Abstract
Thus far, liver, intestine, heart, and placenta have been shown to secrete apolipoprotein (apo)B-containing lipoproteins. In the present study, we first investigated lipoproteins in human follicular fluid (FF), surrounding developing oocytes within the ovary, as well as in corresponding plasma samples (n = 12). HDL cholesterol within FF correlated well with plasma HDL cholesterol (r = 0.80, P < 0.01), whereas VLDL cholesterol did not, indicating that VLDL in FF might originate directly from the granulosa cells producing FF. Primary human granulosa cells expressed apoB, microsomal triglyceride transfer protein, and apoE, but not the apoB-editing enzyme apobec-1. Using 3H-leucine, we show that granulosa cells secrete apoB100-containing lipoproteins and that secretion can be stimulated by adding oleate to the medium (+83%). With electron microscopy, apoB-containing lipoproteins within the secretory pathway of human granulosa cells were directly visualized. Finally, we found a positive relationship between apoB levels in FF and improved fertility parameters in a population of 27 women undergoing in vitro fertilization. This study demonstrates that human granulosa cells assemble and secrete apoB100-containing lipoproteins, thereby identifying a novel cell type equipped with these properties. These results might have important implications for female infertility phenotypes as well as for the development of drugs targeting the VLDL production pathway.
Keywords: triglycerides, cholesterol, VLDL, infertility, follicular fluid, MTP, metabolism
The ability to assemble and secrete apolipoprotein (apo) B-containing lipoproteins has long been known for enterocytes as well as hepatocytes (1, 2). These cells use chylomicrons to secrete resorbed cholesterol and triglycerides (TG) in the case of enterocytes (3), and VLDL to export endogenously synthesized or internalized cholesterol and TG in the case of hepatocytes (4, 5). ApoB-containing lipoproteins are also secreted by the human placenta, supposedly to transfer lipids from the maternal circulation to the fetus (6). Recently, another cell type in the body has been recognized to be capable of producing apoB-containing lipoproteins, namely cardiomyocytes (7). In the heart, VLDL secretion is thought to represent a means of protecting the organ against toxicity associated with TG accumulation (8, 9). In contrast, other peripheral cells utilize apoB-containing lipoproteins to meet their supply with TG and cholesterol.
Developing oocytes within ovarian follicles grow rapidly and require supply with energy and cholesterol (10). Oocytes are surrounded by follicular fluid (FF) that in contrast to human plasma mainly contains HDL cholesterol, the smallest lipoprotein subclass (11, 12). Although expression of the LDL receptor as well as of LDL receptor-related protein 4 has been reported for mammalian oocytes (13, 14), most research at present has been focused on HDL within FF. Granulosa cells, the major estrogen-producing cell type of the follicle, line the follicle and are shielded against the blood compartment by a basal membrane (15–17). Whereas HDL are thought to enter the FF by diffusion (11), apoB-containing lipoproteins, which are considerably larger in size, are not expected to do so. However, given the presence of receptors for apoB-containing lipoproteins on oocytes, we hypothesized that granulosa cells might display an intrinsic ability to assemble and secrete apoB-containing lipoproteins. Therefore, we tested this hypothesis in the present study. Our results provide evidence that human granulosa cells represent a novel cell type that is capable of assembly and secretion of apoB-containing lipoproteins.
MATERIALS AND METHODS
Generation of human FF and isolation of granulosa cells
Human FF was obtained from women who were undergoing in vitro fertilization (IVF) (Center of Sterility, CHU Dijon or Klinik fuer Frauenheilkunde und Geburtshilfe, Luebeck) and were following a follicle-stimulation regimen, including the injection of 10,000 IU of human chorionic gonadotrophin 36 h before transvaginal follicle puncture under ultrasound guidance exactly as previously described (18, 19). After oocyte isolation, FFs were obtained, centrifuged for 5 min at 1,500 rpm, and stored at −80°C until analysis. Granulosa cells were then isolated and cultured in DMEM medium supplemented with 10% FBS and antibiotics as previously published (19). At the same time, a fasting peripheral venous blood sample was obtained from the patients, and plasma was stored frozen at −80°C. All patients had given informed consent, and the protocol was approved by the respective responsible local ethics committees.
Lipid and lipoprotein analysis
To assess the cholesterol distribution over the different lipoprotein subclasses, plasma as well as FF samples were subjected to fast protein liquid chromatography (FPLC) gel filtration using a superose 6 column (GE Healthcare, Uppsala, Sweden) as described (20). Total cholesterol levels were enzymatically measured within individual fractions using commercially available reagents (Wako Pure Chemical Industries, Neuss, Germany). To correlate plasma and FF levels of lipoproteins, the respective values obtained for all fractions of a given subclass (VLDL, LDL, HDL) were added.
Analysis of gene expression by real-time quantitative PCR
Total RNA from granulosa cells, HepG2 cells (obtained from LGC Standards, Middlesex, UK) and human livers (samples from healthy donor livers intended for liver transplantation but not used due to technical reasons, provided by Dr. Matthias Bahr, Dept. of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Germany) was isolated using Trizol (Invitrogen) and quantified with a NanoDrop ND-100 UV-Vis spectrophotometer. cDNA synthesis was performed from 1 μg of total RNA using reagents from Applied Biosystems (Darmstadt, Germany). Real-time quantitative PCR was carried out on an ABI-Prism 7700 (Applied Biosystems) sequence detector with the default settings (21). PCR primers and fluorogenic probes were designed with the Primer Express Software (Applied Biosystems) and synthesized by Eurogentec (Seraing, Belgium). mRNA expression levels presented were calculated relative to the average of the housekeeping gene cyclophilin and further normalized to the relative expression level determined in granulosa cells.
