Abstract
The heart of leptin-deficient ob/ob mice is characterized by pathologic left ventricular hypertrophy along with elevated triglyceride (TG) content, increased stearoyl-CoA desaturase (SCD) activity, and increased myocyte apoptosis. In the present study, using an ob/ob;SCD1−/− mouse model, we tested the hypothesis that lack of SCD1 could improve steatosis and left ventricle (LV) function in leptin deficiency. We show that disruption of the SCD1 gene improves cardiac function in ob/ob mice by correcting systolic and diastolic dysfunction without affecting levels of plasma TG and FFA. The improvement is associated with reduced expression of genes involved in FA transport and lipid synthesis in the heart, as well as reduction in cardiac FFA, diacylglycerol, TG, and ceramide levels. The rate of FA β-oxidation is also significantly lower in the heart of ob/ob;SCD1−/− mice compared with ob/ob controls. Moreover, SCD1 deficiency reduces cardiac apoptosis in ob/ob mice due to increased expression of antiapoptotic factor Bcl-2 and inhibition of inducible nitric oxide synthase and caspase-3 activities. Reduction in myocardial lipid accumulation and inhibition of apoptosis appear to be one of the main mechanisms responsible for improved LV function in ob/ob mice caused by SCD1 deficiency.
Keywords: heart steatosis, apoptosis, ceramide, leptin
Obesity-related heart disease, the most serious complication of human obesity, generally is attributed to coexisting disorders such as coronary artery disease and hypertension. However, an increasing body of evidence suggests that cardiac dysfunction, arrhythmias, cardiomyopathy, and congestive heart disease could be direct consequences of obesity and leptin resistance, i.e., fatty acid (FA) overload of cardiomyocytes and lipid accumulation in the heart (1, 2). Studies performed on humans using magnetic resonance spectroscopy indicate that cardiomyocyte fat correlates well with impaired diastolic filling, even in seemingly asymptomatic obese volunteers (2). One of the proposed reasons is that excess FAs or/and their metabolites, i.e., ceramides, induce apoptosis of cardiomyocytes, which is a direct cause of lipotoxic cardiomyopathy and heart failure (2, 3). The important role for ceramide-induced apoptosis in obesity-related cardiomyopathies has been shown in many animal models, including leptin-insensitive ZDF rats (4), leptin-deficient ob/ob mice (5, 6), and a mouse model of heart disease induced by cardiomyocyte-specific overexpression of acyl-CoA synthase (7). Many maneuvers that reduce ectopic deposition of lipids, i.e., exercise, caloric restriction, and troglitazone, were shown to significantly improve cardiac function in obesity, which additionally implies that steatosis might be a primary reason for cardiac dysfunction (2, 8).
Stearoyl-CoA desaturase (SCD) is the rate-limiting enzyme catalyzing the biosynthesis of monounsaturated FAs and has been shown to be a key regulatory factor of body adiposity (9). Mice with a targeted disruption in the SCD1 gene have increased energy expenditure and insulin sensitivity and are resistant to diet-induced obesity (10, 11). SCD1 is involved in regulation of ceramide metabolism. Lack of SCD1 reduces the mRNA level and activity of serine palmitoyltransferase (SPT), the rate-limiting enzyme of de novo ceramide synthesis. Reduced SPT activity together with decreased long-chain fatty acyl-CoA levels (mainly palmitoyl-CoA) leads to reduced ceramide content in oxidative muscles of SCD1−/− mice (12). SCD1 deficiency also reduces ceramide synthesis in skeletal muscles of ob/ob mice. Moreover, SCD1 is a major target gene of leptin in liver and was found to be specifically repressed during leptin-mediated weight loss, and leptin-deficient ob/ob mice lacking SCD1 showed markedly reduced adiposity despite higher food intake (13). In addition, SCD1 deficiency completely corrects the hypometabolic phenotype and hepatic steatosis of leptin-deficient ob/ob mice, thus mimicking the effect of leptin treatment (13).
Three isoforms of SCD are expressed and regulated in a hormone-dependent fashion in the heart (14), the molecular and metabolic implications of which are practically unknown. Our recent study showed that the lack of SCD1 decreases FA uptake and oxidation while increasing glucose transport and oxidation in the heart (15). This shift in cardiac substrate utilization from FA to glucose is caused by upregulation of insulin signaling, decreased FA availability, and reduced expression of FA oxidation genes in the heart (15). Also, lipogenesis is decreased in the heart of SCD1−/− mice, which is accompanied by a reduction in intracellular FA and triglyceride (TG) content (15). We have also shown that the activity of SCD is significantly increased in the heart of leptin-deficient ob/ob mice (14), characterized by pathologic left ventricular hypertrophy along with elevated TG content and increased myocyte apoptosis (5, 16). Taken together, these results suggest that SCD might be involved in pathogenesis of lipotoxic cardiomyopathy induced by lack of leptin action.
Therefore, in the present study, we used the ob/ob;SCD1−/− double knock-out mouse model to test the hypothesis that loss of SCD1 function could be beneficial in the treatment of lipid-induced heart disease. Here, for the first time, we show that SCD1 deficiency significantly improves systolic and diastolic function of left ventricle (LV) in leptin-deficient ob/ob mice. Lack of SCD1 action resulted in decreased neutral lipids and ceramide accumulation in the heart of ob/ob mice. Decreased ceramide levels led to a reduction in inducible oxide synthase (iNOS) and caspase-3 activities and decreased the cardiac apoptosis rate. Inhibition of the apoptotic pathways appears to be one of the key mechanisms responsible for improved cardiac function in ob/ob mice caused by SCD1 deficiency, although a reduction in the whole body adiposity in ob/ob;SCD1−/− mice may also contribute to the protective effects of SCD1 deletion.
MATERIALS AND METHODS
Animals
The generation of SCD1−/− and ob/ob;SCD1−/− mice has been described previously (17, 18). Pure-bred homozygous (SCD1−/−) and wild-type (WT) mice on a B6 background were used. To generate the SCD1 mutation in ob/ob mice, male SCD1−/− mice were bred with female B6 ob/+ mice. The resulting SCD1+/−;ob/+ were inter-bred to produce set of ob/ob;SCD1−/− mice that were identified by PCR using appropriate DNA primers. Mice were maintained on a 12:12 h dark-light cycle and fed a normal nonpurified diet (5008 test diet; PMI Nutrition International, Richmond, IN). The breeding of these animals was in accordance with the protocols reviewed and approved by the Animal Care Research Committees of the University of Wisconsin-Madison. Male mice of each group (N = 6) at 24 weeks of age were used in the study. Animals were euthanized 2 days after complete echocardiographic examination was performed. Heart was rapidly excised, and LVs were dissected and flash frozen in liquid nitrogen. Fat pads were also collected and weighed.
Echocardiography
Transthoracic echocardiography was performed using a Sonos 5500 ultrasonograph with a 12 MHz transducer (Philips). Noninvasive acquisition of two-dimensional guided M-mode images at the tip of papillary muscles and Doppler studies were recorded on anesthetized mice (50 mg/kg ketamine). The thickness of the posterior and anterior walls of the LV chamber and the LV chamber diameter during systole and diastole were measured using the leading edge-to-leading edge convention. All parameters were measured over at least three consecutive cardiac cycles. These parameters were used to calculate LV mass and fractional shortening as previously described (19). Pulse-wave Doppler was used to measure the velocity of blood through the mitral and aortic valves. From these images, heart rate and the time between closure of the mitral valve and the opening of the aortic valve were calculated.
Blood sampling
Mice were euthanized by cervical dislocation. Blood was collected aseptically by direct cardiac puncture and centrifuged (13,000 g, 5 min, 4°C) to collect plasma. Plasma cholesterol, TG, FFA, insulin, and glucose levels were measured by using commercial kits (Roche Applied Science, Indianapolis, IN; Linco Research, St. Charles, MI; Wako Chemicals, Richmond, VA; and Sigma, St. Louis, MO).
