Abstract
Introduction
Heterogeneity is observed in the patterns of cognition in Alzheimer's disease (AD). Such heterogeneity might suggest the involvement of different aetiological pathways or different host responses to pathology.
Method
627 subjects with mild/moderate AD underwent cognitive assessment with the Mini-Mental Status Exam (MMSE) and the Dementia Rating Scale-2 (DRS-2). Latent class analysis (LCA) was performed on cognition subscale data to identify and characterise cognitive subgroups. Clinical, demographic and genetic factors were explored for association with class membership.
Results
LCA suggested the existence of four subgroups; one group with mild and another with severe global impairment across the cognitive domains, one group with primary impairments in attention and construction, and another group with primary deficits in memory and orientation. Education, disease duration, age, APOE ε4 status, gender, presence of grasp reflex, white matter changes and early or prominent visuospatial impairment were all associated with class membership.
Conclusions
Our results support the existence of heterogeneity in patterns of cognitive impairment in AD. Our observation of classes characterised by predominant deficits in attention/construction and memory respectively deserves further exploration as does the association between membership in the attention/construction class and APOE ε4 negative status.
Keywords: dementia, latent class analysis, cognition, Mattis Dementia Rating Scale-2, Mini-Mental State Examination, apolipoprotein E
Introduction
The diagnosis of Alzheimer's Disease (AD) in clinical and research settings relies primarily on the observation of progressive cognitive and functional decline in the absence of other causes of dementia (McKhann et al., 1984; American Psychiatric Association, 1994). New research criteria for the diagnosis of AD were recently proposed which suggest that early and progressive decline in episodic memory with additional supporting suggestive features should form the core clinical basis for diagnosis (Dubois et al., 2007).
The pattern of cognitive decline in AD typically involves early episodic memory impairment, followed by deficits in attention, visuospatial abilities and language (Alladi et al., 2007; Petersen & Authors, 1998) which is believed to correspond to initial pathology in the medial temporal lobe (particularly the entorhinal cortex and the hippocampal formation) with subsequent spread to other neocortical association regions (Alladi et al., 2007; Hodges & Authors, 2006; Braak et al., 1991). However, heterogeneity is observed both in brain regions affected by AD pathology and in clinical cognitive symptoms at presentation. Understanding the basis for this heterogeneity could lead to the elucidation of different etiological pathways amenable to intervention for the prevention and eventually treatment of AD.
AD primarily presents as an amnestic syndrome in contrast to other dementias such as frontotemporal dementia which may present with prominent speech or behavioural disturbances (Alladi et al., 2007). Recent studies, however, indicate that considerable AD pathology can be observed in individuals presenting with focal syndromes (ie specific cognitive disturbances thought to originate from damage to certain cortical regions) (Alladi et al., 2007; von Gunten et al., 2006) and that these atypical presentations may be more common than previously thought (Galton et al., 2000). Focal presentations described include posterior cortical atrophy, biparietal syndrome, progressive aphasia, cortical basal syndrome and “frontal” presentations involving marked behavioural symptoms and deficits in executive function (Alladi et al., 2007; Schott et al., 2006). Contrary to the pattern attributed to “typical AD”, amnesia may be a less prominent symptom in individuals with focal presentations suggesting relatively lower pathological burden of the medial temporal lobe (Alladi et al., 2007).
A number of studies have aimed to characterise the heterogeneity in the cognitive presentation of AD and to associate this heterogeneity with pathologic features (Kanne et al., 1998; Pappas et al., 2000), imaging profile (Stopford et al., 2008; Snowden et al., 2007), regional metabolism (Martin et al., 1986), or clinical, demographic and genetic features (Sevush et al., 1993; Sevush et al., 2003; Jacobs et al., 1994; Snowden et al., 2007; Fisher et al., 1996; Fisher et al., 1999). These studies often relied on factor or principal components analysis of data from cognitive tests believed to sample specific functions, or subscales of global scales of cognition. While some studies used individuals' factor scores as the basis for tests of association with clinical or pathologic factors (Kanne et al., 1998; Pappas et al., 2000; Sevush et al., 1993; Sevush et al., 2003; Jacobs et al., 1994), others used cluster analysis of factor scores to create homogenous groups of patients for analysis (Martin et al., 1986; Fisher et al., 1996; Fisher et al., 1999; Stopford et al., 2008). Findings on the number of cognitive subgroups identified and the nature of these groups were not consistent.
Factor analysis has certain limitations in exploring subgroupings of individuals based on the analysis of cognition scores. Factor analysis should be performed on normally distributed data (Hatcher, 1994) and it is likely that data from cognitive test scores, and particularly test subscales, may be extremely skewed due to floor and ceiling effects. Further, the scaling of the different cognition tests or subscales may differ, with certain tests having greater inherent variance and sensitivity to differences between subjects than others. This might lead to difficulties in interpreting the results of factor analysis.
The current study aimed to explore the existence of cognitive subgroups of individuals with AD through the use of latent class analysis of cognitive subscale data collected from a large sample of subjects with AD. Latent class analysis (LCA) is a probabilistic technique used to detect homogenous subgroupings of individuals based on responses across a number of measured categorical variables (McCutcheon, 1987). Like factor analysis, it involves measurement of a latent variable but unlike factor analysis, an assumption is made that this variable is categorical in nature rather than continuous. An additional study aim was to explore potential associations of cognitive class membership with clinical, demographic and genetic factors. In particular, we were interested in investigating the role of age, disease duration, education and Apolipoprotein E- ε4 (APOE ε4) genotype in predicting class membership and in exploring the role of further covariates after adjustment for these factors.