In vitro VLDL production experiments and Western blot for apoB
Human primary granulosa cells cultured for 3 days were preincubated for 20 h in serum-free DMEM containing 1.5% BSA alone or 1.5% BSA + 0.4 mM oleate. Following a wash with PBS, cells were pulsed for 25 min with 3H-Leu (100 µCi/ well) in leucine-free MEM, washed three times with PBS, and chased for 1 h in serum-free medium. Then media were collected and centrifuged for 3 h at 100,000 rpm in a Beckman TLX tabletop ultracentrifuge with the density adjusted to 1.006 g/ml with KBr solution. Counts within the supernatant representing apoB-containing lipoproteins produced were measured by liquid scintillation counting. To the cells 0.1M NaOH was added, protein content was determined by the bicinchoninic acid method (Pierce, Rockford, IL), and counts were corrected for cellular protein content. For Western blots, samples were mixed with a loading buffer containing SDS and a reducing agent (Invitrogen, Carlsbad, CA) and then incubated for 10 min at 70°C. Samples were subsequently applied onto 4–12% polyacrylamide gradient gels (NuPage, Invitrogen) in a X-Cell SureLock system (Invitrogen) and then blotted to nitrocellulose membranes (Protran, Schleicher and Schuell, Dassel, Germany). The resulting blots were blocked for 1 h in 5% low-fat dried milk in PBS (100 mM, pH 7.4) containing 0.1% Tween and then washed with PBS/Tween. Human apoB was detected by incubation with an anti-apoB antibody (Boehringer Mannheim, Germany) followed by the appropriate horseradish peroxidase-coupled secondary antibody (Sigma Life Sciences, St Louis, MO). Blots were finally developed using the ECL Plus Detection System (GE Healthcare) and were analyzed with a GelDoc 2000 system (BioRad, Hercules, CA) and the QuantityOne software. VLDL purified from fasting human plasma by ultracentrifugation (d < 1.006) were used as positive control for apoB100. The composition of granulosa- derived VLDL (d < 1.006) was determined using enzymatic methods for total cholesterol (Diasys, Holzheim, Germany) and free cholesterol (Wako), TGs (Diasys), phospholipids (Wako), and bicinchoninic acid for proteins.
Electron microscopy
Transmission electron microscopy was performed on primary human granulosa cells after 3 days of culture. Briefly, cells were detached from culture plates after trypsin incubation and pelleted with a short spin. The cells were then fixed for 30 min with 4% paraformaldehyde and 1.5% glutaraldehyde in 0.1 M phosphate buffer solution (pH 7.4), postfixed in 2% osmium tetroxide for 1 h, dehydrated with graded ethanol series, and finally embedded in Epon. Sections were stained with uranyl acetate and lead citrate prior to examination with a H-7500 electron microscope (Hitachi, Bron, France).
ApoB quantitation in FF from patients undergoing IVF
FFs were obtained from follicular aspirates of 27 women (age, 25–41 years; BMI, 17–29) undergoing IVF and embryo transfer (ET) at the Center of Sterility, CHU, Dijon. Infertility was due to polycystic ovary syndrome, ovulatory dysfunction, endometriosis, tubal abnormalities, male infertility, or was unexplained. All patients gave informed consent.
The stimulation protocol was standard and included downregulation with a gonadotropin-releasing hormone agonist and hyperstimulation with recombinant follicle stimulating hormone. Administration of recombinant chorionic gonadotropin (Ovitrelle, Serono, Boulogne, France) was performed when at least three follicles exceeded 17 mm in diameter. Oocytes were retrieved approximately 36 h after hCG administration by transvaginal ultrasound-guided aspiration. After retrieval, intracytoplasmic sperm injection (ICSI; n = 15) or conventional IVF (n = 12) was performed. Fertilization was assessed 16–18 h after insemination or microinjection by the presence of two pronuclei and two polar bodies. The fertilized oocytes were maintained in culture medium (Global medium, LifeGlobal, USA) and transferred to the uterus transcervically under transabdominal ultrasound guidance 48 h after oocyte retrieval. All embryos were scored on day 2 according to the Giorgetti classification system (22). Embryo scoring (1–4 points) was determined as follows: segmented embryo, 1 point; 4-cells embryo, 1 point; absence of irregular cells, 1 point; absence (0–20%) of cytoplasmic remainders, 1 point. For each oocyte retrieval, the number of top-quality embryos was determined. An embryo was considered a top-quality embryo if there were four or five blastomeres on day 2 with <20% of fragments and the total absence of multinucleated blastomeres (23). All ETs were performed 2 days after oocyte retrieval using Frydman catheters (CCD Laboratories, Paris, France). A clinical pregnancy was defined as the observation of a gestational sac on ultrasound scanning between 6 and 7 weeks after ET.
In parallel with IFV-ET, FF were analyzed for apoB content. By the day of oocyte retrieval, FF aliquots were collected and examined by an embryologist to detect and remove cumulus-oocyte complexes, centrifuged at 3,000 g for 15 min to eliminate cellular elements, and supernatants were stored at – 80°C before analysis. ApoB concentration in FF was determined by an immunoturbidimetric method (24) using a commercial kit with a goat anti- human apoB antibody (Apolipoprotein B FS kit, DiaSys, Holzheim, Germany) on a Dimension Xpand automated device (Siemens) according to the manufacturer's instructions. Briefly, samples were diluted 1:100 in a 100 mM Tris solution containing polyethylenglycol and detergent, and incubated for 5 min before reading background absorbance at 340 nm. Diluted samples were then incubated for 5 min at 37°C in the presence of the anti-apoB antibody prior to endpoint absorbance reading at 340 nm. ApoB concentrations in samples were calculated from a calibration curve obtained with the TruCal calibrator set (DiaSys).
Statistical analysis
Statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL). Data are presented as means ± SEM unless otherwise indicated. Student's t-test was used to compare values of two different groups and the Pearson correlation coefficient to assess possible associations between different parameters. For the studies on human infertility, values were compared by using Mann-Whitney U-test or by the chi-square test, as appropriate. Statistical significance for all comparisons was assigned at P < 0.05.
