The Essential ingredients for the transplantation of any organ include perfection of surgical techniques, adequate organ procurement and preservation, development of methods to prevent rejection, and definition of the role of tissue matching. This framework for practical application was worked out almost exclusively with the simple kidney model, and then applied with certain modifications to the transplantation of the extrarenal organs. Consequently, it is no surprise that almost all of the contributors to the transplantation of extrarenal organs have had a background in the renal field, and frequently have made significant contributions to surgical nephrology.
A marriage of interests is obvious in the development of liver transplantation. The first descriptions of liver replacement in experimental animals were published less than 25 years ago,1,2 from groups with an interest—then and subsequently—in renal transplantation. The first clinical effort at liver transplantation was made on March 1, 1963,3 by a group devoted to the development of renal transplantation. Activities in liver transplantation continued at a very modest level for the next 16 years, largely because the succeses rate was so small. The great wave of human liver transplantations worldwide began in the early 1980s, following the introduction of the new immunosuppressive agent, cyclosporine, by the kidney transplanter, Roy Calne of Cambridge, England. In the following sections, the influence of cyclosporine, as well as other factors which made liver transplantation practical, will be described.
IMMUNOSUPPRESSION
More than 20 years ago, the possibility of obtaining long-term survival after liver transplantation was demonstrated in mongrel dogs treated with azathioprine.4 Although only about 10% of the animals achieved survival for more than four postoperative months, many of these dogs lived for long periods5 and one survived for a full canine lifetime. Similar results were obtained shortly afterward with heterologous antilymphocyte serum (ALS) and its globulin derivative (ALG).6
Although liver replacement in humans was first attempted in 1963, the first clinical trials all failed and extended survival was not accomplished until the summer of 1967.7 The first successfully treated patient eventually died of metastases from the hepatoma for which treatment was provided. The longest survivor in the world today is a young woman who was treated in Jan 1970 for biliary atresia. It is interesting that her excised liver contained an incidental hepatoma which obviously was completely eliminated,7 allowing a cure. Immunosuppression was with azathioprine, prednisone, and ALG.
Clinical Immunosuppressive Regimens Before Cyclosporine
Development with renal transplantation
The first step in pharmacologic immunosuppression was the use of azathioprine as the sole or principal immunosuppressive agent in the Boston trials of 1962.8,9 There were no long-term survivors, and since that time, it has been recognized that cadaver organ transplantation could rarely, if ever, be successful using azathioprine alone.
The so-called modern era of whole organ transplantation began in 1962 and 1963 with the demonstration that azathioprine and steroids had at least additive, if not synergistic, actions.10 The introduction of this so-called double-drug therapy10 was quickly adopted in other centers,11,12 and large numbers of patients began to emerge from renal transplantation clinics with chronically functioning grafts.13 However, satisfactory results were obtained for many years only with living related donors, and the mortality and morbidity from the transplantation of cadaver kidneys kept case accrual at a relatively low level.14
The number of modifications of the original double-drug therapy in the succeeding years was large (Table 1), the most important change being the addition of ALG as a third and short-term immunosuppressive adjunct.6,22 Most centers in which this expedient was tried reported improved results, but the use of ALG was limited by the inability to standardize the agent and by its undesirable side effects.5,22 A new era of ALG therapy has been made possible with the development of monoclonal antibody techniques, pioneered by Kohler and Milstein.23 Using potent and highly standardized antilymphoid monoclonal antibodies, Cosimi et al21 and others24,25 have shown regular reversal of otherwise intractable rejections.
Table 1.
Immunosuppressive Drug Regimens and Adjuncts for Kidney Transplantation and Applied Later for Extrarenal Organs
| Agents | Year Described and Reported |
Place | Deficiencies | Used for Livers |
|---|---|---|---|---|
| Azathioprine | 19628,9 | Boston | Ineffective, dangerous | No |
| Azathioprine-steroids | 19639 | Denver | Suboptimal | Yes |
| Thoracic duct drainage as adjunct | 196310* | Stockholm | Nuisance: requires 20–30 d pre- treatment |
Yes |
| ALG as adjunct | 19666 | Denver | Suboptimal | Yes |
| Cyclophosphamide substitute for azathioprine |
197016 | Denver | No advantage except for patients with azathioprine toxicity |
Yes |
| Total lymphoid irradiation | 197917,18 | Palo Alto, Minn | Dangerous; extensive preparation; not quickly reversible |
Yes |
| Cyclosporine alone | 1978– 197919 |
Cambridge | Suboptimal | Yes |
| Cyclosporine-steroids | 198020 | Denver | Under evaluation | Yes |
| Monoclonal ALG as adjunct | 198121 | Boston | Under evaluation | Yes |
It was not realized until much later that pretreatment for three to four weeks before transplantation was a necessary condition.15
Other variations in immunosuppression between 1962 and 1979 are summarized in Table 1, including the substitution of cyclophosphamide for azathioprine,16 and the use of thoracic duct drainage15,26 or total lymphoid irradiation17,18 as an alternative to ALG for lymphoid depletion. None of these techniques has had a major impact on clinical transplantation.
Application to liver transplantation
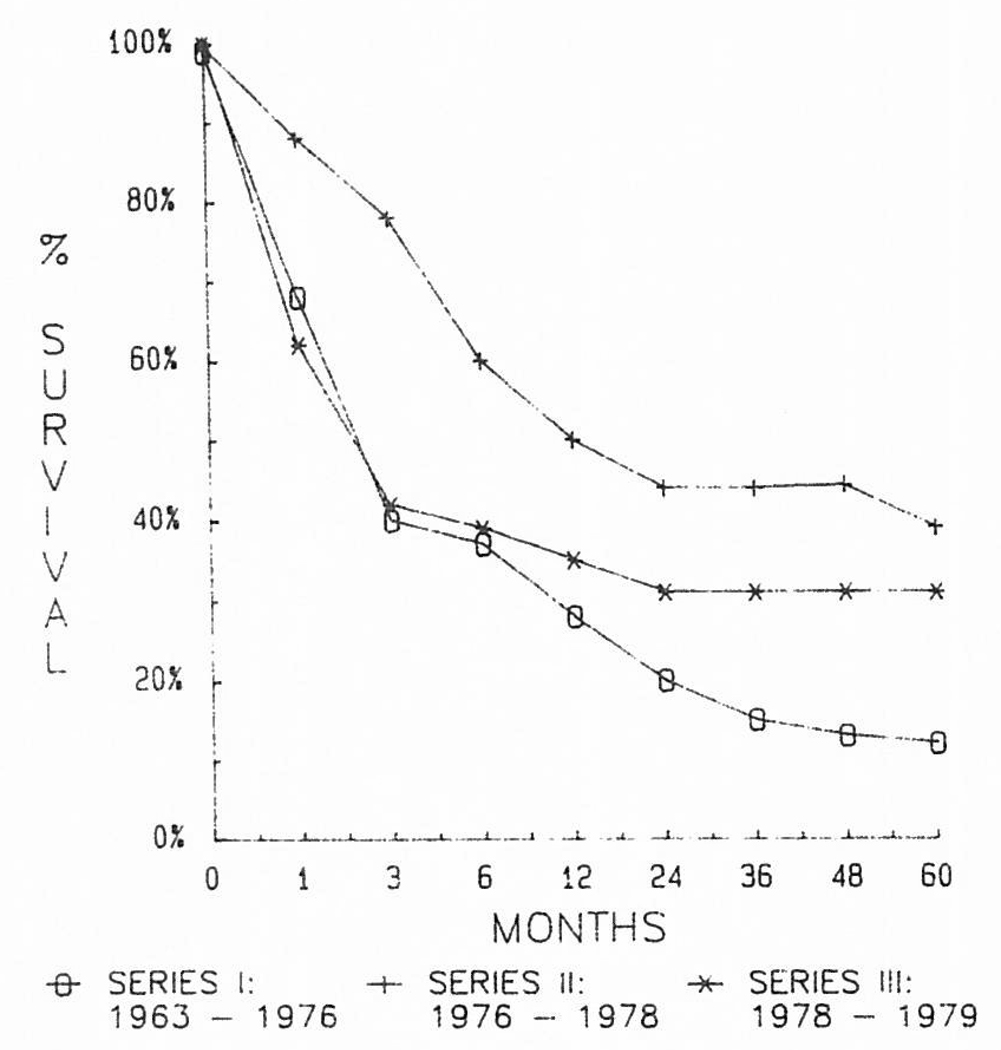
From 1963 through 1969, most of our liver recipients had triple-drug immunosuppression with azathioprine, prednisone, and ALG. In some, cyclophosphamide was substituted for azathioprine, and in a few others, lymphoid depletion was achieved with thoracic duct drainage instead of ALG. Details of these variations are summarized elsewhere.27 The failure of any of the variations to influence survival after liver transplantation is evident in Fig 1.
