FK-506 (FK), like all other known immunosuppressive agents, may be expected to demonstrate certain undesirable side effects, eg, infection, neoplasia. These effects must currently be accepted as common to the immunosuppressed state. The present study was undertaken to explore whether other toxic effects peculiar to FK exist. Because most of our initial transplantation studies were performed in the rat, this animal was chosen as the first model for toxicological studies.
Materials and Methods
Animals
Male Lewis rats were obtained from Harlan Sprague Dawley Co, Indianapolis. The animals were housed in accordance with institutional guidelines and received food and water ad libitum.
Drug
FK was supplied in powder form by the Fujisawa Pharmaceutical Co, Ltd, Osaka, Japan. All concentrations were made up in normal sterile saline and administered to experimental animals per os by feeding syringe.
Experimental Design
One hundred-twenty five rats were divided into five groups of 25 animals each. Group 1 served as a control group and received placebo daily. Group 2 received FK per os at 0.5 mg/kg/d. Group 3 received 1.0 mg/kg/d, Group 4 received 2.0 mg/kg/d, and group 5 received 4.0 mg/kg/d. Five randomly chosen animals from each group were killed on days 7, 14, 21, 28, and 34.
Baseline body weights were obtained, and the animals were weighed at three- to four-day intervals thereafter. At time of sacrifice, blood was collected for chemical and hematologic analysis. Preliminary analysis of some of these studies is available for the current communication.
Autopsies were performed on all killed animals. The spleen, thymus, kidney, and liver were weighed and processed for light microscopy. Tissue was also stored for future electron microscopy.
Results
Body Weight
The mean body weight for each group is graphically demonstrated in Fig 1. It is apparent that group 5 animals sustained a net weight loss at the outset of the experiment in contrast to all other groups. This process reached its nadir on day 13 when this group dropped to a mean 6% loss of original body weight. However, these animals recovered their original body weight by day 13 and continued to gain normally thereafter. At the conclusion of the experiment, the mean weight for the remaining animals in group 5 was 350.6 g and that for control animals, 357.6 g.
Fig 1.
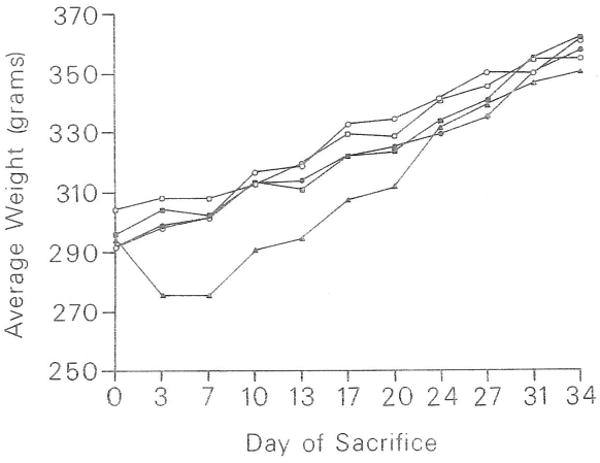
Effect of FK on rat body weight. Group 1 (●); placebo; group 2 (○); 0.5 mg/kg/d; group 3 (■); 1.0 mg/kg/d; group 4 (□); 2.0 mg/kg/d; group 5 (▲); 4.0 mg/kg/d. Weights are represented as means. Number of animals per group: days 0, 3, 7, n = 25; days 10, 13, n = 20; days 17, 20, n = 15; days 24, 27, n = 10; days 31, 34, n = 5.
Gross and Histopathologic Studies
No gross organ abnormalities were apparent at autopsy. Thymus weights showed slight reductions, which appeared related to drug dosage (Fig 2). No similar weight changes in the kidney or liver were apparent. Spleen weights will require further statistical analysis before offering preliminary comments.
Fig 2.