RESULTS
Human FF HDL but not VLDL cholesterol concentrations correlate with plasma levels
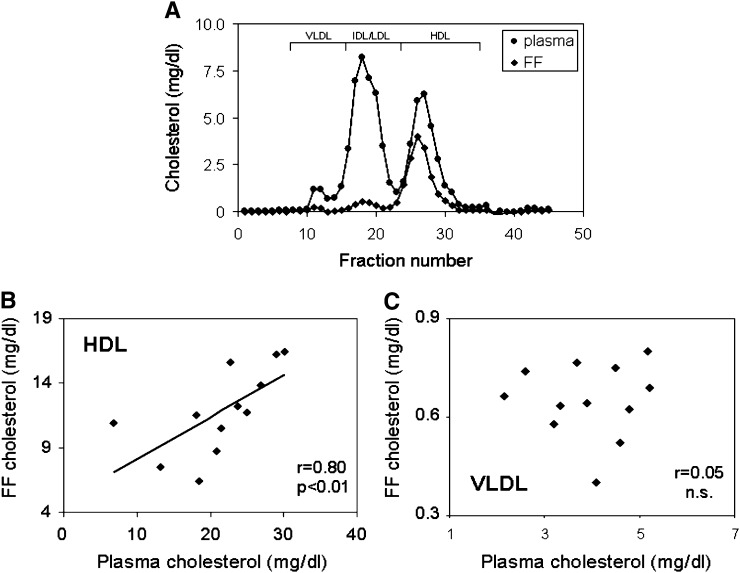
FPLC analysis of lipoprotein distribution over the different subclasses revealed that 83% of the cholesterol is contained within the HDL fraction, indicating that the major cholesterol carrier within FF is HDL (Fig. 1A). However, a small but consistently present VLDL peak was discernible in all FPLC profiles performed on FF (Fig. 1A). In contrast, in plasma, 61% of the cholesterol is found within apoB-containing lipoproteins and on average only 39% within HDL (Fig. 1A). Relating plasma lipoprotein cholesterol to FF cholesterol levels, plasma contains 5.6-fold more VLDL, 15.4-fold more LDL, and 1.8-fold more HDL cholesterol compared with FF. Interestingly, HDL cholesterol levels in plasma were correlated with FF HDL cholesterol (r = 0.80, P < 0.01, Fig. 1B) consistent with diffusion being the conceivable mechanism of entry for HDL lipoproteins into FF (11, 25). On the other hand, VLDL cholesterol levels in plasma and FF were not correlated (r = 0.05, n.s., Fig. 1C). These data demonstrate that VLDL is present in human FF and indicate that, in contrast to HDL, FF-VLDL might not be derived directly from plasma.
Fig. 1.
Lipoprotein profiles in plasma and FF. A: Samples were subjected to gel filtration using a Superose 6 column, and cholesterol levels in each fraction were measured as described in “Materials and Methods.” Relative elution positions of different lipoprotein subclasses are indicated. Shown is a representative example from one patient. Correlation between plasma and FF levels of HDL cholesterol (B) and VLDL cholesterol (C). Respective cholesterol values within subfractions were derived from FPLC profiles. n = 12 patients.
Human granulosa cells express MTP and apoB
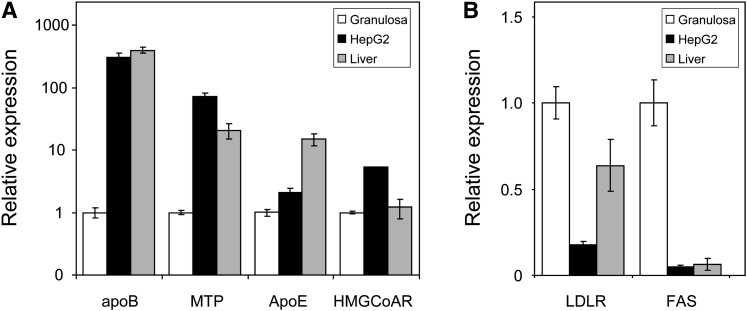
To further test the hypothesis that FF-VLDL are assembled and secreted on site, we first investigated if genes relevant for the production of apoB-containing lipoproteins are expressed in granulosa cells, which are in direct contact with FF and have a major impact on the composition of FF (16, 17). As shown in Fig. 2, granulosa cells express apoB and microsomal triglyceride transfer protein (MTP), the two genes absolutely required for VLDL production (26–28), as well as apoE, a major modifier of VLDL secretion (29, 30). However, the expression level of these respective genes was by far lower in granulosa cells compared with the human hepatoma cell line HepG2 (apoB: 303-fold, MTP: 72-fold, apoE: 2.1-fold; Fig. 2) as well as human liver (apoB: 395-fold, MTP: 21-fold, apoE: 15.2-fold; Fig. 2). Expression of apobec-1 (31), the editing enzyme responsible for the generation of apoB48, was absent in granulosa cells. In addition, granulosa cells expressed 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase in comparable amounts to human liver, but less than HepG2 cells, while mRNA expression of the LDL receptor and fatty acid synthase was greatly increased in granulosa cells (Fig. 2). These results indicate that primary human granulosa cells express the relevant genes required for assembly and secretion of apoB-containing lipoproteins.
Fig. 2.
Gene expression profiles in primary human granulosa cells in relation to HepG2 cells and normal human liver. Shown are genes with decreased (A) and increased (B) expression in granulosa cells. Please note that for A, the y axis is in logarithmic scale. n = 9 for granulosa and HepG2 and n = 4 for human liver. Data are presented as means ± SEM.