Method
The analysis dataset of AD cases was drawn from a large case-control study of 875 AD patients and 850 non-demented control subjects recruited from nine Memory Referral Clinics in Canada between 6/2002 and 3/2005 described elsewhere (Li et al., 2008). The study protocol included neurological, neuropsychological and laboratory assessments plus medical record review of dementia history (including neuroimaging) where available.
Inclusion criteria required that AD patients fulfilled criteria outlined in DSM-IV (American Psychiatric Association, 1994)and by NINCDS-ADRDA (McKhann et al., 1984) criteria for probable AD, with a Global Deterioration Scale (GDS) of 3-7 (ranging from mild to very severe cognitive decline) (Reisberg B et al., 1982) Subjects were excluded if they were in a major depressive episode, acute psychosis, or acute manic or depressive episode of bipolar disorder at the time of recruitment. Neuroimaging was not required as part of the study protocol although imaging at the time of AD diagnosis to rule out vascular and other causes of dementia would have been expected clinical practice. The study protocol was reviewed and approved by the appropriate ethics committee (EC) or investigational review board (IRB) for each study site prior to subject recruitment. Informed consent was obtained from study participants in accordance with all applicable IRB/EC and regulatory requirements.
The present study sample was restricted to 627 mild/moderate AD cases based on a total Mini-Mental Status Examination (MMSE) (Folstein et al., 1975) score of ≥15, to limit the influence of floor effects on the cognitive scales in severe AD.
Cognitive Assessment
Cognitive function was assessed with the MMSE (Folstein et al., 1975) and the Mattis Dementia Rating Scale-2 (DRS-2) (Mattis, 1976; Jurica et al., 2001) scales. Scores on a total of 11 subscales from these tests were used in LCA to derive subgroups of cognitively similar patients based on impairment in specific cognitive domains. The DRS-2 subscales were defined according to Jurica et al (2001) as Attention, Conceptualization, Construction, Initiation/Perseveration and Memory. The MMSE questions were grouped into the following categories: attention (spell “WORLD” backwards), language (object naming, sentence repetition, writing a sentence, read and follow command “Close your eyes”), orientation (for time and place), memory (registration and recall of “apple”, “penny”, “table”), praxis (3-stage command) and construction (pentagon copy).
Data Analysis
Latent class analysis (LCA) of the 11 cognition subscale items was used to examine the latent structure of cognition in the sample of AD cases. In order to correct both for differences in range of possible scores on each subscale (1-37) which might affect weighting of the variable in the analysis, and for skewness of subscale score distributions, median total sample scores for each subscale were used as cut points to create dichotomous indicators for each subscale, corresponding to high/low scores based on the sample distribution. Low scores on both the MMSE and DRS-2 indicate greater impairment.
LCA is a probability-based clustering method which assumes that associations between individuals, based on responses for the observed items, can be explained by an underlying class structure (McCutcheon, 1987). This structure can be characterised through observation of the structural model consisting of latent class probabilities (gamma parameters which correspond to latent class prevalence) and the measurement model or item response probabilities, conditional on class membership (rho parameters) (Lanza S.T. et al., 2007a). In the current analysis, the gamma parameters will correspond to the prevalence of each cognition class and the rho parameters, corresponding to probability of low scores in each of the measured cognitive domains, can be used to infer the cognitive profile associated with class membership. It is assumed that within each latent class, individual items will be uncorrelated (McCutcheon, 1987). Selection of the number of latent classes was based on consideration of model parsimony, measures of goodness of fit (Bayesian Information Criterion (Schwartz, 1978), and the Lo-Mendell-Rubin likelihood ratio test (Lo et al., 2001) as per Nylund (2007)) and substantive interpretation of class meaning. Consistency of model identification was examined by altering the seed that generates sets of random starting values.
Association between class membership and covariates was assessed using multinomial logistic regression. Odds ratios reflect the increase in odds of class membership for each class (relative to a reference class) corresponding to a one-unit increase in the covariate. To characterize the classes, a large number of demographic, clinical and disease-related factors were tested for association with class membership. These are described in Table 1. Variables found to improve model fit significantly (p<0.05) in univariate regression models were then assessed via multiple regression models. Firstly, we constructed a “minimal” regression model comprising variables believed to have the highest potential to confound univariate relationships. This “minimal” model included age, education, duration and APOE ε4 genotype. Additional variables that were significant (p<0.05) in univariate analyses, were then assessed in turn for association with class membership, after adjustment for the minimal model covariates.
Table 1.