Fig 1.
Results obtained over a 16-year period using the conventional immunosuppression shown in Table 1. Note the failure to improve the results despite the acquisition of considerable experience.
The Impact of Cyclosporine
The experimental studies of Borel et al28 and the first clinical trials by Calne et al19 of cyclosporine A opened a new era in transplantation.
Development with renal transplantation
The most important encouraging observation by Calne et al19 was that almost half of their whole organ recipients had achieved chronic graft function with no other agent than cyclosporine. However, the development of lymphomas in nearly 10% of their recipients, the fact that none of the kidney recipients achieved normal graft function, and a high patient mortality rate militated against the wide use of cyclosporine until these adverse findings had been explained or minimized in subsequent trials.
The lymphoma question was resolved with increased information about the etiology and appropriate treatment of these lesions. The lymphomas were proved to be complications of primary or secondary infection with Epstein-Barr virus20,29 and it was learned that the lesions would undergo spontaneous resolution with discontinuance or even reduction of the immunosuppressive doses.30 This involution occurred whether the lesions were polyclonal or monoclonal, overthrowing a previous hypothesis of the effect of monoclonality that almost had become dogma.29
The development of de novo malignancies in immunosuppressed patients has not been unique to cyclosporine. Since the late 1960s.31,32 an association of epithelial cancers with conventional immunosuppression has been well known, the epithelial lesions out-numbering the lymphomas by a ratio of about 4:1.33 Fortunately, there has been little or no increase in the incidence of epithelial tumors using cyclosporine–steroid therapy. Thus, the risk of the development of malignancies is probably less overall with cyclosporine–steroid therapy than with azathioprine and prednisone (with or without ALG), even if one considers the lymphomas to be true tumors, a concession that has been challenged.34
Understanding of the effects of cyclosporine on renal function has been central to the effective use of cyclosporine. The fact that the agent is nephrotoxic was first reported by Calne et al19 and confirmed in many subsequent reports including our own.20,35,36 With the nephrotoxic problem, the full exploitation of the drug was not possible without combining it with other agents, of which prednisone was most important.20,36 By so doing, it was possible to minimize the contribution of homograft rejection to poor renal function while at the same time ameliorating the nephrotoxicity by reducing the requisite doses of cyclosporine. Other drugs have been proposed and/or tried in modifications of the pharmacologic cocktail concept,37,38 always using the cyclosporine–steroid combination as the baseline. Normal renal transplant function has become the rule. We have recently used the OKT3 antibody originally tested by Cosimi et al in patients who developed intractible rejection in spite of cyclosporine–steroid therapy, and with a high incidence of reversal.
The high mortality rate with the first use of cyclosporine apparently was a reflection of a learning experience in which drug overdosage was common. Even in our first trials with the far safer cyclosporine–steroid combination, the one-year mortality rate after cadaveric renal transplantation was 13.6%,39 but in the following year, the one-year mortality rate was reduced to 2%.40 Since then, most groups using cyclosporine–steroid therapy have had a mortality rate of <5%.
Application to liver transplantation
The systematic use of cyclosporine–steroid therapy in liver transplantation was begun in early 1980.41 Almost immediately, a doubling of one-year patient survival was noted. Each subsequent year, the case load in our center has increased until the calendar year of 1984, when 166 liver replacements were performed at the University of Pittsburgh. Augmented activity in other centers throughout the world has been documented.42
It was surprising in the early trials of cyclosporine–steroid therapy that such good kidney or liver graft survival could be achieved without knowing what the cyclosporine blood levels actually were. The clinical judgment in managing such patients reflected a deliberate effort to balance the possibilities of rejection against those of nephrotoxicity36 and to treat both.
Nowadays, the assessment of whole blood or plasma cyclosporine concentration is possible with radioimmunoassay (RIA) or high-performance liquid chromatography (HPLC). Heavy reliance is now placed on the results of these tests for management decisions. This has been a particularly important development in liver recipients, since the intestinal absorption of cyclosporine postoperatively has been unpredictable and to some extent dependent on the quality of graft function. To smooth out the recovery period and to assure continuity of therapeutic levels of the drug, cyclosporine has been administered both intravenously and orally for several days, weeks, or even months postoperatively.42 As absorption improves with the oral drug, the intravenous doses are weaned and eventually discontinued.
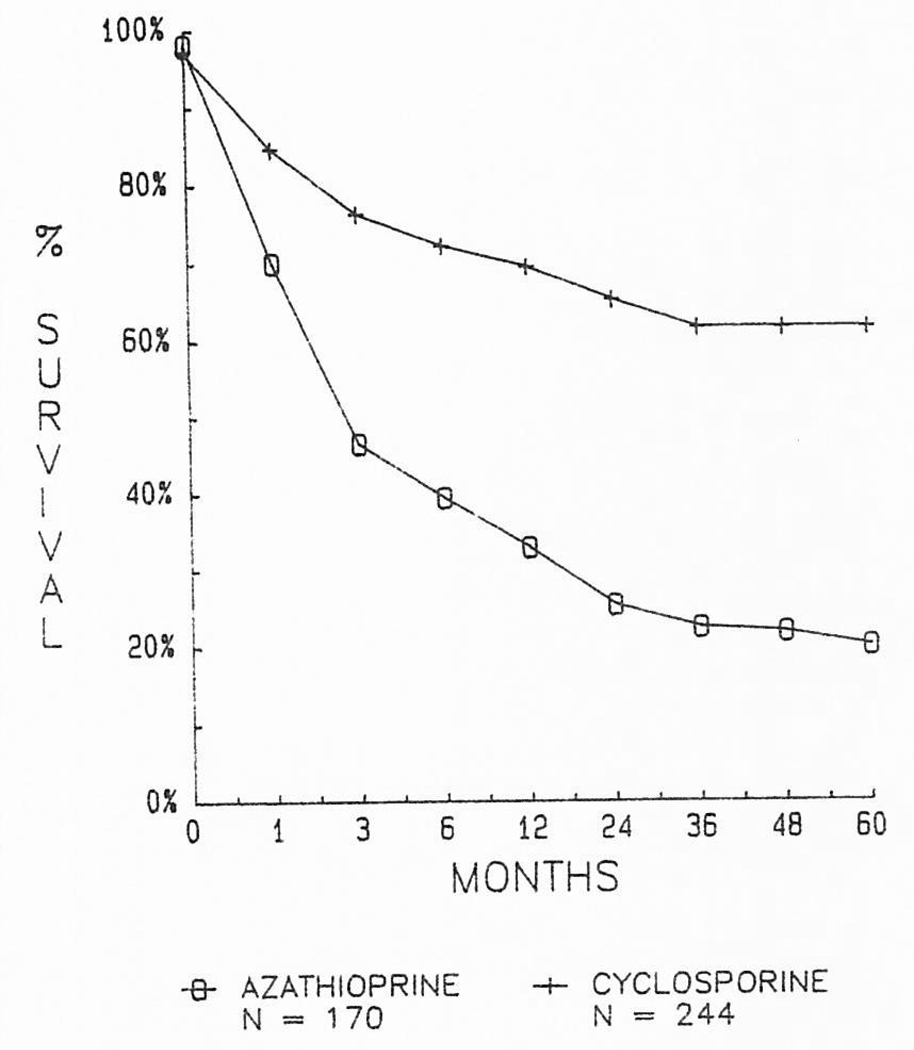
From our first efforts in 1963 through 1979, 170 patients were treated with conventional immunosuppression. The chances for living one year after liver replacement were only about one in three (Fig 2). Subsequently, 244 liver recipients were provided with cyclosporine–steroid therapy between March 1980 and July 1, 1984, allowing follow-up periods of one to more than five years. The chances of one-year survival were more than doubled. Actuarial projections beyond one year indicate that these gains will be sustained for at least one-half decade (Fig 2).
