The pathogenesis of hyperacute organ rejection involves a specific initiating immunologic reaction that consists of antibody-mediated injury directed against antigens on the vascular endothelium of transplanted organs.1,2 This unleashes a very intense and complex nonspecific effector cascade that involves most of the classic mediator systems of the acute inflammatory process.3 The final response can be described as a failure or breakdown of the microvasculature that results in intravascular coagulation and ischemic necrosis of the organ, with a characteristic pathological appearance.4-6
Immunosuppressive agents, such as cyclosporine or FK-506, which inhibit classic cell-mediated rejection, should therefore not be expected to have a significant and consistent effect on hyperacute rejection when used alone. However, one new potential approach for controlling the effector cascade of hyperacute rejection by pharmacological intervention exploits the role of acetyl glycerol ether phosphorylcholine or platelet-activating factor (PAF) as a central candidate in the overall evolution of this inflammatory process.7,8 There is now clear evidence that establishes the candidacy of PAF as a key biologic mediator in the pathogenesis of antibody-mediated hyperacute allograft rejection.9-11 Therefore, inhibition of PAF-induced reactions offers a potentially significant therapeutic approach to this entity.
SRI 63-441 is currently the most potent and specific PAF receptor antagonist available.12 Our recent studies represent the first known report to describe the therapeutic efficacy of a PAF antagonist (SRI 63-441) in any model of hyperacute rejection.13 The combination of SRI 63-441 with either prostacyclin or prostaglandin E1 resulted in a six- to nine-fold increase in kidney survival and a three- to 30-fold increase in urine output when compared with control animals in a pig-to-dog renal xenograft model.13 Furthermore, subsequent studies have demonstrated that a single bolus injection of SRI 63-441 alone, before revascularization, could significantly improve rat cardiac allograft survival in sensitized recipients (unpublished results). Histological evaluation of hearts that had functioned normally and then ceased at three and four days demonstrated a very aggressive acute cellular rejection compared with a classic picture of hyperacute cardiac rejection consisting of myonecrosis and intravascular coagulation in control rats. These findings suggested that the addition of potent immunosuppression could further abrogate this entire process. The present studies evaluated combination therapy with FK-506 and SRI 63-441 on cardiac allograft survival in presensitized rats.
Materials and Methods
Animals
Commercially available inbred male Lewis (L) (RTI1) and ACI (RTIa) strain rats weighing 200 to 220 g were purchased from Harlan Sprague Dawley Co, Indianapolis. This strain combination was selected on the basis of a strong histoincompatibility to provide a reproducible and reliable model of hyperacute rejection.14
Surgical Procedures
Skin grafting
Presensitization of L recipients was carried out under ether anesthesia by removing full-thickness ventral abdominal skin from ACI donor rats.14 A 2.5-cm circular skin graft was transplanted to the dorsum of the L recipient and fastened with Clay Adams wound clips. Casting material was then applied for four days after the grafting procedure to immobilize the tissue. Grafts were evaluated visually for rejection. A second and third graft were placed at intervals of 14 days in a similar manner.
Heterotopic heart transplantation
Heterotopic heart transplantation was performed 17 to 20 days after the third skin graft.
The animals were anesthetized with 40 mg/kg sodium pentobarbital intraperitoneally and supplemented with methoxyflurane. Surgical procedures for primarily vascularized cardiac transplants in rats have been well described previously.15 Briefly, an end-to-side anastomosis is performed between the donor and recipient aorta as well as the donor pulmonary artery and recipient vena cava.
Impulses of the transplanted hearts were monitored every 30 minutes for the initial 15 hours and then twice daily thereafter by palpation through the recipient abdominal wall. Rejection was considered complete when there was no palpable contractions and confirmed visually at laparotomy and by histological evaluation. All experimental animals were weighed daily.
Drug Administration
PAF antagonist
SRI 63-441 was supplied by Sandoz Research Institute (East Hanover, NJ). SRI 63-441 (molecular weight [mol wt], 662) was reconstituted in 0.68% sodium acetate and 0.9% sodium chloride (pH 5.3) to a final concentration of 10 mg/mL and placed in a warm water bath (26 to 28°C) for five minutes.
FK-506
Crystalline powder (mol wt, 882) was supplied by Fujisawa Pharmaceutical Co, Ltd, Osaka, Japan. A final concentration of 1.28 mg/mL was prepared in 0.9% sodium chloride.
Experimental Design
Contemporaneous controls were performed for each experimental group. All recipients (L) animals were sensitized to donor (ACI) antigen.
Experimental groups evaluated were as follows: group 1, control (vehicle only); group 2, FK-506 alone; group 3, SRI 63-441 alone (10 mg/kg); group 4, SRI 63-441 (10 mg/kg) plus FK-506; and group 5, SRI 63-441 (15 mg/kg) plus FK-506.
A single bolus injection of SRI 63-441 was administered via the inferior vena cava four minutes before revascularization.