Summary of covariates
| Demographic | Age (yrs), male gender (n/y), education level, caffeine use (caffeinated drinks per day), ever drank 5 or more drinks of any kind of alcohol almost everyday? (n/y), ever told by doctor that overweight? (n/y), smoking (pack years) |
| Comorbidities | Hypertension (n/y) : blood pressure > 140/90 or antihypertensive use or reported history of hypertension Cerebrovascular disease (n/y): history of myocardial infarction, angina, stroke or transient ischemia attack Diabetes Type 1 or 2 (n/y) : history of Diabetes Type 1 or Type 2 or use of diabetes medications or HbA1c > 6%) Metabolic syndrome (n/y): history o/0 type 2 diabetes or hba1c >=5.7 and 2 of the following: a) antihypertensive medication and/or high blood pressure (≥140mm Hg systolic or ≥90mm Hg diastolic), b) Plasma triglycerides ≥2.27 mmol/L, c) HDL cholesterol ≤35 mg/dL (<0.9 mmol/L) in men or <39 mg/dL (1.0 mmol/L) in women, c) BMI >30kg/m2 and/or waist:hip ratio >0.9 in men, >0.85 in women. |
| AD features | Duration of disease since symptom onset (yrs), current use of AD medication (n/y), ever use of AD medication (n/y), early apraxia (n/y), early/ prominent visuospatial impairment (n/y), early speech abnormalities (n/y), AD with psychotic features (defined as meeting DSM-IV criteria for AD with delusions or use of antipsychotic medication), AD with depression (defined as meeting DSM-IV criteria for AD with depression or use of antidepressant medication) |
| MRI/ CT features (from most recent scan in medical record) | Global atrophy (n/y), focal atrophy (n/y), evidence of white matter change (n/y), evidence of infarcts (n/y) |
| Neurologic exam | Carotid bruits (n/y), frontal gait (n/y), frontal release signs – grasp (n/y), frontal release signs – other (n/y), deep tendon reflexes (normal/ abnormal), plantar responses (normal/abnormal), parkinsonian features (y/n) |
| Genetic/heritability factors | Family history of memory loss (n/y), APOE ε4 alleles (0,1,2) |
The possible confounding effect of disease duration on class membership was further assessed by stratifying the sample using cutpoints based on quartiles of disease duration. The latent class model was run again using duration quartile as a grouping variable to allow assessment of the probability of class membership within each duration stratum. A test for measurement invariance across groups was performed (see Lanza et al, 2007) to ensure that the measurement model held across the groups and that this analysis was valid.
LCA was performed with PROC LCA Version 1.1.3 (Lanza S.T. et al., 2007a) using SAS software, Version 9.1 for Windows1 and was replicated using MPLUS Version 5.1 (Muthen & Muthen, 2007). Covariate modelling was performed using PROC LCA. If data sparseness was highlighted as causing an error in the modelling process, the “Stabilize” function of Proc LCA was employed which added a data-derived prior to stabilize the model (Lanza S.T. et al., 2007b). Only PROC LCA results will be reported here as the class solutions derived were identical.
Results
The study sample characteristics are summarised in Table 2. Review of the model fit statistics for LCA models fit sequentially with increasing numbers of classes indicated that a 4-class solution was optimal. The BIC was lowest for this solution and the Lo-Mendell-Rubin test for five classes failed to reach statistical significance suggesting that the addition of a fifth class did not improve model fit. The 4-class solution was substantively interpretable and variations in the seed value produced the same solution, indicating that the model estimation procedure identified the valid 4-class solution.
Table 2. Sample characteristics – Total Study Sample (N=627).
| Age, mean (sd) | 76.9 (8.48) |
| Duration of AD symptoms, mean (sd) | 4.9 (2.63) |
| GDS score, mean (sd) | 4.1 (0.70) |
| MMSE score, mean (sd) | 22.5 (3.73) |
| DRS-2 score, mean (sd) | 111.2 (15.34) |
| Male gender, N (%) | 267 (42.6) |
| Education level | |
| > 15 y, N (%) | 121 (19.3) |
| 11-15 y, N (%) | 395 (43.7) |
| < 10 y, N (%) | 232 (37.0) |
| APOE ε4 alleles | |
| 0, N (%) | 226 (38.2) |
| 1, N (%) | 290 (49.0) |
| 2, N (%) | 76 (12.8) |
| Current use of medications for AD, N (%) | 187 (29.8) |
Class descriptions
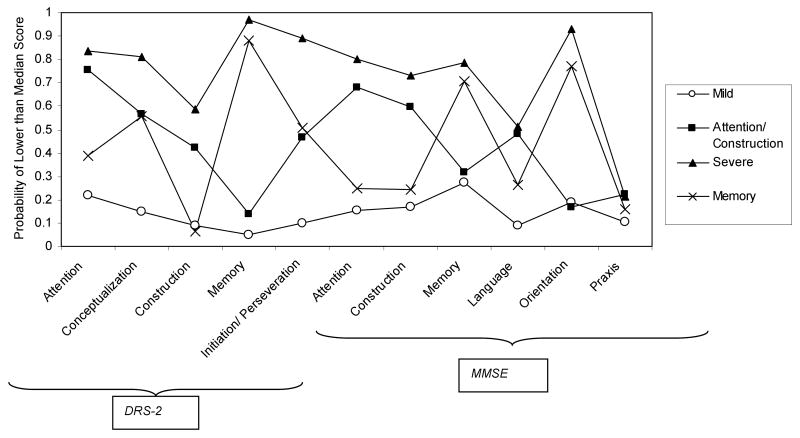
The prevalence of each class and the probabilities, conditional on class membership, of being in the lowest scoring 50% of the study sample for each cognition subscale are presented in Table 3 and graphically in Figure 1.
