We have been interested in the induction of whole organ graft acceptance using a brief course of intramuscular FK 506 in the early postoperative period.1,2 We use the term graft acceptance advisedly, since the long-term surviving animals we describe in this paper are not tolerant in the classical sense.
EXPERIMENTAL MODELS
Our experimental models, using Lewis rat recipients, were (1) heterotopic heart transplantation according to Ono and Lindsey,3 (2) orthotopic liver transplantation with a modification of Kamada and Calne’s technique,4 and (3) multivisceral transplantation by our own techniques.5
LIVER TRANSPLANTATION
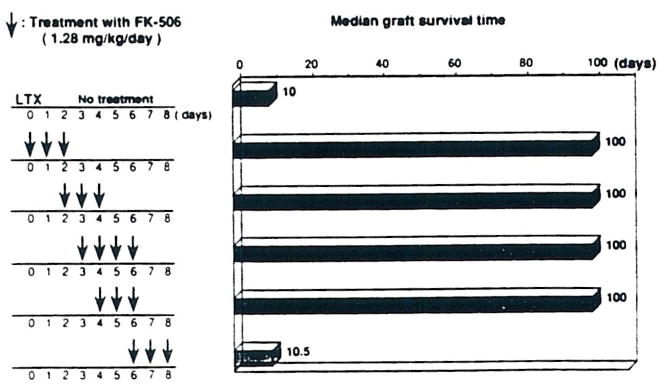
In some respects, the orthotopic liver transplantation was the most interesting preparation. The key observations, culled from more complete studies to be reported elsewhere,6,7 are summarized in Fig 1. The ACI to Lewis liver transplantation is across a major histocompatibility barrier. Yet, it is possible with a 3-day course of intramuscular FK 506, started on the day of operation or at any time up to the fourth postoperative day, to induce permanent acceptance of the majority of the liver grafts.
Fig 1.
Liver transplantation.
What are the changes in the Lewis recipient that permit this acceptance of ACI livers? The recipients, by 100 and 300 days, were not immunodepressed because they could vigorously reject skin grafts, not only from third-party (Brown Norway) donors, but also from ACI rats. Thus, acceptance of the liver grafts did not endow privilege to other tissues from the original donor strain. Whether another ACI liver would be accepted has not been determined. This will require removal of a chronically accepted liver and retransplantation with a second ACI liver.
It has been shown that the lymphocyte subset population does not differ from normal samples in these animals.7 The immunologic status of the recipients has been assessed in other ways,6,7 including one-way mixed lymphocyte reactions using mesenteric lymph node cells obtained from Lewis recipients who were bearing long-surviving liver grafts. Irradiated spleen cells from ACI or third-party Brown Norway rats were added. The Lewis lymphocytes retained a normal to weakened ability to respond to the ACI alloantigens. The potency of the reaction to the Brown Norway splenocytes was normal. In the tissue culture experiments, suppressor cells in the recipient lymphoid tissue could not be demonstrated.7 Similarly, failure to affect rejection in adoptive transfer experiments, using the spleen of long-surviving liver recipients as the cell source, suggested that splenic suppressor cells either were unimportant or absent as a factor in this form of liver graft acceptance.7
It was noteworthy that the transplanted animals remained capable of mounting a graft-versus-host (GVH) reaction against ACI donor histocompatibility antigens in vivo. This was demonstrated with the popliteal lymph node assay technique.7
HEART TRANSPLANTATION
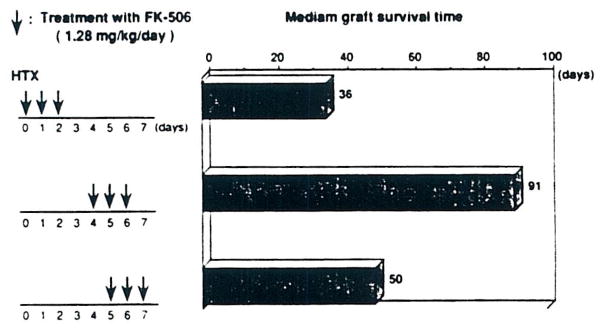
It was more difficult with hearts to induce graft acceptance, and the exact details of dose timing were more critical (Fig 2).6 The best time to give a 1.28 mg/kg/d intramuscular course was on postoperative days 4, 5, and 6. A further difference, compared with the liver, was that graft acceptance usually was not permanent (Fig 2). Even in the best groups, the hearts were gradually rejected, long after transplantation. Nevertheless, a second ACI heart transplanted to the neck 4 weeks after the first (intraabdominal) one had prolongation of its survival. This was not true with third-party Brown Norway hearts, indicating that the temporary heart graft acceptance was strain-specific.7 However, survival was not enhanced for skin grafts from either ACI or Brown Norway donors at 4 weeks after primary heart transplantation.7
Fig 2.
