Abstract
The study of Alzheimer’s disease (AD) pathogenesis requires the use of animal models that develop some amount of amyloid pathology in the brain. Aged canines (beagles) naturally accumulate human-type amyloid β-peptide (Aβ) and develop parallel declines in cognitive function. However, the type and quantity of biochemically extracted Aβ in brain and cerebrospinal fluid (CSF), its link to aging and similarity to human aging, has not been examined systematically. Thirty beagles, aged 4.5–15.7 years, were studied. Aβ40 and Aβ42 were measured in CSF by ELISA, and from SDS and formic acid extracted prefrontal cortex. A sample of the contralateral hemisphere, used to assess immunohistochemical amyloid load, was used for comparison. In the brain, increases in Aβ42 were detected at a younger age, prior to increases in Aβ40, and were correlated with an increased amyloid load. In the CSF, Aβ42 decreased with age while Aβ40 levels remained constant. The CSF Aβ42/40 ratio was also a good predictor of the amount of Aβ in the brain. The amount of soluble oligomers in CSF was inversely related to brain extractable Aβ, whereas oligomers in the brain were correlated with SDS soluble Aβ42. These findings indicate that the Aβ in the brain of the aged canine exhibits patterns that mirror Aβ deposited in the human brain. These parallels support the idea that the aged canine is a useful intermediate between transgenic mice and humans for studying the development of amyloid pathology, and is a potentially useful model for the refinement of therapeutic interventions.
Keywords: beagle, dog, cerebrospinal fluid, amyloid β-peptide, amyloid β-protein precursor, oligomer, Alzheimer’s Disease
Introduction
Alzheimer’s disease (AD) is a progressive and gradual decline in cognitive function accompanied by extensive neurodegeneration. It is the pattern of this neurodegeneration within the brain, the loss of specific populations of neurons and synapses, which distinguishes the clinical presentation of AD from other, less common, forms of dementia. Within the brain itself, the hallmark signs of AD include both extracellular deposition of the amyloid-β peptide (Aβ) and the intracellular accumulation of an abnormally phosphorylated form of the microtubule associated protein tau. Abnormal tau, found in neurofibrillary tangles, is also found in a number of other neurodegenerative diseases. These other diseases, the broader class of tauopathies, do not feature Aβ deposits as a primary feature of pathology, indicating that Aβ deposition is relatively specific to AD [1].
The Aβ peptide, derived from the larger amyloid-β precursor protein (APP), was first identified more than 25 years ago [2–4]. APP is sequentially cleaved by two membrane-bound endoprotease activities, β- and γ-secretase. β-secretase (primarily BACE1 in the brain [5]) first cleaves APP to release an amino terminal derivative, sAPPβ. A fragment of 99 amino acids (CTFβ, which begins with the N-terminal aspartyl residue of Aβ) remains membrane bound, and is in turn rapidly cleaved by γ-secretase to generate Aβ. Cleavage by γ-secretase occurs within the membrane, and is relatively unselective, resulting in the production of a population of heterogenous peptides. The most common form of Aβ, 40 amino acids long (Aβ40), is the most abundant (~80–90%), followed by a more hydrophobic 42 amino acid form (Aβ42, ~5–10%) [6].
The issue of how much Aβ contributes directly to neurodegeneration has been a topic of debate for some years. It is currently believed that the toxic form of the peptide may be a higher molecular weight, oligomeric form that can readily diffuse within the brain and either directly damage neurons or interfere with their normal function [7, 8]. Oligomers form readily from the Aβ42 peptide, less well so from the more abundant Aβ40 [9, 10], suggesting that the C-terminus is critical for oligomer formation to occur. Aβ dimers isolated from AD brain and CSF disrupt synaptic function [11, 12]. Just how oligomeric Aβ causes these effects has yet to be determined, although both receptor-mediated effects as well as direct interactions with the membrane bilayer are possibilities [13, 14].