Table 3. Latent Class Model.
| Class 1 Mild | Class 2 Attention/Construction | Class 3 Memory | Class 4 Severe | |
|---|---|---|---|---|
| Latent Class prevalence (gamma parameters) | ||||
| 24.4% | 14.3% | 35.4% | 25.9% | |
| Probability of <median score on cognition subscale (rho parameters) | ||||
| DRS-2 subscales | ||||
| Attention | 0.2165 | 0.7574 | 0.3904 | 0.8376 |
| Conceptualization | 0.1469 | 0.5675 | 0.5596 | 0.8132 |
| Construction | 0.0897 | 0.4224 | 0.0647 | 0.5863 |
| Memory | 0.0493 | 0.1409 | 0.8802 | 0.9683 |
| Initiation/Perseveration | 0.0981 | 0.4669 | 0.5095 | 0.8916 |
| MMSE subscales | ||||
| Attention | 0.1523 | 0.6834 | 0.2504 | 0.7988 |
| Construction | 0.1716 | 0.598 | 0.2443 | 0.731 |
| Memory | 0.2747 | 0.3173 | 0.7047 | 0.7863 |
| Language | 0.0885 | 0.4801 | 0.2616 | 0.5108 |
| Orientation | 0.1902 | 0.1702 | 0.7729 | 0.9324 |
| Praxis | 0.1051 | 0.2228 | 0.1596 | 0.2128 |
Probabilities greater than 0.5 presented in bold to illustrate important items
Figure 1. Graphical Representation of Table 2 (Conditional Probability of < Median Score on Cognition Subtest by Class).
Based on review of the pattern of impairment across the cognition subscales, the classes were named “Mild”, “Attention/Construction”, “Memory” and “Severe”. The Mild class was considered to represent a group of subjects with uniformly low probabilities of being in the lowest scoring half of the sample across all cognitive domains. The Severe class was also characterised by a fairly uniform level of impairment across all domains with consistently higher impairment across the domains relative to all the other classes. The Mild and the Severe classes were of nearly equal sizes with each accounting for approximately 25% of cases.
The other two classes showed distinct patterns of impairment in certain cognitive domains, rather than global mild or severe impairment. The Attention / Construction class was distinguished by predominant deficits in the attention and, to a lesser extent, the construction subscales of both the MMSE and the DRS-2 tests. Memory and orientation abilities appeared to be spared relative to attention and constructional abilities. Conversely the “Memory” class was associated with predominant deficits on the DRS-2 memory and the MMSE memory and orientation subscales with relative sparing of attention, constructional and language abilities. Probabilities for impairment on the conceptualization and initiation/perseveration DRS-2 subscales were fairly high both for the Attention/ Construction class and the Memory class and these scales thus did not differentiate between these two classes. The Attention / Construction class was also characterised by impairment in the MMSE language subscale relative to the Mild and Memory classes with probabilities of impairment similar to those for the Severe class. The Memory class was the most prevalent, accounting for 35% of cases, while the Attention/ Construction class had the lowest prevalence at 14%.
Covariates associated with class membership
Univariate multinomial regression models indicated a significant role for age, duration, education and APOE ε4 genotype (the minimum model covariates) in predicting class membership. These variables were entered into a multiple regression model (Table 4) and each covariate retained statistical significance after adjustment for all other variables. The Mild class were on average younger than the Severe and the Memory classes and slightly older than the Attention/Construction class. The Mild class was also the most highly educated with the Severe class having the greatest probability of lower education. The Severe class had the longest disease duration while the Mild class the shortest.
Table 4. Multiple Multinomial Logistic Regression Analysis: Minimum Model (N=590).
| Odds Ratios (ORs)† | |||||
|---|---|---|---|---|---|
| Class 1: Mild | Class 2: Attention/Construction | Class 3: Memory | Class 4: Severe | p-value | |
| Age (yrs) | Ref | 0.99 | 1.07 | 1.05 | <0.001 |
| Education (>15 years (ref), 11-15years, <10years) | . | 2.91 | 2.32 | 3.42 | <0.001 |
| Disease duration (yrs since symptom onset) | . | 1.10 | 1.37 | 1.43 | <0.001 |
| APOE ε4 (alleles) | . | 0.26 | 0.77 | 0.85 | <0.001 |
Odds ratio is odds of class membership relative to Class 1 associated with a one unit increase (continuous variables) or relative to reference value (categorical variables), adjusted for all other covariates
Individuals in the Mild class were more likely than the other classes to have an APOE ε4 allele after adjustment for age, education and disease duration. The Attention/Construction class were the least likely to be APOE ε4 positive. These findings were tested further with binary logistic regression modelling which compared each class to all the other classes. After adjustment for age, education and disease duration, membership in the Mild class was associated with a higher likelihood of an APOE ε4 allele (OR 1.53, p<0.05) than membership in any other class, and membership in the Attention/Construction class was associated with a lower likelihood (OR 0.32, p<0.001).
Of the additional variables explored, male sex, frontal gait and frontal release signs as observed in the neurological examination, early/prominent visuospatial impairment, white matter changes as indicated on most recent MRI/CT scan, current use of medications for AD, and the presence of psychotic features were associated with class membership in univariate modelling and were therefore examined further after adjustment for the minimum model covariates (Table 5). Male sex, frontal release signs, early/prominent visuospatial impairment and white matter changes remained statistically significant (p<0.05) after adjustment for age, education, disease duration and APOE ε4 status while the other variables failed to contribute significantly to model fit.