Heart transplantation.
The total number of lymphocytes in the heart grafts was reduced, but the prolongation of heart grafts could not be explained by a change in the lymphocyte subset proportions, which were unchanged from normal.6 Failure of splenectomy to influence the outcome, and failure to demonstrate adoptive transfers with the spleens of chronic surviving heart recipients, were construed as evidence against the existence of a suppressor cell component to this form of graft acceptance.
MULTIVISCERAL AND INTESTINAL TRANSPLANTATION
Consistent chronic survival after multivisceral transplantation has not been achieved in animals. In a previous study in unmodified Lewis recipients of Brown Norway multivisceral grafts,5 the small bowel was found to be selectively vulnerable to rejection, which killed all animals between 10 to 13 days (Table 1). With 14 intramuscular doses of 0.32 or 0.64 mg/kg FK 506, we now have six animals living for 24 to 72 days (Table 1). These surviving rats are gaining weight and have not yet exhibited evidence of GVH disease. These results, as well as other reports at this meeting on intestinal transplantation, have encouraged us to believe that FK 506 may permit successful transplantation of the previously forbidden hollow gastrointestinal viscera.
Table 1.
Effect of FK 506 on Multivisceral Transplantation
| Donor | Recipient | Treatment With FK 506 | N | Survival |
|---|---|---|---|---|
| LEW | LEW | — | 6 | >100 × 5, 7,* 72,† 81† |
| BN | LEW | — | 5 | 10, 10, 10, 10, 13 |
| BN | LEW | 0.32 mg/kg; 14 d | 3 | >32, >25, >24 |
| BN | LEW | 0.64 mg/kg; 14 d | 3 | >72, >67, >66 |
Abbreviations: LEW, Lewis: BN, Brown Norway.
Technical problem.
Killed in healthy condition.
DISCUSSION
With short courses of intramuscular FK 506, long-term heart, liver, multivisceral, and small bowel homograft acceptance can be induced in rats. The acceptance is long-term for liver, partial and variable for hearts, and as yet undetermined for intestinal and multivisceral grafts. Those recipients bearing functioning allografts do not have classical tolerance. Although the mechanism(s) of this type of organ acceptance have not been fully determined, the practical clinical importance of such acceptance may be considerable.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MD.
References
- 1.Murase N, Todo S, Lee PH, et al. Transplant Proc. 1987;19:1284. [PMC free article] [PubMed] [Google Scholar]
- 2.Todo S, Ueda Y, Demetris AJ, et al. Surgery. 1988;104:239. [PMC free article] [PubMed] [Google Scholar]
- 3.Ono K, Lindsey ES. J Thorac Cardiovasc Surg. 1969;7:225. [PubMed] [Google Scholar]
- 4.Kamada N, Calne R. Transplantation. 1979;28:47. [PubMed] [Google Scholar]
- 5.Murase N, Demetris AJ, Kim DG, et al. Surgery. (in press) [Google Scholar]
- 6.Murase N, Kim DG, Nakajima Y, et al. Transplantation. (in press) [Google Scholar]
- 7.Murase N, Kim DG, Todo S, et al. Transplantation. (in press) [Google Scholar]