Abstract
The long-chain omega-3 fatty acids (n-3 FA) eicosapentaenoic acid (EPA) and docosahexaenoic acids (DHA) have beneficial health effects, but the molecular mediators of these effects are not well characterized. Oxygenated n-3 FAs (oxylipins) may be an important class of mediators. Members of this chemical class include epoxides, alcohols, diols, and ketones, many of which have bioactivity in vitro. Neither the presence of n-3 oxylipins in human plasma nor the effect of n-3 FA ingestion on their levels has been documented. We measured plasma oxylipins derived from both the n-3 and n-6 FA classes in healthy volunteers (n = 10) before and after 4 weeks of treatment with prescription n-3 FA ethyl esters (4 g/day). At baseline, EPA and DHA oxylipins were detected in low (1–50 nM) range, with alcohols > epoxides ≥ diols. Treatment increased n-3 oxylipin levels 2- to 5-fold and reduced selected n-6 oxylipins by ∼20%. This is the first documentation that endogenous n-3 oxylipin levels can be modulated by n-3 FA treatment in humans. The extent to which the beneficial cardiovascular effects of n-3 FAs are mediated by increased n-3 and/or reduced n-6 oxylipin levels remains to be explored.
Keywords: omega-3 fatty acid, P-OM3, inflammation, EPA, DHA
Omega-3 fatty acids (n-3 FA) have beneficial effects in cardiovascular (1), inflammatory (2), renal (3), and neuropsychiatric diseases (4, 5). In Japan, increased serum n-3 FA levels appear to explain the lower prevalence of atherosclerosis in middle-aged men compared with their Caucasian counterparts (6), and higher n-3 FA intakes are associated with reduced mortality from heart failure (7). Administration of prescription omega-3 acid ethyl esters (P-OM3; 1 g/day) in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI)-Prevenzione and the GISSI heart failure studies (8, 9) reduced total mortality as well as major adverse cardiac events. It is well established that therapeutic doses of P-OM3 reduce serum triglycerides (TG) (10), and lower doses appear to have anti-arrhythmic properties (11), but the specific molecular mechanisms responsible for these clinical effects are not well understood.
There has been considerable speculation that “eicosanoids” (oxygenated metabolites of 20-carbon FAs; e.g., prostaglandins, leukotrienes, thromboxane) may mediate some of the effects of n-3 FAs, but there is little direct evidence in humans to support this hypothesis. Other mechanisms relating to alterations in membrane function (12) or gene regulation (13) may also be involved.
Eicosanoids belong to a larger class of molecules known as oxylipins, subsets of which are shown in Figs. 1 and 2 and supplemental Fig. I (5-LOX pathways). Many of these molecules or their direct precursors can be derived by either specific enzymatic reactions or nonspecific autooxidative rearrangements. Oxylipins include oxygenated metabolites of unsaturated FAs from both the n-3 [e.g., α-linolenic (aLA), eicosapentaenoic (EPA), docosahexaenoic (DHA)] and n-6 [e.g., linoleic (LA), dihomo-γ-linolenic (dgLA), arachidonic (AA)] series. Enzymatically generated prostaglandins and thromboxanes (Tx) are produced from cyclooxygenase (COX)-derived metabolites, while leukotrienes are 5-lipoxygenase (5-LOX) products. In addition, various alcohols, epoxides, and diols can be produced from the actions of LOXs, cytochrome P-450s (CYP), and epoxide hydrolases. Although n-6 oxylipins have been reasonably well described (14), reports of endogenous levels of n-3 oxylipins in humans are rare (15) and focus only on COX or LOX metabolites; epoxy-n3 has not been reported. Moreover, the effects of n-3 FA administration on the levels of a wide array of n-6 and n-3 oxylipins remain unreported to the best of our knowledge. To understand how biochemical and cellular effects of individual n-3 oxylipins may relate to conditions affected by fish-oil treatment, it is critical to establish the normal concentration ranges of these metabolites in humans and to document the responsiveness of these levels to increased intakes of their parent n-3 FAs. In this study, we report that increased n-3 FA intake impacts a wide array of oxygenated lipid metabolites of 18-22 carbon fatty acids in the blood of a small group of human subjects.
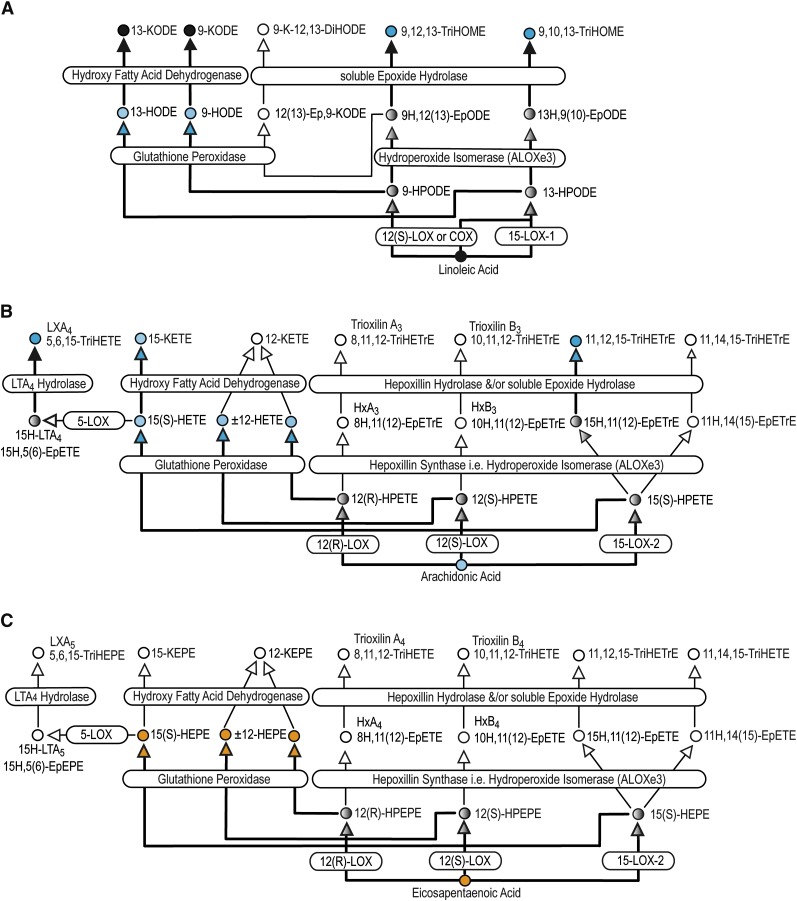
Fig. 1.