Fig 2.
Marked improvement in results of liver transplantation after the introduction to cyclosporine–steroid therapy in early 1980.
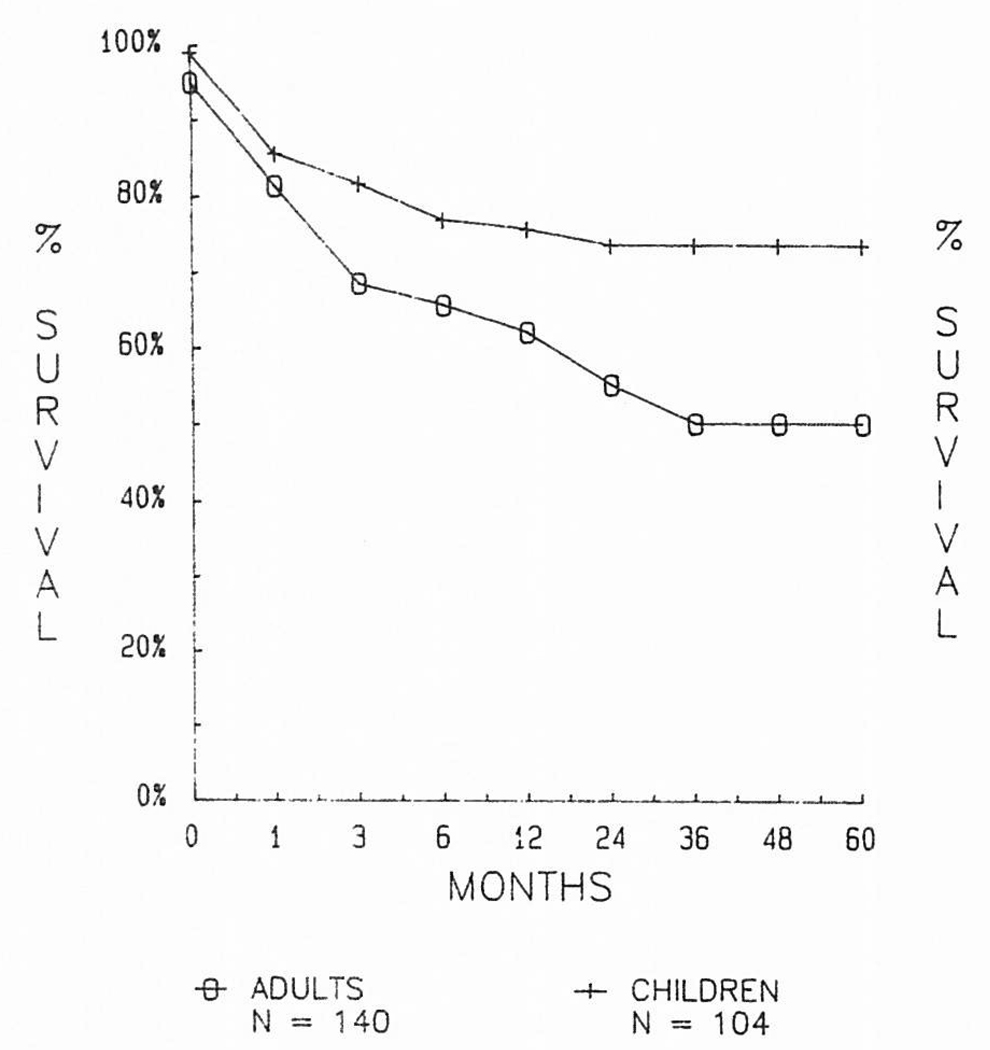
Certain risk factors have been carefully looked at for their effect on survival curves. Among the more important has been age. Pediatric recipients throughout the entire history of liver transplantation have fared better by ten to 25 percentage points than adults, and in the cyclosporine era, the age factor has been particularly important (Fig 3).
Fig 3.
Comparison of results in adult and pediatric recipients during the cyclosporine era of 1980 through 1984.
Somewhat surprisingly, specific diseases that have destroyed the native liver have not, for the most part, influenced survival. In adults, for example, the outcome has been about the same with such diverse diseases as primary biliary cirrhosis, sclerosing cholangitis, and inborn errors of metabolism. Two high-risk diseases have been identified so far. The results with postnecrotic cirrhosis and with primary hepatic tumors have been inferior. With cirrhosis, the principal explanations have been the technical difficulties of the operation caused by the pathologic process, the generally poor condition of the cirrhotic patient, and the almost universal recapitulation of their original chronic active hepatitis in B-virus carriers.
In patients whose reason for liver replacement was primary hepatic malignancy that could not be removed by conventional subtotal hepatic resection, the early mortality rate has been quite low with >80% of the recipients alive at six months. A steady decline thereafter has been caused by recurrent tumor, which can be expected in 80% or more of patients who live long enough for metastases to be detected. The only acceptable results thus far have been in patients with the slow-growing and nonaggressive fibrolamellar hepatomas, which recently have been recognized to be a favorable variant within the larger hepatoma category.43,44
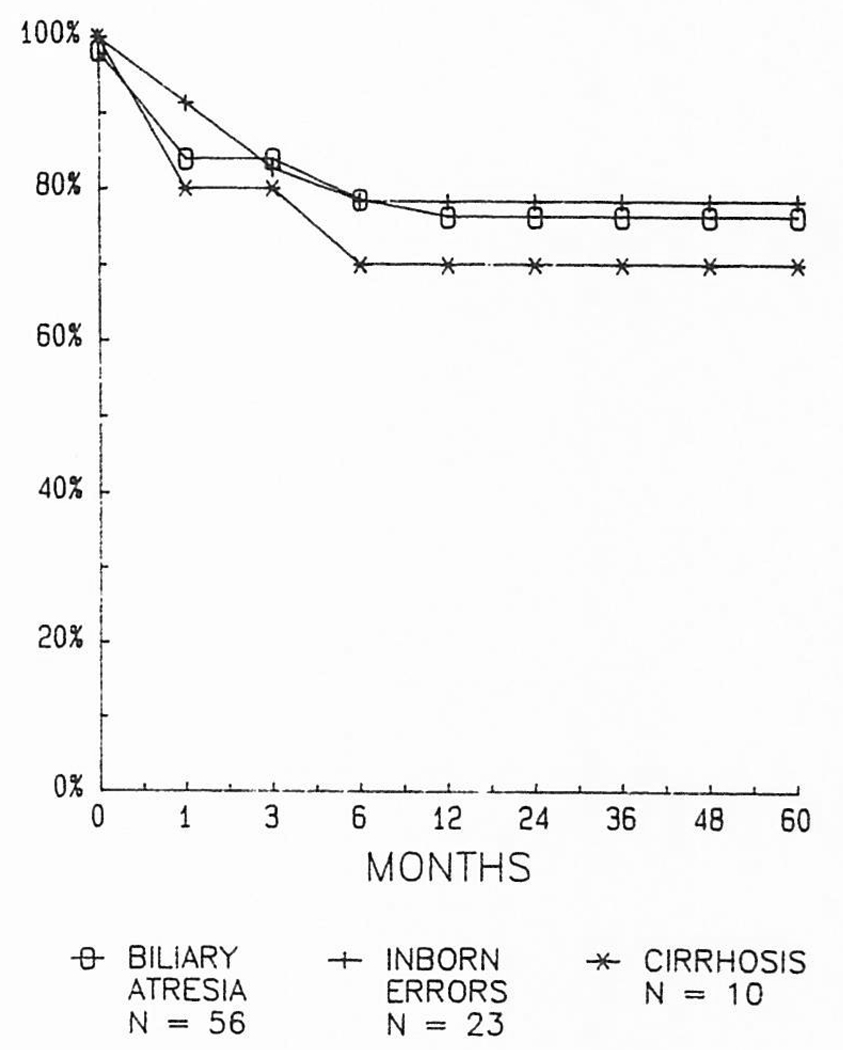
In children, the results have been about the same in all of the main disease categories. It has been interesting that the survival in children with biliary atresia has been competitive with that obtained with other diseases. Transplantation is technically much more difficult in children previously submitted to Kasai operations and re-explorations, but there has not been a demonstrable penalty in terms of either early or late survival (Fig 4).