Alzheimer’s disease is specific to humans – even our most closely related primate relatives do not develop anything that can be considered to be bona fide AD. Hence, much of our understanding of how Aβ pathology develops has been driven by studies in animal models. Although there is no model that captures all the hallmarks of AD, we are able to routinely model Aβ deposition. The study of AD pathogenesis in animal models is primarily focused on transgenic mice over expressing human mutant APP at high levels, typically under the control of a heterologous promoter [15], and insights from these models have been indispensible for our understanding of amyloid deposition in vivo [16–18]. Even though over expression of APP is not an absolute requirement for the development of amyloid pathology [19, 20], the introduction of some combination of familial AD mutations into APP or presenilin 1 (or both) does appear essential for the development of robust Aβ deposition. Although nonhuman primates (NHPs) have near identical APP sequence to humans, and are our closest evolutionary cousins, they develop surprisingly little AD-like neuropathology with age. While older NHPs typically show small amounts of amyloid deposition, this is quite modest compared to cases of AD [21–23], and is far less than that of the aged canine. True neurofibrillary tangle pathology is not a typical feature of pathology in NHPs [22], although chimpanzees [24] and baboons [25] may be an exception.
Aged canines also develop substantial amyloid deposition with age. Unlike aged NHPs, which may require several decades, canines develop pathology at a much younger age [26]. Aged canines (beagles) naturally accumulate human-type Aβ [27, 28] with age [29, 30] and this parallels a decline in cognitive function [31, 32]. Aβ42 accumulation within diffuse plaques in the brain correlates with more severe learning and memory deficits in aged canines [31–33]. The quantity of deposited Aβ is often comparable to AD cases, and a substantial proportion is highly insoluble [34]. This suggests that the amyloid in the aged canine quantitatively overlaps with the amount of amyloid in human disease. Thus, canines may represent a useful intermediate between genetically modified mouse models and AD. In this regard it is worth noting that immunization with fibrillar Aβ in aged canines may be a better parallel to human trials with this therapeutic approach than preclinical mouse models [34, 35].
In spite of significant progress in understanding the development of amyloid pathology in the brain of the aged dog, we still know very little about the interplay between the amount of Aβ in the brain and the levels of soluble oligomers in both the brain and CSF, and their link to age. This is an interesting question, since CSF Aβ42 continues to undergo extensive evaluation as a potential biomarker of AD and the development of amyloid pathology in the brain [36]. From the perspective of a useful preclinical model, determining whether or not CSF Aβ levels predict brain levels would establish a biomarker for the selection of “at risk” animals for use in the development of novel therapeutics.
Materials and Methods
Animals
Thirty beagles, ranging in age from 4.5 – 15.7 years, were included in the study. All animals were from the colony at the Lovelace Respiratory Research Institute (Albuquerque, NM), and were housed either singly or in pairs in kennel buildings with indoor/outdoor runs measuring 91 cm × 600 cm. Animals were fed Wayne Mini Lab Dog Diet 8759 dog food once daily (Teklad Pioneer Lab Diets, Madison, WI) and water was available at all times. All dogs received regular veterinary care.
Aβ Immunohistochemistry
Animals were anesthetized with sodium pentobarbital (Nembutal) in accordance with approved IACUC protocols. The brains were removed and alternating 0.5 cm coronal sections were frozen at −80°C or fixed in 4% paraformaldehyde at 4°C for 72 hours. The fixed tissue was then transferred to phosphate buffered saline (pH 7.4, with 0.02% sodium azide) and stored at 4°C. Aβ was detected using a rabbit polyclonal antibody, designated anti-Aβ42, raised against a synthetic Aβ peptide containing amino acids 1 through 42 [32]. Fifty μm vibratome sections were taken from the dorsolateral prefrontal cortex and pretreated for 4 minutes with 90% formic acid (FA) prior to overnight incubation at room temperature in anti-Aβ 42 (1:500) in Tris-saline with 2.0% bovine serum albumin and 0.1% Triton X-100. Bound antibody was detected using a biotinylated anti-rabbit ABC peroxidase kit and visualized with DAB substrate (Vector Labs, Burlingame, CA).
To determine Aβ load, five semi-random fields from each section (2 sections in total from each animal) were digitized and the cross-sectional area occupied by Aβ quantified using gray scale thresholding as described previously [32]. Briefly, a random region within a random 10X field with Aβ deposition was centered and digitized at 20X. Additional fields were semi-randomly selected by moving laterally by one field. A Sony high-resolution CCD video camera (XC-77) and the built-in video capture capabilities of a Macintosh 8100/80AV were used to digitize images. All sections from a given region were captured sequentially during one session and were analyzed blind with respect to age. Files were saved as gray scale (256 level) images that were subsequently binarized with a cutoff value of 110. This eliminates the background along with some faint Aβ immunoreactivity, and is hence a conservative estimate of the actual amount [29]. Public domain image analysis software (NIH Image 1.55) was used to calculate the percentage area occupied by Aβ.