Table 5. Further Multinomial Logistic Regression Models: Additional Variables Significant in Univariate Testing (p<0.05) after Adjustment for Minimum Model Covariates.
| Odds Ratios (ORs) | ||||||
|---|---|---|---|---|---|---|
| Analysis Population (N) | Class 1: Mild | Class 2: Attention/Construction | Class 3: Memory | Class 4: Severe | p-value | |
| Male gender | 590 | . | 2.09 | 0.80 | 0.70 | 0.01 |
| Frontal gait* | 578 | . | 2.66 | 0.95 | 3.00 | 0.09 |
| Frontal release signs - grasp | 580 | . | 0.76 | 1.05 | 3.88 | 0.01 |
| Early/ prominent visuospatial impairment* | 510 | . | 2.13 | 0.92 | 2.02 | 0.00 |
| White matter changes | 438 | . | 0.72 | 0.39 | 1.16 | 0.02 |
| AD with psychotic features | 590 | . | 1.90 | 2.16 | 3.26 | 0.14 |
| Current use of medications for AD | 590 | . | 1.29 | 0.69 | 0.62 | 0.14 |
Due to data sparseness, a data-derived prior was applied to stabilize logistic regression model
Odds ratio is odds of class membership relative to Class 1 associated with a one unit increase (continuous variables) or relative to reference value (categorical variables), adjusted for Minimum Model covariates
Those in the Attention/Construction class were more likely to be male than those in the other classes. Those in the Severe class were more likely than those in other classes to show grasp reflexes; a sign consistent with their more advanced disease status even in this mild/moderate population. Early/prominent visuospatial impairment was most common in the Attention/Construction class and the Severe class and least common in the Memory class. The likelihood of white matter changes was lowest in the Memory class and greatest in the Severe class.
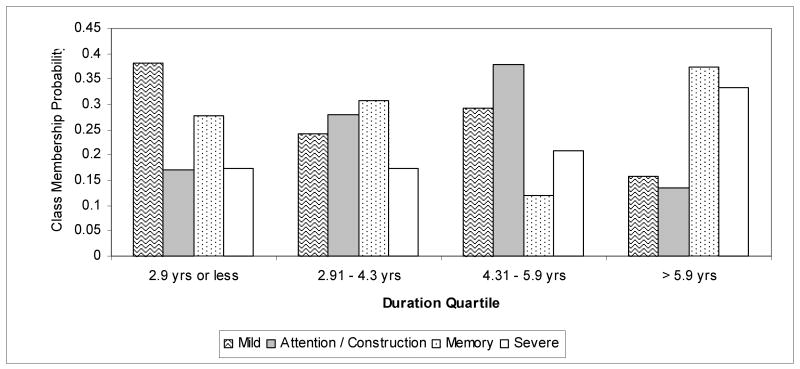
Class prevalence by duration subgroup
Figure 2 shows class membership probabilities (or class prevalence) generated from LCA conducted with disease duration quartile as a grouping variable. The test for measurement invariance was not statistically significant (difference in G2 log-likelihood = 156, df =132, p=0.08) indicating that comparison of class prevalence across duration categories was valid because the classes had the same composition across the different categories. Prevalence of the Mild class dropped across quartiles of disease duration although this pattern was not monotonic, with a slightly increased prevalence in the third quartile compared to the second quartile. Conversely, prevalence of the Severe class increased with increased disease duration although this increase was not particularly marked between quartiles 1 and 2. Prevalence of the Attention/Construction class increased with increasing disease duration but appeared to peak in the third quartile before dropping off in the highest quartile, possibly indicating that as duration progresses those with an Attention/Construction cognitive pattern assume a more globally severe profile. No clear pattern was observed for probability of membership in the Memory class; the prevalence appeared fairly constant across the quartiles of duration although lower in the third quartile.
Figure 2. Class Membership Probabilities as a Function of Duration.
Discussion
Latent class analysis suggested the existence of four cognitive classes in our cross-sectional sample population. Two of the classes were characterised by a global pattern of impairment – one class with mild impairment and the other with severe. We did not find separate classes for predominant deficits in visuospatial construction and language respectively; instead individuals with deficits in these domains were grouped together with those who had predominant deficits in attention in our study, in the class designated Attention/Construction. This profile might be considered to represent a class with primary deficits in executive function, possibly associated with greater frontal pathology. The final, and largest, class comprised patients with a profile of predominant memory/orientation impairment, potentially representing predominant medial temporal lobe pathology.
Results from previous studies which used statistical methods to define typologies of AD based on patterns of cognitive impairment in AD differed markedly in their findings. Martin et al (1986) applied cluster analytic methods to factor analysis of cognition tests from 42 AD patients and detected two clusters representing primary deficits in visuospatial function and in naming abilities. Using a similar methodology in two larger studies, Fisher et al (1996,1999) identified a globally impaired group in addition to groups with predominant impairments in visuospatial and naming abilities. A recent cluster analysis study identified global presentation types and focal presentations with disproportionate impairment in perceptuospatial ability, executive skills, praxis and language (Stopford et al., 2008). Studies using factor analysis scores alone to characterise cognitive subtypes also differed in their findings, in terms of both the number of unique factors identified and the constructs believed to be measured by the factors. However, in keeping with our findings, a number of studies identified a memory (or memory/naming, memory/orientation) factor (Kanne et al., 1998; Jacobs et al., 1994; Pappas et al., 2000; Sevush et al., 1993; Sevush et al., 2003) and a factor indicating deficits in attention or mental control (Kanne et al., 1998; Pappas et al., 2000; Jacobs et al., 1994).
In a principal components analysis of subscale data from the MMSE and DRS from 236 AD patients, Jacobs et al (1994) identified an attentional factor and a recall/naming factor. Early onset AD cases performed worse on the attentional items while late onset AD cases performed worse than early onset cases on measures of recall/naming. These findings correspond to our observation of a younger group with attentional deficits and an older group with predominant memory deficits. The younger group may indeed represent a group with younger age at onset, as disease duration did not differ markedly between the Attention/Construction class and the Memory class; however it is not possible to conclude from the current analysis that membership in these classes was driven primarily by age at onset.