Fig 4.
The lack of influence of underlying disease on the survival of children undergoing liver transplantation.
More complete accounts of underlying disease and other risk factors are being published everywhere.45,46 The improved survival that has been achieved in recent years has been made possible, in part, by an aggressive use of retransplantation to rescue patients whose first grafts have failed because of rejection or for any other reason. Retransplantation was not a successful enterprise under conventional immunosuppression,27 but in the modern times defined by the availability of cyclosporine, the picture has drastically changed.47
THE ROLE OF TECHNICAL IMPROVEMENTS WITH SPECIAL REFERENCE TO VENOVENOUS BYPASSES
The technical principles of liver transplantation have been well established for almost two decades,3,5 but until the 1980s, the most significant development was standardization of biliary tract reconstruction.27,48 More recently, pump-driven venovenous bypasses have been introduced clinically, and in the following account, the influence of the bypasses on the actual operation will be described.
When the technique of liver transplantation was developed in dogs,1,2 operative survival required venovenous bypasses that transmitted blood from the inferior vena cava and the portal vein to the upper part of the body while the venous systems were obstructed during the anhepatic phase of the procedure. Without bypasses, the capillary beds were ruined in dogs by acute venous hypertension even with occlusion times as short as 30 minutes. The original bypasses were used without heparinization or pumps.
From the first experience with liver transplantation in humans, it was concluded that venovenous bypasses were not obligatory for survival. Because the bypass tubing was the source of intraoperative pulmonary emboli in at least three patients,5 passive venovenous bypasses were virtually abandoned. Pump-driven venovenous27,49 or venoarterial50 by-passes under systemic heparinization were impractical because reversal of the heparin effect was often difficult or impossible in the presence of multiple other coagulation defects caused by liver disease.
Without provision to decompress the obstructed splanchnic and systemic venous beds, every liver replacement in patients was carried out in a crisis atmosphere comparable to that with open cardiac surgery under inflow occlusion. When time was such a precious commodity, efforts were made to mobilize the diseased liver as completely as possible before venous occlusion. Even so, hemostasis in the bare areas that were opened up during the final stages of hepatectomy usually had to be put off. Augmented hemorrhage from these bare areas was one of the most troublesome problems caused by venous occlusion. High-pressure bleeding from the thin-walled collaterals in the raw surfaces of the operative wound often could not be controlled by any mechanical means until the obstructed venous systems could be decompressed by revascularizing the new liver.
In 1982 and 1983, a pump-driven venovenous bypass system without recipient heparinization was developed, tested in dogs,51 and eventually brought to the clinical operating rooms.52,53 Then it became possible to modify and improve several aspects of the recipient operation, including the technique of hepatectomy.
Preliminary Steps
The principles of recipient hepatectomy have been thoroughly described.5,54 However, the extent of preliminary dissection can be greatly decreased if venovenous bypass is to be used. The individual structures of the hepatic hilum usually are skeletonized, but no other areas need to be invaded. Specifically, efforts to mobilize the liver from the hepatic fossa usually are deferred.
In patients whose hepatic hilum has been dissected before, it may be easiest to approach the hilar structures from the left side. Even when scarring is extreme, the lesser omental sac can be entered if the left triangular ligament is incised and the lateral segment of the liver is retracted into the wound. By following the lesser sac toward the hilum, the proper plane can be assured.
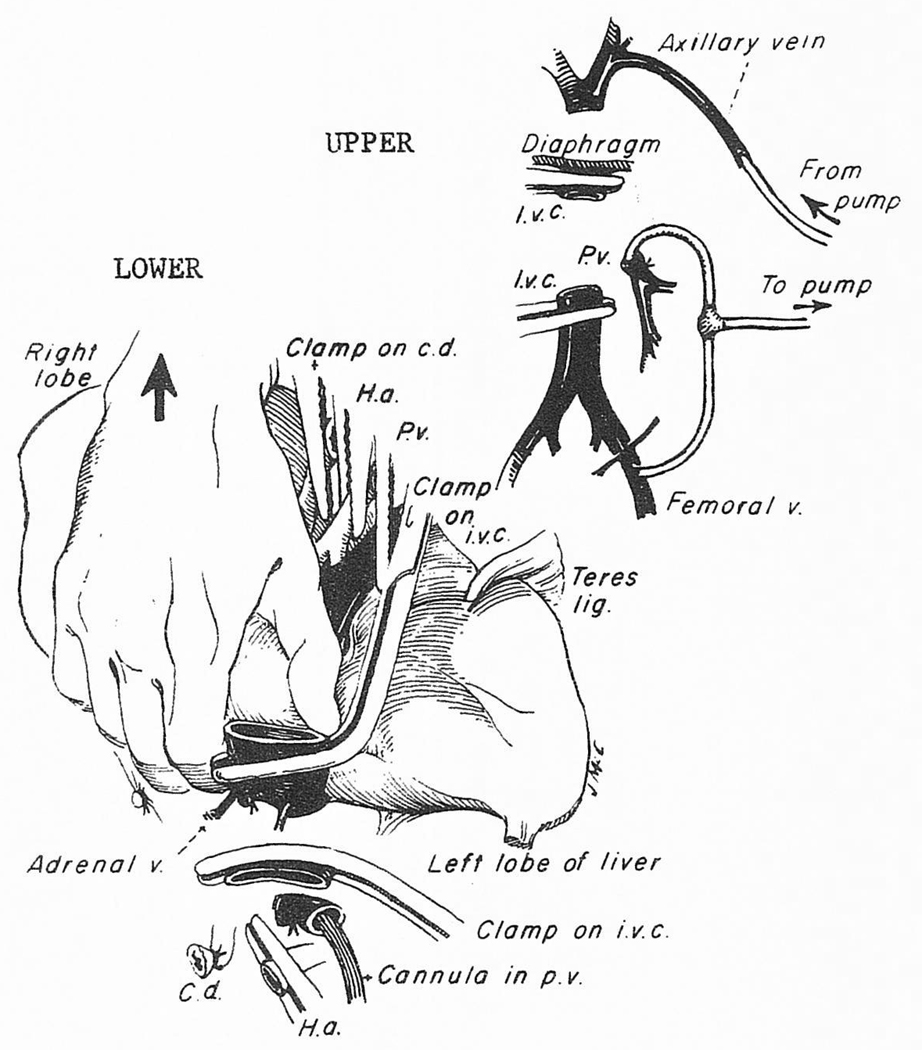
When the bypass is ready for implementation, the hepatic artery and the common duct are ligated and divided (Fig 5, upper portion). The portal vein cannula for the venovenous bypass is inserted as well as the femoral cannula, allowing both the splanchnic and systemic systems to be brought into the pump-driven venovenous circuit that is usually directed into the axillary vein (Fig 5, upper portion). In adults, 1 to 6 L of blood per minute are bypassed. With bypass, simultaneous obstruction of the portal vein and inferior vena cava causes little change in blood pressure, cardiac output or other measures of cardiopulmonary function.53 The operative field is tranquil.
Fig 5.
