Abstract
While expression of C-C chemokine ligand 2 (CCL2) in the central nervous system (CNS) is associated with numerous neuroinflammatory conditions, the critical cellular sources of this chemokine responsible for disease processes – as well as associated pathogenic mechanisms – remain unresolved. As the potential for anti-CCL2 therapeutics in treating neuroinflammatory disease is likely to be contingent upon effective drug delivery to the source(s) and/or target(s) of CCL2 action in the CNS, tools to highlight the course of CCL2 action during neuroinflammation are imperative. In response to this need, we used the Cre/loxP and FLP-FRT recombination system to develop the first two, cell-conditional CCL2 knockout mice – separately targeting CCL2 gene elimination to astrocytes and endothelial cells, both of which have been considered to play crucial though undefined roles in neuroinflammatory disease. Specifically, mice containing a floxed CCL2 allele were intercrossed with GFAP-Cre or Tie2-Cre transgenic mice to generate mice with CCL2-deficient astrocytes (astrocyte KO) or endothelial cells (endothelial KO), respectively. PCR, RT-PCR/qRT-PCR and ELISA of CCL2 gene, RNA and protein, respectively, from cultured astrocytes and brain microvascular endothelial cells (BMEC), established efficiency and specificity of the CCL2 gene deletions and a CCL2 null phenotype in these CNS cells. Effective cell-conditional knockout of CCL2 was also confirmed in an in vivo setting, wherein astrocytes and BMEC were retrieved by immune-guided laser capture microdissection from their in situ positions in the brains of mice experiencing acute, lipopolysaccharide-mediated endotoxemia to induce CCL2 gene expression. In vivo analysis further revealed apparent cross-talk between BMEC and astrocytes, regarding regulation of astrocyte CCL2 expression. Use of astrocyte KO and endothelial KO mice should prove critical in elaborating pathogenic mechanisms of, and optimizing treatments for, neuroinflammatory disease.
Keywords: astrocytes, endothelial cells, CCL2, neuroinflammation, cell-conditional knockout, blood-brain barrier
INTRODUCTION
From its earliest descriptions nearly two decades ago, C-C chemokine ligand 2 (CCL2; formerly called monocyte chemoattractant protein, MCP-1) was heralded as a critical factor in inflammation (Leonard and Yoshimura, 1990; Kunkel, 1991; Rollins, 2001). Soon thereafter, CCL2 was recognized to be elevated in the central nervous system (CNS) in association with a myriad of neuroinflammatory conditions of diverse etiologies (Hulkower et al., 1993; Ranshohoff et al., 1993; Wang and Feuerstein, 1995; Kim, 1996; Glabinski et al., 1996; Berman et al., 1996; Larhtz et al., 1998). In light of its clear chemotactic properties toward mononuclear leukocytes, demonstrated convincingly in Boyden chamber-type in vitro assays (Rollins et al., 1991; Yoshimura et al., 1991), CCL2 has been considered since early on to play a major role in attracting leukocytes across the blood-brain barrier (BBB) (Hulkower et al., 1993; Ransohoff et al., 1993). And such opinion is further bolstered by reports showing that mice with global knockout (KO) of CCL2, as well as wild-type (W.T.) mice treated with anti-CCL2 antibodies, are refractory to experimentally-induced neuroinflammatory disease and correspondingly exhibit greatly reduced inflammatory infiltrates (Huang et al., 2001; Karpus et al., 2003).
Perivascular astrocytes have been shown to be the most common and prominent CNS source of CCL2 in various neuroinflammatory conditions, notable among these being multiple sclerosis and its animal model experimental autoimmune encephalomyelitis (EAE) (Hulkower et al., 1993; Glabinski et al., 1995; McManus et al., 1998; Simpson et al., 1998; Van der Voorn, 1999), viral encephalotides (So et al., 2003; Peterson et al., 2004), brain ischemia (Sakurai-Yamashita et al., 2006), and neurotrauma (Glabinski et al., 1996; Babcocl et al., 2003). And therein lay the conundrum regarding the actual role of CCL2 in promoting CNS inflammatory disease: How could this chemokine - a protein that is hydrophilic and approximately 8.7 kilodaltons in native molecular weight (Leonard and Yoshimura, 1990) - come to interact with blood-born leukocytes, from which it is physically separated by the highly impervious BBB? To put this issue in stark perspective, the BBB typically limits effective diffusion into the CNS to lipophilic molecules less than 600 daltons in size (Pardridge, 2005).