Aβ Immunoassay
Frozen tissue samples were directly homogenized in 70% formic acid (FA; 1.0 ml / 150 mg of tissue), with complete protease inhibitor cocktail (PIC; Roche). Samples were centrifuged at 100,000 × g for 1 hour, and the aqueous layer collected, aliquoted and frozen at −80°C. For the assay [20, 37, 38], samples were initially neutralized by a 1:20 dilution in TP buffer (1 M Tris base, 0.5 M Na2HPO4), followed by a further dilution as needed (but at least 1:1) in capture buffer [0.02 M sodium phosphate buffer (pH = 7), 0.4 M NaCl, 2 mM EDTA, 0.4% Block Ace (Serotec), 0.2% bovine serum albumin, 0.05% CHAPS, and 0.05% sodium azide]. CSF samples were diluted in capture buffer, as necessary. Standard curves of either synthetic Aβ40 or Aβ42 were prepared in the same buffer as the samples. All samples and standards were run at least in duplicate on Ab3160 coated ELISA plates, followed by detection with either HRP conjugated BA27 (for Aβ40) or BCO5 (for Aβ42). Aβ values were determined by comparison to the appropriate standard curve. An additional subset of samples (N=14) across a range of ages were extracted first in 2% SDS (+PIC), centrifuged for 30 minutes at 20,000 × g, and the supernatant collected and frozen. The resulting pellet was then extracted in 70% FA, as described above.
Oligomer Blots
PBS soluble fractions were tested for total protein concentration by the BCA protein assay and diluted with double-distilled water to a standardized concentration. 7.6 μg of total protein was spotted onto a nitrocellulose membrane (Protran BA83, 0.2 μm pore size). Samples were dried and then blocked (10% Non-Fat Milk in Low-Tween TBS, pH 7.6 (20 mM Tris, 137 mM NaCl, 0.001% Tween 20) for 1 hour at room temperature. After blocking, the blot was washed in Low-Tween TBS, pH 7.6 (20 mM Tris, 137 mM NaCl, 0.001% Tween 20) three times for 5 minutes each. The blot was then incubated in anti-oligomer antibody (A11-1:2000 [8]) in 5% Milk- Low Tween TBS overnight at 4°C. After washing the blot 3 times for 5 minutes each, the blot was transferred into goat-anti-rabbit IgG (Jackson Immuno Research), diluted 1:12000 in Low-Tween TBS. Secondary antibody incubation was for 1 hour at room temperature. After the final 3 washes (5 min each), the blot was exposed to ECL reagent (Amersham) for 1 minute, exposed to film and the film developed. The film was scanned and pixel density was determined using Scion Imaging Software (Version: Beta 4.0.2). Experiments were repeated twice and the average densities used in data analyses.
For additional comparison, SDS extracts were run on 10–20% SDS-PAGE gels, transferred to nitrocellulose, and blotted with 1 μg/ml of 6E10 (which will detect fragments of the amyloid-β precursor protein that contain Aβ, as well as Aβ oligomers). Analyses were performed on the A11 signal since this represents “total oligomers”, and since it cannot be determined which bands should be summed for a comparable measure with 6E10.
Data Analysis
SPSSR® for Windows was used to test for effects of age, and to determine the significance of all correlations and regression analyses.