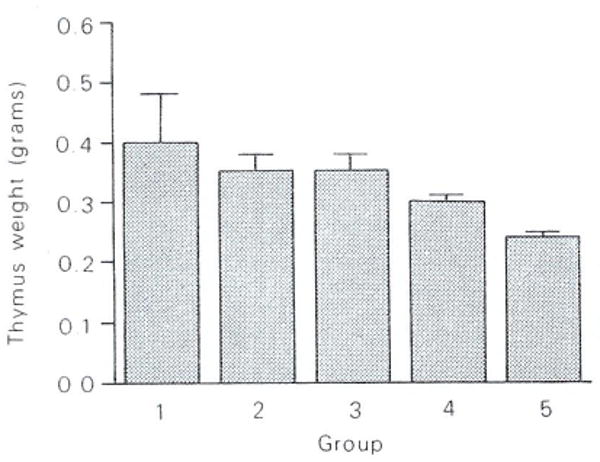
Effect of FK on thymus weight at day 34. Each bar represents the mean and SE of five animals per group killed on the last day of the experiment (day 34). Groups are as in Fig 1.
Histologically, the changes in the thymus were most striking (Fig 3). An increased corticomedullary ratio was observed that appeared proportional to drug dosage. This change was accompanied by increased phagocytic activity in the cortex.
Fig 3.
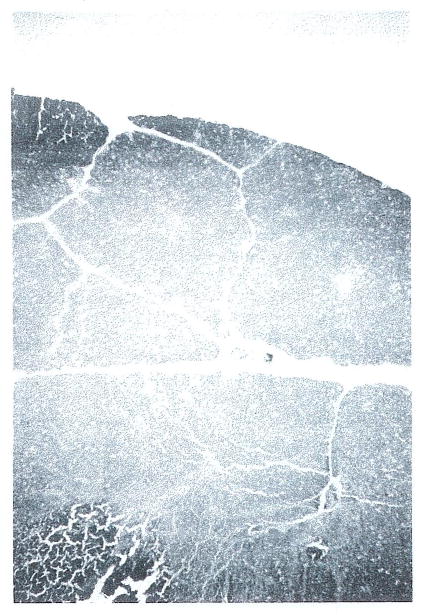
Thymus from FK-treated animal on day 7 shows a reduction of the medullary regions. This loss was progressive over time (H&E; original magnification ×32).
The pancreas demonstrated small numbers of isolated acinar cells undergoing necrosis (Fig 4). In some cases, rare mitoses of acinar cells were also observed.
Fig 4.
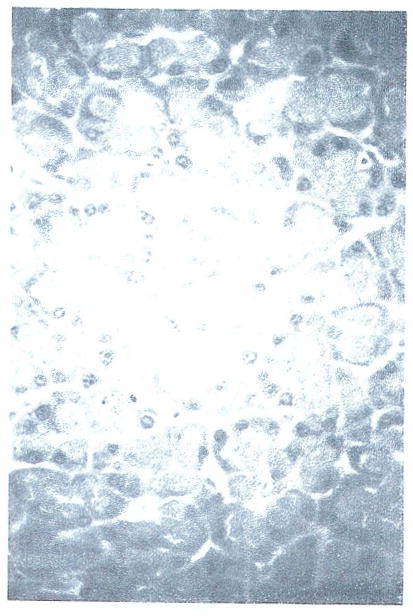
Pancreas from FK-treated animal showing small focus of necrotic acinar cells (H&E; original magnification ×325).
With high doses of drug, a perivascular eosinophilia was observed to be localized in most cases to vessels in the lung (Fig 5). Close histological examination failed to reveal any evidence of vascular damage in association with this finding.
Fig 5.
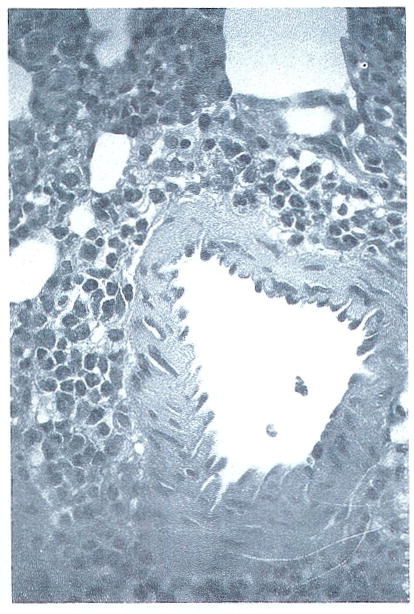
Lung from FK-treated rat showing perivascular eosinophilic infiltration. No direct vessel damage can be appreciated in this section. (H&E: original mangification ×325).
Sections of skin, muscle, fat, intestine, liver, kidney, spleen, and heart were also examined. No reproducible changes were seen in these organs at the drug dosages administered.