Members of the Attention / Construction class were more likely to be male than those in other classes after adjustment for age, duration, education and APOE ε4 genotype. This could reflect premorbid differences in cognitive style due to social or genetic factors although previous research suggests that males might be expected to outperform females in visuospatial tasks such as those required in the MMSE and DRS-2 construction subscales (Voyer et al., 1995). It is also possible that there is an interaction between male sex and a potential AD disease pathway which manifests as a subphenotype with predominant attentional and/or constructional deficits.
The role of APOE ε4 as a risk factor for AD is consistently demonstrated but no clear association of this locus with a particular cognitive phenotype has been established. In studies of normal aging or mild cognitive impairment, associations were observed between APOE ε4 and emergent deficits in episodic memory (Bondi et al., 1995), spatial ability and naming (Bretsky et al., 2003), working memory/ visuospatial attention (Greenwood et al., 2005), and orientation and language (Tsai et al., 2008). There is also evidence from imaging studies that APOE ε4 is associated with greater hippocampal atrophy in the normally aging population (den Heijer et al., 2002); an area that is believed to be involved in memory function.
In a sample of 157 AD patients, Smith et al (1998) found an APOE ε4 effect on measures of memory (learning) and verbal comprehension but not on attention, perceptual organisation or naming. Marra et al (2004) found APOE ε4 positive status to be associated with worse performance on measures of learning, verbal memory and general intelligence but only in those with early onset disease. Van der Flier et al (2006) retrospectively classified 100 consecutive AD patients presenting to their clinic into those with memory and nonmemory phenotype based on description of earliest symptoms. The memory phenotype was associated with a higher likelihood of APOE ε4 positivity while those with the nonmemory phenotype were less likely to be APOE ε4 positive. Schott et al (2006) observed that some patients present with “biparietal” AD which is characterised by relatively preserved memory function but with impairment in calculation, spelling, praxis, visuoperceptual and visuospatial abilities. They assessed the presentation of 39 AD patients and deemed 10 to have biparietal presentation according to neuropsychological criteria. Those with biparietal presentation were unlikely to have an APOE ε4 allele leading the authors to suggest that an alternative pathological pathway might be involved.
We did not find clear evidence that probability of membership in the Memory / Orientation class increased with increasing numbers of APOE ε4 alleles as might have been expected if APOE ε4 is primarily associated with a pathway that induces deficits in memory function; instead membership in the Mild class was most highly associated with increased numbers of APOE ε4 alleles. However, our finding that probability of membership in the Attention / Construction class decreased with APOE ε4 allele number is consistent with results from van der Flier et al (2006) and Schott et al (2006) in that we have identified a phenotype with less memory involvement, which may be associated with a pathway that is independent of the APOE ε4 allele.
In addition to the biparietal presentation described by Schott et al (2006), frontal presentations of AD, characterised by behavioural symptoms and early executive dysfunction, were described in neuropathological studies (Alladi et al., 2007; von Gunten et al., 2006; Johnson et al., 1999; Kanne et al., 1998). It is conceivable that the Attention/Construction class may represent those with biparietal AD or early frontal or prefrontal lobe involvement. It is also possible that this group included misclassified cases of frontotemporal or other types of dementia, even with the strict adherence to AD diagnostic algorithms in our study and at the time of AD diagnosis. Investigation of the pathologic or neuroimaging profile of those with APOE ε4-independent disease and nonmemory phenotype would be of interest in future studies.
Although our analyses of neuroimaging data were limited to those with information in the patient record, those in the memory class were least likely of any class to have evidence of white matter changes on MRI or CT and those with globally severe disease were most likely to have changes of this kind. It would be interesting to assess the role of vascular pathology as a source of heterogeneity in AD cognitive presentation and, specifically, to assess whether a persistent, singular memory impairment is indicative of a “purer” AD pathology.
Age and education were both significant predictors of class membership in our study. We chose to use unadjusted raw scores because age- and education-adjusted norms were not available for our MMSE subscales and because we were interested in exploring the contribution of these variables to class membership rather than in controlling their impact a priori. Age-associated decline in cognitive performance, independent of the decline in cognitive abilities associated with the AD disease process, may be associated with a specific pattern of deficits which may have obscured the pattern of deficits attributed to AD. After adjustment for age, education duration was highest in the mild group and lowest in the severe group. It is possible that high education may have masked deficits in certain cognitive domains and lower education may have led to the spurious finding of impairment. However the extent to which education introduces a misclassification bias which should be controlled or is actually implicated in the pathological processes of AD is unclear (Tombaugh et al., 1992).
Although the presence of psychotic features appeared to be associated with membership in the severe class, this association did not hold after adjustment for age, duration, education, and APOE ε4 status. We were unable to detect any association between depression in AD and class membership. It is possible, however, that our capture of these features was insufficiently sensitive or, conversely, that the inclusion of medication use in the criteria for identifying patients with these features led to the inclusion of individuals with completely controlled symptoms, masking any potential effect. Furthermore, the study excluded subjects with acute depression or psychosis, which could potentially reflect behavioural subgroups.
While no association was detected between reported current use of medications indicated for the treatment of AD and class membership after adjustment for the minimum model covariates, a role for medication use in contributing to latent class membership cannot be ruled out. We did not analyse the effects of individual drugs or drug classes and it is possible that these contributed to a certain cognitive profile to the extent that they may improve performance or reduce decline differentially across the cognitive domains. It is also possible that other medications commonly taken by the elderly may have affected cognitive performance, and the effect of this is difficult to ascertain.