Human granulosa cells secrete apoB100-containing lipoproteins, which is stimulated by the addition of oleic acid
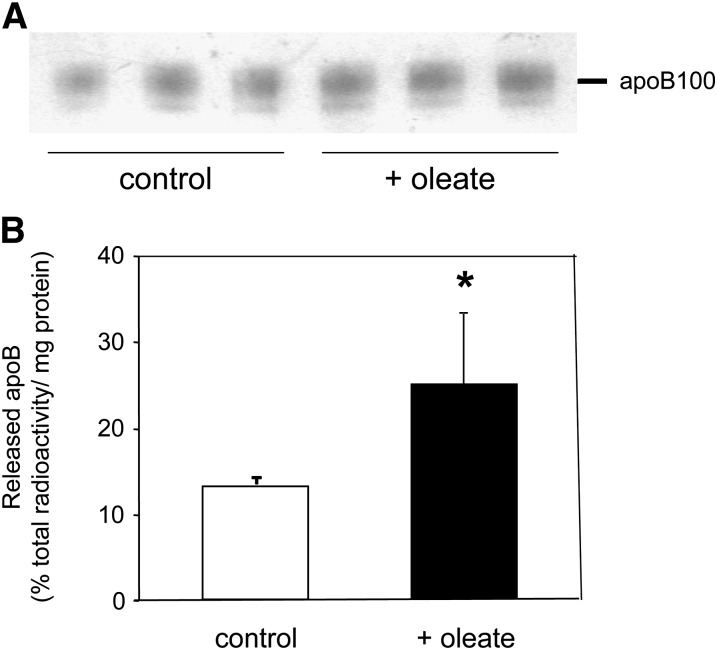
Next, we explored if the expression of the relevant genes for assembly and secretion of apoB-containing lipoproteins in granulosa cells also translates into actual VLDL production. Therefore, we initially performed Western blots on cell culture supernatants of granulosa cells grown in FBS-free medium. As shown in Fig. 3A, an apoB100 band could be detected; consistent with the absent expression of apobec1, no immunoreactive apoB48 was found. Interestingly, the intensity of the apoB band appeared to be increased following incubation of the cells in the presence of 0.4 mM oleate. VLDL present in the media were subsequently isolated by ultracentrifugation and analyzed for their lipid and protein content. The relative composition (as percent of total weight) of granulosa-derived VLDL was 5.83 ± 0.86% for total cholesterol, 3.69 ± 1.59% for unesterified cholesterol, 2.80 ± 0.26% for cholesteryl esters, 73.23 ± 2.27% for TGs, 5.70 ± 0.92% for phospholipids, and 10.62 ± 1.91% for proteins. This composition pattern closely resembled native plasma VLDL but with a slightly higher TG enrichment, mainly at the expense of cholesterol content (32).
Fig. 3.
Primary human granulosa cells secrete apoB100-containing lipoproteins. A: Representative Western blot for human apoB on the supernatant of granulosa cells cultured either in the absence (control) or presence (+oleate) of 0.4 mM oleate as detailed in “Materials and Methods.” B: Pulse/chase experiment performed separately in granulosa cells isolated from six individual donors preincubated for 20 h in FBS-free medium containing either 1.5% BSA or 1.5% BSA complexed with 0.4 mM oleate using a 25 min pulse with 3H-leucine followed by a 1 h chase as detailed in “Materials and Methods.” ApoB-containing lipoproteins were isolated from the medium, and incorporated radioactivity was counted and expressed in relation to cellular protein content. Data are presented as means ± SEM. * Indicates statistically significant differences compared with granulosa cells incubated without oleate as assessed by Student's t-test.
Subsequently, a pulse-chase experiment was performed using a pulse of 3H-leucine for 25 min on primary human granulosa cells preincubated either in the absence or the presence of oleate. After a chase for 1 h, supernatants were collected, and counts within apoB-containing lipoproteins produced were assessed. Using this approach, we could demonstrate that significant amounts of tracer are incorporated into apoB secreted by granulosa cells and that the addition of oleate resulted in a 83% increase of apoB protein secretion from these cells (Fig. 3B).
VLDL-like particles can be directly visualized by electron microscopy within primary human granulosa cells
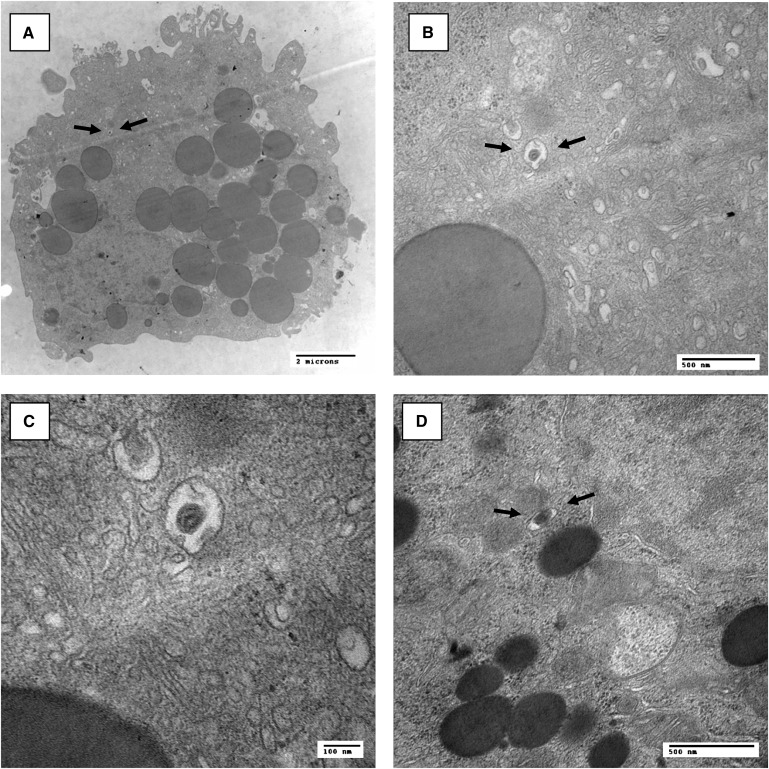
In addition to demonstrating VLDL production by granulosa cells, we also investigated these cells by electron microscopy. Interestingly, small lipid-staining particles with a diameter ranging from 50 to 150 nm, which in terms of appearance were consistent with the presence of apoB-containing lipoproteins in the secretory pathway (33), were detected within the Golgi apparatus of granulosa cells (Fig. 4A–D). These results demonstrate that apoB-containing lipoproteins can be directly visualized by electron microscopy within granulosa cells, providing further evidence that these cells assemble and secrete VLDL.
