Abstract
Plasma lipoproteins and glucose homeostasis were evaluated after marked weight loss before and over 12 months following Roux-en-Y gastric-bypass (RYGBP) surgery in 19 morbidly obese women. Standard lipids, remnant-lipoprotein cholesterol (RLP-C); HDL-triglyceride (TG); apolipoproteins (apo) A-I, A-II, E, and A-I-containing HDL subpopulations; lecithin-cholesterol acyltransferase (LCAT) and cholesteryl ester transfer protein (CETP) mass and activity; plasma glucose and insulin levels were measured before and at 1, 3, 6, and 12 months after GBP surgery. Baseline concentrations of TG, RLP-C, glucose, and insulin were significantly higher in obese than in normal-weight, age-matched women, whereas HDL cholesterol (HDL-C), apoA-I, apoA-II, α-1 and α-2 levels were significantly lower. Over 1 year, significant decreases of body mass index, glucose, insulin, TG, RLP-C, HDL-TG, and preβ-1 levels were observed with significant increases of HDL-C and α-1 levels (all P < 0.05). Changes of fat mass were correlated with those of LDL cholesterol (P = 0.018) and LCAT mass (P = 0.011), but not with CETP mass (P = 0.265). Changes of fasting plasma glucose concentrations were inversely correlated with those of CETP mass (P = 0.005) and α-1 level (P = 0.004). Changes of fasting plasma insulin concentrations were positively correlated with those of LCAT mass (P = 0.043) and inversely with changes of α-1 (P = 0.03) and α-2 (P = 0.05) concentrations. These results demonstrate beneficial changes in HDL remodeling following substantial weight loss induced by RYGBP surgery and that these changes are associated with improvement of glucose homeostasis in these patients.
Keywords: high density lipoprotein particles, cardiovascular disease risk, lipoprotein metabolism, glucose homeostasis
Cardiovascular disease (CVD) is the leading cause of death and disability in the United States. Obesity is associated with hypertension, diabetes, elevated triglyceride (TG) levels, and decreased HDL-cholesterol (HDL-C) levels, all acknowledged as independent CVD risk factors by the AHA (1). Obesity, defined by body mass index (BMI) ≥ 30 kg/m2, often coexists with the metabolic syndrome, which is associated with increased CVD risk. High BMI has been associated with increased risk for the development of coronary, cerebral, and peripheral vascular disease; however, due to the coexistence of multiple risk factors, the quantitative assessment of CVD risk exclusively associated with obesity is complicated.
Bariatric surgery is an effective intervention to achieve long-term marked weight reduction in patients with morbid obesity (2, 3). It has recently been published that calculated 10 year CVD risk (based on the Framingham equation) in morbidly obese subjects decreased by 1.3% 12 months after laparoscopic Roux-en-Y gastric bypass (RYGBP) surgery (4) and that substantial weight loss, following RYGBP surgery, decreased overall mortality (5) and often resulted in significant improvements in lipid parameters and CVD risk (6–10). Increased circulating adiponectin concentrations, improvements of insulin sensitivity, decreased heart rate and hypertension, and other comorbidities of severe obesity have also been shown to accompany substantial weight loss after RYGBP surgery (11–16).
Elevated LDL cholesterol (LDL-C) and TG as well as low levels of HDL-C are well-documented CVD risk factors. Lowering total and LDL-C levels has clearly been shown to reduce CVD risk. However, almost one-half of myocardial infarctions and strokes occur in people with normal LDL-C levels (17), leading to a search for more specific markers for risk of CVD. Emerging data indicate that more detailed characterization of plasma apolipoproteins (apo) and lipoprotein subpopulations, including apoA-I, apoA-II, apoE, apoB, remnant lipoprotein cholesterol (RLP-C), and HDL subpopulations, provides additional information regarding CVD risk (18–21). Specific apoA-I-containing HDL particles (large lipid-rich α-1 and α-2, and small lipid-poor preβ-1) were proven to be superior to standard lipid measurements in CVD risk assessment in large epidemiological studies (19–21).
In this study, we tested the hypothesis that significant weight loss in morbidly obese women following RYGBP surgery induces beneficial effects not only on body weight, body fat, and established CVD risk markers, but also on emerging CVD risk markers and HDL remodeling.
METHODS
Study population
We have studied 19 morbidly obese women (mean age 41 ± 8 years) who underwent RYGBP surgery and 19 age-matched lean female controls who had no history of diabetes or cardiovascular, kidney, or thyroid disease. The surgery was performed at the University of California Davis Medical Center or at Mercy San Juan Hospital in Sacramento, CA. Fasting blood samples were collected prior to and at 1, 3, 6, and 12 months after surgery; body composition was assessed at 0, 1, and 12 months at the University of California Davis Clinical and Translational Science Center's Clinical Research Center (Mather Field Department of Veterans Affairs Medical Center, Rancho Cordova, CA). The Institutional Review Board of the University of California Davis approved the experimental protocol, and all subjects provided written informed consent to participate in the study.
Anthropometric and biochemical measurements
Body composition was determined using air-displacement plethysmography (BodPod Body composition system, Life Measurements, Concord, CA). Body weight, height, and waist and hip circumference were measured by a trained nurse using standard methods.