Isolation and analysis of RNA
Total RNA was isolated from hearts of WT, ob/ob, and ob/ob;SCD1−/− mice using TRIzol reagent (Invitrogen, Carlsbad, CA) and then treated with DNase. cDNA was prepared by reverse transcription with random hexamer primers and amplified by PCR using gene-specific primers in the presence of SYBR Green (Applied Biosystems, Foster City, CA) on an ABI Prism 7500 Fast Instrument. Relative abundance of SCD1, SCD4, peroxisome proliferator-activated receptor (PPAR)γ, DAG acyltransferase (DGAT), glycerol-3-phosphate acyltransferase (GPAT), FA translocase CD36 (CD36), FA transport protein (FATP)-1, PPARα, carnitine palmitoyltransferase 1 (CPT1), acyl-CoA oxidase (ACO), LCB1, and LCB2 mRNA was calculated by normalizing to cyclophilin. Primer sequences are available upon request.
SCD activity assay
Microsomes were isolated from hearts of WT, ob/ob, and ob/ob;SCD1−/− mice by differential centrifugation and suspended in a 0.1 M potassium phosphate buffer (pH 7.2). SCD activity was assayed with 3 μM [14C]stearoyl-CoA (American Radiolabeled Chemicals, St. Louis, MO), 2 mM NADH, and 100 μg of microsomal protein (15). After 5 min of incubation, 200 μl of 2.5 M KOH in 75% ethanol was added, and the reaction mixture was saponified at 85°C for 1 h. The samples were cooled and acidified with 280 μl of formic acid. FFA were extracted with 700 μl of hexane and separated on a 10% AgNO3-impregnated TLC plate using chloroform:methanol:acetic acid:H2O (90:8:1:0.8). The TLC plates were analyzed with Instant Imager (Packard).
Western blot analysis
To measure CD36 and FATP protein levels, 100 μg of clarified homogenate protein was loaded onto 9% SDS-PAGE. The separated proteins were transferred to nitrocellulose membranes that were blotted using antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The proteins were visualized using ECL as described by the manufacturer (Pierce, Rockford, IL) and quantified by densitometry.
Measurement of lipids
Heart lipids were extracted by the method of Bligh and Dyer (20) and measured as described (12). Briefly, the lipids were separated by TLC on silica gel-60 plates (Merck) in heptane-isopropyl ether-glacial acetic acid (60:40:3, v/v/vol) with authentic standards. The bands corresponding to TG, diacylglycerol (DAG), phospholipids (PL), ceramide, and FFA standards (Sigma) were scraped off the plate and transferred to screw-cap glass tubes containing pentadecanoic acid as an internal standard. FA were then transmethylated in the presence of 14% boron trifluoride in methanol. The resulting methyl esters were extracted with hexane and analyzed by gas-liquid chromatography. Total contents were calculated from individual FA content in each fraction.
SPT activity
Activity of SPT in isolated microsomes was measured with L-[3-14C]serine (American Radiolabeled Chemicals, St. Louis, MO) as substrate, as described (21). The reaction was terminated by addition of 1.5 ml of chloroform-methanol (2:1, v/v). The radiolabeled product, 3-ketosphinganine, was separated from radiolabeled serine by phase partitioning. Sphinganine (25 µg) was added as a carrier, followed by the addition of 1 ml of chloroform and 2 ml of 0.5 N NH4OH. The chloroform layer was counted in a scintillation counter (Packard 1900).
[14C]palmitic acid incorporation into ceramide
The mice were anesthetized, and 0.8 µCi of [14C]palmitic acid (55 mCi/mmol; American Radiolabeled Chemicals) per 20 g body weight, conjugated to albumin, was administered into the tail vein (12). Ten minutes after administration of the label, heart samples were taken. The cardiac lipids were extracted, and ceramide was isolated as described above. The lipid bands corresponding to ceramide were scraped off the plates, and the radioactivity was counted in a liquid scintillation counter (Packard 1900).
Caspase-3 and iNOS activities
To measure caspase-3 activity the heart was homogenized in 10 mM HEPES buffer (pH 7.5). Reaction mixture contained 100 mM HEPES (pH 7.5), 5 mM DTT, 0.1% CHAPS, 10% sucrose, 16 mM caspase 3 substrate Ac-ASP-Glu-Val-Asp-p-aniline, and enzyme. The amount of p-nitroaniline reversed by caspase-3 activity was quantitated at 37°C by measuring the optical density at 405 nm (22). iNOS activity was assayed using Bioxytech 22113 kit (Oxox International Inc., Portland, OR) (23).
Measurement of FA oxidation
Mitochondria were isolated as described by Scaduto (24). Mitochondrial FA oxidation was measured as described using [14C]palmitoyl-CoA as substrate (25). Labeled CO2 was trapped in 10 M KOH and then counted in a liquid scintillation counter (Packard 1900).
Statistical analysis
Results were analyzed using one-way ANOVA with student-Newman posthoc test. A difference of P < 0.05 was considered significant. Values are presented as means ± SD (N = 6 mice per group).
RESULTS
Reduced body weight and epididymal fat mass in SCD1-deficient ob/ob mice
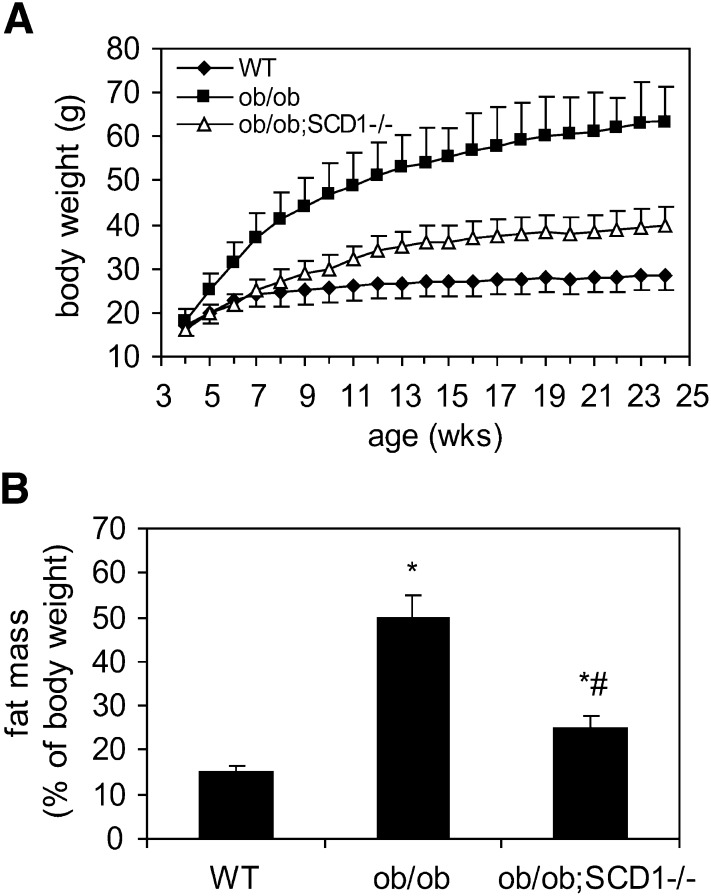
The knock-down of SCD1 gene in ob/ob mice caused significant reduction in body weight (Fig. 1A). Also the epididymal fat pad mass was markedly reduced in ob/ob;SCD1−/− relative to ob/ob mice (24.8% of body weight vs. 50.2%; P < 0.05) (Fig. 1B). Both body and fat mass were significantly higher in ob/ob;SCD1−/− than in WT mice (Fig. 1A, B). The concentrations of glucose, insulin, FFA, TG, and cholesterol in plasma were significantly increased in ob/ob mice relative to WT mice (Table 1). SCD1 deficiency decreased plasma cholesterol level in ob/ob mice; however concentrations of plasma glucose, insulin, FFA, and TG were not significantly different between ob/ob;SCD1−/− and ob/ob mice (Table 1).