Fig. 4.
Electron micrographs of primary human granulosa cells cultured for 3 days demonstrating lipid-staining particles within the Golgi compartment. Arrows point toward lipid-staining particles consistent in appearance with apoB-containing lipoproteins. A–C follow a specific particle with increasing magnifications, and D provides an example from a different cell. Magnifications are indicated on the pictures.
ApoB levels in human FF are associated with parameters of female fertility
FFs from 27 women undergoing IVF were collected during oocyte retrieval and analyzed for their apoB content. ApoB was readily detectable in all patients, although the concentration in FF (mean, 14.0 ± 3.1 mg/L) was 40–50 times lower than in normolipidemic plasma [640 ± 90 mg/L, (34)].
Patients were subsequently divided into two groups according to the median apoB level in FF, with values ≤ 13.6 mg/L and > 13.6 mg/L in the low apoB group and in the high apoB group, respectively (Table 1). Age distribution, body mass index, and the IVF to ICSI ratio were not statistically different between groups. When apoB stratification was applied to the whole population (n = 27), the number of grade 4 embryos was significantly higher in high apoB patients compared with low apoB patients (2.8 ± 2.2 vs. 1.6 ± 2.7, respectively; P< 0.05), and this was accompanied by a higher pregnancy rate in the high apoB group compared with the low apoB group (69.1% vs. 23.1%, respectively; P < 0.05) (Table 1, left). Because the population studied includes patients with direct ovarian disorders that might introduce confounding factors to follicular function and oocyte quality, the same stratification was performed after exclusion of patients with polycystic ovary syndrome, ovarian endometriosis, ovulatory dysfunction, or those who underwent ovarian surgery. Again, in the remaining population (n = 18), high apoB levels in FF were associated with a higher number of grade 4 embryos (3.6 ± 1.5 vs. 0.6 ± 0.9 in the low apoB group; P < 0.05), a higher number of top-quality embryos (4.0 ± 1.6 vs. 1.2 ± 1.3 in the low apoB group; P < 0.05), and also a strikingly higher clinical pregnancy rate (60.0% vs. 0.0% in the low apoB group; P < 0.05, Table 1, right). These data suggest that the concentration of apoB present in FF might predict oocyte quality for the generation of viable embryos and further successful implantation and pregnancy.
TABLE 1.
Patient characteristics, embryo quality, and fertility in groups with low and high apoB concentration in FF
| All Patients |
Patients Without Direct Ovarian Disorders |
|||||
|---|---|---|---|---|---|---|
| ApoB (mg/L) |
ApoB (mg/L) |
|||||
| Low (≤13.6) (n = 14) | High (>13.6) (n = 13) | P | Low (≤13.6) (n = 9) | High (>13.6) (n = 9) | P | |
| Age | 35.3 ± 3.5 | 33.6 ± 4.7 | 0.366 | 34.8 ± 3.3 | 34.2 ± 5.9 | 0.832 |
| BMI | 21.2 ± 2.1 | 21.3 ± 3.8 | 0.417 | 22.2 ± 1.8 | 22.0 ± 4.6 | 0.647 |
| IVF/ICSI (n) | 8 / 6 | 7 / 6 | 0.863 | 5 / 4 | 4 / 5 | 0.637 |
| ApoB (mg/L) | 11.6 ± 0.8 | 16.6 ± 2.5 | <0.0001 | 11.5 ± 0.7 | 16.4 ± 3.0 | 0.009 |
| Top-quality embryos (n) | 2.2 ± 2.8 | 3.0 ± 2.5 | 0.174 | 1.2 ± 1.3 | 4.0 ± 1.6 | 0.027 |
| Grade 4 embryos (n) | 1.6 ± 2.7 | 2.8 ± 2.2 | 0.034 | 0.6 ± 0.9 | 3.6 ± 1.5 | 0.011 |
| Clinical pregnancy rate (%) | 23.1 | 69.2 | 0.013 | 0.0 | 60.0 | 0.038 |
Continuous variables are means ± SD and were compared using Mann-Whitney U-test. Distribution of fertilization methods (IVF and ICSI) and clinical pregnancy data were compared by the chi-square test. BMI, body mass index. Boldface type indicates significant differences between low and high apoB groups.
DISCUSSION
The results of this study demonstrate that human granulosa cells assemble and secrete apoB100-containing lipoproteins. Thereby, granulosa cells represent a novel cell type with these properties in addition to enterocytes (3), hepatocytes (1, 2), cardiomyocytes (7), placenta (herein presumably trophoblasts) (6), and, at least in the chicken, the kidney (35). Thus far, secretion of apoB-containing lipoproteins has been shown to serve two different purposes that might, however, be related as in the case of hepatocytes: i) supply distant cells via the blood stream with TGs and cholesterol; and ii) prevent cellular stress and toxicity associated with TG overload.