Total cholesterol (total-C), HDL-C, LDL-C, and TG were measured by standard enzymatic methods with kits from Roche Diagnostics (Indianapolis, IN). RLP-C was assessed with an immuno-separation method using kits from Kyowa Medex (Tokyo, Japan). HDL-TG was measured after the apoB-containing lipoproteins were precipitated by dextran-sulfate-MgCl. Total plasma apoA-I, apoA-II, and apoE concentrations were measured with turbidimetric immunoassay kits from Wako Diagnostics (Richmond, VA). Lecithin-cholesterol acyltransferase (LCAT) and cholesterol ester (CE) transfer protein (CETP) activities were determined as described by Fielding (22, 23). The principle of this method is that the increased CE in plasma, incubated in vitro, as a result of LCAT activity is associated with an equivalent molar decrease in free cholesterol (FC). The rate of CETP-mediated transfer of CE from HDL to very low density lipoprotein (VLDL)and LDL is then the difference between the rate of decrease in FC in whole plasma, and the rate of increase of CE in HDL, as a function of time. This assay provides unique information on the interplay between LCAT and CETP by measuring total-C and FC in total plasma and in apoB-depleted plasma at two time points [time 0 (initial) and after 3 h of incubation at 37°C (final)]. Molar CETP activity = [(initial plasma FC – final plasma FC)] – [(final HDL-TC – final HDL-FC) – (initial HDL-TC – initial HDL-FC)]. LCAT activity was assessed as the difference between initial plasma FC and final plasma FC as a function of time. All measurements were performed on a Hitachi 911 analyzer with inter-assay coefficients of variation <5% in the Lipid Metabolism Laboratory at Tufts University in Boston. CETP and LCAT masses were measured by ELISA kits obtained from Wako Diagnostics (Richmond, VA) and Alpco Diagnostics (Salem, NH), respectively.
Insulin was measured by radioimmunoassay (Linco, St. Charles, MO), and glucose was measured with a glucose analyzer (YSI, Yellow Springs, OH). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as previously described (24).
ApoA-I-containing HDL subpopulations were determined by 2-dimensional nondenaturing gel electrophoresis, immunoblotting, and image analysis as described (25, 26). ApoA-I levels in the individual HDL subpopulations were calculated by multiplying plasma apoA-I levels by the subpopulation percentiles. Because each HDL particle has a fixed number of apoA-I molecules, the change in apoA-I levels in each HDL subpopulation is proportional to changes in particle numbers. The inter- and intra-assay coefficients of variation were <10% for the HDL subpopulation determinations. All plasma samples were stored at –80°C and were never thawed until analysis. ApoA-IV- and apoE-containing HDL subpopulations were determined by 2-dimensional nondenaturing gel electrophoresis followed by immunoblotting for apoA-IV and apoE, respectively, and image analysis. ApoE-containing HDL particles were calculated as “large apoE HDL” with sizes >11 nm and “small apoE HDL” with sizes <11 nm. It is worth noting that all large apoE HDL are probably highly lipidated, whereas the tight band appearance of the small apoE HDL particles indicates that those are aggregates of lipid-poor apoE.
Statistical analysis
All data were calculated as mean ± SD. Due to nonparametric distribution, TG data were log-transformed before analyses. Differences were determined from analyses of log-transformed data. Differences between means were calculated by the two-sided P < 0.05, Tukey's Honestly Significant Difference test. Overall changes were calculated by 32 repeated ANOVAs (adjusted for BMI) for the hypothesis of no change over time. Matrix analyses (nonparametric) were used for calculating associations between selected variables. The SAS statistical package was used in all statistical analyses. The best fit curve was calculated using the StatMost statistical program.
RESULTS
The anthropometric characteristics and parameters related to lipid and glucose metabolism measured in 19 morbidly obese women before (baseline) and 12 months after RYGBP surgery compared with age-matched, healthy, normal-weight control subjects as reference are presented in Table 1. RYGBP patients, at baseline, had significantly lower plasma concentrations of total-C, LDL-C, HDL-C, apoA-I, and apoA-II and significantly higher plasma concentrations of TG and RLP-C than control subjects. At 12 months after surgery, RYGBP patients exhibited marked reductions of BMI, fat mass, and lean mass. In addition, plasma TG, RLP-C, and apoE levels, as well as glucose, insulin, and HOMA-IR, were also reduced, whereas HDL-C level was significantly increased compared with baseline levels. Among the apoA-I-containing HDL subpopulations, mean baseline concentrations of large α- and preα-mobility HDL particles (α-1, α-2, preα-1, and preα-2) were significantly lower in RYGBP patients before surgery than in control subjects. Of these parameters, mean concentrations of α-1 and preα-1 increased significantly at 12 months after surgery. Plasma concentrations of small HDL particles (α-3, α-4, preα-3, and preα-4) were comparable between controls and RYGBP subjects at baseline, despite the significantly lower plasma apoA-I level in RYGBP subjects, and did not change after surgery/weight loss.
TABLE 1.