Fig. 1.
Body weight (A) and adipose tissue mass (B) of 6-month-old WT, ob/ob, and ob/ob;SCD1−/− mice. Results are mean ± SD. *P < 0.05 versus WT mice; #P < 0.05 versus ob/ob mice; N = 6.
TABLE 1.
Plasma parameters in 6-month mice
| WT | ob/ob | ob/ob;SCD1−/− | |
|---|---|---|---|
| Glucose (mg/dl) | 104.21 ± 8.2 | 230.25 ± 5.5* | 265.62 ± 28.1* |
| Insulin (ng/ml) | 1.23 ± 0.2 | 9.87 ± 5.2* | 8.51 ± 4.6* |
| FFA (meq/ml) | 0.49 ± 0.1 | 0.68 ± 0.2* | 0.61 ± 0.1* |
| TG (mg/dl) | 62.52 ± 9.1 | 82.53 ± 14.3* | 79.18 ± 10.5* |
| Cholesterol (mg/dl) | 112.08 ± 20.4 | 322.12 ± 28.9* | 196.57 ± 15.8*# |
Results are mean ± SD. *P < 0.05 versus WT mice; #P < 0.05 versus ob/ob mice; N = 8.
SCD1 deficiency improves cardiac function of ob/ob mice
Transthoracic echocardiography with Doppler flow analysis was performed in anesthetized 24-wk-old mice. As we previously showed (15), cardiac structural and functional parameters were not significantly different between WT and SCD1−/− mice (Table 2). The ob/ob mice had a larger LV diameter and significantly increased LV mass than the ob/ob;SCD1−/−, SCD1−/−, and WT mice (Table 2). However, LV wall thickness was not different between groups. Because the body mass of the ob/ob mice was much larger than either WT or ob/ob;SCD1−/− mice (Fig. 1A), the LV mass/body weight ratio was significantly lower than WT and ob/ob;SCD1−/− mice, which were also lower than WT and SCD1−/− mice. Ob/ob mice appeared to have a significant reduction in systolic function as demonstrated by the impaired fractional shortening (Table 2). SCD1 deficiency increased the percent of fractional shortening by 28% in the heart of ob/ob;SCD1−/− compared with ob/ob mice. The percent of fractional shortening in ob/ob;SCD1−/− mice was not different from WT mice. The myocardial performance index, a Doppler-based measure of LV function, was marginally different between ob/ob and ob/ob;SCD1−/− mice. On Doppler flow analysis, the E/Ea ratio was reduced by 57% in ob/ob mice compared with WT, indicating a diastolic dysfunction in ob/ob mice. Although the E/Ea ratio in the heart of ob/ob;SCD1−/− mice was still significantly lower than in the WT, SCD1 deficiency increased the E/Ea ratio by 27% in the heart of these mice compared with ob/ob mice (Table 2).
TABLE 2.
Echocardiographic analysis of heart function and structure of WT, SCD1−/−, ob/ob, and ob/ob;SCD1−/− mice
| WT | SCD1−/− | ob/ob | ob/ob;SCD1−/− | |
|---|---|---|---|---|
| HR (bpm) | 453.13 ± 23.1 | 473.15 ± 28.4 | 515.13 ± 36.0 | 481.33 ± 67.1 |
| AWd (mm) | 0.83 ± 0.2 | 0.85 ± 0.2 | 0.89 ± 0.1 | 0.85 ± 0.1 |
| PWd (mm) | 0.82 ± 0.1 | 0.86 ± 0.2 | 0.92 ± 0.1 | 0.87 ± 0.1 |
| LVDd (mm) | 3.27 ± 0.2 | 3.56 ± 0.5 | 3.79 ± 0.3* | 3.34 ± 0.2# |
| LV mass (mg) | 100.01 ± 4.4 | 110.64 ± 7.2 | 128.64 ± 3.9*† | 107.60 ± 3.4# |
| LV mass/BW (mg/g) | 3.51 ± 0.3 | 3.98 ± 0.6 | 2.01 ± 0.2*† | 2.69 ± 0.4*†# |
| % Fractional shortening | 52.33 ± 4.6 | 55.02 ± 5.7 | 43.1 ± 3.7*† | 55.3 ± 7.8# |
| IVRT (sec) | 0.014 ± 0.006 | 0.016 ± 0.004 | 0.020 ± 0.002 | 0.020 ± 0.003 |
| MPI | 0.44 ± 0.2 | 0.49 ± 0.1 | 0.57 ± 0.2 | 0.50 ± 0.1 |
| Ea/Aa | 1.78 ± 0.5 | 1.64 ± 0.2 | 1.33 ± 0.2 | 1.07 ± 0.2*†# |
| E/Ea | 29.03 ± 4.3 | 27.36 ± 3.6 | 17.6 ± 5.1*† | 22.3 ± 2.4* |
Results are mean ± SD. *P < 0.05 versus WT mice; †P < 0.05 versus SCD1−/− mice; #P < 0.05 versus ob/ob mice; N = 8. HR, heart rate in beats per minute; AWd, anterior wall in diastole; PWd, posterior wall in diastole; LVDd, LV diameter in diastole; LV mass/BW, LV mass in milligrams/body weight in grams; Fractional shortening, (LVDd − LVDs)/LVDd; IVRT, isovolumic relaxation time in seconds; MPI, myocardial performance index = the ratio of isovolumic contraction and relaxation to ejection time, (MPI = (a − b)/b), where a = the time of mitral value closure and b = aortic ejection time; Ea, early diastolic maximal velocity from tissue Doppler; Aa, late diastolic maximal velocity from tissue Doppler; E, transmitral early filling velocity.
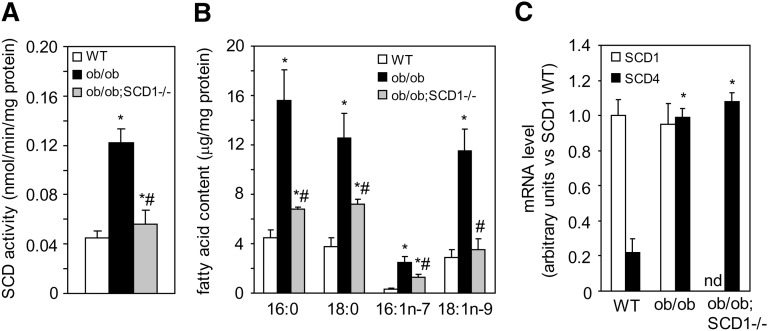
The activity of SCD is decreased in the heart of ob/ob;SCD1−/− mice
The total activity of SCD was more than 2-fold higher in the heart of ob/ob mice compared with WT controls (Fig. 2A), whereas SCD1 deficiency decreased SCD activity by 58% in the hearts of double knock-out ob/ob;SCD1−/− compared with ob/ob mice (Fig. 2A). Changes in SCD activity were coupled with the total levels of cardiac 18:1n9, 16:1n7 FAs (Fig. 2B). We measured the mRNA levels of SCD1 and cardiac-specific SCD4, which are the main isoforms of SCD expressed in the heart (14). In WT mice, mRNA level of SCD1 was about 4-fold higher than the mRNA level of SCD4 (P < 0.001); however, SCD4 gene expression was significantly increased in the heart of ob/ob mice (Fig. 2C). SCD1 deficiency decreased SCD activity without affecting SCD4 gene expression (Fig. 2A–C).
Fig. 2.
SCD activity and expression in the heart of WT, ob/ob, and ob/ob;SCD1−/− mice. A: For SCD activity assay, microsomes from the hearts of WT, ob/ob, and ob/ob;SCD1−/− mice were incubated with a reaction mixture containing [14C]stearoyl-CoA as a substrate. B: Total FAs were extracted from heart of WT, ob/ob, and ob/ob;SCD1−/− mice and quantitated by gas-liquid chromatography. C: SCD1 and SCD4 expression was measured by real-time PCR. Results are mean ± SD. *P < 0.05 versus WT mice; #P < 0.05 versus ob/ob mice; N = 6. nd, not detectable.