12- and 15-Lipoxygenase-associated metabolism of linoleic (A), arachidonic (B), and eicosapentaenoic (C) acids. Reactive oxygen species (ROS) can also produce PUFA hydroperoxides. Metabolites are indicated by circles and enzymes by rounded rectangles. Treatment effects are indicated by color: black = unchanged; blue = decreased; orange = increased; white = not measured. Bolded arrows demarcate metabolic pathways evaluated and gray circles and arrows are unmeasured metabolites along evaluated pathways. Analogous metabolic pathways for α-linolenic acid and docosahexaenoic acid exist, and the metabolites measured within these pathways track the changes observed in linoleic and eicosapentaenoic acids, respectively.
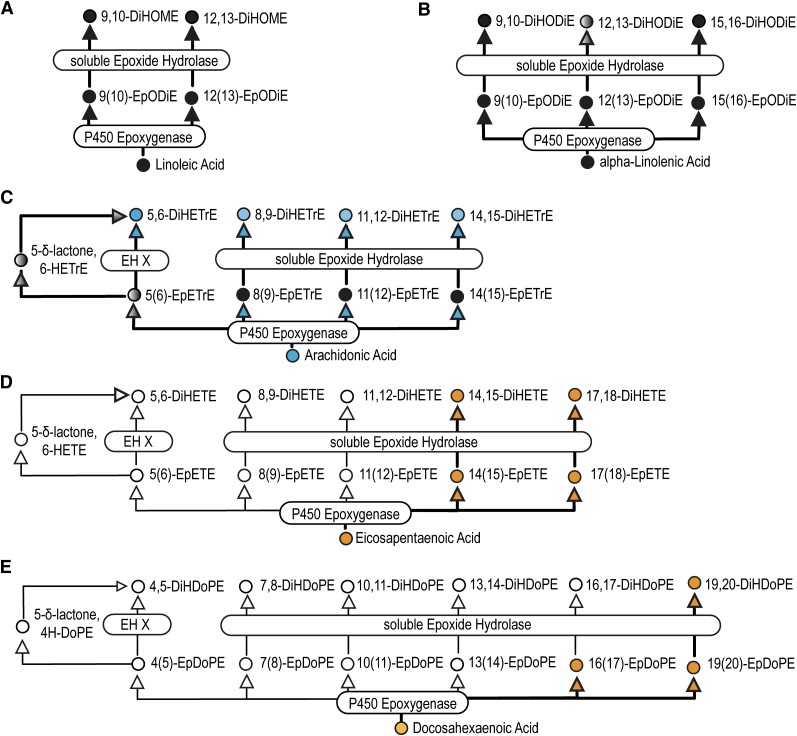
Fig. 2.
Cytochrome P450 epoxygenase-associated metabolism of linoleic (A), α-linolenic (B), arachidonic (C), eicosapentaenoic (D), and docosahexaenoic (E) acids. Metabolites are indicated by circles and enzymes by rounded rectangles. Some reactions may be mediated by multiple enzymes. Treatment effects are indicated by color: black = unchanged; blue = decreased; orange = increased; white = not measured. Bolded arrows demarcate metabolic pathways evaluated and gray circles and arrows are unmeasured metabolites along evaluated pathways.
METHODS
Subjects and design
Ten healthy adults meeting the inclusion and exclusion criteria previously described (16) participated. The four men and six women were all Caucasian, with a median [interquartile range (IQR)] age of 33 (24, 51) years and a BMI of 25.5 (21, 29) kg/m2. Oxylipin levels were measured before and after 4 weeks of treatment with 4 g/day of P-OM3 (Lovaza, GlaxoSmithKline, Philadelphia, PA). The study was approved by the University of South Dakota Institutional Review Board, and written informed consent was obtained from each subject.