Characteristics of morbidly obese women before and 12 months after gastric bypass surgery and of age-matched healthy control subjects
| Gastric Bypass Patients (n = 19) |
|||
|---|---|---|---|
| Variable | Controls (n = 19) | Baseline | 12 months |
| Age (year) | 44 ± 4 | 41 ± 8 | 42 ± 8 |
| BMI (kg/m2) | 25 ± 4 | 46 ± 8a | 31 ± 5ab |
| Fat mass (kg) | NA | 67 ± 15 | 33 ± 8b |
| Lean mass (kg) | NA | 63 ± 14 | 46 ± 5b |
| Waist/hip ratio | 0.82 ± 0.1 | 0.95 ± 0.1a | 0.88 ± 0.1ab |
| Fasting glucose | 84 ± 10 | 100 ± 20a | 83 ± 5b |
| Fasting insulin (IU) | 9 ± 7 | 33 ± 16a | 11 ± 3ab |
| HOMA-IR | 1.7 ± 0.8 | 8.5 ± 5.7a | 2.3 ± 0.7ab |
| Total-C | 203 ± 48 | 147 ± 31a | 133 ± 24a |
| LDL-C | 121 ± 41 | 98 ± 29a | 81 ± 19ab |
| HDL-C | 59 ± 14 | 37 ± 10a | 46 ± 8ab |
| LDL/HDL | 2.2 ± 1.0 | 2.6 ± 0.7 | 1.9 ± 0.5b |
| HDL-TGa | NA | 19.4 ± 7.5 | 15.5 ± 5.3b |
| TGa | 112 ± 58 | 141 ± 105a | 84 ± 51b |
| RLP-C | 4.7 ± 2.4 | 7.4 ± 6.7a | 4.0 ± 3.6b |
| Apo A-I | 156 ± 23 | 108 ± 25a | 117 ± 26 |
| Apo A-II | 37 ± 8 | 24 ± 5a | 23 ± 5a |
| Apo E | 4.0 ± 0.8 | 3.6 ± 0.9 | 2.8 ± 0.6ab |
| LCAT mass (μg/ml) | NA | 9.3 ± 2.1 | 7.4 ± 1.6b |
| LCAT activityb | NA | 3.9 ± 1.0 | 2.7 ± 1.4b |
| CETP mass (μg/ml) | NA | 1.0 ± 0.3 | 1.1 ± 0.3 |
| CETP activityc | NA | 16.5 ± 19.2 | 2.8 ± 18.8b |
| ApoA-I-containing HDL subpopulations | |||
| Preβ-1 | 12.4 ± 6.5 | 14.3 ± 9.7 | 11.5 ± 7.3b |
| Preβ-2 | 2.3 ± 1.4 | 2.2 ± 1.7 | 1.9 ± 1.4 |
| α-1 | 27.4 ± 9.2 | 9.0 ± 4.7a | 17.3 ± 5.1ab |
| α-2 | 55.9 ± 11.0 | 39.1 ± 11.2a | 42.7 ± 11.0 |
| α-3 | 28.9 ± 4.7 | 24.8 ± 7.3 | 20.8 ± 7.9 |
| α-4 | 10.0 ± 3.1 | 10.4 ± 3.8 | 9.6 ± 4.0 |
| Preα-1 | 7.2 ± 3.2 | 1.9 ± 1.6a | 4.2 ± 2.4ab |
| Preα-2 | 8.0 ± 2.8 | 3.6 ± 1.8a | 4.8 ± 2.4a |
| Preα-3 | 1.9 ± 0.4 | 1.9 ± 0.8 | 1.7 ± 0.8 |
| Preα-4 | 1.0 ± 0.3 | 0.9 ± 0.5 | 0.9 ± 0.3 |
Values are mean ± SD (mg/dl) or as indicated. P ≤ 0.01 was selected as significantly different (Student's t-test). Letters indicate statistically significant difference from: (a) controls, (b) baseline. Conversion factor from mg/dl to mmol: for cholesterol, divide numbers by 38.88; for TG, divide numbers by 86.88.
Statistical test performed using log transformed values.
μg/ml/h CE increase in plasma.
μg/ml/h CE decrease in HDL.
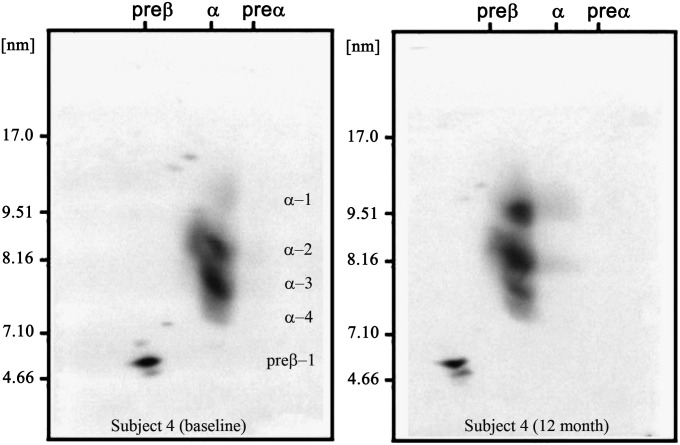
The mean of individual changes of the measured parameters from baseline over time (at 1, 3, 6, and 12 months) are presented in Table 2. As expected and reported earlier (16, 27), BMI decreased progressively after RYGBP surgery. Plasma glucose decreased significantly at 3 months (−10%) and remained lowered. Insulin (−36%) and HOMA-IR (−38%) decreased at 1 month after surgery and progressively decreased further over the 12 month follow-up period. LDL-C (−8%, −4%, −6%, and −15%), TG (−3%, −9%, −15%, and −20%), and HDL-TG (−14%, −24%, −27%, and −34%) levels also showed improvement during the studied time course. RLP-C decreased by ∼20% at 1 month and remained decreased by this proportion over 12 months after surgery. Postoperative changes of HDL-C followed a U-shaped curve: there was a significant decrease from baseline at 1 month, followed by increases at each time point, reaching significantly elevated levels compared with baseline at 12 months. ApoA-I and apoA-II levels initially decreased then returned to baseline levels at 3 months and remained at that level. The apoE level was significantly decreased at 3 months and remained significantly lower than baseline for the remaining 9 months. CETP mass first decreased (mean change −31%) at 1 month, then continually increased with a 13% increase [nonsignificant (NS)] above baseline level at 12 months. At the same time, CETP activity continually decreased after the surgery (mean changes from baseline −17%, −27%, −57%, and −83%). Both LCAT mass and activity decreased significantly at 1 month and then remained at that level. Concentrations of all apoA-I-containing HDL subpopulations (except preβ-2) were lower at 1 month compared with baseline, reflecting the reduction in total plasma apoA-I level at that time. At 12 months, HDL particle concentrations increased, although not uniformly. The mean individual changes of small, lipid-poor preβ-1, α-4, and α-3 particles from baseline to any sampling time were not significant, whereas the mean individual changes of large, lipid-rich α-1 and α-2 particles from baseline were significant at each sampling time and increased by 177% and 22% at 12 months, respectively. Figure 1 illustrates changes in the HDL subpopulation profile of a representative subject at baseline and at 12 months. At baseline, the average α-1 level of obese subjects was 9 mg/dl versus 27.4 mg/dl of lean subjects. After an average 34 kg fat mass loss, the mean α-1 concentration increased to 17.3 mg/dl, which was still lower than normal, but the subjects’ weights were still significantly above normal.
TABLE 2.