Within a growing follicle in the ovary, the oocyte is surrounded by concentric layers of cells and tissue, which are, from the periphery inwards: i) theca externa; ii) theca interna; iii) basal membrane; and iv) granulosa cells (17). The oocyte has, as a rapidly growing cell, a considerable demand for energy as well as cholesterol (10). Although most earlier studies detected only HDL within FF (11, 36), there are single reports on the presence of VLDL in older literature in a variable percentage of subjects investigated (12, 37). A very recent study reported apoB to be consistently detectable within FF in agreement with our results (38). However, the possibility of granulosa cells secreting apoB-containing lipoproteins has not been investigated previously. Although the precise role of apoB-containing lipoprotein secretion by granulosa cells is presently unclear, it appears conceivable that apoB-containing lipoproteins might have a nourishing function that the TG-poor HDL lipoproteins are not able to fulfill properly. In favor of such a theory is on the one hand the expression of receptors from the LDLR family on oocytes (13, 14) and on the other the correlation of FF-apoB levels to the outcome of IVF-ET. Because VLDL also contain lipophilic components such as vitamins, e.g., vitamin E (39), or steroids (40), delivery of these components to oocytes might also be a function of apoB-containing lipoprotein secretion by granulosa cells. However, it cannot be excluded that granulosa cells utilize the secretion of apoB-containing lipoproteins to prevent TG accumulation, because they might be sensitive toward metabolic stress.
An interesting question to explore is the relevance of reduced or increased apoB-containing lipoprotein production by granulosa cells for reproductive physiology. Thus far in humans, to our knowledge, no association between genetic variation within the apoB locus and female infertility has been published. In addition, there are also no patients reported completely lacking apoB. Consistent with this notion is the observation that homozygous apoB knockout mice die in utero due to severe neurodevelopmental abnormalities (41–43). In female heterozygous mice, no apparent infertility has been described, but male heterozygous apoB knockout mice display reduced fertility, which has been directly related to the expression of apoB within the male reproductive tract (43, 44). An initial study indicated that at least the insertion/deletion polymorphism of the apoB gene might be related to male infertility in humans (45). However, apoB expression has not been demonstrated thus far in the female reproductive tract. Our data on the outcome of IVF-ET suggest that an association between reduced apoB expression and decreased fertility might also exist in female human patients that has thus far escaped recognition. In accordance with this hypothesis, we show here for the first time in humans a positive relationship between the apoB levels in FF and fertility parameters as reflected by embryo quality and pregnancy rate in a small cohort of women undergoing IVF-ET. Thereby, our observations support a physiological relevance of apoB expression by follicular cells during oocyte maturation.
The second essential gene for apoB-containing lipoprotein production is MTP (1). Whereas homozygous MTP knockout mice die in utero, no obvious female fertility phenotype has been published in heterozygous knockouts (46). Also, reports on fertility are lacking for human patients suffering from abetalipoproteinemia, a rare autosomal recessive disease with the complete absence of MTP due to genetic mutations (47). However, conditional knockout mice have been generated for MTP (48, 49). Therefore, the pathophysiological consequences of genetic ablation of MTP and thereby production of apoB-containing lipoproteins specifically in granulosa cells are testable at least in the mouse model. We would also recommend to consider investigating whether there is an association between genetic variations within apoB and MTP loci and female infertility in human patients. In addition, it would be relevant to learn about the effects of MTP inhibition on reproductive physiology, because MTP inhibitors have been developed to reduce elevated levels of apoB-containing lipoproteins in patients for the prevention and treatment of atherosclerotic cardiovascular disease (50).
In summary, our results demonstrate that human granulosa cells represent a novel cell type equipped to assemble and secrete apoB-containing lipoproteins. Supplementation with oleate stimulates production rates of apoB-containing lipoproteins in these cells. Furthermore, we show a positive relationship between the apoB content of FF and fertility parameters in women undergoing IVF-ET. These data might have important implications for our understanding of female infertility phenotypes as well as for the further development of MTP inhibitors for the treatment of atherosclerotic cardiovascular disease in humans and the possible side effects of this strategy on reproductive biology.
Acknowledgments
The authors are indebted to Liliane Princep and Elizabeth Niot (Centre Hospitalier Universitaire de Dijon) for excellent technical assistance.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- ET
- embryo transfer
- FF
- follicular fluid
- FPLC
- fast protein liquid chromatography
- ICSI
- intracytoplasmic sperm injection
- IVF
- in vitro fertilization
- MTP
- microsomal triglyceride transfer protein
- TG
- triglyceride
This work was supported by a grant from the Netherlands Organization for Scientific Research (VIDI Grant 917-56-358) (to U.J.F.T.), by the INSERM (Institut National de la Santé Et de la Recherche Médicale) (to T.G., L.L., and D.M.), and by the Fondation pour la Recherche Médicale (to T.G.).