Results
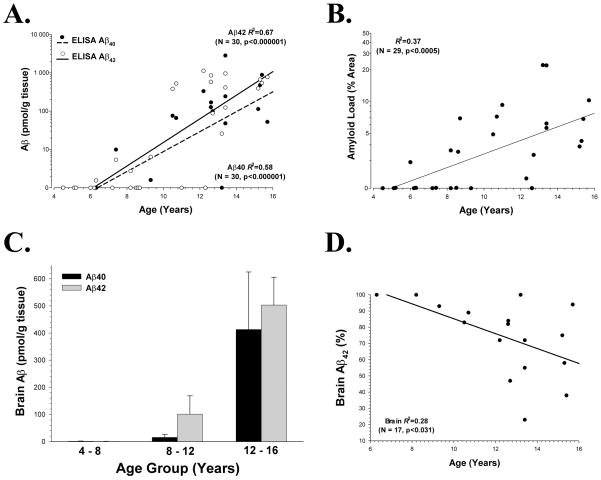
In the brain, both Aβ40 (p<0.001) and Aβ42 (p<0.001) increased exponentially with age (Fig. 1A), as did the Aβ42 load (p<0.001) as determined by immunohistochemical analysis (Fig. 1B). Immunohistochemical Aβ42 load and total Aβ42 determined by ELISA were significantly correlated with each other (R2=0.23, p<0.01). Aβ42 deposition began at a slightly earlier age (7.6 years) as compared to Aβ40 (8.1 years), based on examination of the x-intercept data. The extractable Aβ in younger animals was primarily Aβ42, with Aβ40 appearing only in the older subjects, similar to what has been observed in humans. To examine this point further, animals were grouped into three age groups of approximately equal width (aged 4 to 8 years, N = 8; aged 8 to 12 years, N = 9; aged 12 to 16 years, N = 13); this illustrates that Aβ42 is much more prominent in middle aged (ranging from 8–12 years of age) canines, with Aβ40 appearing in substantial quantities only in later years (Fig. 1C). This is also reflected in the ratio of Aβ42/Aβ40, which tended to decrease with age in the brain (p<0.04; Fig. 1D). As with a previous report in canine brain, there are a small number of individual aged dogs (>10 years) that show low levels of Aβ [39].
Figure 1. Age related changes in Aβ from the Canine Prefrontal Cortex.
(A) Formic acid extractable Aβ40 and Aβ42, as measured by ELISA, each increase exponentially with age (note the logarithmic scale); (B) amyloid load (using rabbit anti-Aβ42; see text), as determined immunohistochemically, also increases exponentially with age in brain, but the correlation is not as strong as Aβ ELISA measurements; (C) Aβ42 can be seen as the prominent peptide species deposited in middle age when animals are grouped into approximately equal age categories (young, middle-aged, and old), whereas Aβ40 appears in significant quantities only in older animals; (D) Aβ42, as a percentage of total Aβ(Aβ40 + Aβ42) in the brain, decreases with age (note the increasing individual variability after the age of 12 years).
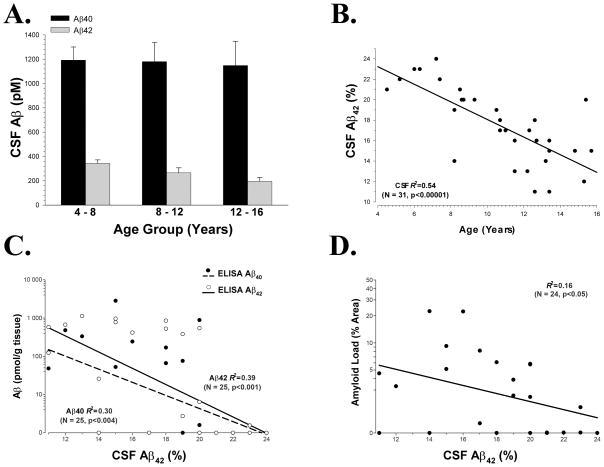
In CSF, Aβ40 remained remarkably stable with age while Aβ42 decreased slightly (Fig. 2A). We expressed the Aβ42 data as a percentage of the total, a common method of examining Aβ in biological fluids that better controls for individual variability. The percentage (or ratio) of Aβ42 undergoes a linear decrease with increasing age (p<0.001; Fig. 2B). Lower percentages of CSF Aβ42 were correlated with larger amounts of formic acid extractable Aβ42 in the brain (p<0.001), as well as brain Aβ40 (p<0.005; Fig. 2C). Similarly, higher prefrontal immunohistochemical Aβ42 loads were correlated with lower CSF Aβ42 percentages (p<0.05; Fig. 2D).
Figure 2. Age related changes in Aβ in Canine CSF.
(A) Aβ40 remains relatively constant with age while Aβ42 decreases slightly; (B) Aβ42, expressed as a percentage of total Aβ, decreases linearly with age in the CSF; (C) The percentage of CSF Aβ42 is inversely correlated to the amount of formic acid extractable Aβ in the brain; (D) Although the relationship is weaker, CSF Aβ42 percentage is also inversely correlated with prefrontal immunohistochemical Aβ42 load.