Mean percent changes (± SD) of variables of 19 severely obese women following gastric bypass surgery compared with baseline values
| Variable | 1 Month | 3 Month | 6 Month | 12 Month |
|---|---|---|---|---|
| BMI | −10 ± 2a | −18 ± 4ab | −25 ± 5abc | −32 ± 6bcd |
| Fat mass | −12 ± 3a | NA | NA | −51 ± 11ab |
| Lean mass | −10 ± 4a | NA | NA | −16 ± 13ab |
| Waist/hip ratio | −5 ± 9 | −6 ± 7a | −7 ± 9ab | −6 ± 9a |
| Glucose | −7 ± 12 | −10 ± 12a | −13 ± 12ab | −14 ± 14ab |
| Insulin | −36 ± 37a | −49 ± 20ab | −57 ± 17ab | −59 ± 18ab |
| HOMA-IR | −38 ± 42a | −53 ± 22a | −62 ± 18ab | −63 ± 18ab |
| Total-C | −10 ± 24 | −3 ± 26 | −4 ± 23 | −7 ± 15 |
| LDL-C | −8 ± 31 | −4 ± 30 | −6 ± 28 | −15 ± 19 |
| HDL-C | −16 ± 24a | −2 ± 20b | 8 ± 20bc | 15 ± 20abcd |
| LDL/HDL | 12 ± 29 | −11 ± 15b | −10 ± 19b | −10 ± 20b |
| HDL-TGa | −14 ± 29 | −24 ± 27a | −27 ± 26a | −34 ± 27ab |
| TGa | −3 ± 51 | −9 ± 67 | −15 ± 56ab | −20 ± 61ab |
| RLP-C | −19 ± 50a | −16 ± 57a | −22 ± 56a | −21 ± 72a |
| Apo A-I | −24 ± 20a | −6 ± 26b | 3 ± 25b | 9 ± 31bc |
| Apo A-II | −21 ± 15a | −14 ± 30a | −4 ± 19b | −6 ± 25b |
| Apo E | −11 ± 19 | −19 ± 28a | −15 ± 17a | −16 ± 18a |
| CETP activity | −17 ± 19 | −27 ± 28a | −53 ± 48a | −83 ± 66ab |
| LCAT activity | −33 ± 17a | −22 ± 26a | −17 ± 33a | −24 ± 53a |
| CETP mass | −31 ± 13a | −19 ± 19ab | −13 ± 21 | 13 ± 29bcd |
| LCAT mass | −18 ± 16a | −13 ± 17a | −12 ± 20a | −22 ± 13a |
| ApoA-I-containing HDL subpopulations | ||||
| Preβ-1 | −20 ± 46a | −12 ± 67 | −4 ± 57 | −1 ± 4b |
| Preβ-2 | 70 ± 224 | 112 ± 228 | 12 ± 60 | 30 ± 70 |
| α-1 | −7 ± 43 | 68 ± 99b | 115 ± 160ab | 177 ± 180abc |
| α-2 | −23 ± 22a | −6 ± 26b | 8 ± 31b | 22 ± 42abc |
| α-3 | −19 ± 32a | −10 ± 29 | −7 ± 28 | −10 ± 30 |
| α-4 | −20 ± 38a | −12 ± 36 | 2 ± 36 | 4 ± 51 |
| Preα-1 | −52 ± 238 | 159 ± 319 | 251 ± 416b | 580 ± 920abc |
| Preα-2 | −6 ± 54 | 20 ± 81b | 47 ± 63ab | 75 ± 99abc |
| Preα-3 | −5 ± 48 | 2 ± 48 | 9 ± 64 | −2 ± 51 |
| Preα-4 | −7 ± 45 | 9 ± 55 | 5 ± 69 | 17 ± 58 |
Letters indicate statistically significant difference from: (a) baseline, (b) 1 month, (c) 3 month, (d) 6 month (two-sided P < 0.05, Tukey's Honestly Significant Difference).
Differences were determined from analyses of log-transformed data.
Fig. 1.
ApoA-I-containing HDL subpopulations of a representative subject before and 12 month after GBP surgery. Baseline values: HDL-C, 41.5 mg/dl; apoA-I, 124 mg/dl; TG, 214 mg/dl; α-1 HDL, 10.4 mg/dl; and α-2 HDL, 28.2 mg/dl. Values at 12 months: HDL-C, 48.1 mg/dl; apoA-I, 129 mg/dl; TG, 128 mg/dl; α-1, 22.7 mg/dl; and α-2 HDL, 34.5 mg/dl. While HDL-C and apoA-I levels increased 16% and 4%, there was a redistribution of apoA-I-containing HDL particles toward large particles with 117% and 22% increases in α-1 and α-2 levels.
Correlations between proportional (percent) changes from baseline to 12 months for lipoprotein, obesity, and glucose homeostasis variables based on matrix analysis are presented in Table 3. There were significant positive associations between changes of BMI and LDL-C (r = 0.520; P = 0.018), changes of fat mass and LDL-C (r = 0.52; P = 0.018), and changes of fat mass and LCAT mass (r = 0.569; P = 0.011). Changes of plasma insulin level were positively correlated with changes of LCAT mass (r = 0.475; P = 0.043) and inversely with changes of large α-1 (r = −0.498; P = 0.030) and α-2 (r = −0.445; P = 0.050) HDL particle levels. Changes of plasma glucose concentration were inversely correlated with changes of CETP mass (r = −0.629; P = 0.005) and α-1 HDL particle levels (r = −0.641; P = 0.004). Changes of insulin resistance (HOMA-IR) were inversely correlated with changes of CETP mass (r = −0.520; P = 0.031) and α-1 HDL particle levels (r = −0.548; P = 0.018) and positively with changes of LCAT mass (r = 0.472; P = 0.044). The changes in TG were positively associated with RLP-C (r = 0.719; P = 0.002), apoE (r = 0.529; P = 0.029) and LCAT mass (r = 0.476; P = 0.040) and inversely with α-2 HDL particle (r = −0.458; P = 0.042) levels.
TABLE 3.