REFERENCES
- 1.Shelness G. S., Ledford A. S. 2005. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr. Opin. Lipidol. 16: 325–332. [DOI] [PubMed] [Google Scholar]
- 2.Blasiole D. A., Davis R. A., Attie A. D. 2007. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol. Biosyst. 3: 608–619. [DOI] [PubMed] [Google Scholar]
- 3.Hussain M. M., Fatma S., Pan X., Iqbal J. 2005. Intestinal lipoprotein assembly. Curr. Opin. Lipidol. 16: 281–285. [DOI] [PubMed] [Google Scholar]
- 4.Olofsson S. O., Boren J. 2005. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 258: 395–410. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg I. J., Ginsberg H. N. 2006. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology. 130: 1343–1346. [DOI] [PubMed] [Google Scholar]
- 6.Madsen E. M., Lindegaard M. L., Andersen C. B., Damm P., Nielsen L. B. 2004. Human placenta secretes apolipoprotein B-100-containing lipoproteins. J. Biol. Chem. 279: 55271–55276. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen L. B., Veniant M., Boren J., Raabe M., Wong J. S., Tam C., Flynn L., Vanni-Reyes T., Gunn M. D., Goldberg I. J., et al. 1998. Genes for apolipoprotein B and microsomal triglyceride transfer protein are expressed in the heart: evidence that the heart has the capacity to synthesize and secrete lipoproteins. Circulation. 98: 13–16. [DOI] [PubMed] [Google Scholar]
- 8.Veniant M. M., Nielsen L. B., Boren J., Young S. G. 1999. Lipoproteins containing apolipoprotein B-100 are secreted by the heart. Trends Cardiovasc. Med. 9: 103–107. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen L. B., Bartels E. D., Bollano E. 2002. Overexpression of apolipoprotein B in the heart impedes cardiac triglyceride accumulation and development of cardiac dysfunction in diabetic mice. J. Biol. Chem. 277: 27014–27020. [DOI] [PubMed] [Google Scholar]
- 10.Stouffer R. L., Xu F., Duffy D. M. 2007. Molecular control of ovulation and luteinization in the primate follicle. Front. Biosci. 12: 297–307. [DOI] [PubMed] [Google Scholar]
- 11.Jaspard B., Fournier N., Vieitez G., Atger V., Barbaras R., Vieu C., Manent J., Chap H., Perret B., Collet X. 1997. Structural and functional comparison of HDL from homologous human plasma and follicular fluid. A model for extravascular fluid. Arterioscler. Thromb. Vasc. Biol. 17: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 12.Simpson E. R., Rochelle D. B., Carr B. R., MacDonald P. C. 1980. Plasma lipoproteins in follicular fluid of human ovaries. J. Clin. Endocrinol. Metab. 51: 1469–1471. [DOI] [PubMed] [Google Scholar]
- 13.Sato N., Kawamura K., Fukuda J., Honda Y., Sato T., Tanikawa H., Kodama H., Tanaka T. 2003. Expression of LDL receptor and uptake of LDL in mouse preimplantation embryos. Mol. Cell. Endocrinol. 202: 191–194. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi Y. L., Tanaka S. S., Kasa M., Yasuda K., Tam P. P., Matsui Y. 2006. Expression of low density lipoprotein receptor-related protein 4 (Lrp4) gene in the mouse germ cells. Gene Expr. Patterns. 6: 607–612. [DOI] [PubMed] [Google Scholar]
- 15.Azhar S., Tsai L., Medicherla S., Chandrasekher Y., Giudice L., Reaven E. 1998. Human granulosa cells use high density lipoprotein cholesterol for steroidogenesis. J. Clin. Endocrinol. Metab. 83: 983–991. [DOI] [PubMed] [Google Scholar]
- 16.Havelock J. C., Rainey W. E., Carr B. R. 2004. Ovarian granulosa cell lines. Mol. Cell. Endocrinol. 228: 67–78. [DOI] [PubMed] [Google Scholar]
- 17.Okamura H., Katabuchi H., Ohba T. 2003. What we have learned from isolated cells from human ovary? Mol. Cell. Endocrinol. 202: 37–45. [DOI] [PubMed] [Google Scholar]
- 18.von Otte S., Paletta J. R., Becker S., Konig S., Fobker M., Greb R. R., Kiesel L., Assmann G., Diedrich K., Nofer J. R. 2006. Follicular fluid high density lipoprotein-associated sphingosine 1-phosphate is a novel mediator of ovarian angiogenesis. J. Biol. Chem. 281: 5398–5405. [DOI] [PubMed] [Google Scholar]
- 19.Drouineaud V., Sagot P., Garrido C., Logette E., Deckert V., Gambert P., Jimenez C., Staels B., Lagrost L., Masson D. 2007. Inhibition of progesterone production in human luteinized granulosa cells treated with LXR agonists. Mol. Hum. Reprod. 13: 373–379. [DOI] [PubMed] [Google Scholar]
- 20.Tietge U. J. F., Maugeais C., Cain W., Grass D., Glick J. M., de Beer F. C., Rader D. J. 2000. Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J. Biol. Chem. 275: 10077–10084. [DOI] [PubMed] [Google Scholar]
- 21.Nijstad N., Wiersma H., Gautier T., van der Giet M., Maugeais C., Tietge U. J. F. 2009. Scavenger receptor BI-mediated selective uptake is required for the remodeling of high density lipoprotein by endothelial lipase. J. Biol. Chem. 284: 6093–6100. [DOI] [PubMed] [Google Scholar]
- 22.Giorgetti C., Terriou P., Auquier P., Hans E., Spach J. L., Salzmann J., Roulier R. 1995. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum. Reprod. 10: 2427–2431. [DOI] [PubMed] [Google Scholar]
- 23.Van Royen E., Mangelschots K., De Neubourg D., Valkenburg M., Van de Meerssche M., Ryckaert G., Eestermans W., Gerris J. 1999. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum. Reprod. 14: 2345–2349. [DOI] [PubMed] [Google Scholar]
- 24.Riepponen P., Marniemi J., Rautaoja T. 1987. Immunoturbidimetric determination of apolipoproteins A-1 and B in serum. Scand. J. Clin. Lab. Invest. 47: 739–744. [PubMed] [Google Scholar]
- 25.Le Goff D. 1994. Follicular fluid lipoproteins in the mare: evaluation of HDL transfer from plasma to follicular fluid. Biochim. Biophys. Acta. 1210: 226–232. [DOI] [PubMed] [Google Scholar]
- 26.Hussain M. M., Shi J., Dreizen P. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44: 22–32. [DOI] [PubMed] [Google Scholar]