Sample collection and analysis
Blood was drawn into sodium EDTA after a 10 h overnight fast, and whole plasma FA composition was measured using gas chromatography as described for erythrocyte extracts (17). Briefly, FAs were extracted from 100 μl plasma aliquots using methylene chloride, methanol, and water (2:2:1), followed by treatment with 14% boron trifluoride in methanol at 100°C for 10 min. The resulting FA methyl esters were analyzed in a GC2010 (Shimadzu, Columbia, MD) using a 100 m SP2560 capillary column (Supelco, Bellefonte, PA). Oxylipin isolation and determination were performed using modifications of previously reported procedures, which included expansion of the previous analyte list (18, 19) (see supplemental data for more detail). Briefly, plasma (200 μl) was spiked with butylated hydroxytoluene/EDTA in 10 deuterated prostanoid, eicosanoid, and octadecanoid surrogates (see supplemental Table II), and subjected to overnight hydrolysis in 1 M methanolic sodium hydroxide to release ester linked oxygenated lipids (18). Samples were extracted on 60 mg Oasis HLB solid phase extraction columns (Waters, Milford, MA), dried for 30 min, wetted with 0.5 ml methanol, eluted with 2 ml ethyl acetate into 6 µL of 30% glycerol, and reduced to dryness under vacuum. We have confirmed oxylipid stability for more than 5 months at −80°C under these conditions (unpublished observations). Residues were reconstituted in 75 µL of a 400 nM solution of 1-cyclohexyluriedo-3-dodecanoic acid (CUDA) and 1-phenylurea-3-hexanoic acid (PUHA) in methanol, vortexed, and filtered for 3 min by centrifugation at 0.1 µm using Amicon Ultrafree-MC durapore PVDF filters (Millipore). Analytes within the filtered elutes were separated by reverse-phase ultra-performance liquid chromatography (UPLC) on a 1.7 μm Acquity BEH column (Waters) using a 25 min two-solvent gradient (solvent A = 0.1% acetic acid; solvent B = 90:10 v/v acetonitrile/isopropanol; see supplemental Table I). Oxylipids were detected by negative mode electrospray ionization tandem quadrupole mass spectroscopy using modifications of the previously reported method (19). Ionization and fragmentation energies for the reported precursor-product ions were optimized for analysis on an API 4000 QTrap (Applied Biosystems Inc., Foster City, CA). Collision induced dissociation mass transitions and analytical surrogates for all target analytes are reported in supplemental Tables II–V.
Statistics
The effects of P-OM3 on the plasma concentrations of n-3 FA oxylipins were tested using multiway ANOVA (ANOVA). Epoxides of n3 FAs and the DHA-derived diol 19,20-DiHDPA were obtained in a second analysis of the sample extracts (n = 7; insufficient extract volumes from 3 subjects). Data from all 10 subjects were available for all other oxylipins. Oxylipin concentrations were log-transformed to achieved normal distributions with equal variance. Tukey's honest significant difference test was used in posthoc analyses. The least square mean (95% CI) of individual regioisomers is reported. Results were considered significant at P < 0.05. Analyses were performed using JMP software (version 7.0.2). All model assumptions were verified and model fit was confirmed.
Oxylipin nomenclature
See supplemental data.
RESULTS
The baseline and final levels of whole plasma polyunsaturated FAs (PUFA) are given in Table 1. As expected, EPA and DHA levels increased with treatment by ∼8- and ∼3-fold, respectively. Neither LA nor aLA was altered by P-OM3; however, small decreases in dgLA and AA were noted.
TABLE 1.
Plasma content (% of total FA) of the oxylipin parent FA
| Oxylipin Parent FA | Pre P-OM3 | Post P-OM3 | Fold Change | P |
|---|---|---|---|---|
| LA | 34.2 (32, 36) | 33.5 (32, 35) | – | >0.05 |
| aLA | 0.558 (0.48, 0.65) | 0.551 (0.47, 0.64) | – | >0.05 |
| dgLA | 0.204 (0.19, 0.22) | 0.174 (0.16, 0.19) | 0.85 | 0.015 |
| AA | 7.24 (6.8, 7.8) | 6.34 (5.9, 6.8) | 0.88 | 0.012 |
| EPA | 0.425 (0.31, 0.59) | 3.6 (2.6, 5) | 8.46 | 0.0004 |
| DHA | 1.47 (1.2, 1.8) | 3.97 (3.3, 4.8) | 2.71 | 0.0001 |
Before and after 4 weeks of treatment with prescription omega-3 acid ethyl esters (P-OM3) in healthy volunteers (n = 10). Abbreviations: AA, arachidonic acid; aLA, α-linolenic acid; dgLA, dihommo-γ-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FA, fatty acid; LA, linolenic acid; P-OM3, prescription omega-3 ethyl esters.
Alcohols and ketones
As Tables 2 and 3 show, alcohols derived from each of the six parent PUFAs were present at baseline in concentrations ranging from ∼1–100 nM, except LA alcohols, which were present in much greater concentrations (∼100–1000 nM). FA alcohols derived from each of the measured parent PUFAs (except aLA) were altered by P-OM3 treatment. In the case of AA metabolites, 15-HETE was present in the highest concentration, with progressively decreasing concentrations as the site of oxygenation moves toward the carboxyl group. The exception to this trend is 5-HETE, the other AA regioisomer with a single proximate double bond, which was present at the same concentration as 15-HETE. As for EPA metabolites, 5-HEPE (with a single proximate double bond) was present in the highest concentration, while both 12- and 15-HEPE (with two proximate double bonds) were present at lower concentrations. P-OM3 increased the measured EPA and DHA alcohols by 5.7- and 2.1-fold, respectively, regardless of regioisomer and reduced AA and LA alcohols by ∼20% and dgLA alcohols by 34%. Neither the aLA alcohols nor the AA terminal alcohol 20-HETE were affected by P-OM3.
TABLE 2.
Plasma octadecanoic acid alcohol and ketone concentrations (nM)a
| Alcohol Oxylipin | Isomer Comparisonb | Pre P-OM3 | Post P-OM3 | Fold Change | Pc |
|---|---|---|---|---|---|
| LA – HODE | |||||
| 9-HODE | A | 296 (250, 340) | 252 (220, 290) | 0.86 | 0.047 |
| 13-HODE | B | 741 (640, 860) | 641 (550, 750) | ||
| aLA – HOTE | |||||
| 9-HOTE | – | 8.07 (5.6, 12) | 7.65 (5, 12) | – | >0.05 |
| 13-HOTE | – | 6.82 (5.6, 8.2) | 5.84 (4.8, 7.1) | ||
| LA – KOTE | |||||
| 9-KODE | A | 88.2 (75, 100) | 74.9 (64, 88) | – | >0.05 |
| 13-KODE | B | 206 (180, 240) | 182 (160, 210) | ||
The mean and 95% CI of individual regioisomers after adjustment for subject and extraction batch. Abbreviations: AA, arachidonic acid; aLA, α-linolenic acid; EPA, eicosapentaenoic acid; HODE, hydroxyoctadecadienoic acid; HOTE, hydroxyoctadecatrienoic acid; KODE, ketooctadecadienoic acid; KOTE, ketooctadecadienoic acid; LA, linolenic acid; LOX, lipoxygenase; P-OM3, prescription omega-3 ethyl esters.