The current study has a number of limitations. First the MMSE and the DRS-2, while widely used screening tools for cognition, are crude instruments for measuring the cognitive domains in their subscales. Scaling in the latent class models as binary variables with cutoffs at the median potentially loses information relative to other approaches that consider the scores as continuous variables. Incorporating further neuropsychological testing as well as other cognitive domains may lead to a more refined classification. The cross-sectional study design and enrolment of subjects with different disease durations is a further limitation as it is not possible to ascertain whether individuals in the Attention/Construction and Memory classes had demonstrated a globally mild profile earlier in their disease or would develop a globally severe profile over time. Future longitudinal studies could enrol AD patients with recent onset of disease and could assess changes in class membership over time using latent transition analysis, an extension of the latent class analysis method (Lanza S.T. et al., 2007a). Ceiling and floor effects of cognitive scales may make patterns difficult to detect at early and late stages of disease, and learning effects on cognitive tests may compromise accurate description of affected and unaffected domains over time (Zehnder et al., 2007). Finally, use of the DSM-IV and NINCDS-ADRDA criteria to define our AD sample may have yielded a research sample that was specific for AD but not fully representative of AD in clinical settings where mixed dementia and atypical AD presentations may be more common. This might have led to the exclusion of other latent classes which might be identified in a clinical setting.
An interesting point to consider is whether the underlying latent variable(s) inferred to exist through latent class analysis of cognition measures would truly be categorical in nature. Traditional factor analysis assumes a continuously distributed latent variable whereas latent class analysis assumes the latent variable to be categorical. The question is equivalent to asking whether inferred cognitive subtypes represent truly distinct categories, or arbitrary subdivisions of a spectrum. The answer to this is currently unclear given our current understanding of functional neuroanatomy and the aetiology of cognitive deficits in AD. To the extent that a pattern of cognition, for example a deficit in attentional abilities, is driven by pathology in a certain brain region or the presence of a certain type of pathology, it is reasonable to suggest that the latent variable is categorical. It may also be that a certain threshold of pathology in an area must be reached for symptoms of loss to be detectable through tests of cognitive ability and this would give rise to a categorical latent variable. Finally, if unique patterns of cognitive deficits derive from pathology which is caused by genetic or other categorical risk factors, the latent variable could be categorical in nature.
In summary, our latent class analysis finding of multiple classes with unique cognitive profiles confirms the existence of heterogeneity in patterns of cognitive impairment in AD. To our knowledge this is the first application of latent class analysis to cognitive measures in AD. A number of hypotheses have been suggested through this exploratory study that could be tested in future studies. In particular, class membership transitions should be explored over time, and the cognitive phenotype, pathological correlates and potential aetiology associated with APOE ε4 negative AD should be further investigated.
Acknowledgments
We thank Daniella Dhalla, and Natalie Coletta (GlaxoSmithKline Inc., Mississauga, Ontario, Canada) for study management; Allen Roses (GlaxoSmithKline R&D, Research Triangle Park, North Carolina, USA), Lefkos Middleton and Linda Surh (GlaxoSmithKline R&D, Harlow, UK) for GenADA study design and concept, and George Quartey (GlaxoSmithKline R&D, Harlow, UK) for analysis advice.
This study was funded by GlaxoSmithKline R&D. Dr Bray's contribution to the project described was supported by Award Number P50-DA-010075 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Copyright © 2002-2003 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Reference List
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, et al. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: 1994. [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, et al. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Authors FN, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [Review] [63 refs] [DOI] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE, MacArthur Studies of Successful Aging et al. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60:1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM, et al. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [see comment]. [Review] [143 refs] [DOI] [PubMed] [Google Scholar]
- Fisher NJ, Rourke BP, Bieliauskas L, Giordani B, Berent S, Foster NL, et al. Neuropsychological subgroups of patients with Alzheimer's disease. Journal of Clinical & Experimental Neuropsychology: Official Journal of the International Neuropsychological Society. 1996;18:349–370. doi: 10.1080/01688639608408993. [DOI] [PubMed] [Google Scholar]
- Fisher NJ, Rourke BP, Bieliauskas LA, Authors FN, Rourke BP, Bieliauskas LA. Neuropsychological subgroups of patients with Alzheimer's disease: an examination of the first 10 years of CERAD data. Journal of Clinical & Experimental Neuropsychology: Official Journal of the International Neuropsychological Society. 1999;21:488–518. doi: 10.1076/jcen.21.4.488.887. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, Authors FN, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Xuereb JH, Hodges JR, Authors FN, Patterson K, et al. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(Pt 3):484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R, Authors FN, Lambert C, et al. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health's BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher L. A Step-by-Step Approach to using SAS for Factor Analysis and Structural Equation Modelling. Cary, NC: SAS Insitute Inc.; 1994. [Google Scholar]