(Upper) Cannulas inserted for decompression of the inferior vena cava (IVC) and portal venous (PV) systems. The blood is usually pumped back into the axillary vein. (Lower) Technique of removing the liver by peeling it out from below upward. Abbreviations: HA, hepatic artery; CD, common duct.
The Hepatectomy
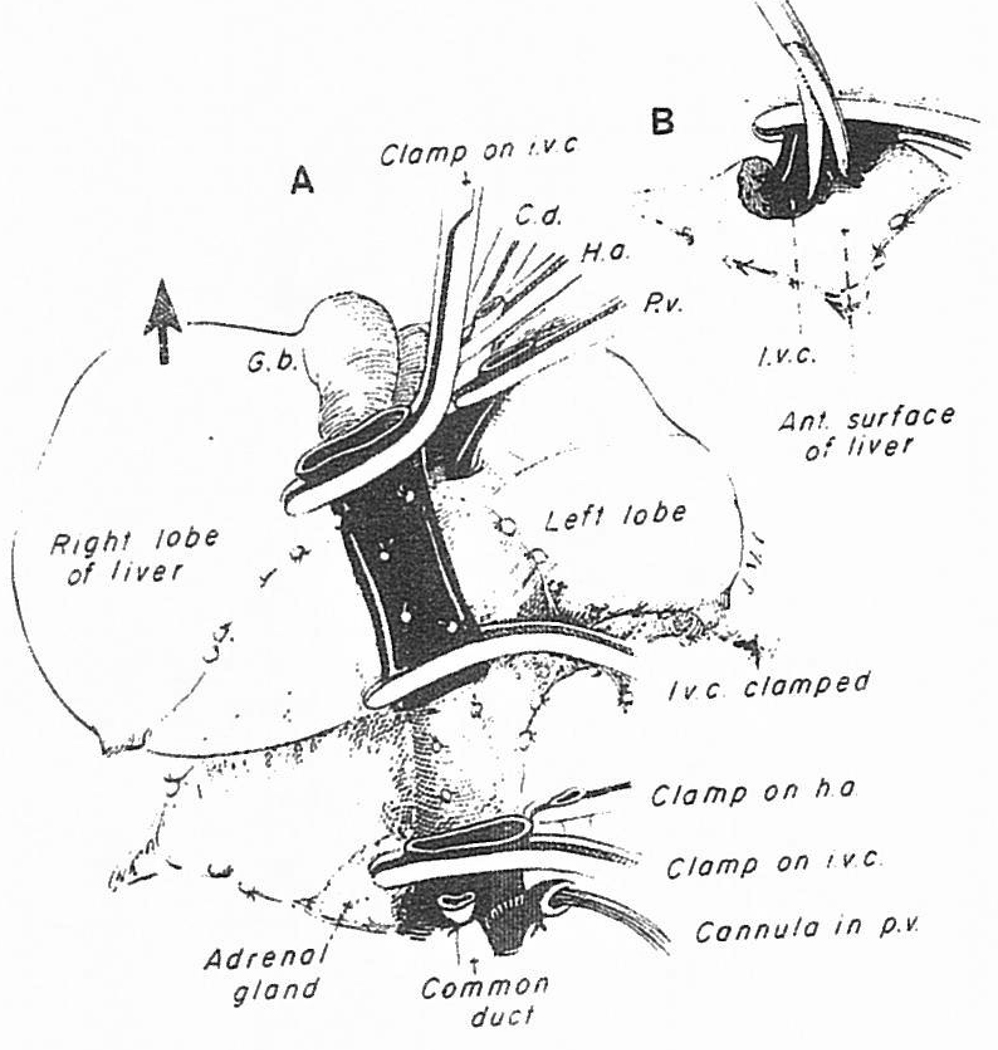
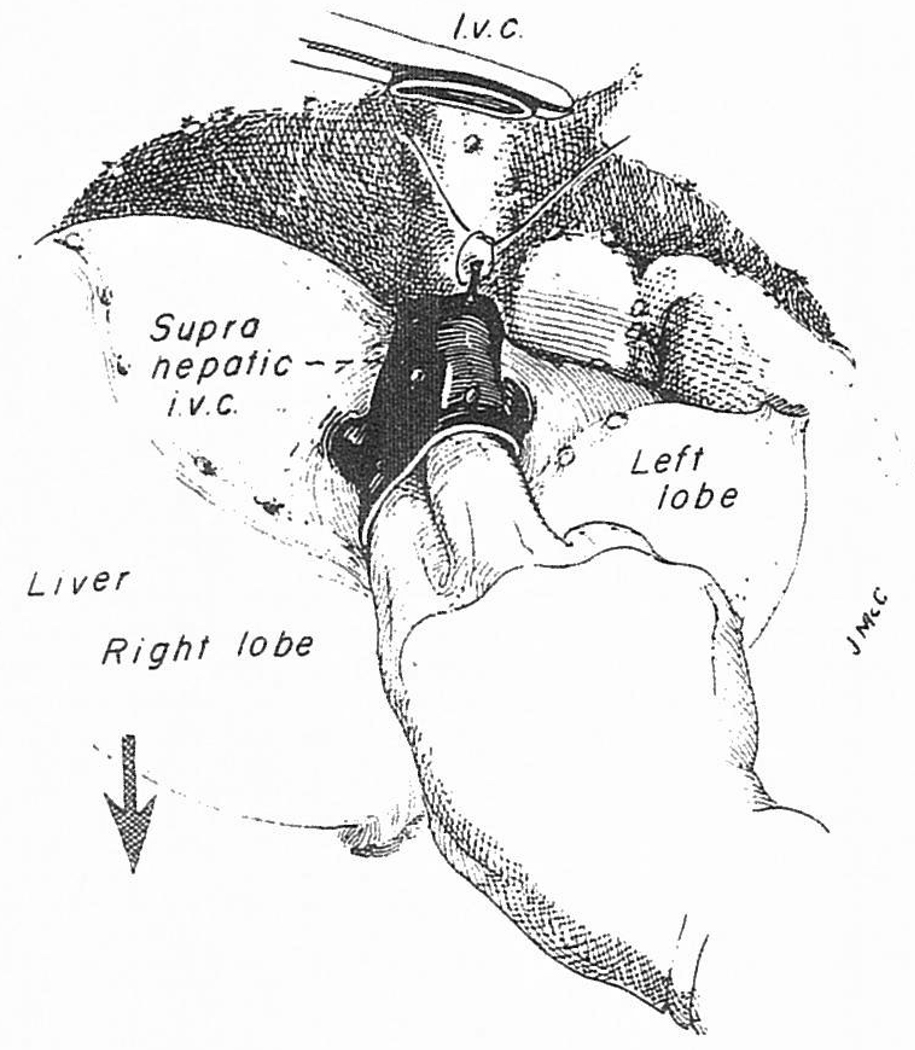
On venovenous bypass, the right hepatic lobe can be retracted into the wound (Fig 5, lower portion, and Fig 6). If it has been difficult to encircle the inferior vena cava, this can now be done either just below or above the liver, and eventually at both locations.
Fig 6.
(A) Continuation of the maneuver by which the liver is peeled out of the hepatic fossa from below upward. (B) The suprehepatic vena cava has been crossclamped and the liver is dissected away from the vena cava in order to increase the length of the cuff.
Once the venovenous bypass is started, all of the structures that are still holding the liver, including the infrahepatic vena cava, can be divided (Fig 5, lower portion). The triangular ligaments are cut if these have not been incised already, as well as the leaves of peritoneal reflection which make up the coronary ligament (Fig 6). The bare areas are entered on both the right and left sides (Fig 6). As described before,54 the liver can then be shelled out on the stalk formed by the supra-hepatic vena cava connection (Fig 6A). The vena caval cuff for eventual anastomosis can be developed (Fig 6B).