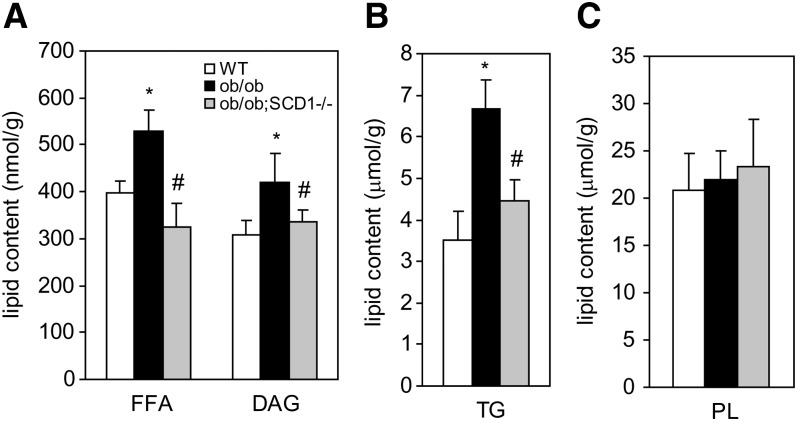
Decreased cardiac steatosis in ob/ob;SCD1−/− mice
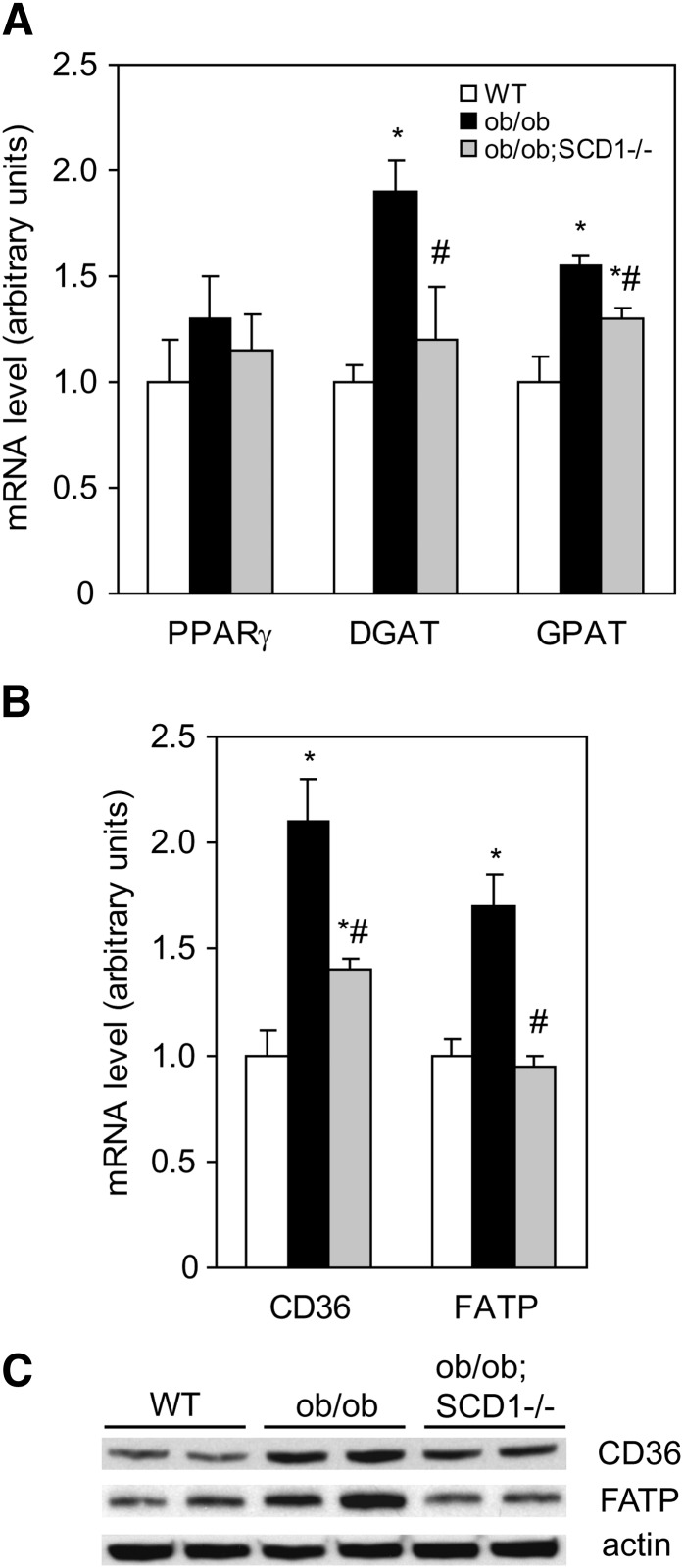
To test whether improvement in LV function in ob/ob;SCD1−/− mice was associated with a reduction in heart steatosis, we analyzed intracellular neutral lipid accumulation. The levels of FFA, DAG, and TG in the heart of ob/ob;SCD1−/− were significantly reduced by 39%, 20%, and 33%, respectively, compared with ob/ob mice but comparable to values noted in WT mice (Fig. 3A, B). Heart PL content was similar in all studied groups (Fig. 3C). The deletion of SCD1 gene resulted in changes in FA composition in each of the analyzed lipid fractions. The levels of monounsaturated FA in FFA, TG, and PL were reduced by 20%, 35%m and 28%, respectively, in ob/ob;SCD1−/− compared with ob/ob mice. SCD1 deficiency also resulted in a 23% increase in polyunsaturated FA content in PL fraction in the heart of ob/ob mice (data not shown). To address reasons for decreased heart steatosis observed in ob/ob;SCD1−/− mice, we assessed mRNA levels of PPARγ, a transcription factor that contributes to increased cellular assimilation of lipids (26). SCD1 deficiency did not change PPARγ mRNA levels (Fig. 4A), suggesting that PPARγ is not involved in regulation of fat content in the heart of these animals. Next, we measured mRNA levels of GPAT and DGAT, which catalyze the first and the final step in TG synthesis, respectively. GPAT and DGAT mRNA levels were significantly increased in hearts of ob/ob mice, consistent with their increased lipid accumulation (Fig. 4A). However, SCD1 deletion decreased the expression of these two genes in ob/ob mice (Fig. 4A). FA uptake by cardiac myocytes occurs by two main transport processes: protein-mediated transport, which accounts for ∼80% of total FA uptake, and simple diffusion. CD36 and FATP are the major proteins responsible for membrane FA transport. We measured both CD36 and FATP mRNA and protein levels by real-time PCR and Western blotting, respectively. The mRNA and protein levels of both CD36 and FATP were significantly lower in the heart of ob/ob;SCD1−/− mice than in ob/ob controls (Fig. 4B, C). These results suggest that lower rates of FA transport and reduced lipogenesis contribute to the decreased lipid content observed in hearts of SCD1-deficient ob/ob mice.
Fig. 3.
The total content of FFA and DAG (A), TG (B), and PL (C) in the heart of WT, ob/ob, and ob/ob;SCD1−/− mice. Lipids were extracted, separated by TLC, and quantitated by gas-liquid chromatography as described in “Materials and Methods.” Results are mean ± SD. *P < 0.05 vs WT mice; #P < 0.05 vs ob/ob mice; N = 6.
Fig. 4.
Expression of lipogenic genes in the heart of WT, ob/ob, and ob/ob;SCD1−/− mice. A: PPARγ, DGAT, GPAT, and CD36 (B), and FATP mRNA level was measured by real-time PCR. C: CD36 and FATP protein levels were assayed by Western blotting. Results are mean ± SD. *P < 0.05 versus WT mice; #P < 0.05 versus ob/ob mice; N = 6.
