitatively important. Overall, these findings support the conclusion that skeletal muscle capacity for oxidative metabolism influences the propensity to gain or lose weight.
Acknowledgments
The authors are thankful to our research volunteers for their generous participation in this study. The authors also are grateful for the exceptional support from Heather Doelle, Brenda Bradley, and Sybil Hebert for their work in recruitment and clinical data collection; from Linda Jui and Mahmoud Salkhordeh for their technical expertise in histology; and for Dr. Mark Tarnopolsky (McMaster University) for technical advice on muscle biopsy procedures. The antibody for type IIx fibers was generously provided by Dr. Peter Merrifield (University of Western Ontario).
Footnotes
Abbreviations:
- BMI
- body mass index
- FRZB
- frizzled-related protein B
- GSEA
- gene set enrichment analyses
- ODR
- obese diet-resistant
- ODS
- obese diet-sensitive
- PAE
- physical activity energy expenditure
- PLS-DA
- partial least squares discriminant analysis
- SDH
- succinate dehydrogenase
- SLC24A3
- sodium/potassium/calcium exchange member 3
REFERENCES
- 1.Bell C. G., Walley A. J., Froguel P. 2005. The genetics of human obesity. Nat. Rev. Genet. 6: 221–234. [DOI] [PubMed] [Google Scholar]
- 2.Sims E. A., Danforth E., Jr., Horton E. S., Bray G. A., Glennon J. A., Salans L. B. 1973. Endocrine and metabolic effects of experimental obesity in man. Recent Prog. Horm. Res. 29: 457–496. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard C., Tremblay A., Despres J. P., Nadeau A., Lupien P. J., Theriault G., Dussault J., Moorjani S., Pinault S., Fournier G. 1990. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 322: 1477–1482. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C., Tremblay A. 1997. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J. Nutr. 127: 943S–947S. [DOI] [PubMed] [Google Scholar]
- 5.Harper M. E., Dent R., Monemdjou S., Bezaire V., Van Wyck L., Wells G., Kavaslar G. N., Gauthier A., Tesson F., McPherson R. 2002. Decreased mitochondrial proton leak and reduced expression of uncoupling protein 3 in skeletal muscle of obese diet-resistant women. Diabetes. 51: 2459–2466. [DOI] [PubMed] [Google Scholar]
- 6.Zurlo F., Larson K., Bogardus C., Ravussin E. 1990. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Invest. 86: 1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley D. E., He J., Menshikova E. V., Ritov V. B. 2002. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 51: 2944–2950. [DOI] [PubMed] [Google Scholar]
- 8.Mootha V. K., Lindgren C. M., Eriksson K. F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstrale M., Laurila E., et al. 2003. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34: 267–273. [DOI] [PubMed] [Google Scholar]
- 9.Patti M. E., Butte A. J., Crunkhorn S., Cusi K., Berria R., Kashyap S., Miyazaki Y., Kohane I., Costello M., Saccone R., et al. 2003. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA. 100: 8466–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simoneau J. A., Veerkamp J. H., Turcotte L. P., Kelley D. E. 1999. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 13: 2051–2060. [DOI] [PubMed] [Google Scholar]
- 11.Toledo F. G., Menshikova E. V., Azuma K., Radikova Z., Kelley C. A., Ritov V. B., Kelley D. E. 2008. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 57: 987–994. [DOI] [PubMed] [Google Scholar]
- 12.Karjalainen J., Tikkanen H., Hernelahti M., Kujala U. M. 2006. Muscle fiber-type distribution predicts weight gain and unfavorable left ventricular geometry: a 19 year follow-up study. BMC Cardiovasc. Disord. 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanner C. J., Barakat H. A., Dohm G. L., Pories W. J., MacDonald K. G., Cunningham P. R., Swanson M. S., Houmard J. A. 2002. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 282: E1191–E1196. [DOI] [PubMed] [Google Scholar]