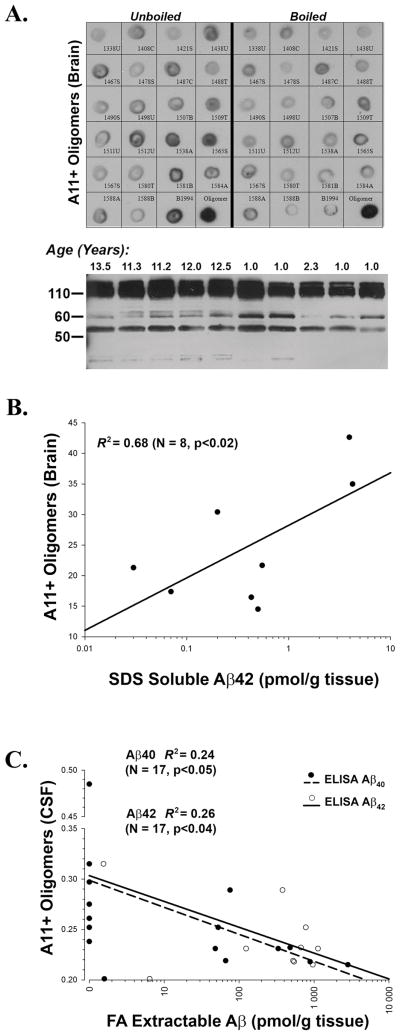
Finally, we performed an examination of A11 positive oligomers in the brain and CSF within a subset of the subjects (Fig. 3A). As expected, 6E10 immunblots run for comparison purposes displayed bands corresponding to full length APP, possible fragments of APP, and various oligomeric species of Aβ. Brain A11 immunoreactivity did not correlate with the total amount of either Aβ40 or Aβ42 (data not shown). However, although the number of subjects that overlapped on this measure was small, A11 immunoreactivity against soluble oligomers did correlate with SDS extractable Aβ42 (R2=0.68, p<0.02) (Fig. 3B). In the CSF, A11 immunoreactivity was inversely correlated with both Aβ40 (R2=0.24, p<0.05) and Aβ42 (R2=0.26, p<0.04) in the prefrontal cortex, indicating that as the total amount of brain Aβ increases, the amount of soluble oligomers in the CSF decreases (Fig. 3C).
Figure 3. Relationship Between Aβ and Oligomers in the Brain and CSF.
(A) Dot (top; A11) and Western (bottom; 6E10) blots illustrating oligomers in the canine brain (animal numbers are indicated adjacent to each dot). Species quantified from dot blots represent a population of Aβ oligomers from the brain that can vary over a wide range of sizes. For instance, the intense set of bands at ~110 kDa is mostly full length APP, and 6E10 positive bands between 50 and 60 kDa could represent either fragments of APP or oligomeric Aβ. Hence, analyses are focused on A11 positive material only. (B) Soluble, A11 positive oligomers in brain are correlated with the amount of SDS extractable Aβ42, but with nothing else. (C) Soluble, A11 positive oligomers in CSF are inversely correlated to the amount of Aβ40 and Aβ42 in the brain.
Discussion
Formic acid extractable Aβ40 and Aβ42 increases in the brain with age in canines, and parallels similar changes in immunohistochemically detectable Aβ. In contrast, in the CSF, Aβ42 decreased with age while Aβ40 showed little change. Decreasing Aβ42 in the CSF is consistent with the concept of a “peripheral pool effect”: as Aβ is deposited in brain, soluble CSF pools decrease [40]. The association between brain and CSF Aβ is similar to that observed for both AD patients [41–44] and transgenic mice [38]. Brain extractable Aβ from younger animals was almost exclusively Aβ42, with Aβ40 appearing later. This extends earlier immunohistochemical studies in the canine showing that Aβ40 accumulation occurs at later ages than Aβ42, paralleling observations in human brain [33, 45].