See Fig. 1 for associated 12- and 15-LOX pathways and the analogous AA and EPA regioisomers.
Regioisomers derived from the same parent FA with different letters differ significantly (P < 0.05) by Tukey's Honest Significant Differences test.
P-value of treatment effects on plasma concentrations.
TABLE 3.
Plasma eicosanoic and docosanoic acid alcohol and ketone concentrations (nM)a
| Alcohol Oxylipin | Isomer Comparisonb | Pre P-OM3 | Post P-OM3 | Fold Change | Pc |
|---|---|---|---|---|---|
| dgLA – HETrE | |||||
| 15-HETrE | – | 13.7 (12, 16) | 8.98 (7.6, 11) | 0.66 | 0.003 |
| AA – HETE | |||||
| 5-HETE | A | 81.9 (72, 93) | 66 (58, 75) | 0.8 | <0.0001 |
| 8-HETE | B | 33.9 (30, 38) | 26.1 (23, 29) | ||
| 9-HETE | C, B | 36.3 (32, 41) | 29.7 (26, 34) | ||
| 11-HETE | C | 43.4 (38, 49) | 34.1 (30, 39) | ||
| 12-HETE | D | 52.6 (47, 59) | 43.4 (38, 49) | ||
| 15-HETE | A | 84.7 (75, 96) | 67.4 (60, 76) | ||
| ω-alcohol | |||||
| 20-HETE | – | 4.35 (3.6, 5.3) | 4.27 (3.5, 5.2) | – | – |
| EPA – HEPE | |||||
| 5-HEPE | A | 12.4 (10, 15) | 71.1 (58, 88) | 5.7 | <0.0001 |
| 12-HEPE | B | 3.54 (2.9, 4.4) | 20.1 (16, 25) | ||
| 15-HEPE | B | 3.48 (2.8, 4.3) | 19.2 (16, 24) | ||
| DHA – HDoHE | |||||
| 17-HDoHE | – | 26.8 (21, 34) | 56 (44, 71) | 2.1 | 0.001 |
| AA – KETE | |||||
| 5-KETE | A | 19.7 (17, 23) | 16.3 (14, 19) | 0.83 | 0.03 |
| 15-KETE | B | 63.1 (54, 74) | 53.8 (46, 63) | ||
Mean and 95% CI of individual regioisomers after adjustment for subject and extraction batch. Abbreviations: AA, arachidonic acid; aLA, α-linolenic acid; dgLA, dihomo-γ-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FA, fatty acid; HDoHE, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; HETrE, hydroxyeicosatrienoic acid; HODE, hydroxyoctadecadienoic acid; KETE, ketoeicosatetraenoic acid; LA, linolenic acid; LOX, lipoxygenase; P-OM3, prescription omega-3 ethyl esters.
See Fig. 1 and supplemental Fig. I (5-LOX) for associated pathways for each oxylipin regioisomer.
Regioisomers derived from the same parent FA with different letters differ from each other significantly (P < 0.05) by Tukey's Honest Significant Differences test.
P value of treatment effects on plasma.
Ketones of LA and AA were present at concentrations ranging from 10–200 nM. P-OM3 treatment reduced AA-derived ketones by about 20% but had no effect on LA-derived ketones. In both sets of ketones, the concentrations of the most distal alcohols (15-KETE and its LA analog 13-KODE) were about 2–3 times more abundant than more proximal alcohols (5-KETE and 9-KODE). Standards for EPA- and DHA-derived ketones were not commercially available and, therefore, not quantified.
Epoxides and vicinal diols
As shown in Table 4, baseline concentrations of PUFA epoxides decreased in the following order: LA > AA > DHA > EPA ≥ aLA epoxides. Of the AA-derived epoxides, 11(12)-EpETrE was 2–3 times more abundant than the others. P-OM3 increased EPA- and DHA- derived epoxides 5- and 2-fold, respectively, as seen in the alcohols.
TABLE 4.