Correlations between changes (percentile) from baseline to 12 months of lipoprotein parameters with BMI, fat mass, TG, fasting glucose and insulin, and HOMA-IR
| Variable | BMI | Fat Mass | TG | Insulin | Glucose | HOMA-IR |
|---|---|---|---|---|---|---|
| LDL-C | 0.520(0.018) | 0.520(0.018) | −0.133 | 0.047 | −0.409 | −0.102 |
| HDL-C | 0.171 | 0.160 | −0.270 | 0.025 | 0.022 | 0.030 |
| TG | 0.410 | 0.251 | — | 0.065 | 0.154 | 0.100 |
| RLP-C | 0.453 | 0.054 | 0.719(0.002) | 0.170 | 0.171 | 0.056 |
| apoA-I | 0.053 | −0.040 | 0.022 | −0.233 | 0.041 | −0.062 |
| apoA-II | 0.159 | 0.117 | −0.079 | −0.159 | 0.234 | −0.083 |
| Apo E | 0.319 | −0.215 | 0.523(0.029) | −0.081 | 0.092 | 0.019 |
| CETP mass | 0.060 | 0.034 | 0.112 | −0.322 | –0.629(0.005) | –0.520(0.0031) |
| CETP activity | 0.019 | 0.012 | 0.049 | 0.030 | −0.217 | −0.110 |
| LCAT mass | 0.392 | 0.569(0.011) | 0.476(0.040) | 0.475(0.0043) | 0.324 | 0.472(0.044) |
| LCAT activity | 0.111 | 0.050 | 0.069 | 0.100 | 0.010 | 0.078 |
| Preβ-1 | 0.035 | 0.015 | 0.205 | −0.194 | −0.111 | −0.200 |
| Preβ-2 | 0.242 | 0.112 | −0.216 | −0.367 | −0.108 | −0.333 |
| α-1 | −0.030 | −0.033 | −0.353 | -0.498(0.030) | -0.641(0.004) | -0.548(0.018) |
| α-2 | −0.140 | −0.165 | –0.458(0.042) | -0.445(0.050) | −0.076 | −0.430 |
| α-3 | −0.100 | −0.206 | 0.032 | −0.187 | −0.113 | −0.174 |
| α-4 | 0.085 | −0.100 | 0.414 | −0.171 | −0.228 | −0.209 |
| Preα-1 | 0.010 | 0.038 | −0.071 | −0.339 | −0.244 | −0.339 |
| Preα-2 | 0.076 | 0.047 | −0.376 | −0.445 | 0.242 | −0.365 |
| Preα-3 | −0.086 | −0.208 | 0.013 | −0.114 | 0.171 | −0.054 |
| Preα-4 | 0.028 | −0.140 | 0.010 | −0.096 | −0.084 | −0.106 |
Values represent r values; values in parenthesis represent P-values. An r value > 0.44 was considered significant with a P-value < 0.05.
Moreover, changes in waist circumference were associated positively with changes in HDL-TG (r = 0.457; P = 0.043) and tended to be inversely associated with changes in HDL-C (r = −0.376; NS). Changes in HDL-TG had a significant positive association with changes in total TG (r = 0.671; P = 0.008) and NS positive associations with changes in HDL-C (r = 0.225), apoA-I (r = 0.425), apoE (r = 0.443), and insulin (r = 0.316) levels. Changes in HDL-TG had a tendency to be associated positively with changes in the levels of small α-4 HDL particles (r = 0.316, NS) and inversely with changes in the large α-1 HDL particles (r = −0.333, NS) (data for these associations are not shown).
The distribution of apoA-IV- and apoE-containing HDL particles was also assessed by nondenaturing 2-dimensional gel analysis, immunoblotting, and image-analysis. The distribution of apoA-IV-containing particles in obese subjects at baseline was similar to those in controls and did not change in the 12 month follow-up (data not shown). On the other hand, morbidly obese RYGBP patients had more small apoE-containing HDL particles at baseline than controls, and the dominance of small apoE-containing particles remained unchanged (data not shown), although plasma apoE concentrations decreased significantly at 12 months postsurgery (Table 1).
DISCUSSION
Previous epidemiologic studies have reported that obesity is an independent CVD risk factor (28, 29). However, the pathophysiological mechanisms underlying this association are not clear. The goal of this study was to follow 19 morbidly obese women prior to and for 12 months following RYGBP surgery and examine changes in markers of lipid and glucose metabolism associated with substantial weight loss. Samples were collected from RYGBP patients before (baseline) and at 1, 3, 6, and 12 months after surgery, providing an opportunity to investigate the time course of changes of the measured parameters.
In accordance with others (30, 31), we observed normal baseline total cholesterol and LDL-C levels (mean = 98 mg/dl) in these obese women. Baseline TG concentrations, although significantly higher than in normal-weight control subjects, were generally within the normal range (<150 mg/dl). However, we observed a 25% higher preβ-1 level along with a 66% lower α-1 HDL level in morbidly obese subjects before surgery compared with control subjects, indicating an abnormal HDL remodeling and maturation process. Overall, the HDL subpopulation profile of obese subjects was similar to that we have reported in subjects with established CVD (25). We have documented in several populations that an HDL subpopulation profile characterized by low levels of α-1 and α-2 and high levels of preβ-1 HDL is significantly associated with increased initial and recurrent CVD events (18–21). Furthermore, in the HDL-Atherosclerosis Treatment Study, simvastatin + niacin combination therapy increased concentrations of large HDL particles (α-1 and α-2) and decreased the concentration of the small HDL particle (preβ-1), and this shift was significantly associated with a slower progression of coronary stenosis assessed by angiography (18).