Such a paradox lead us to postulate that parenchymally-derived CCL2 is likely to interact with the CNS microvascular endothelium, a major constituent of the BBB, in ways that would allow the chemokine to breach this obstacle. Using cultured brain microvascular endothelial cells (BMEC), this laboratory provided two lines of evidence to support this hypothesis. On the one hand, CCL2 was demonstrated to cause diminished expression and altered sub-cellular localization of tight junction- and adherens junction-associated proteins ZO-1 and occludin, and β-catentin and VE-cadherin, respectively (Song and Pachter, 2004; Song et al., 2007). Such actions were further shown to culminate in heightened transcellular permeability of CCL2 (Ge et al., 2008), a feature that could conceivably lend towards establishing transendothelial chemokine gradients. On the other hand, CCL2 was also shown to undergo transcellular transport across BMEC from the abluminal to the luminal surface - possibly within caveolae (Ge et al., 2008). This latter property could possibly reflect CCL2’s function as an arrest chemokine (Gerszten et al., 1999; dos Santos et al., 2005), promoting heightened adhesion of leukocytes to the microvascular endothelium as a prelude to their diapedesis into the CNS.
Notwithstanding the numerous in vitro observations of CCL2 action, the in vivo situation during neuroinflammation offers far more complexity than can be modeled in culture. And while CCL2 transgenic, over-expressing mice have significantly added to our understanding of the seminal participation of this chemokine in neuroinflammatory events (Fuentes et al., 1995; Huang et al., 2002, 2005; Bennet et al., 2003; Elhofy et al., 2005; Yamamoto et al., 2005; Menetski et al., 2007), the precise mechanisms and venues in vivo by which CCL2 promotes leukocyte accumulation in the CNS, as well as associated inflammatory damage, have continued to escape resolution. In part, this may be due to transgenic over-expression being directed to cell types other than those known to express CCL2 during neuroinflammation (Fuentes et al., 1995). Another reason may be that chronic over-expression of this chemokine – even in appropriately targeted cells – results in desensitized effects. Indeed, as expression of CCL2 is typically given only to paroxysms in the CNS (Dawson et al., 2003), and the cognate CCL2 receptor, CCR2, is subject to downmodulation upon chronic exposure to this chemokine (Andjelkovic and Pachter, 2000; Andjelkovic et al., 2002), it is not surprising that both transgenic over-expression and global deletion of CCL2 have been described to mitigate inflammation and disease severity in EAE (Huang et al., 2001; Elhofy et al., 2005). It may further hold that the physiological and pathophysiological actions of CCL2 are determined by the biochemical milieu in both time and space. Without knowledge of the full biological context in which CCL2 is expressed, efforts to discern its mechanism(s) of action in the CNS will necessarily be hampered.
These factors prompted us to take a different tact to begin clarifying the pathways by which CCL2 mediates neuroinflammation. Specifically, CCL2 gene deletion was targeted to two cell types that arguably play a prominent role in CNS inflammatory processes: astrocytes and endothelial cells. Astrocytes were chosen because of their identity as the major source of CCL2 during neuroinflammation (Hulkower et al., 1993; Glabinski et al., 1995, 1996; McManus et al., 1998; Simpson et al., 1998; Van der Voorn, 1999; Babcock et al., 2003; So et al., 2003; Peterson et al., 2004, Sakurai-Yamashita et al., 2006), and the intimate relationship of terminal astrocyte foot processes with the CNS microvasculature (Abbott, 2006). And endothelial cells were selected as BMEC have been widely described as up-regulating expression of CCL2 in culture in response to a variety of inflammatory mediators (Crane et al., 2000; Zhang et al., 2000; Prat et al., 2002; Harkness et al., 2003; Sequin et al., 2003; Omari et al., 2004), as well as in vivo during some neuroinflammatory conditions (Berman et al., 1996; Adamus et al., 1997; Fujioka et al., 1999; Toborek et al., 2003). Importantly, as global elimination of CCL2 has underscored the preeminent, non-redundant role of this chemokine in several neuroinflammatory conditions (Huang et al., 2001; Hughes et al., 2002; Rankine et al., 2006), these cell-conditional knockout mice are apt to reveal indispensable pathways of CCL2 involved in disease pathogenesis.
Herein we describe the first two, cell-conditional chemokine KO mice, and present in vitro and in vivo evidence that independent elimination of the CCL2 gene from astrocytes and endothelial cells in two separate mouse lines, was both selective and efficient.