Concentration of plasma epoxides and vicinal diols in nMa
| Epoxide Oxylipin | Baseline | n-3 FA | Fold Change | Pc | Vicinal Diol Oxylipin | Baseline | n-3 FA | Fold Change | Pc | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LA – EpOME | LA – DiHOME | ||||||||||
| 9(10)-EpOME | 83.4 (72, 97) | 79.5 (69, 92) | – | >0.05 | 9,10-DiHOME | A | 197 (170, 230) | 220 (190, 260) | – | >0.05 | |
| 12(13)-EpOME | 83.1 (72, 96) | 76.9 (66, 89) | >0.05 | 12,13-DiHOME | B | 18.1 (16, 21) | 16.7 (14, 19) | ||||
| aLA – EpODE | aLA – DiHODE | ||||||||||
| 9(10)-EpODE | Ab | 5.5 (4.4, 6.9) | 5.32 (4.3, 6.6) | – | >0.05 | 9,10-DiHODE | A | 1.38 (1.1, 1.7) | 1.6 (1.3, 2) | – | >0.05 |
| 12(13)-EpODE | B | 1.44 (1.2, 1.8) | 1.39 (1.1, 1.7) | 12,13-DiHODEd | – | – | |||||
| 15(16)-EpODE | C | 9.19 (7.4, 11) | 8.09 (6.5, 10) | 15,16-DiHODE | B | 14.5 (12, 18) | 16.9 (13, 21) | ||||
| AA – EpETrE | AA – DiHETrE | ||||||||||
| 5(6)-EpETrEe | – | – | 5,6-DiHETrE | A | 20.1 (18, 23) | 15.1 (13, 17) | 0.81 | <0.0001 | |||
| 8(9)-EpETrE | A | 16.4 (14, 19) | 14.7 (13, 17) | – | >0.05 | 8,9-DiHETrE | B | 11.7 (10, 13) | 9.3 (8.2, 11) | ||
| 11(12)-EpETrE | B | 61.7 (54, 71) | 56.4 (49, 64) | 11,12-DiHETrE | C | 3.98 (3.5, 4.5) | 3.31 (2.9, 3.8) | ||||
| 14(15)-EpETrE | C | 28.5 (25, 33) | 26.1 (23, 30) | 14,15-DiHETrE | D | 2.57 (2.3, 2.9) | 2.28 (2, 2.6) | ||||
| EPA – EpETE | EPA – DiHETE | ||||||||||
| 14(15)-EpETE | A | 3.78 (2.8, 5.2) | 17.5 (13, 24) | 4.7 | <0.0001 | 14,15-DiHETE | A | 2.14 (1.8, 2.6) | 8.6 (7.2, 10) | 4.1 | <0.0001 |
| 17(18)-EpETE | B | 5.81 (4.2, 8) | 28 (20, 38) | 17,18-DiHETE | B | 8.85 (7.4, 11) | 36.3 (30, 43) | ||||
| DHA – EpDoPE | DHA – DiHDoPE | ||||||||||
| 16(17)-EpDoPE | A | 11.1 (7.8, 16) | 24.6 (17, 35) | 2.1 | 0.0003 | 16,17-DiHDoPEf | – | – | 2.1 | 0.017 | |
| 19(20)-EpDoPE | A | 11.9 (8.3, 17) | 24.2 (17, 35) | 19,20-DiHDoPE | 3.33 (2.4, 4.6) | 7.00 (5.1, 9.7) | |||||
Means and 95% CI of individual regioisomers after adjustment for subject and extraction batch. Abbreviations: AA, arachidonic acid; aLA, α-Linolenic acid; DHA, docosahexaenoic acid; DiHDoPE, dihydroxydocosapentaenoic acid; DiHETE, dihydroxyeicosatetraenoic acid; DiHETrE, dihydroxyeicosatrienoic acid; DiHODE, dihydroxyoctadecadienoic acid; DiHOME, dihydroxyoctadecamonoenoic acid; EPA, eicosapentaenoic acid; EpDoPE, epoxyedocosapentaenoic acid; EpETE, epoxyeicosatetraenoic acid; EpETrE, epoxyeicosatrienoic acid; EpOME, epoxyoctadecamonoenoic acid; LA, linoleic acid.
See Fig. 2 for associated pathways for each oxylipin regioisomer.
Regioisomers derived from the same parent FA with different letters differ from each other significantly (P < 0.05) by Tukey's Honest Significant Differences test.
P value of treatment effects on plasma concentrations.
Below limit of detection.
Surrogate recovery failed quality control due to lactone formation (See Fig. 2).
No commercial standard available.
Among vicinal diols, the baseline concentrations of the EPA-derived DiHETEs and DHA-derived DiDPA were comparable to those of the AA-derived counterpart DiHETrEs. Among the AA-derived DiHETrEs, the baseline concentrations decreased continuously from the proximal diol (5,6-DiHETrE) to the distal diol (14,15-DiHETrE). The most abundant diol was 9,10-DiHOME, which was among only three diols that were more abundant than their parent epoxide.
Other oxylipins
A small number of nonvicinal diols and triols of LA, AA, and EPA were available as calibration standards, allowing the quantitative determination of these compounds in plasma (see supplemental Table VI). Concentrations of these oxylipins ranged 0.5–10 nM. P-OM3 treatment uniformly reduced the measurable n-6 nonvicinal diols by ∼20% and triols (e.g., lipoxin A4) by ∼35%. The EPA-derived triol resolvin E1 was not detected above 0.5 nM.
Plasma prostaglandin F2a (PGF2a) and thromboxane (TxB2) were detected in the ∼1–10 nM range and were unaffected by P-OM3 treatment. The influence of POM-3 on PGE2 and PGD2 derived metabolites could not be determined, as these metabolites are unstable under the alkaline hydrolysis procedures used to liberate oxylipins in this study. Levels of LTB5, 6-keto PGF1α, 20-Hydroxy-LTB4, 20-carboxy-LTB4, and 12,13-DiHODE were below detection limits in our assay.
DISCUSSION
Many facts point to omega-3 FA oxidation products as normal components of the human metabolome. These include the presence of n3-FA biosynthetic pathways, the promiscuous nature of FA oxygenating enzymes, and the defined role of many oxygenated n3-FAs in inflammatory homeostasis, not to mention the nonspecific nature of reactive oxygen-dependent modifications of unsaturated lipids in both membranes and bulk lipid pools. Moreover, the broad occurrence of unsaturated FA oxidation products in nature would suggest that omega-3 FA oxidation products may also be present in the diet. While these facts are suggestive, information regarding either plasma concentrations of the suite of potential metabolites or their sensitivity to manipulation is limited. For instance, to the best of our knowledge, this article is the first to document the presence of EPA- and DHA-derived n-3 epoxides and diols in human plasma and to demonstrate that the concentrations of multiple n-3 long chain oxylipin classes are increased by EPA/DHA ethyl ester ingestion.