FK-506 was administered at a dosage of 1.28 mg/kg/d for a duration of 14 days (or until rejection) by intramuscular injection commencing three days before transplantation.
Histology
Tissues were formalin-fixed, paraffin-embedded, cut at 3 μm, and stained with hematoxylin and eosin (H + E).
Statistical Analysis
Where available, mean survival times (MST) for experimental groups were compared by using the Wilcoxon rank sum test. A P value of < .05 was considered statistically significant.
It should be noted that in group 4 and 5 many of the animals were alive with functioning grafts at the time of manuscript preparation.
Results
Survival
The survival rates for the five groups evaluated are listed in Table 1. Sensitized L control recipients consistently rejected ACI donor hearts in a hyperacute fashion (MST 0.27 ± 0.09 days). Treatment of sensitized animals with SRI 63-441 alone as a single intravenous bolus of 10 mg/kg before revascularization resulted in a significant prolongation of cardiac allograft survival (MST, 1.70 ± 0.66 days; P < .003) when compared with control rats.
Table 1. The Effect of FK-506 and SRI 63-441 on Hyperacute Rejection of Rat Cardiac Allografts.
| Group | Treatment | n | Graft Survival (d) | MST ± SE |
|---|---|---|---|---|
| 1 | Control (vehicle only) | 13 | 0.002, 0.005, 0.007, 0.01, 0.02, 0.08, 0.15, 0.24, 0.26, 0.27, 0.67, 0.85, 0.97 | 0.27 ± 0.09 |
| 2 | FK-506 alone | 5 | 0.06, 1.4, 2, >3, >6 | |
| 3 | SRI 63-441 alone (10 mg/kg) | 5 | 0.06, 1.13, 1.19, 2.19, 4.0 | 1.70 ± 0.66* |
| 4 | SRI 63-441 (10 mg/kg) + FK-506 | 5 | 4, 4, † 13, 21, >28 | |
| 5 | SRI 63-441 (15 mg/kg) + FK-506 | 6 | 8. 9‡, >4, >10, >22, >24 |
P < .003 as compared with group 1 (Mann-Whitney).
Died with a functioning graft.
Killed with a functioning graft for histological evaluation.
Of even greater significance was the response in cardiac allograft survival observed when SRI 63-441 treatment (at two dosage levels) was combined with immunosuppression by FK-506 (groups 4 and 5). It is important to note that five of 11 animals were still alive with functioning grafts at the time of preparation of this manuscript, with three animals approaching 1-month survival. In fact, out of the 11 animals evaluated, one was killed at nine days for histological evaluation (Fig 1), and four grafts ceased to function at 4, 8, 13, and 20 days after transplantation. These grafts demonstrated similar morphological features to those shown in Fig 1. One rat in group 4 died with a functioning graft four days after transplantation. This survival far exceeds allograft survival in nonsensitized recipients (6.3 ± 0.5 days). There was no significant change in weight in rats surviving long-term.
Fig 1.
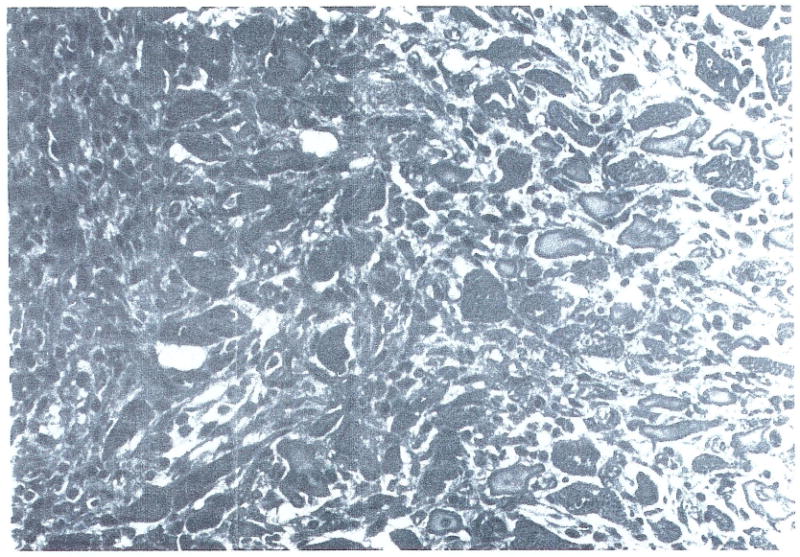
Myocardium (H + E; original magnification ×200) demonstrating slight mononuclear interstitial infiltrate and replacement by fibroblasts. This specimen is taken from a rat treated with SRI 63-441 and FK-506 and sacrificed at day 9 after transplantation with a functioning graft. It should be noted that a simitar picture of minimal cellular rejection was evident in the grafts that ceased to function in similarly treated rats.