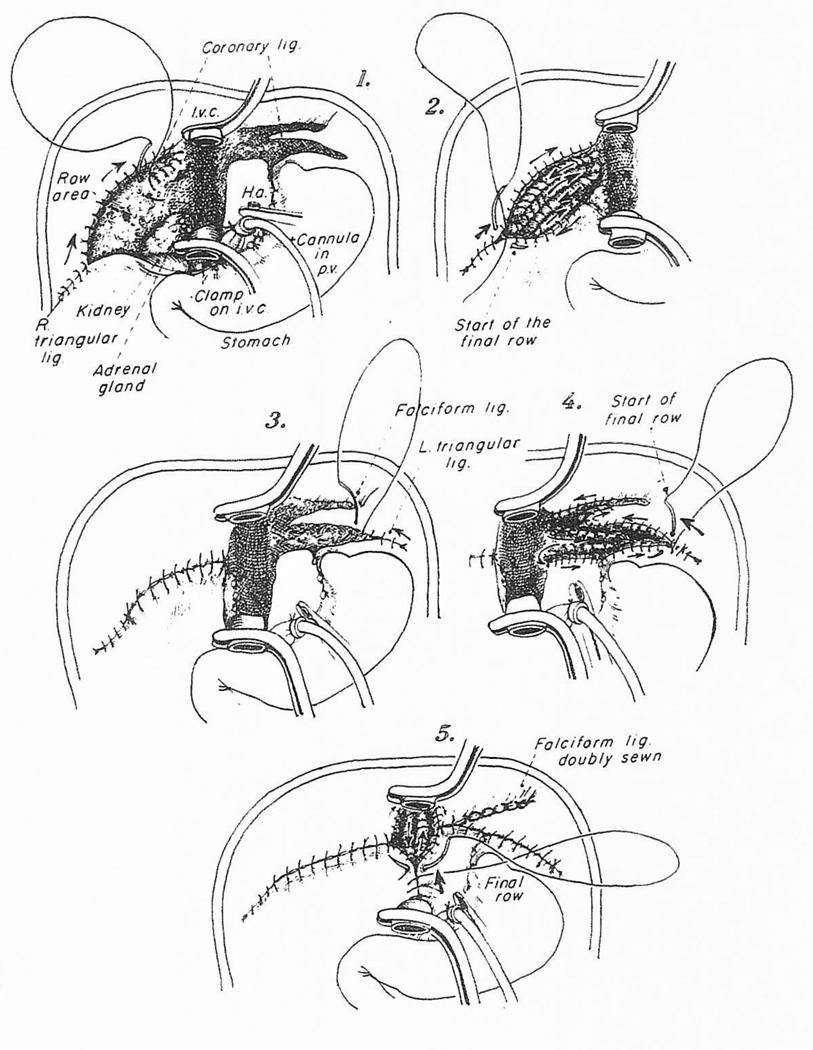
Once the liver has been taken out, it is possible while using venovenous bypass to close all of the raw surfaces that were created during the hepatectomy. This is usually done with a continuous monofilament polypropolene (Prolene) suture, beginning at the tip of the right triangular ligament and continuing this centrally in rows that eventually are connected (Fig 7-1). The superior leaf of the coronary ligament can be the starting point, with continuation into the bare area itself (Fig 7-2) and finally to the inferior portion of the coronary ligament. When these continuous suture lines are eventually incorporated into a single suture line, all of the right bare area is eliminated (Fig 7-3). The same principle is followed in dealing with the left triangular and falciform ligaments (Fig 7-4)
Fig 7.
Elimination of the raw areas in the hepatic fossa with continuous Prolene suturing.
Of vital importance is the bare area behind the excised recipient inferior vena cava, where the right adrenal gland is left behind (Fig 7-1). This region is sewed in a superior-inferior orientation and as the continuous suture is placed, an effort is made to place at least a double layer by sewing first down and then up (Fig 7-5). By the time this final suture line has been completed, virtually all of the bare areas have been eliminated. The time necessary for these hemostatic maneuvers is 30 to 90 minutes. The investment is rewarded later when, if major hemorrhage occurs from the hepatic fossa after the new liver is revascularized, it can be assumed with some degree of assurance that the bleeding is from the graft itself or from one of the anastomoses rather than from raw recipient tissues.
Alternative Approaches
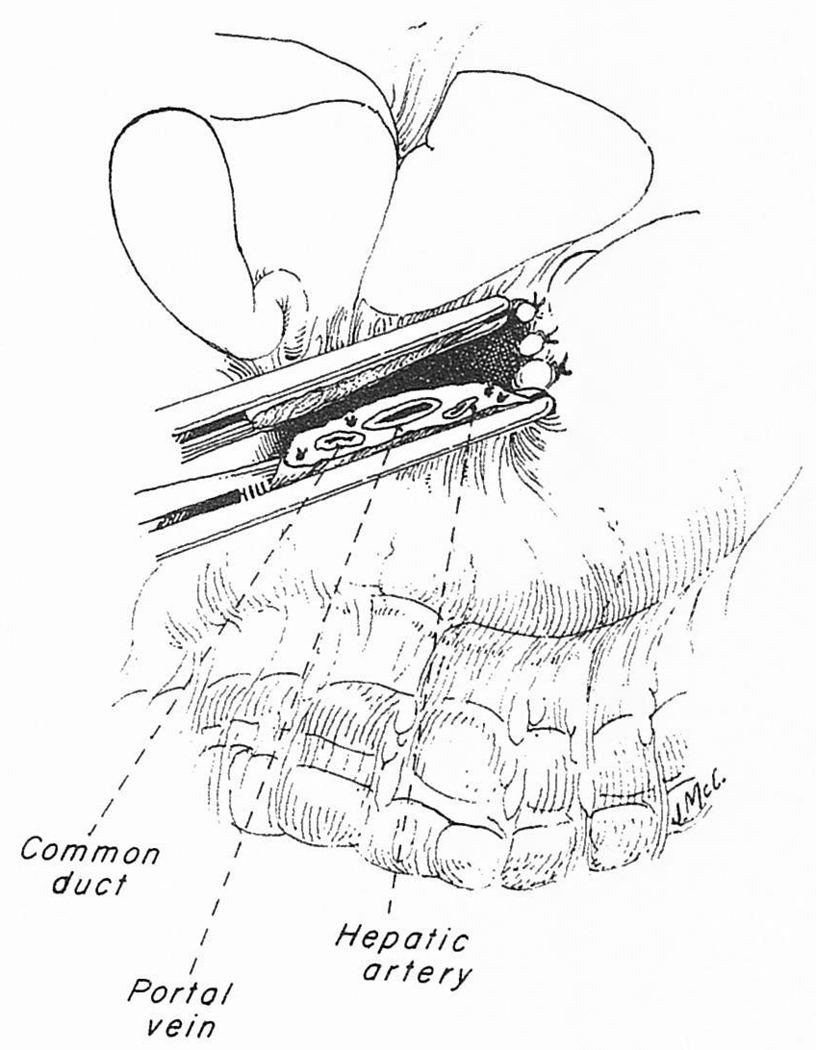
There is no single best way to remove a disease native liver. At the beginning of the operation, and after exposure has been obtained, it is important to assess the situation, to decide upon whatever technical approach the basic pathology will permit, and to determine if the operation just described needs to be modified. It may be impossible in some patients because of scarring or massive formation of variceal collaterals to individually dissect the structures of the portal triad. If so, a vascular clamp can be placed across all of the portal triad structures, which are transected simultaneously (Fig 8). The portal vein can be dissected free for cannulation, and the other triad structures are dissected back. If the portal vein contains new or old thrombus, the placement of a portal cannula could be dangerous, and only the vena cava should be bypassed.
Fig 8.
Mass clamping of the portal structures. The maneuver is indicated if there is great difficulty in individually dissecting the structures of the triad. These structures can then be dissected back from the cut ends.
In a few patients, encirclement of the vena cava below the liver at an early stage of the operation may be difficult or impossible, whereas encirclement of the suprahepatic vena cava can be easy. We have then cross-clamped the suprahepatic vena cava, boldly cut across it, and stripped the liver out from above downward (Fig 9). Fatal hemorrhage is prevented with occluding fingers thrust down the vena cava (Fig 9). The vena cava below the liver is clamped and transected as the final step.