As the proportion of Aβ42 in the CSF decreased, brain Aβ accumulation increased. The percentage of Aβ42 in the CSF was not only able to predict the amount of formic acid soluble Aβ42 in the brain, but also formic acid extractable Aβ40, as determined by ELISA. Also, the amount of Aβ42 measured as a percentage of occupied cortical area, determined by immunohistochemical techniques using a different antibody, was strongly related to the percentage of Aβ42 in the CSF. It is not surprising that the percentage of Aβ42 in the CSF is able to predict not only Aβ42 in the brain, but also Aβ40, since there is a high degree of correlation between the amounts of each peptide in the brain. Although CSF Aβ has yet to fulfill its potential as a biomarker of AD, these data suggest another possible use: measures of Aβ derived from CSF samples may be useful for predicting the levels of Aβ in brain as well as the extent of plaque formation in animal models of amyloid deposition. This measure could be particularly useful for selecting animals with extensive amounts of pre-existing Aβ pathology to use in targeted intervention studies aimed at reducing Aβ levels (e.g., immunization, statin therapy, nonsteroidal anti-inflammatories, etc.) and to potentially monitor the effects of ongoing treatments. This is particularly important in animal model research, since in vivo imaging of Aβ is expensive and usually impractical for most studies and investigators. Also, at least in the case of the most widely studied amyloid imaging agent, Pittsburg Compound B, amyloid deposited in the brain of species other than humans does not image nearly as well, likely due to differences in the interaction between the dye and the proportion of high affinity binding sites on the amyloid fibrils in vivo [46–48]. Since there is a current lack of good in vivo imaging agents that can assess the amount of Aβ in the brain from non-humans, CSF Aβ42 may prove a useful tool that behaves similarly across all species.
We have previously shown that Aβ in diffuse deposits is correlated with cognitive test error scores [32, 33]. For example, visual reversal learning scores, a measure of frontal function correlates with the prefrontal cortex Aβ load [32]. Cognitive impairment in humans is associated with a decline in the level of Aβ42 in the CSF [43, 49], with an increase in the amount of brain extractable Aβ40 and Aβ42 [44], and with some aspects of plaque pathology [50]. A similar relationship is seen between FA extractable Aβ and water maze performance in Tg2576 mice [51]. It would thus seem that extracted Aβ is a functionally relevant measure that reflects soluble species of Aβ in addition to the accumulation of insoluble Aβ detected by immunohistochemistry. It will be interesting to see whether or not CSF Aβ42 is able to predict aspects of cognitive function in the aging beagle.
Finally, the amount of soluble oligomers in CSF was inversely related to brain extractable Aβ, whereas oligomers in the brain were correlated with SDS soluble Aβ42. It is now widely believed that soluble oligomeric forms of Aβ are responsible for the biological, and neurotoxic, effects of the peptide [7, 8]. Oligomeric Aβ species, which can exist at concentrations in the pM range in the CSF or brain interstitial fluid [52], are orders of magnitude more neurotoxic than either monomeric or fibrillar Aβ [53]. It is also likely that soluble oligomeric Aβ pools exist in equilibrium with other forms of Aβ in the brain; for example, neuritic plaques and other amyloid deposits may serve as stable reservoirs [54]. It is possible that the decrease in CSF soluble A11 positive oligomers in canine CSF relates to the exponential increase in the amount of insoluble Aβ deposited in the brain; this is a similar argument to that proposed above to account for the decreasing proportion of Aβ42 in the CSF. We also observed in a small subset of animals that A11 immunoreactivity in the brain is related to the amount of SDS soluble Aβ42. SDS-stable Aβ oligomers with size corresponding to a dimer have been isolated from AD brain and CSF, and have been put forward as likely candidates for the major form of toxic Aβ [11, 12]. However, A11 positive prefibrillar oligomers did not increase with age or with AD whereas fibrillar oligomers were higher in AD tissue [55] suggesting that future studies to measure the latter pool of oligomers would be interesting in the canine model.
The canine has proven to be a useful model for preclinical drug development due to similarities with humans in terms of absorption, toxicity and other pharmacokinetics. This contrasts to that of drugs developed in rodents and subsequently translated to the human [56]. Several recent potential AD therapeutic agents, with a heavy reliance on preclinical data developed in transgenic mouse models of Aβ deposition, have ultimately failed in clinical trials. In light of this lack of success, it may be justified to include a complementary animal model in addition to mice in order to bolster the justification for proceeding into human trials. The present findings combined with previous data suggest that the aged canine provides a useful intermediate between transgenic mice and humans for the development of AD therapeutic interventions, particularly those aimed at reducing brain Aβ.