- 14.Abou Mrad J., Yakubu F., Lin D., Peters J. C., Atkinson J. B., Hill J. O. 1992. Skeletal muscle composition in dietary obesity-susceptible and dietary obesity-resistant rats. Am. J. Physiol. 262: R684–R688. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian A., Kuehn H., Gould J., Tamayo P., Mesirov J. P. 2007. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics. 23: 3251–3253. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., et al. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramakrishnan S. N., Lau P., Burke L. J., Muscat G. E. 2005. Rev-erbbeta regulates the expression of genes involved in lipid absorption in skeletal muscle cells: evidence for cross-talk between orphan nuclear receptors and myokines. J. Biol. Chem. 280: 8651–8659. [DOI] [PubMed] [Google Scholar]
- 18.Wilson P. W., Paffenbarger R. S., Jr., Morris J. N., Havlik R. J. 1986. Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am. Heart J. 111: 1177–1192. [DOI] [PubMed] [Google Scholar]
- 19.Hall K. D. 2008. What is the required energy deficit per unit weight loss? Int J Obes (Lond). 32: 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross R., Janssen I., Dawson J., Kungl A. M., Kuk J. L., Wong S. L., Nguyen-Duy T. B., Lee S., Kilpatrick K., Hudson R. 2004. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes. Res. 12: 789–798. [DOI] [PubMed] [Google Scholar]
- 21.He J., Watkins S., Kelley D. E. 2001. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 50: 817–823. [DOI] [PubMed] [Google Scholar]
- 22.Malenfant P., Joanisse D. R., Theriault R., Goodpaster B. H., Kelley D. E., Simoneau J. A. 2001. Fat content in individual muscle fibers of lean and obese subjects. Int. J. Obes. Relat. Metab. Disord. 25: 1316–1321. [DOI] [PubMed] [Google Scholar]
- 23.Goodpaster B. H., Theriault R., Watkins S. C., Kelley D. E. 2000. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 49: 467–472. [DOI] [PubMed] [Google Scholar]
- 24.Howald H., Hoppeler H., Claassen H., Mathieu O., Straub R. 1985. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch. 403: 369–376. [DOI] [PubMed] [Google Scholar]
- 25.van Loon L. J., Schrauwen-Hinderling V. B., Koopman R., Wagenmakers A. J., Hesselink M. K., Schaart G., Kooi M. E., Saris W. H. 2003. Influence of prolonged endurance cycling and recovery diet on intramuscular triglyceride content in trained males. Am. J. Physiol. Endocrinol. Metab. 285: E804–E811. [DOI] [PubMed] [Google Scholar]
- 26.Anakwe K., Robson L., Hadley J., Buxton P., Church V., Allen S., Hartmann C., Harfe B., Nohno T., Brown A. M., et al. 2003. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development. 130: 3503–3514. [DOI] [PubMed] [Google Scholar]
- 27.Wang S., Krinks M., Moos M., Jr. 1997. Frzb-1, an antagonist of Wnt-1 and Wnt-8, does not block signaling by Wnts -3A, -5A, or -11. Biochem. Biophys. Res. Commun. 236: 502–504. [DOI] [PubMed] [Google Scholar]
- 28.Delp M. D., Duan C. 1996. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J. Appl. Physiol. 80: 261–270. [DOI] [PubMed] [Google Scholar]
- 29.Saltin B., Henriksson J., Nygaard E., Andersen P., Jansson E. 1977. Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann. N. Y. Acad. Sci. 301: 3–29. [DOI] [PubMed] [Google Scholar]
- 30.Zierath J. R., Hawley J. A. 2004. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol. 2: e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lexell J., Taylor C. C., Sjostrom M. 1988. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 84: 275–294. [DOI] [PubMed] [Google Scholar]