Fig 9.
Removal of the liver from above downward, with prevention of hemorrhage by fingers thrust down the lumen of the vena cava. The maneuver may be indicated if it is difficult or impossible to safely encircle the inferior vena cava below the liver.
Vascular Anastomotic Cuffs
With the relative leisure provided by the venous bypass, good venous cuffs can be fashioned after the liver is out. If the cuffs are adequate, the anastomoses of the vena cava above and below the liver are easy to construct in the quiet operative field that exists under venovenous bypass. During performance of the lower vena caval anastomosis, the liver is flushed with cold lactated Ringer’s or saline solution to remove entrapped air from the major veins and to rid the graft of the high potassium solution used for preservation.55 The portal bypass cannula is now clamped, leaving the patient on vena caval venous bypass during construction of the portal anastomosis; then the first three anastomoses are released. The liver is now revascularized with portal blood. The bypass is discontinued and all bypass cannulas are removed. After reasonable hemostasis is obtained, the liver is arterialized by previously described techniques5,56,57 and the biliary tract is reconstructed with choledochocholedochostomy or Roux-en-Y choledochojejunostomy.27 There may be an occasional reason to perform all four vascular anastomoses before restoring blood flow to the liver, but the potential advantage of complete revascularization at one time is usually outweighed by the even greater desirability of early restoration of portal flow.
Bypasses for Children
Venovenous bypasses have been routinely used only for recipients of adult size. Infants and small children tolerate occlusion of the portal vein and inferior vena cava reasonably well. Nevertheless, low-flow bypasses are being used with increasing frequency in pediatric recipients,58 as it has been realized that bypasses with low flows can be conducted with safety.
Simplication of Bypass Techniques
The low-flow atraumatic centrifugal pump has proved to be the most important ingredient of the bypass system, as emphasized in the original canine studies of Denmark et al.51 The later addition, for safety purposes, of more expensive heparin-bonded (Gott) cannulas and tubing52,53 may not be necessary, even for the low-flow bypasses used in pediatric recipients.58 Research on the issue of the heparin-bonded extracorporeal equipment is going on in our laboratories and elsewhere.
THE ROLE OF TISSUE MATCHING
In patients treated with cyclosporine and steroids after renal transplantation, the antigen matching at the A, B, or D loci has had little influence on the results. Such matching has not even been attempted for liver recipients. Tissue matching will play a significant role in further developments in liver transplantation.
The remarkable resistance of the liver to hyperacute rejection has been reported before.59,60 There has been no obvious penalty with transplantation of livers to recipients whose sera contain the cytotoxic anti-graft antibodies that almost invariably lead to immediate loss of kidney grafts. Furthermore, many liver transplantations have been and are being carried out across the ABO blood group barriers that frequently (although not invariably) cause hyperacute rejection of kidneys as the consequence of anti-graft isoagglutinins.13 Recognition that matching is nonrelevant in liver transplants has simplified some of the logistic problems of liver transplantation.
ORGAN REMOVAL AND PRESERVATION
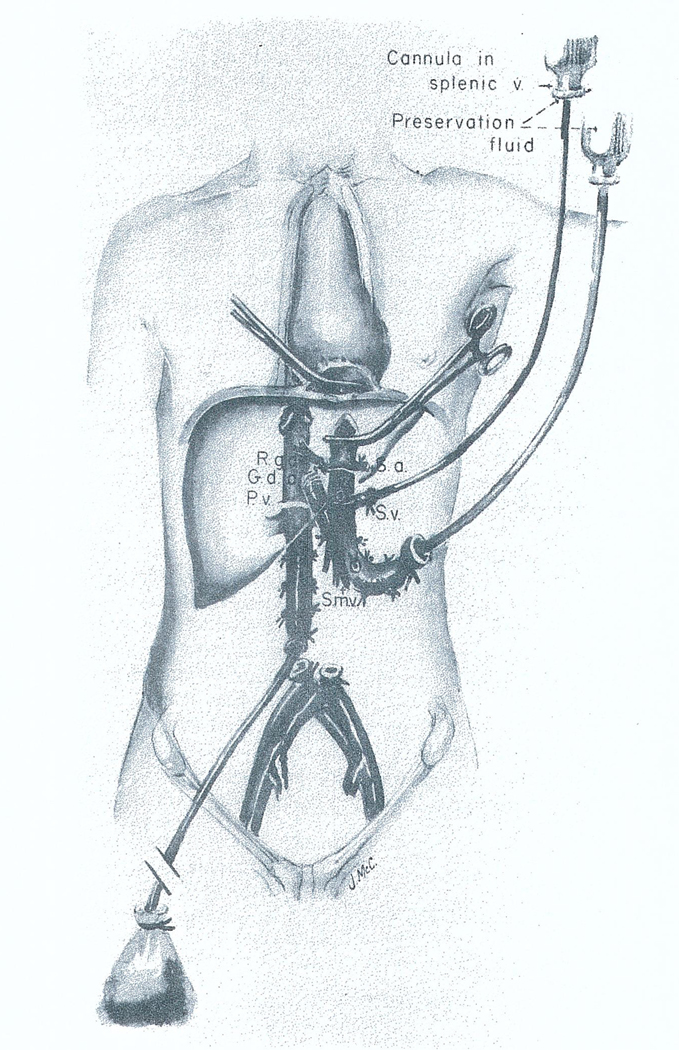
Great advances have been made in multiple organ removal, and a relatively standard procedure is being used throughout most of the United States.61 The operation is done through a complete midline incision from the suprasternal notch to the pubis, including splitting of the sternum. The principle followed is to dissect the aorta for crossclamping at a level that will allow intraaortic infusion of cold fluids, which will pass into the organs to be removed. If the liver is to be one of these organs, dissection of the liver hilum is carried out, after which the liver can be infused through both the aorta and portal vein (Fig 10). The kidneys are also cooled by the aortic perfusion. With minor modifications, the heart can also be excised.
Fig 10.
In situ infusion technique used when the kidneys and liver are removed from the same donor. Abbreviations: R.g.a., right gastric artery; G.d.a., gastroduodenal artery; S.a., splenic artery; S.v., splenic vein; P.v., portal vein; and S.m.v., superior mesenteric vein. Reproduced by permission.61
This procurement technique requires “brain death” conditions and stable cardiovascular function. An alternative with which we have had recent experience can be done swiftly, and in donors who have had cardiac arrest.42 With this so-called fast method, a crossclamp is placed on the aorta near the diaphragm and cold solutions (usually the high-potassium high-magnesium concentration Collin’s solution) are infused rapidly. Blood enters the liver through the normal celiac axis route but also through the portal vein after passing through the splanchnic capillary bed. The portal venous blood quickly becomes almost red cell free.
The cold ischemia limit permissible for a human liver graft has been set arbitrarily at ten hours, but great efforts are made to work within a five- or six-hour time frame. One of the most urgent needs in liver transplantation is the development of better methods of preservation. Any technique that would allow safe preservation of livers for the better part of a day would revolutionize the field overnight.
SUMMARY
Liver transplantation has become an accepted service during the last five years. The introduction of cyclosporine–steroid therapy has been the most important factor in making this possible. Improvements in surgical technique, including perfection of intra-operative venovenous bypasses and the standardization of biliary tract reconstruction have also contributed. Tissue typing and matching have played no role in improving the results of liver transplantation. With the demonstration that preformed antibody states are irrelevant, even avoidance of positive cross-matches caused by cytotoxic antibodies and observance of ABO blood group barriers have become unnecessary if the recipient’s needs are great. The nature of the underlying hepatic disease has not profoundly influenced the results, with the exceptions of malignancy and cirrhosis. Retransplantation has played an important role in improving survival. The development of better methods of preservation that will allow the recipient operations to be done in a more leisurely manner and at more convenient times is the most pressing need for further application of liver transplantation at a national and international level.
Acknowledgments
Supported by research grants from the Veterans Administration and by Project Grant No. AM-29961 from the National Institutes of Health.