One important mechanism that could produce the anti-inflammatory benefits of long chain omega-3 FA ingestion is through alteration of 5-LOX-dependent metabolism. The 5-LOX-dependent pathways produce powerful proinflammatory effects, with the AA-based oxylipins playing a major role in mediating inflammation (23). Here, P-OM3 decreased 5-HETE and 5-KETE by 20% while increasing 5-HEPE, leading to ∼30% increase in measured 5-LOX products. Powell et al. demonstrated that 5-HEPE is able to stimulate immune cells, but to a lesser degree than 5-KETE or 5-HETE (24), so the absolute impact of these changes in 5-LOX products in terms of inflammatory tone are difficult to establish. These intricacies and metabolic interactions highlight the fact that care must be taken when extrapolating in vitro effects of oxylipin species (each studied in the absence of the others) to the in vivo setting, where multiple oxylipins are likely to be simultaneously changing.
As opposed to the relatively clear proinflammatory effects of the 5-LOX AA metabolites, it is more difficult to uniformly categorize other AA-derived LOX metabolites, as there is a spectrum of activity from pro- to anti-inflammation depending on the regioisomer (25–27). The midchain octadecanoid products derived from LA were the most abundant oxylipins detected in plasma. P-OM3 lowered the concentration of both HODE isomers by 16% without significant affects on the aLA-derived HOTEs. The 13-HODE has been reported to have numerous physiological effects, including potent inhibition of platelet binding to endothelial cells (28) and antiproliferation (29), suggesting that the reduction in these metabolites should not be overlooked when considering the overall effects of dietary omega-3 FAs.
In general, CYP epoxygenase products have potent anti-inflammatory (30, 31) and vasodilatory (32) effects. AA epoxides function as endothelium-derived hyperpolarizing factors with roles in vascular homeostatic maintenance and capacity to block cardiac hypertrophy (33). While less well characterized, the hydrolysis products of these epoxylipids, the 1,2- or vicinal-diols, are putative PPARα agonists (34) and, thus, may also modulate inflammation through PPAR-dependent pathways (35). In vitro, EPA and DHA epoxides are 10- to 100-fold more potent than their AA counterparts with respect to vascular relaxation (36). As with the 5-LOX products, POM-3 intake increased n3 epoxide and diols and decreased AA-dependent products, but it had no impact on the linoleate-derived epoxides or diols. Thus, raising n-3 epoxide concentrations with agents like P-OM3 may promote an anti-inflammatory processes.
Together, these finding demonstrate shifts in specific metabolite classes of oxylipins, namely FA alcohols and epoxides, as well as many of their downstream metabolites, with P-OM3 treatment. The finding that P-OM3 treatment affected not only EPA- and DHA-derived oxylipins but also AA- and LA-derived species suggests that at least some of the beneficial effects of n-3 FA supplementation could be mediated by this shift. As there was a general absence of an acute inflammatory condition in the study population and we used strong alkaline conditions to prepare samples, this study has little power to assess POM-3 impacts on COX-dependent metabolism.
While oxylipins can be derived by either coordinated enzymatic reactions, autooxidative processes, or a combination of the two, the effects of these compounds will depend on their local concentration rather than their source. While investigation of the role of each metabolite may provide insight into the effect of P-OM3, future investigations should not ignore the fact that these changes occur in the context of a coordinated and concerted shift in the levels of a vast array of oxylipins; almost every one reported here. For example, the final effect of increasing EPA alcohols cannot be considered without regard to concurrent increases in DHA alcohols and/or decreasing concentrations of AA and LA alcohols.
In the current study, base hydrolysis was used to release esterified oxylipids, resulting in measures of whole plasma concentration without regard to their status as free or esterified lipids. Because over 95% of alcohols, ketones, epoxides, diols, and triols are sequestered in plasma lipoproteins (37), it is expected that the downstream effect of these plasma metabolites will be highly influenced by shifts in lipoprotein metabolism. Additionally, given both the effects of P-OM3 on lipoprotein metabolism (10, 38–40) and the shift in the oxylipin distribution among lipoproteins brought on by dyslipidemias (41), future studies should consider the effect of P-OM3 on oxylipins in the context of lipoprotein metabolism as well. Finally, it is expected that tissue oxylipin synthesis, the expression of oxylipin receptors, and differential rates of inactivation of each metabolite will all contribute to the net effect of any intervention that alters oxylipin patterns.
SUMMARY
We have shown for the first time in humans that a wide array of n-3 oxylipins circulate in human plasma and that increasing n-3 FA consumption not only increases levels of n-3 FA-derived oxylipins, but also modulates n-6 FA-derived oxylipin levels. These observations suggest novel pathways that may mediate the beneficial effects of increased n-3 FA consumption and lay the foundation for future studies to define 1) the biological activities of each of the n-3 oxylipins carried in plasma; 2) the role of lipoproteins trafficking oxylipins to tissues; 3) the effects of n-3 FAs on circulating oxylipin profiles in various disease states; and 4) the extent to which increased n-3 FA levels in cell membranes may modulate the activity of putative membrane-bound oxylipin receptors. In light of these findings, it is likely that a full explanation for the beneficial effects of fish oil in human health will involve oxylipin-mediated mechanisms.