This study allowed a detailed analysis of the changes of lipoprotein subclasses and an examination of the relationships between changes of these parameters with changes of BMI, fat mass, and glucose homeostasis following weight loss. Following RYGBP-induced weight loss, we observed significant improvements in the lipoprotein profile in addition to the previously reported significant improvements in inflammation and indices of glucose homeostasis (27). We observed significant decreases in LDL-C, plasma TG and HDL-TG, RLP-C, and significant increases in HDL-C levels, which resulted in marked improvement of the HDL subpopulation profile (a 177% increase in the cardio-protective α-1 particle level). The changes in HDL subpopulation profile in postoperative samples indicated a substantial improvement in HDL remodeling and likely in HDL functions. HDL particles are implicated in different steps of reverse cholesterol transport, a metabolic pathway involved in the removal of cholesterol from macrophages in the vessel wall and transportation back to the liver. Preβ-1 HDL consists of small, lipid-poor particles, containing apoA-I without apoA-II, and interacts with cholesterol transporter ATP-binding cassette 1 to promote cellular-lipid efflux. After picking up FC and phospholipids from cells, preβ-1 continually matures into larger, lipid-rich HDL particles (α-4, α-3, α-2, and α-1), increasing size and lipid content through the action of LCAT and lipoprotein lipase (32). The large, lipid-rich HDL particles, α-1 and α-2, directly transport cholesterol to the bile via the hepatic scavenger receptor class B type I pathway or indirectly via apoB-containing particles with the involvement of CETP and LDL receptors (33). A significant increase in the levels of large HDL particles, observed during the follow-up, requires more CE accumulation in HDL, which might be the result of lower CETP and/or higher LCAT activities. ApoA-I, on the surface of HDL, activates LCAT that esterifies FC into CE, the prime core material for the large spherical HDL particles. CETP transports CE from HDL to VLDL in exchange for TG. The TG-enriched HDL is susceptible for hepatic lipase activity, which can result in the formation of HDL remnants like the lipid-poor preβ-1 and α-4 particles (34). As a result of a decrease in HDL size, the fractional catabolic rate of HDL increases (35), which may provide a possible explanation for the lower baseline levels of key HDL components (HDL-C, apoA-I, and apoA-II) in obese subjects. Because of significant weight loss, HDL-C increased and HDL-TG content decreased. After weight loss, both CETP and hepatic lipase activity decreased and in turn HDL size increased. Changes in waist circumference were associated positively with changes in HDL-TG and inversely with changes in HDL-C. The associations between HDL-TG and HDL particles indicated that only LpA-I HDL particles were associated with HDL-TG level: the large LpA-I α-1 particles displayed an inverse relationship with HDL-TG, while the small α-4 was positively correlated with HDL-TG. A significant positive association observed between HDL-TG and apoE levels indicates a potential role of apoE in TG trafficking.
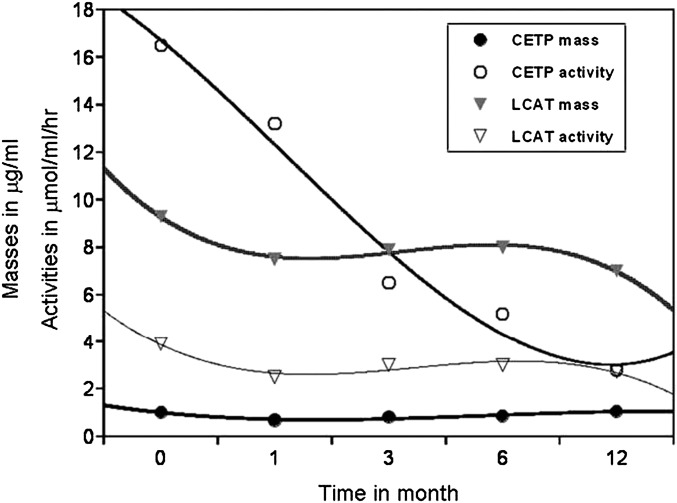
Although, adipose tissue is a major source of CETP protein, in this study, changes of CETP mass were not associated with changes of fat mass. We hypothesize that CETP production is more closely related to adipocyte number than the lipid content (size) of adipocytes, and weight loss does not necessarily promote fat cell loss but mainly decreases adipocyte volume. In vivo, CETP activity is considered to be more influenced by the concentration of its substrates, VLDL and/or specific HDL particles, than by the concentration of CETP protein (32, 36). In agreement with these publications, we observed that changes of CETP activity were independent of changes of CETP mass (Fig. 2). In this study, we observed a significant inverse association between changes in glucose and CETP and α-1 HDL particle levels. This observation indicates that glucose homeostasis may also regulate CETP mass. The continuous decrease in CETP activity, which was independent of change in CETP mass (Fig. 2), was probably secondary to quantitative and qualitative changes in VLDL and HDL, which both are substrates for CETP activity. These findings are in line with those of Dullart (37–39), who has published several papers on associations between glucose homeostasis and PLTP and CETP activities, and of John Albers (40), who published that both mRNA and activity of PLTP was regulated by glucose.
Fig. 2.
Best curve fit of mean values of CETP and LCAT masses and activities obtained from plasma samples collected at baseline and at 1, 3, 6, and 12 months following gastric bypass surgery.
Limitations of the study include: 1) the study was not a randomized intervention study; 2) the study population included only one male subject whose data were omitted; therefore the observations in this manuscript may not be applicable to men; and 3) data on diet, exercise, and alcohol intake were not available to be included in the analyses.