Acknowledgments
All work was conducted with prior IACUC approval, and was performed in accordance with USDA and PHS guidelines. The authors were supported by grants from the National Institutes of Health (M.P.M.: NS058382, AG005119; E.H.: AG032550, AG031764) and the Alzheimer’s Association (IIRG-03-5673). The authors thank Takeda Industries and Dr. Steven G. Younkin for providing the original antibodies used in this study, and Drs. Carl W. Cotman and Todd E. Golde for helpful advice.
Footnotes
The authors declare no financial conflict of interest.
References
- 1.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 2.Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 3.Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer’s disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985;4:2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. PNAS. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 7.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 8.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 9.Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A. 2003;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soreghan B, Kosmoski J, Glabe C. Surfactant properties of Alzheimer’s A beta peptides and the mechanism of amyloid aggregation. J Biol Chem. 1994;269:28551–28554. [PubMed] [Google Scholar]
- 11.Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, Selkoe DJ, Anwyl R, Walsh DM, Rowan MJ. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 14.Sokolov Y, Kozak JA, Kayed R, Chanturiya A, Glabe C, Hall JE. Soluble amyloid oligomers increase bilayer conductance by altering dielectric structure. J Gen Physiol. 2006;128:637–647. doi: 10.1085/jgp.200609533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong PC, Cai H, Borchelt DR, Price DL. Genetically engineered mouse models of neurodegenerative diseases. Nat Neurosci. 2002;5:633–639. doi: 10.1038/nn0702-633. [DOI] [PubMed] [Google Scholar]
- 16.Games D, Buttini M, Kobayashi D, Schenk D, Seubert P. Mice as models: transgenic approaches and Alzheimer’s disease. J Alzheimers Dis. 2006;9:133–149. doi: 10.3233/jad-2006-9s316. [DOI] [PubMed] [Google Scholar]
- 17.McGowan E, Eriksen J, Hutton M. A decade of modeling Alzheimer’s disease in transgenic mice. Trends Genet. 2006;22:281–289. doi: 10.1016/j.tig.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 18.LeVine H, Walker LC. Model’s of Alzheimer’s Disease. In: Conn MP, editor. Handbook of Models for Human Aging. Academic Press / Elsevier; New York: 2006. pp. 121–134. [Google Scholar]
- 19.Flood DG, Reaume AG, Dorfman KS, Lin YG, Lang DM, Trusko SP, Savage MJ, Annaert WG, De Strooper B, Siman R, Scott RW. FAD mutant PS-1 gene-targeted mice: increased A beta 42 and A beta deposition without APP overproduction. Neurobiol Aging. 2002;23:335–348. doi: 10.1016/s0197-4580(01)00330-x. [DOI] [PubMed] [Google Scholar]
- 20.Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol Dis. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Wisniewski HM, Ghetti B, Terry RD. Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropath Exp Neurol. 1973;32:566–584. doi: 10.1097/00005072-197310000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Walker LC, Kitt CA, Schwam E, Buckwald B, Garcia F, Sepinwall J, Price DL. Senile plaques in aged squirrel monkeys. Neurobiol Aging. 1987;8:291–296. doi: 10.1016/0197-4580(87)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.Martin LJ, Sisodia SS, Koo EH, Cork LC, Dellovade TL, Weidemann A, Beyreuther K, Masters C, Price DL. Amyloid precursor protein in aged nonhuman primates. Proc Natl Acad Sci U S A. 1991;88:1461–1465. doi: 10.1073/pnas.88.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, Davis-Turak J, Coppola G, Geschwind DH, Pare JF, Duong TQ, Hopkins WD, Preuss TM, Walker LC. Tauopathy with paired helical filaments in an aged chimpanzee. J Comp Neurol. 2008;509:259–270. doi: 10.1002/cne.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz C, Dehghani F, Hubbard GB, Thal DR, Struckhoff G, Braak E, Braak H. Filamentous tau pathology in nerve cells, astrocytes, and oligodendrocytes of aged baboons. J Neuropathol Exp Neurol. 2000;59:39–52. doi: 10.1093/jnen/59.1.39. [DOI] [PubMed] [Google Scholar]