- 32.Conley K. E., Amara C. E., Jubrias S. A., Marcinek D. J. 2007. Mitochondrial function, fibre types and ageing: new insights from human muscle in vivo. Exp. Physiol. 92: 333–339. [DOI] [PubMed] [Google Scholar]
- 33.Kempen K. P., Saris W. H., Kuipers H., Glatz J. F., Van Der Vusse G. J. 1998. Skeletal muscle metabolic characteristics before and after energy restriction in human obesity: fibre type, enzymatic beta-oxidative capacity and fatty acid-binding protein content. Eur. J. Clin. Invest. 28: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 34.Booth F. W., Thomason D. B. 1991. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol. Rev. 71: 541–585. [DOI] [PubMed] [Google Scholar]
- 35.Jarvis J. C., Mokrusch T., Kwende M. M., Sutherland H., Salmons S. 1996. Fast-to-slow transformation in stimulated rat muscle. Muscle Nerve. 19: 1469–1475. [DOI] [PubMed] [Google Scholar]
- 36.Pette D. 1998. Training effects on the contractile apparatus. Acta Physiol. Scand. 162: 367–376. [DOI] [PubMed] [Google Scholar]
- 37.Olson E. N., Williams R. S. 2000. Calcineurin signaling and muscle remodeling. Cell. 101: 689–692. [DOI] [PubMed] [Google Scholar]
- 38.James G., Olson E. N. 1990. Fatty acylated proteins as components of intracellular signaling pathways. Biochemistry. 29: 2623–2634. [DOI] [PubMed] [Google Scholar]
- 39.Naya F. J., Mercer B., Shelton J., Richardson J. A., Williams R. S., Olson E. N. 2000. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 275: 4545–4548. [DOI] [PubMed] [Google Scholar]
- 40.Lin J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N., et al. 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 418: 797–801. [DOI] [PubMed] [Google Scholar]
- 41.Wu H., Kanatous S. B., Thurmond F. A., Gallardo T., Isotani E., Bassel-Duby R., Williams R. S. 2002. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 296: 349–352. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y. X., Zhang C. L., Yu R. T., Cho H. K., Nelson M. C., Bayuga-Ocampo C. R., Ham J., Kang H., Evans R. M. 2004. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2: e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hultman E. 1995. Fuel selection, muscle fibre. Proc. Nutr. Soc. 54: 107–121. [DOI] [PubMed] [Google Scholar]
- 44.Lexell J., Taylor C. C. 1991. Variability in muscle fibre areas in whole human quadriceps muscle: effects of increasing age. J. Anat. 174: 239–249. [PMC free article] [PubMed] [Google Scholar]
- 45.Lexell J., Henriksson-Larsen K., Winblad B., Sjostrom M. 1983. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 6: 588–595. [DOI] [PubMed] [Google Scholar]
- 46.Sato T., Akatsuka H., Kito K., Tokoro Y., Tauchi H., Kato K. 1984. Age changes in size and number of muscle fibers in human minor pectoral muscle. Mech. Ageing Dev. 28: 99–109. [DOI] [PubMed] [Google Scholar]
- 47.Gregory C. M., Vandenborne K., Dudley G. A. 2001. Metabolic enzymes and phenotypic expression among human locomotor muscles. Muscle Nerve. 24: 387–393. [DOI] [PubMed] [Google Scholar]
- 48.Tarnopolsky M. A., Rennie C. D., Robertshaw H. A., Fedak-Tarnopolsky S. N., Devries M. C., Hamadeh M. J. 2007. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292: R1271–R1278. [DOI] [PubMed] [Google Scholar]
- 49.Pruchnic R., Katsiaras A., He J., Kelley D. E., Winters C., Goodpaster B. H. 2004. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am. J. Physiol. Endocrinol. Metab. 287: E857–E862. [DOI] [PubMed] [Google Scholar]
- 50.Forbes G. B. 2000. Body fat content influences the body composition response to nutrition and exercise. Ann. N. Y. Acad. Sci. 904: 359–365. [DOI] [PubMed] [Google Scholar]
- 51.Hall K. D. 2007. Body fat and fat-free mass inter-relationships: Forbes's theory revisited. Br. J. Nutr. 97: 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]