In this study, a marked loss of fat mass produced significant improvement in the HDL subpopulation profile, which was substantially greater than we had previously reported with drug intervention therapies (18, 41). Moreover, substantial weight loss was accompanied by decreases of plasma TG and of the highly atherogenic RLP-C and HDL-TG levels and by increases of HDL-C. Together with the improvement of glucose homeostasis, these are indicative of the improved cardiovascular health/cardiometabolic function in these weight-reduced patients.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- BMI
- body mass index
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- CVD
- cardiovascular disease
- FC
- free cholesterol
- HDL-C
- HDL cholesterol
- HOMA-IR
- homeostatic model assessment of insulin resistance
- LCAT
- lecithin-cholesterol acyltransferase
- LDL-C
- LDL cholesterol
- NS
- nonsignificant
- RLP-C
- remnant-lipoprotein cholesterol
- RYGBP
- Roux-en-Y gastric bypass
- TG
- triglyceride
- total-C
- total cholesterol
This work was supported by grants from the US Department of Agriculture (Contract 53-3K06-5-10), and by an award from the University of California Davis HEALTH Care System, the UC Davis, Clinical and Translational Science Center supported by NIH (RR-024146), and NIH (HL-64738). Dr Havel's laboratory also received support from the NIH (HL-075675, HL-091333, AT-002599, AT-002933, and AT-003645), and from the American Diabetes Association. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Poirier P., Giles T. D., Bray G. A., Hong Y., Stern J. S., Pi-Sunyer F. X., Eckel R. H. 2006. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 113: 898–918. [DOI] [PubMed] [Google Scholar]
- 2.Brolin R. E. 2002. Bariatric surgery and long-term control of morbid obesity. JAMA. 288: 2793–2796. [DOI] [PubMed] [Google Scholar]
- 3.Steinbrook R. 2004. Surgery for severe obesity. N. Engl. J. Med. 350: 1075–1079. [DOI] [PubMed] [Google Scholar]
- 4.Arterburn D., Schauer D. P., Wise R. E., Gersin K. S., Fischer D. R., Selwyn C. A., Jr., Erisman A., Tsevat J. 2009. Change in predicted 10-year cardiovascular risk following laparoscopic Roux-en-Y gastric bypass surgery. Obes. Surg. 19: 184–189. [DOI] [PubMed] [Google Scholar]
- 5.Christou N. V., Sampalis J. S., Liberman M., Look D., Auger S., McLean A. P., MacLean L. D. 2004. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann. Surg. 240: 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams D. B., Hagedorn J. C., Lawson E. H., Galanko J. A., Safadi B. Y., Curet M. J., Morton J. M. 2007. Gastric bypass reduces biochemical cardiac risk factors. Surg. Obes. Relat. Dis. 3: 8–13. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen N. T., Varela E., Sabio A., Tran C. L., Stamos M., Wilson S. E. 2006. Resolution of hyperlipidemia after laparoscopic Roux-en-Y gastric bypass. J. Am. Coll. Surg. 203: 24–29. [DOI] [PubMed] [Google Scholar]
- 8.Zlabek J. A., Grimm M. S., Larson C. J., Mathiason M. A., Lambert P. J., Kothari S. N. 2005. The effect of laparoscopic gastric bypass surgery on dyslipidemia in severely obese patients. Surg. Obes. Relat. Dis. 1: 537–542. [DOI] [PubMed] [Google Scholar]
- 9.Kligman M. D., Dexter D. J., Omer S., Park A. E. 2008. Shrinking cardiovascular risk through bariatric surgery: application of Framingham risk score in gastric bypass. Surgery. 143: 533–538. [DOI] [PubMed] [Google Scholar]
- 10.Brolin R. E., Bradley L. J., Wilson A. C., Cody R. P. 2000. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J. Gastrointest. Surg. 4: 464–469. [DOI] [PubMed] [Google Scholar]
- 11.Faraj M., Havel P. J., Phélis S., Blank D., Sniderman A. D., Cianflone K. 2003. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J. Clin. Endocrinol. Metab. 88: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 12.Kopp H. P., Krzyzanowska K., Möhlig M., Spranger J., Pfeiffer A. F., Schernthaner G. 2005. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes (Lond). 29: 766–771. [DOI] [PubMed] [Google Scholar]
- 13.Sjöström C. D., Lissner L., Wedel H., Sjöström L. 1999. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes. Res. 7: 477–484. [DOI] [PubMed] [Google Scholar]
- 14.Polyzogopoulou E. V., Kalfarentzos F., Vagenakis A. G., Alexandrides T. K. 2003. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 52: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 15.Buchwald H., Avidor Y., Braunwald E., Jensen M. D., Pories W., Fahrbach K., Schoelles K. 2004. Bariatric surgery: a systematic review and meta-analysis. JAMA. 292: 1724–1737. [DOI] [PubMed] [Google Scholar]
- 16.Swarbrick M. M., Austrheim-Smith I. T., Stanhope K. L., Van Loan M. D., Ali M. R., Wolfe B. M., Havel P. J. 2006. Circulating concentrations of high-molecular-weight adiponectin are increased following Roux-en-Y gastric bypass surgery. Diabetologia. 49: 2552–2558. [DOI] [PubMed] [Google Scholar]
- 17.Braunwald E. 1997. Shattuck lecture. Cardiovascular medicine at the turn of the milenneum: triumphs, concerns, and opportunities. N. Engl. J. Med. 337: 1360–1369. [DOI] [PubMed] [Google Scholar]
- 18.Asztalos B. F., Batista M., Horvath K. V., Cox C. E., Dallal G. E., Morse J. S., Brown G. B., Schaefer E. J. 2003. Change in alpha1 HDL concentration predicts progression in coronary artery stenosis. Arterioscler. Thromb. Vasc. Biol. 23: 847–852. [DOI] [PubMed] [Google Scholar]
- 19.Asztalos B. F., Cupples L. A., Demissie S., Horvath K. V., Cox C. E., Batista M. C., Schaefer E. J. 2004. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler. Thromb. Vasc. Biol. 24: 2181–2187. [DOI] [PubMed] [Google Scholar]
- 20.Asztalos B. F., Collins D., Cupples L. A., Demissie S., Horvath K. V., Bloomfield H. E., Robins S. J., Schaefer E. J. 2005. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler. Thromb. Vasc. Biol. 25: 2185–2191. [DOI] [PubMed] [Google Scholar]