Histologic Evaluation
Tissue sections stained with H + E were reviewed without knowledge of treatment protocols. Morphological features interpreted as cardiac hyperacute rejection included extensive interstitial hemorrhage, occlusion of vessels by platelets, endothelial cell damage, and myocyte necrosis. Polymorphonuclear cell infiltration was not a constant feature. Sections exhibiting predominately a mononuclear cell infiltrate were interpreted as acute cellular rejection. Typically, there was much less interstitial hemorrhage and little or no platelet plugging of vessels noted. Myocyte degeneration and fibroblast proliferation were frequently extensive. No overlap with hyperacute rejection was recognized.
The native hearts of the animals in all groups were consistently without morphological change. The extent and quality of the mononuclear infiltrate observed in the grafts of those animals treated with SRI 63-441 alone and SRI 63-441 and FK-506 was quite different. The grafts of those animals treated with SRI 63-441 alone contained a more abundant mononuclear cell infiltrate, many of which exhibited features of blast formation.
Discussion
The present study represents the first known report describing a therapeutic approach that completely overcomes the phenomenon of experimental hyperacute rejection. We had already demonstrated that significant abrogation of a very rapid and violent form of hyperacute rejection in a xenograft model could be achieved solely by the pharmacological manipulation of the inflammatory mediator response.13 This relied on the antagonism of PAF, perhaps the most important inflammatory mediator to be nominated as a central candidate in the overall evolution of hyperacute rejection.9-11 In a model of heterotopic cardiac transplantation into sensitized rats, we were able to demonstrate that the phenomenon of hyperacute rejection, previously thought to be refractory and uncontrollable, could be converted into a potentially more manageable form of classic rejection by a single bolus administration of a PAF antagonist, SRI 63-441, before revascularization. Indeed, hearts in treated animals that ceased to function at two to four days after transplantation demonstrated a very aggressive histological picture of acute cellular rejection with marked edema and extensive mononuclear cell infiltration (unpublished results). This suggested the prospect of adding classic immunosuppression in an attempt to overcome the cellular rejection.
It was clear that very potent immunosuppression would be required because the cellular rejection of hearts in the SRI 63-441–treated animals was much more aggressive and was occurring much earlier than is usually seen in the same donor-recipient combination without prior sensitization. This probably reflects the aggressive rejection of an organ made vulnerable by antibody attack. Other reports in this symposium have already demonstrated the potency and efficacy of FK-506 as an immunosuppressant in standard transplant models. The combination of FK-506 and SRI 63-441 treatment in this model of hyperacute cardiac rejection resulted in remarkable prolongation of graft survival in healthy animals. In fact, at the time of manuscript preparation, 45% of the grafts were still surviving at various times after transplantation, with three grafts each approaching 30-day survival.
These studies provide further insight into the complex immunologic and inflammatory reactions involved in the process of hyperacute rejection. Moreover, we introduce a novel and potentially effective therapeutic approach that relies on a combined attack on the very potent and unusually diverse spectrum of biologic activities of a key inflammatory mediator as well as on a very aggressive form of classic cell-mediated rejection.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, MD. Dr Makowka is a recipient of a Centennial Fellowship from the Medical Research Council of Canada.
References
- 1.Giles GR, Boehmig JJ, Lilly J, et al. Transplant Proc. 1970;2:522. [PMC free article] [PubMed] [Google Scholar]
- 2.Boehmig HJ, Giles GR, Amemiya H, et al. Transplant Proc. 1971;3:1105. [PMC free article] [PubMed] [Google Scholar]
- 3.Pinckard RN. Monogr Pathol. 1982;23:38. [PubMed] [Google Scholar]
- 4.Starzl TE, Lerner RA, Dixon FJ, et al. N Engl J Med. 1968;278:642. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kissmeyer-Nielsen F, Olsen S, Petersen VP, et al. Lancet. 1966;2:662. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg JC, Broersma RJ, Bullemer G, et al. Transplantation. 1969;8:152. doi: 10.1097/00007890-196908000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Venuti MC. Annu Rev Med Chem. 1985;20:193. [Google Scholar]
- 8.Braquet P, Varagaftig BB. Transplant Proc. 1986;18:10. [Google Scholar]
- 9.Ito S, Camussi G, Tetta C, et al. Lab Invest. 1984;51:148. [PubMed] [Google Scholar]
- 10.Camussi G, Aglietta M, Malavasi F, et al. J Immunol. 1983;131:2397. [PubMed] [Google Scholar]
- 11.Camussi G. Transplant Proc. 1986;18:91. [Google Scholar]
- 12.Handley DA, Tomesch JC, Saunders RN. Thromb Haemost. 1986;56:40. [PubMed] [Google Scholar]
- 13.Makowka L, Miller C, ChapChap P, et al. Ann Surg. doi: 10.1097/00000658-198710000-00009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttman RD. Transplantation. 1974;17:383. [Google Scholar]
- 15.Ono K, Lindsey E. J Thorac Cardiovasc Surg. 1969;57:225. [PubMed] [Google Scholar]