- 26.Satou T, Cummings BJ, Head E, Nielson KA, Hahn FF, Milgram NW, Velazquez P, Cribbs DH, Tenner AJ, Cotman CW. The progression of beta-amyloid deposition in the frontal cortex of the aged canine. Brain Res. 1997;774:35–43. doi: 10.1016/s0006-8993(97)81684-8. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP. Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Research Molecular Brain Research. 1991;10:299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- 28.Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science. 1987;235:873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- 29.Cummings BJ, Su JH, Cotman CW, White R, Russell MJ. Beta-amyloid accumulation in aged canine brain: a model of early plaque formation in Alzheimer’s disease. Neurobiol Aging. 1993;14:547–560. doi: 10.1016/0197-4580(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 30.Wisniewski T, Lalowski M, Bobik M, Russell M, Strosznajder J, Frangione B. Amyloid beta 1–42 deposits do not lead to Alzheimer’s neuritic plaques in aged dogs. Biochem J. 1996;313 ( Pt 2):575–580. doi: 10.1042/bj3130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learning Memory. 1996;66:11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- 32.Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–425. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 33.Cummings BJ, Satou T, Head E, Milgram NW, Cole GM, Savage MJ, Podlisny MB, Selkoe DJ, Siman R, Greenberg BD, Cotman CW. Diffuse plaques contain C-terminal A beta 42 and not A beta 40: evidence from cats and dogs. Neurobiol Aging. 1996;17:653–659. doi: 10.1016/0197-4580(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 34.Head E, Pop V, Vasilevko V, Hill M, Saing T, Sarsoza F, Nistor M, Christie LA, Milton S, Glabe C, Barrett E, Cribbs D. A two-year study with fibrillar beta-amyloid (Abeta) immunization in aged canines: effects on cognitive function and brain Abeta. J Neurosci. 2008;28:3555–3566. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 36.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, Foster NL, Petersen RC, Weiner MW, Price JC, Mathis CA. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki N, Cheung TT, Cai X-D, Odaka A, Otvos L, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid β protein is secreted by familial amyloid β protein precursor (β APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 38.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF and plasma amyloid beta protein in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21:89–96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 40.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer’s disease from normal and pathologic aging. Exp Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 42.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 43.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. Jama. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 45.Wisniewski T, Frangione B. Molecular biology of brain aging and neurodegenerative disorders. Acta Neurobiologiae Experimentalis. 1996;56:267–279. doi: 10.55782/ane-1996-1132. [DOI] [PubMed] [Google Scholar]
- 46.Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, Abrahamson EE, Debnath ML, Holt DP, Huang GF, Shao L, DeKosky ST, Price JC, Mathis CA. Binding of the positron emission tomography tracer Pittsburg compound-B reflects the amount of amyloid-beta in Alzheimer’s disease brain but not in transgenic mouse brain. J Neurosci. 2005;25:10598–10606. doi: 10.1523/JNEUROSCI.2990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda J, Ji B, Irie T, Tomiyama T, Maruyama M, Okauchi T, Staufenbiel M, Iwata N, Ono M, Saido TC, Suzuki K, Mori H, Higuchi M, Suhara T. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer’s disease enabled by positron emission tomography. J Neurosci. 2007;27:10957–10968. doi: 10.1523/JNEUROSCI.0673-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosen RF, Walker LC, LeVine H., 3rd Binding in Aged Primate Brain: Unique Enrichment of High Affinity Sites in Humans with AD. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.02.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maruyama M, Arai H, Sugita M, Tanji H, Higuchi M, Okamura N, Matsui T, Higuchi S, Matsushita S, Yoshida H, Sasaki H. Cerebrospinal fluid amyloid beta(1–42) levels in the mild cognitive impairment stage of Alzheimer’s disease. Exp Neurol. 2001;172:433–436. doi: 10.1006/exnr.2001.7814. [DOI] [PubMed] [Google Scholar]
- 50.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR. Modeling the Association between 43 Different Clinical and Pathological Variables and the Severity of Cognitive Impairment in a Large Autopsy Cohort of Elderly Persons. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, Mirkin CA. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2005;102:2273–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid {beta} associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomic JL, Pensalfini A, Head E, Glabe CG. Soluble fibrillar oligomer levels are elevated in Alzheimer’s disease brain and correlate with cognitive dysfunction. Neurobiol Dis. 2009;35:352–358. doi: 10.1016/j.nbd.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alberts AW. HMG-CoA reductase inhibitors - the development. In: Stokes J, Mancini M, editors. Atherosclerosis Reviews. Raven Press, Ltd; New York: 1988. pp. 123–131. [Google Scholar]