Supplementary Material
Acknowledgments
The authors are indebted to Brenda Collison-Schmidt, Andrew Christianson, Joe Ashmore, and Kritsi Fillaus, who provided excellent technical assistance and study coordination.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- aLA
- α-linolenic acid
- COX
- cyclooxygenase
- CYP
- cytochrome p450
- dgLA
- dihomo-γ-linolenic acid
- DHA
- docosahexaenoic acid
- DiHDoPE
- dihydroxydocosapentaenoic acid
- DiHETE
- dihydroxyeicosatetraenoic acid
- DiHETrE
- dihydroxyeicosatrienoic acid
- DiHODE
- dihydroxyoctadeca(di)enoic acid
- DiHOME
- dihydroxyoctadeca(mono)enoic acid
- EPA
- eicosapentaenoic acid
- EpDoPE
- epoxyedocosapentaenoic acid
- EpETE
- epoxyeicosatetraenoic acid
- EpETrE
- epoxyeicosatrienoic acid
- EpODE
- epoxyoctadecadienoic acid
- EpOME
- epoxyoctadecamonoenoic acid
- FA
- fatty acid
- HDoHE
- hydroxydocosahexaenoic acid
- HEPE
- hydroxyeicosapentaenoic acid
- HETE
- hydroxyeicosatetraenoic acid
- HETrE
- hydroxyeicosatrienoic acid
- HODE
- hydroxyoctadecadienoic acid
- HOTE
- hydroxyoctadecatrienoic acid
- HPI
- hydroperoxide isomerase
- IQR
- interquartile range
- KETE (i.e., oxo-ETE)
- ketoeicosatetraenoic acid
- KODE (i.e., oxo-ODE)
- ketooctadecadienoic acid
- KOTE
- ketooctadecadienoic acid
- LA
- linoleic acid
- γLA
- γ-linolenic acid
- LOX
- lipoxygenase
- PGX
- prostaglandin X
- P-OM3
- prescription omega-3 ethyl esters
- PUFA
- polyunsaturated fatty acid
- sEH
- soluble epoxide hydrolase
- TG
- triglyceride
- TxX
- thromboxane X.
This work was supported in part by Project 5306-51530-016-00D from the Agricultural Research Service (ARS), U.S. Department of Agriculture (USDA); by the National Institutes of Health; and by Grant 2-P20-RR016479 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program, National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the U.S. Department of Agriculture.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of supplemental methods, references, six tables, and one figure.
REFERENCES
- 1.Harris W. S., Miller M., Tighe A. P., Davidson M. H., Schaefer E. J. 2008. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 197: 12–24. [DOI] [PubMed] [Google Scholar]
- 2.Calder P. C. 2006. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 83: 1505S–1519S. [DOI] [PubMed] [Google Scholar]
- 3.Donadio J. V., Jr., Larson T. S., Bergstralh E. J., Grande J. P. 2001. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J. Am. Soc. Nephrol. 12: 791–799. [DOI] [PubMed] [Google Scholar]
- 4.Samieri C., Feart C., Letenneur L., Dartigues J. F., Peres K., Auriacombe S., Peuchant E., Delcourt C., Barberger-Gateau P. 2008. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am. J. Clin. Nutr. 88: 714–721. [DOI] [PubMed] [Google Scholar]
- 5.Lin P. Y., Su K. P. 2007. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J. Clin. Psychiatry. 68: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 6.Sekikawa A., Curb J. D., Ueshima H., El-Saed A., Kadowaki T., Abbott R. D., Evans R. W., Rodriguez B. L., Okamura T., Sutton-Tyrrell K., et al. 2008. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J. Am. Coll. Cardiol. 52: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamagishi K., Iso H., Date C., Fukui M., Wakai K., Kikuchi S., Inaba Y., Tanabe N., Tamakoshi A. 2008. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J. Am. Coll. Cardiol. 52: 988–996. [DOI] [PubMed] [Google Scholar]
- 8.Marchioli R., Barzi F., Bomba E., Chieffo C., Di Gregorio D., Di Mascio R., Franzosi M. G., Geraci E., Levantesi G., Maggioni A. P., et al. 2002. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 105: 1897–1903. [DOI] [PubMed] [Google Scholar]
- 9.Tavazzi L., Maggioni A. P., Marchioli R., Barlera S., Franzosi M. G., Latini R., Lucci D., Nicolosi G. L., Porcu M., Tognoni G. 2008. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 372: 1223–1230. [DOI] [PubMed] [Google Scholar]
- 10.Harris W. S., Ginsberg H. N., Arunakul N., Shachter N. S., Windsor S. L., Adams M., Berglund L., Osmundsen K. 1997. Safety and efficacy of Omacor in severe hypertriglyceridemia. J. Cardiovasc. Risk. 4: 385–391. [PubMed] [Google Scholar]
- 11.Leaf A. 1995. Omega-3 fatty acids and prevention of ventricular fibrillation. Prostaglandins Leukot. Essent. Fatty Acids. 52: 197–198. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh S. R., Cherezov V., Caffrey M., Stillwell W., Wassall S. R. 2003. Interaction of cholesterol with a docosahexaenoic acid-containing phosphatidylethanolamine: trigger for microdomain/raft formation? Biochemistry. 42: 12028–12037. [DOI] [PubMed] [Google Scholar]
- 13.Deckelbaum R. J., Worgall T. S., Seo T. 2006. n-3 fatty acids and gene expression. Am. J. Clin. Nutr. 83: 1520S–1525S. [DOI] [PubMed] [Google Scholar]
- 14.Fleming I. 2007. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins Other Lipid Mediat. 82: 60–67. [DOI] [PubMed] [Google Scholar]
- 15.Honstra G., van Houwelingen A. C., Kivits G. A., Fischer S., Uedelhoven W. 1990. Influence of dietary fish on eicosanoid metabolism in man. Prostaglandins. 40: 311–329. [DOI] [PubMed] [Google Scholar]
- 16.Larson M. K., Ashmore J. H., Harris K. A., Vogelaar J. L., Pottala J. V., Sprehe M., Harris W. S. 2008. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb. Haemost. 100: 634–641. [PubMed] [Google Scholar]
- 17.Block R. C., Harris W. S., Reid K. J., Sands S. A., Spertus J. A. 2008. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 197: 821–828. [DOI] [PubMed] [Google Scholar]