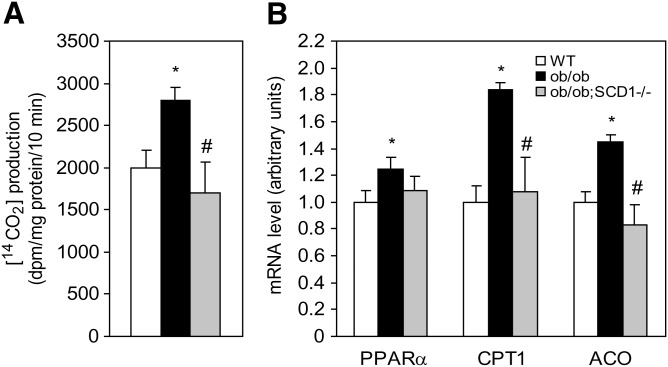
Decreased cardiac FA β-oxidation in SCD1-deficient ob/ob mice
We measured oxidation of [14C]palmitoyl-CoA in heart mitochondria. Palmitoyl-CoA β-oxidation was increased in the heart of ob/ob mice in comparison to WT controls and significantly reduced in ob/ob;SCD1−/− compared with ob/ob mice (Fig. 5A). There were no significant differences in the rate of FA oxidation between WT and ob/ob;SCD1−/− double knock-out mice (Fig. 5A). To address the possible reasons for decreased FA oxidation in the myocardium of SCD1-deficient ob/ob mice, we measured mRNA levels of PPARα, a primary nuclear factor known to stimulate genes of lipid oxidation. mRNA levels of PPARα were increased by 25% in the hearts of ob/ob mice compared with WT animals. Interestingly, PPARα levels were not elevated in the myocardium of ob/ob;SCD1−/− mice relative to WT mice (Fig. 5B). We also measured expression of CPT1 and ACO, both of which are target genes of PPARα. The levels of both CPT1 and ACO were significantly increased in ob/ob mice relative to WT mice. Interestingly, however, they were significantly reduced in the hearts of ob/ob;SCD1−/− mice relative to ob/ob mice (Fig. 5B). These results suggest that the reduced expression of oxidative genes in the myocardium of ob/ob;SCD1−/− mice is associated with decreased FA oxidation measured in these animals.
Fig. 5.
The effect of SCD1 deficiency on the rate of FA β-oxidation in the heart of ob/ob mice. A: Oxidation of palmitoyl-CoA was measured in mitochondria and is presented as amount of labeled CO2 released during oxidation. B: PPARα, CPT1, and ACO mRNA level was measured by real-time PCR. Results are mean ± SD. *P < 0.05 versus WT mice; #P < 0.05 versus ob/ob mice; N = 6.
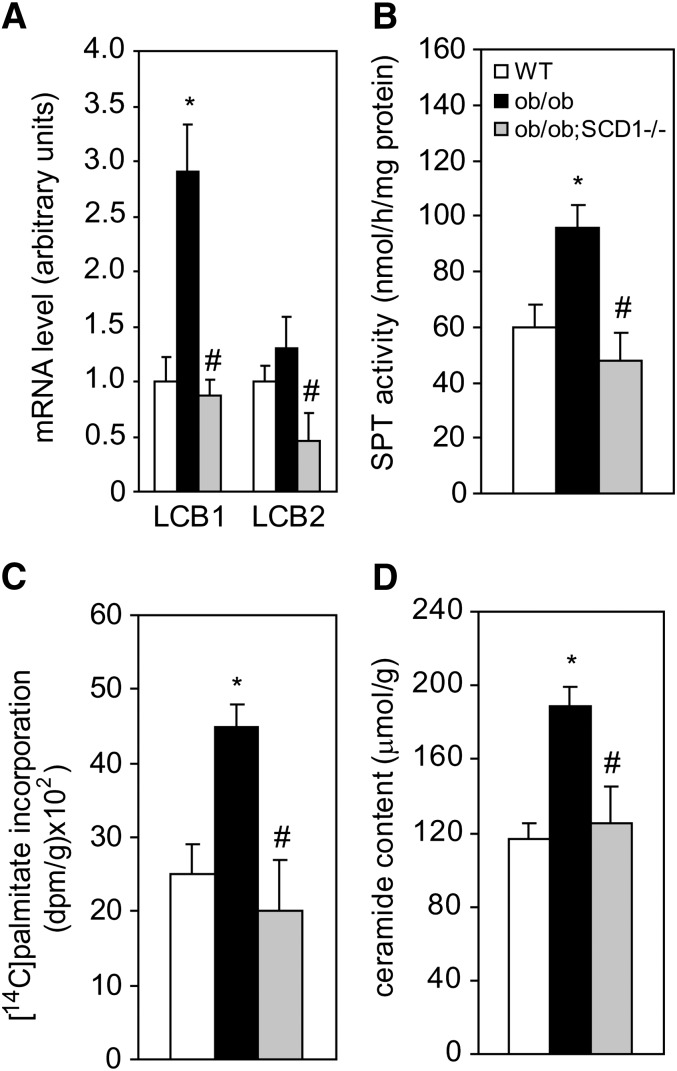
Decreased rate of de novo synthesis of cardiac ceramide caused by SCD1 knock-down
SPT is the rate-limiting enzyme in de novo synthesis of ceramide (27). The mRNA levels of both SPT subunits (LCB1 and LCB2) were reduced by 70% and 65%, respectively, in the heart of double mutant ob/ob;SCD1−/− mice compared with ob/ob mice and were even lower than values measured in WT mice (Fig. 6A). SPT activity was increased in the hearts of ob/ob mice by 60% when compared with WT mice and SCD1 deficiency reduced the enzyme activity in the hearts of ob/ob;SCD1−/− mice to the levels measured in WT animals (Fig. 6B). The differences in the activity of SPT in all of the experimental groups paralleled the changes in intracellular level of palmitate (Fig. 2B), i.e., a preferred substrate of SPT. To determine whether SCD1 deficiency affects de novo synthesis of ceramide in the heart, we analyzed the incorporation of [14C]palmitic acid into the ceramide fraction. The rate of incorporation of labeled palmitate into ceramide was decreased in the heart of ob/ob;SCD1−/− mice by 55% compared with ob/ob mice and was comparable to WT (Fig. 6C). The total content of ceramide was increased by 62% in the heart of ob/ob mice compared with WT controls (Fig. 6D). SCD1 deficiency decreased the ceramide content by 34% in the heart of ob/ob;SCD1−/− mice compared with ob/ob mice (Fig. 6D).
Fig. 6.
mRNA levels of the two SPT subunits (LCB1 and LCB2 (A), SPT activity (B), [14C]palmitic acid incorporation into ceramide fraction (C), and total ceramide content (D) in the heart of WT, ob/ob, and ob/ob;SCD1−/− mice. LCB1 and LCB2 mRNA levels were measured by real-time PCR. Activity of SPT in isolated microsomes was measured with L-[3-14C]serine as substrate. To asses [14C]palmitic acid incorporation into ceramide, [14C]palmitic acid was administered into the tail vein of mice, heart samples were taken, ceramide was isolated as described in “Materials and Methods,” and the radioactivity was counted in a liquid scintillation counter. Results are mean ± SD. **P < 0.05 versus WT mice; #P < 0.05 versus ob/ob mice; N = 6.