- 21.Lamon-Fava S., Herrington D. M., Reboussin D. M., Sherman M., Horvath K. V., Cupples L. A., White C., Demissie S., Schaefer E. J., Asztalos B. F. 2008. Plasma levels of HDL subpopulations and remnant lipoproteins predict the extent of angiographically-defined coronary artery disease in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 28: 575–579. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa Y., Fielding C. J. 1985. Assay of cholesteryl ester transfer activity and purification of a cholesteryl ester transfer protein. Methods Enzymol. 111: 274–285. [DOI] [PubMed] [Google Scholar]
- 23.Fielding C. J., Havel R. J., Todd K. M., Yeo K. E., Schloetter M. C., Weinberg V., Frost P. H. 1995. Effects of dietary cholesterol and fat saturation on plasma lipoproteins in an ethically diverse population of healthy young men. J. Clin. Invest. 95: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews D. R., Hosker J. P., Rudensky A. S., Naylor B. A., Treacher D. F., Turner R. C. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 25.Asztalos B. F., Roheim P. S., Milani R. L., Lefevre M., McNamara J. R., Horvath K. V., Schaefer E. J. 2000. Distribution of ApoA-I-containing HDL subpopulations in patients with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 20: 2670–2676. [DOI] [PubMed] [Google Scholar]
- 26.Asztalos B., Lefevre M., Wong L., Foster T. A., Tulley R., Windhauser M., Zhang W., Roheim P. S. 2000. Differential response to low-fat diet between low and normal HDL-cholesterol subjects. J. Lipid Res. 41: 321–328. [PubMed] [Google Scholar]
- 27.Swarbrick M. M., Stanhope K. L., Austrheim-Smith I. T., Van Loan M. D., Ali M. R., Wolfe B. M., Havel P. J. 2008. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 51: 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manson J. E., Willett W. C., Stampfer M. J., Colditz G. A., Hunter D. J., Hankinson S. E., Hennekens C. H., Speizer F. E. 1995. Body weight and mortality among women. N. Engl. J. Med. 333: 677–685. [DOI] [PubMed] [Google Scholar]
- 29.Bengtsson C., Björkelund C., Lapidus L., Lissner L. 1993. Associations of serum lipid concentrations and obesity with mortality in women: 20 year follow up of participants in prospective population study in Gothenburg, Sweden. BMJ. 307: 1385–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt S. C., Williams R. R., Adams T. D. 1995. Biochemical and anthropometric characterization of morbid obesity in a large Utah pedigree. Obes. Res. 3 (Suppl. 2): 165S–172S. [DOI] [PubMed] [Google Scholar]
- 31.Dixon J. B., O'Brien P. 2000. A disparity between conventional lipid and insulin resistance markers at body mass index levels greater than 34 kg/m2. Int. J. Obes. Relat. Metab. Disord. 25: 793–797. [DOI] [PubMed] [Google Scholar]
- 32.Asztalos B. F., Schaefer E. J., Horvath K. V., Yamashita S., Miller M., Franceschini G., Calabresi L. 2007. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J. Lipid Res. 48: 592–599. [DOI] [PubMed] [Google Scholar]
- 33.Asztalos B. F., de la Llera-Moya M., Dallal G. E., Horvath K. V., Schaefer E. J., Rothblat G. H. 2005. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J. Lipid Res. 46: 2246–2253. [DOI] [PubMed] [Google Scholar]
- 34.Xiao C., Watanabe T., Zhang Y., Trigatti B., Szeto L., Connelly P. W., Marcovina S., Vaisar T., Heinecke J. W., Lewis G. F. 2008. Enhanced cellular uptake of remnant high-density lipoprotein particles: a mechanism for high-density lipoprotein lowering in insulin resistance and hypertriglyceridemia. Circ. Res. 103: 159–166. [DOI] [PubMed] [Google Scholar]
- 35.Brinton E. A., Eisenberg S., Breslow J. L. 1994. Human HDL cholesterol levels are determined by apoA-I fractional catabolic rate, which correlates inversely with estimates of HDL particle size. Effects of gender, hepatic and lipoprotein lipases, triglyceride and insulin levels, and body fat distribution. Arterioscler. Thromb. 14: 707–720. [DOI] [PubMed] [Google Scholar]
- 36.Mann C. J., Yen F. T., Grant A. M., Bihain B. E. 1991. Mechanism of plasma cholesteryl ester transfer in hypertriglyceridemia. J. Clin. Invest. 88: 2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riemens S. C., Van Tol A., Stulp B. K., Dullaart R. P. 1999. Influence of insulin sensitivity and the TaqIB cholesteryl ester transfer protein gene polymorphism on plasma lecithin:cholesterol acyltransferase and lipid transfer protein activities and their response to hyperinsulinemia in non-diabetic men. J. Lipid Res. 40: 1467–1474. [PubMed] [Google Scholar]
- 38.Riemens S. C., van Tol A., Sluiter W. J., Dullaart R. P. 1998. Plasma phospholipid transfer protein activity is related to insulin resistance: impaired acute lowering by insulin in obese Type II diabetic patients. Diabetologia. 41: 929–934. [DOI] [PubMed] [Google Scholar]
- 39.Van Tol A., Ligtenberg J. J., Riemens S. C., van Haeften T. W., Reitsma W. D., Dullaart R. P. 1997. Lowering of plasma phospholipid transfer protein activity by acute hyperglycaemia-induced hyperinsulinaemia in healthy men. Scand. J. Clin. Lab. Invest. 57: 147–157. [DOI] [PubMed] [Google Scholar]
- 40.Tu A. Y., Albers J. J. 2001. Glucose regulates the transcription of human genes relevant to HDL metabolism: responsive elements for peroxisome proliferator-activated receptor are involved in the regulation of phospholipid transfer protein. Diabetes. 50: 1851–1856. [DOI] [PubMed] [Google Scholar]
- 41.Asztalos B. F., Le Maulf F., Dallal G. E., Stein E., Jones P. H., Horvath K. V., McTaggart F., Schaefer E. J. 2007. Comparison of the effects of high doses of rosuvastatin versus atorvastatin on the subpopulations of high-density lipoproteins. Am. J. Cardiol. 99: 681–685. [DOI] [PubMed] [Google Scholar]