- Hodges JR, Authors FN. Alzheimer's centennial legacy: origins, landmarks and the current status of knowledge concerning cognitive aspects. Brain. 2006;129:2811–2822. doi: 10.1093/brain/awl275. [DOI] [PubMed] [Google Scholar]
- Jacobs D, Sano M, Marder K, Bell K, Bylsma F, Lafleche G, et al. Age at onset of Alzheimer's disease: relation to pattern of cognitive dysfunction and rate of decline. Neurology. 1994;44:1215–1220. doi: 10.1212/wnl.44.7.1215. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Head E, Kim R, Starr A, Cotman CW, Authors FN, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Archives of Neurology. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. see comment. [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2: Professional manual. Lutz,Fl: Psychological Assessment Resources; 2001. [Google Scholar]
- Kanne SM, Balota DA, Storandt M, McKeel DW, Jr, Morris JC, Authors FN, et al. Relating anatomy to function in Alzheimer's disease: neuropsychological profiles predict regional neuropathology 5 years later. Neurology. 1998;50:979–985. doi: 10.1212/wnl.50.4.979. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: A SAS Procedure for Latent Class Analysis. Structural Equation Modeling: A Multidisciplinary Journal. 2007a;14:671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Lemmon DR, Schafer JL, Collins LM. Proc LCA & Proc LTA User's Guide Version 1.1.3 beta. The Methodology Center, The Pennsylvania State University; 2007b. [Google Scholar]
- Li H, Wetten S, Li L, St Jean PL, Upmanyu R, Surh L, et al. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Archives of Neurology. 2008;65:45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Marra C, Bizzarro A, Daniele A, De LL, Ferraccioli M, Valenza A, et al. Apolipoprotein E epsilon4 allele differently affects the patterns of neuropsychological presentation in early- and late-onset Alzheimer's disease patients. Dementia & Geriatric Cognitive Disorders. 2004;18:125–131. doi: 10.1159/000079191. [DOI] [PubMed] [Google Scholar]
- Martin A, Brouwers P, Lalonde F, Cox C, Teleska P, Fedio P, et al. Towards a behavioral typology of Alzheimer's patients. Journal of Clinical & Experimental Neuropsychology: Official Journal of the International Neuropsychological Society. 1986;8:594–610. doi: 10.1080/01688638608405178. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Grune, Stratton, editors. Geriatric Psychiatry. New york: 1976. pp. 77–121. [Google Scholar]
- McCutcheon AL. Latent Class Analysis. Newbury Park: Sage Publications, Inc.; 1987. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User's Guide. Fifth. Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- Nylund KL, Asparouhov T, Muthe?n BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal LJ, Authors FN, et al. Choline acetyltransferase activity and cognitive domain scores of Alzheimer's patients. Neurobiology of Aging. 2000;21:11–17. doi: 10.1016/s0197-4580(00)00090-7. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Authors FN. Clinical subtypes of Alzheimer's disease. Dementia & Geriatric Cognitive Disorders. 1998;9 3:16–24. doi: 10.1159/000051199. [Review] [67 refs] [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. American Journal of Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Schott JM, Ridha BH, Crutch SJ, Healy DG, Uphill JB, Warrington EK, et al. Apolipoprotein e genotype modifies the phenotype of Alzheimer disease. Archives of Neurology. 2006;63:155–156. doi: 10.1001/archneur.63.1.155. [DOI] [PubMed] [Google Scholar]
- Schwartz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:497–511. [Google Scholar]
- Sevush S, Leve N, Brickman A, Authors FN, Leve N, Brickman A. Age at disease onset and pattern of cognitive impairment in probable Alzheimer's disease. Journal of Neuropsychiatry & Clinical Neurosciences. 1993;5:66–72. doi: 10.1176/jnp.5.1.66. [DOI] [PubMed] [Google Scholar]
- Sevush S, Peruyera G, Bertran A, Cisneros W, Authors FN, Peruyera G, et al. A three-factor model of cognition in Alzheimer disease. Cognitive & Behavioral Neurology. 2003;16:110–117. doi: 10.1097/00146965-200306000-00004. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen E, Tangalos EG, Ivnik RJ, et al. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer's disease but not in healthy control subjects. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Stopford CL, Julien CL, Thompson JC, Davidson Y, Gibbons L, et al. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex. 2007;43:835–845. doi: 10.1016/s0010-9452(08)70683-x. [DOI] [PubMed] [Google Scholar]
- Stopford CL, Snowden JS, Thompson JC, Neary D, Authors FN, Snowden JS, et al. Variability in cognitive presentation of Alzheimer's disease. Cortex. 2008;44:185–195. doi: 10.1016/j.cortex.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ, Authors FN, McIntyre NJ. The mini-mental state examination: a comprehensive review. Journal of the American Geriatrics Society. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [see comment]. [Review] [169 refs] [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Gau YT, Liu ME, Hsieh CH, Liou YJ, Hong CJ, et al. Association study of brain-derived neurotrophic factor and apolipoprotein E polymorphisms and cognitive function in aged males without dementia. Neuroscience Letters. 2008;433:158–162. doi: 10.1016/j.neulet.2007.12.057. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Schoonenboom SN, Pijnenburg YA, Fox NC, Scheltens P, Authors FN, et al. The effect of APOE genotype on clinical phenotype in Alzheimer disease. Neurology. 2006;67:526–527. doi: 10.1212/01.wnl.0000228222.17111.2a. [see comment] [DOI] [PubMed] [Google Scholar]
- von Gunten A, Bouras C, Kovari E, Giannakopoulos P, Hof PR, Authors FN, et al. Neural substrates of cognitive and behavioral deficits in atypical Alzheimer's disease. Brain Research Reviews. 2006;51:176–211. doi: 10.1016/j.brainresrev.2005.11.003. [Review] [280 refs] [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP, Authors FN, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Zehnder AE, Blasi S, Berres M, Spiegel R, Monsch AU. Lack of practice effects on neuropsychological tests as early cognitive markers of Alzheimer disease? American Journal of Alzheimer's Disease and other Dementias. 2007;22:416–426. doi: 10.1177/1533317507302448. [DOI] [PMC free article] [PubMed] [Google Scholar]