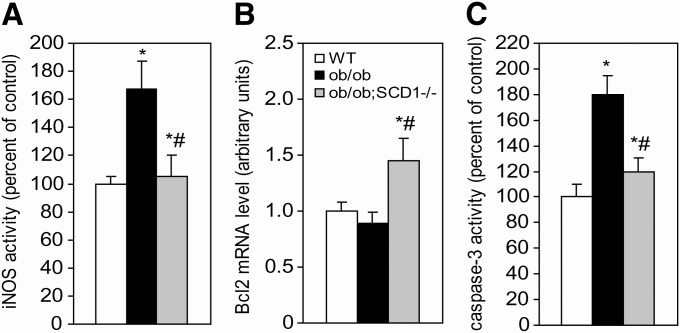
SCD1 deficiency decreases apoptosis in the heart of ob/ob mice
Ceramide has been shown in recent years to be critically involved in cardiac apoptosis (28). There is evidence that ceramide upregulates iNOS and increases NO production, which is believed to result in apoptosis (2, 3). Another mechanism for ceramide-induced apoptosis involves downregulation of the antiapoptotic factor Bcl-2 and activation of caspase-3 (29, 30). To test whether SCD1 deficiency reduces the rate of ceramide-mediated apoptosis in the heart, we analyzed iNOS activity and mRNA levels of Bcl-2 as well as activity of caspase-3 in our mice. We found that iNOS activity was 67% greater in the myocardium of ob/ob mice than in WT controls (Fig. 7A), whereas SCD1 deficiency decreased iNOS activity by 37% in the hearts of double knock-out ob/ob;SCD1−/− mice compared with ob/ob mice (Fig. 7A). Bcl-2 mRNA levels were increased by 63% in the hearts of ob/ob;SCD1−/− mice relative to ob/ob mice (Fig. 7B). Also, activity of caspase-3, an indicator of cardiac apoptosis (31), was decreased by 33% in the heart of ob/ob;SCD1−/− double mutant mice relative to ob/ob mice, which had a significant elevation of caspase-3 activity (Fig. 7C).
Fig. 7.
Apoptotic markers in the heart of WT, ob/ob, and ob/ob; SCD1−/− mice. A: iNOS activity was measured using commercial kit Bioxytech 22113. B: Bcl-2 mRNA level was measured by real time PCR. C: Caspase 3 activity was quantitated by measuring of the conversion of p-nitroaniline as described in “Materials and Methods.” Results are mean ± SD. *P < 0.05 versus WT mice; #P < 0.05 versus ob/ob mice; N = 6.
DISCUSSION
High levels of FA and their fatty acyl-CoA esters are detrimental to myocardial structure and function (32). Ectopic deposition of lipids in myocardium leads to functional impairment observed in obese leptin-resistant ZDF rats (4) and db/db mice as well as leptin-deficient ob/ob mice (1). Ob/ob mice develop pathologic LV hypertrophy along with elevated TG content and increased myocyte apoptosis (5, 14). Here, we show that SCD1 deficiency corrects these known pathologies of leptin deficiency and significantly improves LV function in ob/ob mice even though it does not affect hypertriglyceridemia or glucose intolerance in ob/ob mice (18). Instead, the improvement in cardiac function in ob/ob;SCD1−/− mice was accompanied by decreased intracellular neutral lipid and ceramide contents in the heart and inhibition of the apoptotic pathway(s) regulated by lipids in the LV cardiomyocytes.
Previously, we showed that SCD1 deficiency reduces accumulation of intracellular lipids in skeletal muscle and liver by downregulating lipid synthesis and increasing the rate of β-oxidation (12, 33). The present study reveals that loss of SCD1 function decreases FFA, DAG, and TG levels in the heart of ob/ob mice; however, the decreased cardiac steatosis observed in ob/ob;SCD1−/− mice is not due to increased FA oxidation, as evidenced by a reduced rate of palmitoyl-CoA oxidation and decreased expression of CPT1 and ACO genes. In SCD1 knock-out mice, reduced FA β-oxidation is coupled with increased glucose oxidation in the heart (15). It is thus possible that similar changes in substrate utilization may occur also in ob/ob;SCD1−/− mice, because a decreased cardiac FA oxidation is accompanied by increased oxygen consumption in SCD1-deficient ob/ob mice (13).
To elucidate the mechanisms by which SCD1 deficiency reduces lipid content in the leptin-deficient heart, we analyzed the expression of the transcription factor PPARγ, which contributes to the cellular assimilation of lipids (26), and has been shown to be increased in lipotoxic cardiomyopathies (8). In the present study, we did not find differences in PPARγ expression between ob/ob;SCD1−/− and ob/ob mice; however, mRNA levels of GPAT and DGAT that are involved in TG synthesis were significantly lower in the myocardium of ob/ob;SCD1−/− than in ob/ob mice. In addition, protein and mRNA levels of two membrane FA transporters, CD36 and FATP, were decreased in the heart of ob/ob;SCD1−/− mice compared with ob/ob controls. These results, together with increased plasma TG and FFA levels in ob/ob;SCD1−/− mice, suggest that a lower rate of intracellular FA transport and reduced lipogenesis, rather than FA availability, are coupled to both reduced FA β-oxidation and deceased lipogenesis in the ob/ob;SCD1−/− heart.
It has been proposed that excessive deposition of TG in nonadipose tissues enlarges the intracellular pool of fatty acyl-CoA, thereby providing substrate for nonoxidative metabolic pathways, such as ceramide synthesis. Increased ceramide levels lead to cell dysfunction and death through apoptosis (32). Lipoapoptosis is also observed in cardiomyocytes and leads to the development of obesity-related heart failure (4). This effect was observed in obese ZDF rats, which at 14 weeks of age have ceramide levels 2- to 3-fold higher than in control group. Troglitazone therapy of obese fa/fa rats decreased TG levels, accompanied by reduced ceramide levels (4). In the present study, we observed reduced TG and ceramide levels in the heart of ob/ob;SCD1−/− mice in comparison to control ob/ob mice. Notably, the ceramide level in the hearts of ob/ob;SCD1−/− mice was comparable to values noted in the myocardium of WT controls. Ceramide may be formed by hydrolysis of sphingomyelin, de novo synthesis via condensation of palmitoyl-CoA and serine, glycosphingolipid breakdown, or conversion of other sphingolipids (27). Increased de novo ceramide synthesis, through increased expression of SPT mRNA, has been shown to be the dominant mechanism of lipid-induced damage/death of pancreatic islets of obese ZDF rats (34). The decrease in ceramide content in SCD1-deficient hearts appears to be due to decreased de novo synthesis, as evidenced by decreased SPT activity and gene expression, and reduced incorporation of [14C]palmitate into ceramide. The reduced intracellular palmitate level in the heart of ob/ob;SCD1−/− mice resulting from decreased lipogenesis and reduced FA uptake may also be one of rate-limiting factors in de novo ceramide synthesis. Similar effects, including reduced SCD activity and decreased intramuscular palmitoyl-CoA content, were observed in oxidative skeletal muscles of SCD1−/− mice and SCD1-deficient ob/ob mice concomitantly with downregulation of SPT activity and reduction in ceramide synthesis (12).
Because the ceramide pathway is the most important of the lipoapoptotic routes in cardiomyocytes (35), decreased ceramide content due to SCD1 deficiency may result in a reduced rate of apoptosis in the heart of ob/ob mice. We have shown here that two key markers of ceramide-induced apoptosis, NO production (measured by iNOS activity) and caspase-3 activity, were significantly reduced in the hearts of ob/ob;SCD1−/− double mutant mice. Decreased activities of iNOS and caspase-3 might result from reduced transcription of their genes caused by SCD1 deficiency as well as inhibition of de novo ceramide synthesis. Bielawska et al. (3) proposed that ceramide upregulates iNOS expression and increases NO production, which causes an increase in peroxynitrite and apoptosis. Also, downregulation of caspase-3 was often linked with ceramide action (29, 30). Ravid et al. (29) showed that ceramide-mediated apoptosis is blocked by a general caspase inhibitor, Boc-D-fluoromethylketone. Ruvolo et al. (36) established that exogenous ceramide can downregulate antiapoptotic factor Bcl-2 expression and phosphorylation and thereby activate caspase-3 and apoptosis. It has also been suggested that the antiapoptotic effect of Bcl-2 may occur via the modulation of ceramide production and prevention of ceramide-mediated caspase activation (37, 38). We found increased mRNA levels of Bcl-2 in the heart of ob/ob;SCD1−/− mice compared with ob/ob controls. Thus, increased gene expression of Bcl-2 could be another factor contributing to the downregulation of caspase-3 activity and reduction in apoptosis rate in the heart of ob/ob;SCD1−/− double knock-out mice. Because reduced apoptosis rate in cardiomyocytes was shown to improve cardiac function (1), the results suggest that inhibition of lipid-induced apoptosis could be responsible for improved LV function in ob/ob mice caused by SCD1 deficiency.