- 18.Newman J. W., Kaysen G. A., Hammock B. D., Shearer G. C. 2007. Proteinuria increases oxylipid concentrations in VLDL and HDL, but not LDL particles in the rat. J Lipid Res. 48: 1792–1800. [DOI] [PubMed] [Google Scholar]
- 19.Luria A., Weldon S. M., Kabcenell A. K., Ingraham R. H., Matera D., Jiang H., Gill R., Morisseau C., Newman J. W., Hammock B. D. 2007. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J. Biol. Chem. 282: 2891–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. 1978. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 2: 117–119. [DOI] [PubMed] [Google Scholar]
- 21.Leaf A., Kang J. X., Xiao Y. F., Billman G. E. 2003. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 107: 2646–2652. [DOI] [PubMed] [Google Scholar]
- 22.Fischer S., Weber P. C. 1983. Thromboxane A3 (TXA3) is formed in human platelets after dietary eicosapentaenoic acid (C20:5 omega 3). Biochem. Biophys. Res. Commun. 116: 1091–1099. [DOI] [PubMed] [Google Scholar]
- 23.Patel P., Cossette C., Anumolu J. R., Gravel S., Lesimple A., Mamer O. A., Rokach J., Powell W. S. 2008. Structural requirements for activation of the 5-oxo-6E,8Z, 11Z,14Z-eicosatetraenoic acid (5-oxo-ETE) receptor: identification of a mead acid metabolite with potent agonist activity. J. Pharmacol. Exp. Ther. 325: 698–707. [DOI] [PubMed] [Google Scholar]
- 24.Powell W. S., Gravel S., Gravelle F. 1995. Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J. Lipid Res. 36: 2590–2598. [PubMed] [Google Scholar]
- 25.Hughes M. A., Brash A. R. 1991. Investigation of the mechanism of biosynthesis of 8-hydroxyeicosatetraenoic acid in mouse skin. Biochim. Biophys. Acta. 1081: 347–354. [DOI] [PubMed] [Google Scholar]
- 26.Bolick D. T., Orr A. W., Whetzel A., Srinivasan S., Hatley M. E., Schwartz M. A., Hedrick C. C. 2005. 12/15-lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler. Thromb. Vasc. Biol. 25: 2301–2307. [DOI] [PubMed] [Google Scholar]
- 27.Takata S., Papayianni A., Matsubara M., Jimenez W., Pronovost P. H., Brady H. R. 1994. 15-Hydroxyeicosatetraenoic acid inhibits neutrophil migration across cytokine-activated endothelium. Am. J. Pathol. 145: 541–549. [PMC free article] [PubMed] [Google Scholar]
- 28.Buchanan M. R., Horsewood P., Brister S. J. 1998. Regulation of endothelial cell and platelet receptor-ligand binding by the 12- and 15-lipoxygenase monohydroxides, 12-, 15-HETE and 13-HODE. Prostaglandins Leukot. Essent. Fatty Acids. 58: 339–346. [DOI] [PubMed] [Google Scholar]
- 29.Xi S., Pham H., Ziboh V. A. 2000. Suppression of proto-oncogene (AP-1) in a model of skin epidermal hyperproliferation is reversed by topical application of 13-hydroxyoctadecadienoic acid and 15-hydroxyeicosatrienoic acid. Prostaglandins Leukot. Essent. Fatty Acids. 62: 13–19. [DOI] [PubMed] [Google Scholar]
- 30.Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., Liao J. K. 1999. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 285: 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmelzer K. R., Kubala L., Newman J. W., Kim I. H., Eiserich J. P., Hammock B. D. 2005. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. USA. 102: 9772–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming I. 2004. Cytochrome P450 epoxygenases as EDHF synthase(s). Pharmacol. Res. 49: 525–533. [DOI] [PubMed] [Google Scholar]
- 33.Xu D., Li N., He Y., Timofeyev V., Lu L., Tsai H. J., Kim I. H., Tuteja D., Mateo R. K., Singapuri A., et al. 2006. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc. Natl. Acad. Sci. USA. 103: 18733–18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang X., Hu S., Xu B., Snyder G. D., Harmon S., Yao J., Liu Y., Sangras B., Falck J. R., Weintraub N. L., et al. 2006. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am. J. Physiol. Heart Circ. Physiol. 290: H55–H63. [DOI] [PubMed] [Google Scholar]
- 35.Chinetti G., Fruchart J. C., Staels B. 2000. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 49: 497–505. [DOI] [PubMed] [Google Scholar]
- 36.Ye D., Zhang D., Oltman C., Dellsperger K., Lee H. C., VanRollins M. 2002. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 303: 768–776. [DOI] [PubMed] [Google Scholar]
- 37.Shearer G. C., Newman J. W. 2008. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot. Essent. Fatty Acids. 79: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackness M. I., Bhatnagar D., Durrington P. N., Prais H., Haynes B., Morgan J., Borthwick L. 1994. Effects of a new fish oil concentrate on plasma lipids and lipoproteins in patients with hypertriglyceridaemia. Eur. J. Clin. Nutr. 48: 859–865. [PubMed] [Google Scholar]
- 39.Calabresi L., Villa B., Canavesi M., Sirtori C. R., James R. W., Bernini F., Franceschini G. 2004. An omega-3 polyunsaturated fatty acid concentrate increases plasma high-density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metabolism. 53: 153–158. [DOI] [PubMed] [Google Scholar]
- 40.Chan D. C., Watts G. F., Barrett P. H., Beilin L. J., Redgrave T. G., Mori T. A. 2002. Regulatory effects of HMG CoA reductase inhibitor and fish oils on apolipoprotein B-100 kinetics in insulin- resistant obese male subjects with dyslipidemia. Diabetes. 51: 2377–2386. [DOI] [PubMed] [Google Scholar]
- 41.Newman J. W., Kaysen G. A., Hammock B. D., Shearer G. C. 2007. Proteinuria increases oxylipid concentrations in VLDL and HDL but not LDL particles in the rat. J. Lipid Res. 48: 1792–1800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.