Blood Studies
These studies will be presented more fully in a subsequent publication (in preparation). An early observation of interest is the trend for increased blood glucose levels in treated groups. As shown in Fig 6, this trend appears to be exaggerated with time in the animals. Statistical analysis of this data is currently underway.
Fig 6.
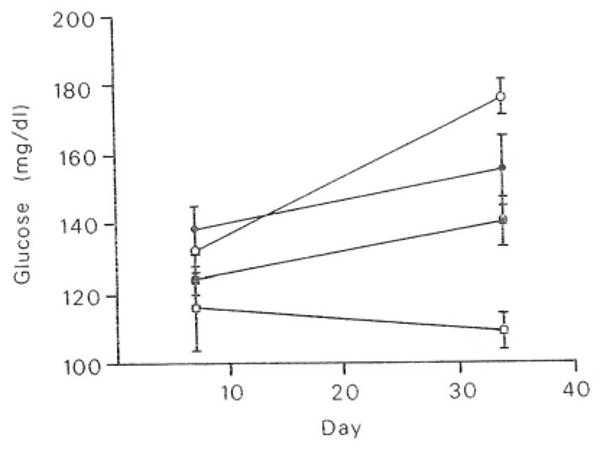
Effect of FK on blood glucose levels. Means and SE of blood glucose values were measured on days 7 and 34. Groups are as in Fig 1. Number of animals per group: day 7, 5; day 34, 5. □, Group 1; ●, group 3; ■, group 4; ○, group 5.
Discussion
FK has been shown to be a potent immunosuppressive agent by Ochiai et al.1 Confirming studies at our institution2,3 have indicated that the drug does indeed possess important in vivo antirejection effects. The current study explores some of the possible toxicological effects of this compound in the Lewis rat.
No spontaneous deaths occurred over the interval of the study. High-dose rats experienced an initial loss of body weight. However, they almost fully recovered from this loss over the next 2 weeks. A dose-dependent reduction of thymus weight indicated that the drug was exerting an influence on the immune system at doses administered. The histological changes in this organ resembled those described for cyclosporine A (CsA),4 which reflects a form of medullary atrophy.
A possible low-grade effect on the pancreas was manifested by histological changes corresponding to an increased pancreatic acinar cell turnover. Pancreatic islets were not specifically evaluated because they are best examined by other means. However, a trend for treated rats to demonstrate higher levels of blood glucose was noted in this study. This finding must be interpreted with caution because specimens were not obtained in a fasting state and may thus be artifactual. Nevertheless, it targets this area as worthy of further study, especially in light of the known effects of CsA on the pancreatic islets.5
No clinicopathologic evidence of vasculitis was seen in the present study. This finding is in contrast to results in canine models in which vascular toxicity represents a real and possibly rate-limiting factor in the use of the drug (Thiru, personal communication; Demetris, unpublished observations).
Some rat histological slides were kindly made available for our review by the Fujisawa Co. In their animals, which received doses up to eight times that administered in our study, additional toxic manifestations were obvious in renal tubules and hepatocytes.
In summary, FK is a new compound with clinically exploitable major activity involving the immune system. It is not without effects in other organ systems, however. The relative importance and species specificity of these effects are currently being explored. The most important of these toxic effects involves vascular damage as seen in the dog. Similar damage has not as yet been documented in the rat. More extensive studies, especially in subhuman primates, will be required to resolve these issues. At present, FK appears to hold considerable promise as an important member in the polypharmaceutical approach to antirejection therapy.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, MD.
References
- 1.Ochiai T, Nakajima K, Nagata M, et al. Transplant Proc. 1987;19:1284. [PubMed] [Google Scholar]
- 2.Lee P, Murase N, Todo S, et al. Surg Res Comm (in press) [PMC free article] [PubMed] [Google Scholar]
- 3.Todo S, Demetris A, Ueda Y, et al. Transplant Proc. this issue. [Google Scholar]
- 4.Beschorner WE, Namnoum JD, Hess A, et al. Am J Pathol. 1987;126:487. [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn JH, Laube F, Lucke S, et al. Transplantation. 1986;41:44. doi: 10.1097/00007890-198601000-00008. [DOI] [PubMed] [Google Scholar]