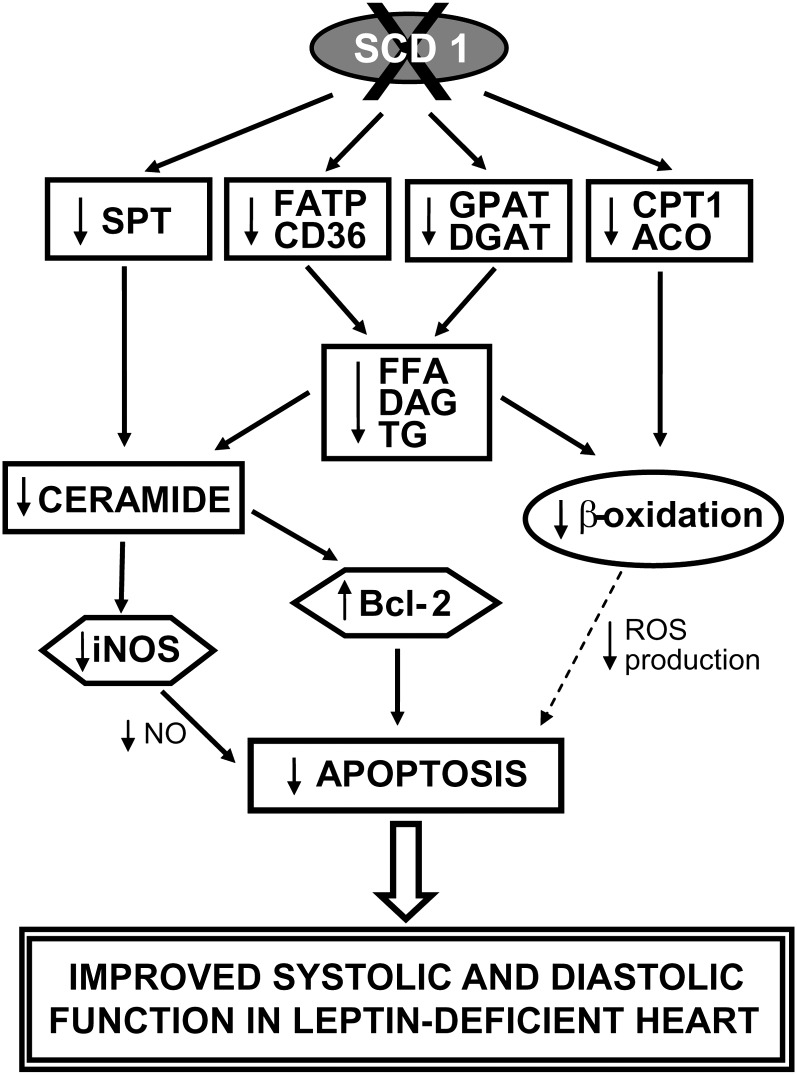
In summary, the results presented herein demonstrate the importance of SCD1 in the pathogenesis of lipotoxic heart disease. SCD1 deficiency reduced heart steatosis and the rate of cardiomyocyte apoptosis and significantly improved systolic and diastolic function of the LV in ob/ob mice. As shown schematically in Fig. 8, the disruption of the SCD1 gene reduces FA transport and expression of lipogenic genes, which leads to reductions in intracellular FFA, DAG, and TG accumulation in the heart. Decreased FA content together with reduced expression of oxidative genes results in reduction in β-oxidation rate, which itself might have positive effect on the heart function (8, 32). However, SCD1 deficiency also reduces the activity and gene expression of SPT and leads to a decrease in de novo ceramide synthesis and its accumulation. Reduced ceramide levels might be accountable for a decrease in cardiac apoptosis via increase in expression of Bcl-2 and inhibition of iNOS pathway (Fig. 8). Reduction in myocardial lipid accumulation and inhibition of lipid-induced apoptosis appear to be one of the main mechanisms responsible for improved LV function in ob/ob mice due to SCD1 deficiency. Decreased myocardial lipid content and improved cardiac function are often coupled with the reduction in systemic lipid balance (4). While SCD1 deficiency does not reduce plasma TG and FFA concentrations, it significantly decreases body weight and adiposity of ob/ob mice. It is thus possible that the protective effects of SCD1 deletion may also be coupled to the lean phenotype of the ob/ob;SCD1−/− mice. Further studies are needed to dissect the contributions of SCD1 to whole body adiposity and the heart function.
Fig. 8.
Proposed model of the effect of SCD1 gene deletion on heart lipid metabolism and LV function in leptin deficiency. Reduced FA transport and decreased expression of lipogenic genes lead to diminish FFA, DAG, and TG intracellular accumulation in the heart. Decreased level of FA, together with reduced SPT activity and gene expression, accounts for decrease in ceramide formation. A drop in intracellular ceramide content activates gene expression of antiapoptotic factor Bcl-2 and inhibits iNOS pathway and thus is accountable for decreased cardiac apoptosis in ob/ob mice. Additionally, decreased intracellular FA content simultaneously with reduced expression of oxidative genes results in reduction in FA β-oxidation, which might also lead to reduction in cardiomyocyte apoptosis, e.g., due to a decrease in ROS production (8). Taken together, reduction in myocardial lipid accumulation and inhibition of lipid-induced apoptosis appear to be the main mechanism responsible for improved cardiac function in leptin-deficient ob/ob mice caused by lack of SCD1 function. However, a reduction in the whole body adiposity in ob/ob;SCD1−/− mice may also contribute to the protective effects of SCD1 deletion.
Acknowledgments
The authors thank Dr. Harini Sampath for critical review of this paper. The authors also thank Dr. Timothy A. Hacker from Cardiovascular Physiology Core Facility, Department of Medicine at UW-Madison for help with echocardiography and Doppler measurements.