- 27.Olofsson S. O., Asp L., Boren J. 1999. The assembly and secretion of apolipoprotein B-containing lipoproteins. Curr. Opin. Lipidol. 10: 341–346. [DOI] [PubMed] [Google Scholar]
- 28.Veniant M. M., Kim E., McCormick S., Boren J., Nielsen L. B., Raabe M., Young S. G. 1999. Insights into apolipoprotein B biology from transgenic and gene-targeted mice. J. Nutr. 129: 451S–455S. [DOI] [PubMed] [Google Scholar]
- 29.Kuipers F., Jong M. C., Lin Y., Eck M., Havinga R., Bloks V., Verkade H. J., Hofker M. H., Moshage H., van Berkel T. J., et al. 1997. Impaired secretion of very low density lipoprotein-triglycerides by apolipoprotein E- deficient mouse hepatocytes. J. Clin. Invest. 100: 2915–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maugeais C., Tietge U. J. F., Tsukamoto K., Glick J. M., Rader D. J. 2000. Hepatic apolipoprotein E expression promotes very low density lipoprotein-apolipoprotein B production in vivo in mice. J. Lipid Res. 41: 1673–1679. [PubMed] [Google Scholar]
- 31.Chan L., Chang B. H., Nakamuta M., Li W. H., Smith L. C. 1997. Apobec-1 and apolipoprotein B mRNA editing. Biochim. Biophys. Acta. 1345: 11–26. [DOI] [PubMed] [Google Scholar]
- 32.Yang C. Y., Gu Z. W., Valentinova N., Pownall H. J., Lee B., Yang M., Xie Y. H., Guyton J. R., Vlasik T. N., Fruchart J. C., et al. 1993. Human very low density lipoprotein structure: interaction of the C apolipoproteins with apolipoprotein B-100. J. Lipid Res. 34: 1311–1321. [PubMed] [Google Scholar]
- 33.Chao F. F., Stiers D. L., Ontko J. A. 1986. Hepatocellular triglyceride synthesis and transfer to lipid droplets and nascent very low density lipoproteins. J. Lipid Res. 27: 1174–1181. [PubMed] [Google Scholar]
- 34.Pont F., Duvillard L., Florentin E., Gambert P., Verges B. 2002. Early kinetic abnormalities of apoB-containing lipoproteins in insulin-resistant women with abdominal obesity. Arterioscler. Thromb. Vasc. Biol. 22: 1726–1732. [DOI] [PubMed] [Google Scholar]
- 35.Walzem R. L., Hansen R. J., Williams D. L., Hamilton R. L. 1999. Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 129: 467S–472S. [DOI] [PubMed] [Google Scholar]
- 36.Enk L., Crona N., Olsson J. H., Hillensjo T. 1986. Lipids, apolipoproteins and steroids in serum and in fluid from stimulated and non-stimulated human ovarian follicles. Acta Endocrinol. (Copenh.). 111: 558–562. [DOI] [PubMed] [Google Scholar]
- 37.Volpe A., Coukos G., Uccelli E., Droghini F., Adamo R., Artini P. G. 1991. Follicular fluid lipoproteins in preovulatory period and their relationship with follicular maturation and progesterone production by human granulosa-luteal cells in vivo and in vitro. J. Endocrinol. Invest. 14: 737–742. [DOI] [PubMed] [Google Scholar]
- 38.Von Wald T., Monisova Y., Hacker M. R., Yoo S. W., Penzias A. S., Reindollar R. R., Usheva A. 2010. Age-related variations in follicular apolipoproteins may influence human oocyte maturation and fertility potential. Fertil. Steril. 93: 2354–2361. [DOI] [PubMed] [Google Scholar]
- 39.Behrens W. A., Thompson J. N., Madère R. 1982. Distribution of alpha-tocopherol in human plasma lipoproteins. Am. J. Clin. Nutr. 35: 691–696. [DOI] [PubMed] [Google Scholar]
- 40.Peinado-Onsurbe J., Blay M., Casadomè L., Fernández-López J. A., Remesar X., Alemany M. 2001. Effect of 24-h food deprivation on lipoprotein composition and oleoyl-estrone content of lean and obese Zucker rats. Eur. J. Nutr. 40: 155–160. [DOI] [PubMed] [Google Scholar]
- 41.Homanics G. E., Smith T. J., Zhang S. H., Lee D., Young S. G., Maeda N. 1993. Targeted modification of the apolipoprotein B gene results in hypobetalipoproteinemia and developmental abnormalities in mice. Proc. Natl. Acad. Sci. USA. 90: 2389–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farese R. V., Jr., Ruland S. L., Flynn L. M., Stokowski R. P., Young S. G. 1995. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc. Natl. Acad. Sci. USA. 92: 1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang L. S., Voyiaziakis E., Markenson D. F., Sokol K. A., Hayek T., Breslow J. L. 1995. apo B gene knockout in mice results in embryonic lethality in homozygotes and neural tube defects, male infertility, and reduced HDL cholesterol ester and apo A-I transport rates in heterozygotes. J. Clin. Invest. 96: 2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang L. S., Voyiaziakis E., Chen H. L., Rubin E. M., Gordon J. W. 1996. A novel functional role for apolipoprotein B in male infertility in heterozygous apolipoprotein B knockout mice. Proc. Natl. Acad. Sci. USA. 93: 10903–10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterlin B., Zorn B., Volk M., Kunej T. 2006. Association between the apolipoprotein B signal peptide gene insertion/deletion polymorphism and male infertility. Mol. Hum. Reprod. 12: 777–779. [DOI] [PubMed] [Google Scholar]
- 46.Raabe M., Flynn L. M., Zlot C. H., Wong J. S., Veniant M. M., Hamilton R. L., Young S. G. 1998. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. USA. 95: 8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon D. A., Jamil H. 2000. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein-B lipoprotein assembly. Biochim. Biophys. Acta. 1486: 72–83. [DOI] [PubMed] [Google Scholar]
- 48.Chang B. H., Liao W., Li L., Nakamuta M., Mack D., Chan L. 1999. Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein/low density lipoprotein production in a viable conditional knockout mouse. J. Biol. Chem. 274: 6051–6055. [DOI] [PubMed] [Google Scholar]
- 49.Raabe M., Veniant M. M., Sullivan M. A., Zlot C. H., Bjorkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnett J. R., Watts G. F. 2007. MTP inhibition as a treatment for dyslipidaemias: time to deliver or empty promises? Expert Opin. Ther. Targets. 11: 181–189. [DOI] [PubMed] [Google Scholar]