REFERENCES
- 1.Starzl TE, Kaupp HA, Brock DR, et al. Surg Gynecol Obstet. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FD, Wheeler HB, Demissianos HV, et al. Ann Surg. 1960;152:374. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Marchioro TL, von Kaulla K, et al. Surg Gynecol Obstet. 1963;117:659. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Marchioro TL, Porter KA, et al. Surgery. 1965;58:131. [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE. (with the assistance of CW Putnam): Experience in Hepatic Transplantation. Philadelphia: Saunders; 1969. [Google Scholar]
- 6.Starzl TE, Marchioro TL, Porter KA, et al. Surg Gynecol Obstet. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Groth CG, Brettschneider L, et al. Ann Surg. 1968;168:392. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray JE, Merrill JP, Dammin GJ, et al. Ann Surg. 1962;156:337. doi: 10.1097/00000658-196209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray JE, Merrill JP, Harrison JH, et al. N Engl J Med. 1963;268:1315. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 10.Starzl TE, Marchioro TL, Waddell WR. Surg Gynecol Obstet. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 11.Hume DM, Magee JH, Kauffman HM, Jr, et al. Ann Surg. 1963;158:608. doi: 10.1097/00000658-196310000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodruff MFA, Robson JS, Nolan B, et al. Lancet. 1963;2:675. doi: 10.1016/s0140-6736(63)90465-3. [DOI] [PubMed] [Google Scholar]
- 13.Starzl TE. Experience in Renal Transplantation. Philadelphia: Saunders; 1964. [Google Scholar]
- 14.Opelz G, Mickey MR, Terasaki PI. Transplantation. 1977;23:490. doi: 10.1097/00007890-197706000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Starzl TE, Weil R, III, Koep LJ, et al. Surg Gynecol Obstet. 1979;149:815. [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Putnam CW, Halgrimson CG, et al. Surg Gynecol Obstet. 1971;133:981. [PMC free article] [PubMed] [Google Scholar]
- 17.Strober S, Slavin S, Fuks Z, et al. Transplant Proc. 1979;11:1032. [PubMed] [Google Scholar]
- 18.Najarian JS, Ferguson RM, Sutherland DER, et al. Ann Surg. 1982;196:442–452. doi: 10.1097/00000658-198210000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calne RY, Rolles K, White DJG, et al. Lancet. 1979;2:1033. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 20.Starzl TE, Weil R, III, Iwatsuki S, et al. Surg Gynecol Obstet. 1980;151:17. [PMC free article] [PubMed] [Google Scholar]
- 21.Cosimi AB, Burton RC, Colvin RB, et al. Transplantation. 1981;36:535. doi: 10.1097/00007890-198112000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Starzl TE, Porter KA, Iwasaki Y, et al. In: Antilymphocytic Serum. Wolstenholme, O’Connor, editors. London: Churchill; 1967. pp. 4–34. [Google Scholar]
- 23.Kohler G, Milstein C. Nature. 1975;256:495. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 24.Norman DJ, Barry JM, Henell K, et al. Transplant Proc. 1985;17:39. [Google Scholar]
- 25.Goldstein G, Schindler J, Sheahan M, et al. Transplant Proc. 1985;17:129. [Google Scholar]
- 26.Franksson C. Lancet. 1964;1:1331. [Google Scholar]
- 27.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borel JF, Feurer C, Gubler HU, et al. Agents Actions. 1976;6:468. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 29.Hanto DW, Gajl-Peczalska KJ, Frizzera G, et al. Ann Surg. 1983;198:356. doi: 10.1097/00000658-198309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Nalesnik MA, Porter KA, et al. Lancet. 1984;1:583. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penn I, Hammond W, Brettschneider’ L, et al. Transplant Proc. 1969;1:106. [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl TE, Penn I, Putnam CW, et al. Transplant Rev. 1971;7:112. doi: 10.1111/j.1600-065x.1971.tb00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penn I. Curr Probl Surg. 1981;18:681. doi: 10.1016/s0011-3840(81)80011-1. [DOI] [PubMed] [Google Scholar]
- 34.Editorial: Lancet. 1984;1:601. [Google Scholar]
- 35.Klintmalm GBG, Iwatsuki S, Starzl TE. Lancet. 1981;1:470. doi: 10.1016/s0140-6736(81)91851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, Hakala TR, Rosenthal JT, et al. Surg Gynecol Obstet. 1982;154:819. [PMC free article] [PubMed] [Google Scholar]
- 37.Slapak M, Geoghegan T, Digard N, et al. Transplant Proc. 1985;17:1222. [PubMed] [Google Scholar]
- 38.Illner W-D, Land W, Habersetzer R, et al. Transplant Proc. 1985;17:1181. [Google Scholar]
- 39.Slarzl TE, Klintmalm GBG, Weil R, III, et al. Surg Gynecol Obstet. 1981;153:486–1981. [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenthal JT, Hakala TR, Iwatsuki S, et al. Surg Gynecol Obstet. 1983;157:309. [PMC free article] [PubMed] [Google Scholar]
- 41.Starzl TE, Klintmalm GBG, Porter KA, et al. N Engl J Med. 1981;305:266. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Transplant Proc. 1985;17:250. [PMC free article] [PubMed] [Google Scholar]
- 43.Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Surg Gynecol Obstet. in press. [PMC free article] [PubMed] [Google Scholar]
- 44.Iwatsuki S, Gordon RD, Shaw BW, Jr, et al. Ann Surg. doi: 10.1097/00000658-198510000-00001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Semin Liver Dis. doi: 10.1055/s-2008-1040683. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw BW, Jr, Wood RP, Gordon RD, et al. Semin Liver Dis. doi: 10.1055/s-2008-1040637. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw BW, Jr, Gordon RD, Iwatsuki S, et al. Transplant Proc. 1985;17:264. [PMC free article] [PubMed] [Google Scholar]
- 48.Starzl TE, Putnam CW, Hansbrough JF, et al. Surgery. 1977;81:212. [PubMed] [Google Scholar]
- 49.Cutropia JC, Coratola F, Spinetta A, et al. Rev Esp Enferm Apar Dig. 1972;38:553. [PubMed] [Google Scholar]
- 50.Calne RY, McMaster P, Smith DP, et al. Lancet. 1979;2:612. doi: 10.1016/s0140-6736(79)91668-4. [DOI] [PubMed] [Google Scholar]
- 51.Denmark SW, Shaw BW, Jr, Griffith BP, et al. Surg Forum. 1983;34:380. [PMC free article] [PubMed] [Google Scholar]
- 52.Griffith BP, Shaw BW, Jr, Hardesty RL, et al. Surg Gynecol Obstet. 1985;160:270. [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Ann Surg. 1984;200:524. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starzl TE, Porter KA, Putnam CW, et al. Surg Gynecol Obstet. 1976;142:487. [PMC free article] [PubMed] [Google Scholar]
- 55.Starzl TE, Schneck SA, Mazzoni G, et al. Ann Surg. 1978;187:236. doi: 10.1097/00000658-197803000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw BW, Jr, Iwatsuki S, Starzl TE. Surg Gynecol Obstet. 1984;159:490. [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon RD, Shaw BW, Jr, Iwatsuki S, et al. Surg Gynecol Obstet. 1985;160:474. [PMC free article] [PubMed] [Google Scholar]
- 58.Kam I, Lynch S, Todo S, et al. Surg Gynecol Obstet. in press. [PMC free article] [PubMed] [Google Scholar]
- 59.Starzl TE, Putnam CW, Ishikawa M, et al. NY Acad Sci. 1975;252:145. doi: 10.1111/j.1749-6632.1975.tb19151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwatsuki S, Iwaki Y, Kano T, et al. Transplant Proc. 1981;13:286–1981. [PMC free article] [PubMed] [Google Scholar]
- 61.Starzl TE, Hakala TR, Shaw BW, Jr, et al. Surg Gynecol Obstet. 1984;158:223. [PMC free article] [PubMed] [Google Scholar]