Footnotes
Abbreviations:
- ACO
- acyl-CoA oxidase
- CD36
- FA translocase
- CPT1
- carnitine palmitoyltransferase 1
- DAG
- diacylglycerol
- DGAT
- diacylglycerol acyltransferase
- FATP
- FA transport protein
- GPAT
- glycerol-3-phosphate acyltransferase
- iNOS
- inducible nitric oxide synthase
- LV
- left ventricle
- PL
- phospholipid
- PPAR
- peroxisome proliferator-activated receptor
- SCD
- stearoyl-CoA desaturase
- SPT
- serine palmitoyltransferase
- TG
- triglyceride
- WT
- wild-type
This work was supported by Polish Ministry of Science and Higher Education Grant No N301 0129 33 (to A.D.), EMBO Installation Grant No.1643 (to A.D.), and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health Grant RO1162388 (to J.M.N.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Barouch L. A., Gao D., Chen L., Miller K. L., Xu W., Phan A. C., Kittleson M. M., Minhas K. M., Berkowitz D. E., Wei C., et al. 2006. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ. Res. 98: 119–124. [DOI] [PubMed] [Google Scholar]
- 2.Szczepaniak L. S., Victor R. G., Orci L., Unger R. H. 2007. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ. Res. 101: 759–767. [DOI] [PubMed] [Google Scholar]
- 3.Bielawska A. E., Shapiro J. P., Jiang L., Melkonyan H. S., Piot C., Wolfe C. L., Tomei L. D., Hannun Y. A., Umansky S. R. 1997. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am. J. Pathol. 151: 1257–1263. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y. T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., Unger R. H. 2000. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. USA. 97: 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch L. A., Berkowitz D. E., Harrison R. W., O'Donnell C. P., Hare J. M. 2003. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 108: 754–759. [DOI] [PubMed] [Google Scholar]
- 6.Christoffersen C., Bollano E., Lindegaard M. L., Bartels E. D., Goetze J. P., Andersen C. B., Nielsen L. B. 2003. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 144: 3483–3490. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y., Naseem R. H., Duplomb L., Park B. H., Garry D. J., Richardson J. A., Schaffer J. E., Unger R. H. 2004. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc. Natl. Acad. Sci. USA. 101: 13624–13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unger R. H. 2003. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 144: 5159–5165. [DOI] [PubMed] [Google Scholar]
- 9.Dobrzyn P., Ntambi J. M., Dobrzyn A. 2008. Stearoyl-CoA desaturase: a novel control point of lipid metabolism and insulin sensitivity. Eur. J. Lipid Sci. Technol. 110: 93–100. [Google Scholar]
- 10.Ntambi J. M., Miyazaki M., Stoehr J. P., Lan H., Kendziorski C. M., Yandell B. S., Song Y., Cohen P., Friedman J. M., Attie A. D. 2002. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA. 99: 11482–11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman S. M., Dobrzyn A., Dobrzyn P., Lee S. H., Miyazaki M., Ntambi J. M. 2003. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc. Natl. Acad. Sci. USA. 100: 11110–11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobrzyn A., Dobrzyn P., Lee S. H., Miyazaki M., Cohen P., Asilmaz E., Hardie D. G., Friedman J. M., Ntambi J. M. 2005. Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 288: E599–E607. [DOI] [PubMed] [Google Scholar]
- 13.Cohen P., Miyazaki M., Socci N. D., Hagge-Greenberg A., Liedtke W., Soukas A. A., Sharma R., Hudgins L. C., Ntambi J. M., Friedman J. M. 2002. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 297: 240–243. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki M., Jacobson M. J., Man W. C., Cohen P., Asilmaz E., Friedman J. M., Ntambi J. M. 2003. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J. Biol. Chem. 278: 33904–33911. [DOI] [PubMed] [Google Scholar]
- 15.Dobrzyn P., Sampath H., Dobrzyn A., Miyazaki M., Ntambi J. M. 2008. Loss of stearoyl-CoA desaturase 1 inhibits fatty acid oxidation and increases glucose utilization in the heart. Am. J. Physiol. Endocrinol. Metab. 294: E357–E364. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi P., Yang R., Barouch L. A. 2008. Decreased p110alpha catalytic activity accompanies increased myocyte apoptosis and cardiac hypertrophy in leptin deficient ob/ob mice. Cell Cycle. 7: 560–565. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki M., Kim H. J., Man W. C., Ntambi J. M. 2001. Oleoyl-CoA is the major de novo product of stearoyl-CoA desaturase 1 gene isoform and substrate for the biosynthesis of the Harderian gland 1-alkyl-2,3-diacylglycerol. J. Biol. Chem. 276: 39455–39461. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki M., Sampath H., Liu X., Flowers M. T., Chu K., Dobrzyn A., Ntambi J. M. 2009. Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem. Biophys. Res. Commun. 380: 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris S. P., Bartley C. R., Hacker T. A., McDonald K. S., Douglas P. S., Greaser M. L., Powers P. A., Moss R. L. 2002. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ. Res. 90: 594–601. [DOI] [PubMed] [Google Scholar]
- 20.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biol. Chem. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 21.Dickson R., Lester R. L., Nagiec M. 2003. Serine palmitoyltransferase. Methods Enzymol. 311: 3–9. [DOI] [PubMed] [Google Scholar]
- 22.Pesant M., Sueur S., Dutartre P., Tallandier M., Grimaldi P. A., Rochette L., Connat J. L. 2006. Peroxisome proliferator-activated receptor delta (PPARdelta) activation protects H9c2 cardiomyoblasts from oxidative stress-induced apoptosis. Cardiovasc. Res. 69: 440–449. [DOI] [PubMed] [Google Scholar]
- 23.Husain K., Hazelrigg S. R. 2002. Oxidative injury due to chronic nitric oxide synthase inhibition in rat: effect of regular exercise on the heart. Biochim. Biophys. Acta. 1587: 75–82. [DOI] [PubMed] [Google Scholar]
- 24.Scaduto R. C., Jr. 1994. Calcium and 2-oxoglutarate-mediated control aspirate formation by rat heart mitochondria. Eur. J. Biochem. 223: 751–758. [DOI] [PubMed] [Google Scholar]
- 25.Lanser A. C., Emken F. A., Ohlrogge J. B. 1986. Oxidation of oleic and elaidic acids in rat and human heart homogenates. Biochim. Biophys. Acta. 875: 510–515. [DOI] [PubMed] [Google Scholar]
- 26.Semple R. K., Chatterjee V. K., O'Rahilly S. 2006. PPAR gamma and human metabolic disease. J. Clin. Invest. 116: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanada K. 2003. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta. 1632: 16–30. [DOI] [PubMed] [Google Scholar]
- 28.Schenck M., Carpinteiro A., Grassmé H., Lang F., Gulbins E. 2007. Ceramide: physiological and pathophysiological aspects. Arch. Biochem. Biophys. 462: 171–175. [DOI] [PubMed] [Google Scholar]
- 29.Ravid T., Tsaba A., Gee P., Rasooly R., Medina E. A., Goldkorn T. 2003. Ceramide accumulation precedes caspase-3 activation during apoptosis of A549 human lung adenocarcinoma cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 284: L1082–L1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillo S. S., Levy M., Wang C., Thaikoottathil J. V., Khan E., Goldkorn T. 2007. Nitric oxide-enhanced caspase-3 and acidic sphingomyelinase interaction: a novel mechanism by which airway epithelial cells escape ceramide-induced apoptosis. Exp. Cell Res. 313: 816–823. [DOI] [PubMed] [Google Scholar]
- 31.Wang J., Zhen L., Klug M. G., Wood D., Wu X., Mizrahi J. 2000. Involvement of caspase 3- and 8-like proteases in ceramide-induced apoptosis of cardiomyocytes. J. Card. Fail. 6: 243–249. [DOI] [PubMed] [Google Scholar]
- 32.Mengi S. A., Dhalla N. S. 2004. Carnitine palmitoyltransferase-I, a new target for the treatment of heart failure: perspectives on a shift in myocardial metabolism as a therapeutic intervention. Am. J. Cardiovasc. Drugs. 4: 201–209. [DOI] [PubMed] [Google Scholar]
- 33.Dobrzyn P., Dobrzyn A., Miyazaki M., Cohen P., Asilmaz E., Hardie D. G., Friedman J. M., Ntambi J. M. 2004. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc. Natl. Acad. Sci. USA. 101: 6409–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unger R. H., Zhou Y. T., Orci L. 1999. Regulation of fatty acid homeostasis in cells: novel role of leptin. Proc. Natl. Acad. Sci. USA. 96: 2327–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimabukuro M., Higa M., Zhou Y. T., Wang M. Y., Newgard C. B., Unger R. H. 1998. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J. Biol. Chem. 273: 32487–32490. [DOI] [PubMed] [Google Scholar]
- 36.Park T. S., Hu Y., Noh H. L., Drosatos K., Okajima K., Buchanan J., Tuinei J., Homma S., Jiang X. C., Abel E. D., et al. 2008. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49: 2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruvolo P. P., Clark W., Mumby M., Gao F., May W. S. 2002. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J. Biol. Chem. 277: 22847–22852. [DOI] [PubMed] [Google Scholar]
- 38.Sawada M., Nakashima S., Banno Y., Yamakawa H., Takenaka K., Shinoda J., Nishimura Y., Sakai N., Nozawa Y. 2000. Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells. Oncogene. 19: 3508–3520. [DOI] [PubMed] [Google Scholar]