MATERIALS AND METHODS
Mice
GFAP-Cre [FVB-Tg(GFAP-cre)25Mes/J], Tie2-Cre [B6.Cg-Tg(Tek-cre)12Flv/J] and Rosa-FLP1 [129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym/J] mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All animal studies were performed, and CO2-mediated euthanasia carried-out, according to the Animal Care and Guidelines of the University of Connecticut Health Center (Animal Welfare Assurance #A3471-01). Mice engineered in this study were backcrossed onto the (C57BL/6) genetic background for at least nine generations.
Generation of CCL2 floxed mice
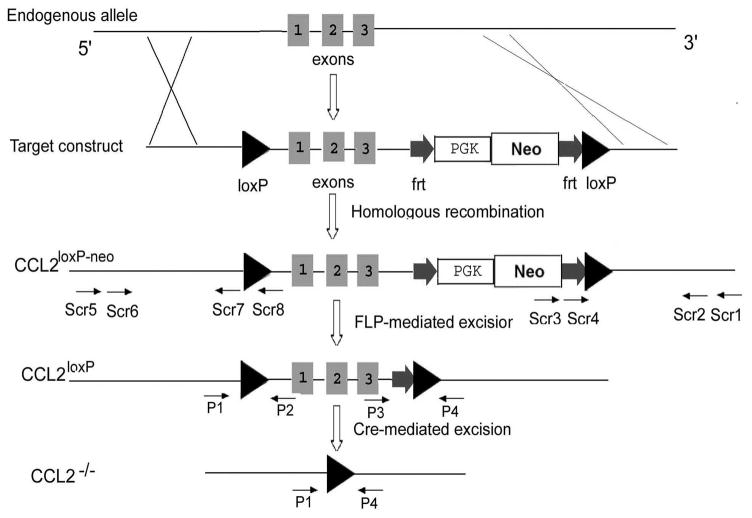
To generate the conditional CCL2 KOs, we utilized the Cre-loxP and FLP-FRT recombination system. CCL2 is encoded by 3 exons and spans ~ 4.5 kb on chromosome 11. To construct the targeting vector, C57BL/6J BAC clones (RP23-460K4) containing the CCL2 gene were obtained from BAC resources. A 10 kb fragment was retrieved from the BAC to a plasmid vector. A loxP site was inserted 2430 bp upstream of exon 1, and an FRT-PGK-neo-FRT-loxP cassette from PL452 plasmid was inserted 190 bp downstream of exon 3, resulting in a floxed 4501 bp fragment containing the complete CCL2 gene (Fig. 1). The targeting vector was linearized and electroporated into the 129/C57BL/6J F1 hybrid ES cell line (generated in the Gene Targeting and Transgenic Facility, University of Connecticut Health Center). Neo-resistant ES cell clones were screened for 3′ and 5′ homologous recombination by using the nested PCR pairs Scr1/Scr4, Scr2/Scr3 and Scr5/Scr7, Scr6/Scr8, respectively. The sense primers Scr1 and Scr2 are located in the 3′ untranslated region of the CCL2 gene but not present in the construct, and the anti-sense primers Scr3 and Scr4 are located in the neo cassette within the construct (Figs. 1, 2A). These primer pairs yield a 3013-bp band as a result of 3′ recombination. The sense primers Scr 5 and Scr6 are located in the 5′ untranslated region of the CCL2 gene but outside the targeting construct, and the anti-sense primers Scr7 and Scr8 are located downstream of the first loxP site within the construct. These primer pairs yield a 2992-bp band as a result of 5′ recombination. Two CCL2loxP-neo/+ (1E5 and 1F7) ES cell clones were expanded, and aggregated with CD-1 embryos (Gene Targeting and Transgenic Facility, University of Connecticut Health Center) to generate chimeras. The chimeras were mated with CD-1 females (The Jackson Laboratory) to detect germ-line transmission. Germ-line chimeras (CCL2loxP-neo/+) were then mated with Rosa-FLP1 females to remove the FRT-neo-FRT cassette, leaving two loxP sites flanking exons 1–3 of the CCL2 gene (CCL2loxP/+).
Fig. 1. Strategy for generation of CCL2 floxed mice and Cre-mediated excision.
Schematic shows the wild-type CCL2 gene targeting construct, and the homologous recombinant allele in ES cells. A loxP site was inserted upstream of exon 1 and an FRT-PGK-neo-FRT-loxP cassette from PL452 plasmid was inserted downstream of exon 3. The positions of PCR primers used for each screening step are displayed at the approximate locations. P1, P2, P3 and P4 show the location of the different primers used in the PCR genotyping analysis. Scr1, Scr2, Scr3 and Scr4 show the location of nested PCR primers for 3′ arm region confirmation, while Scr5, Scr6, Scr7 and Scr8 show the same for 5′ arm region confirmation.
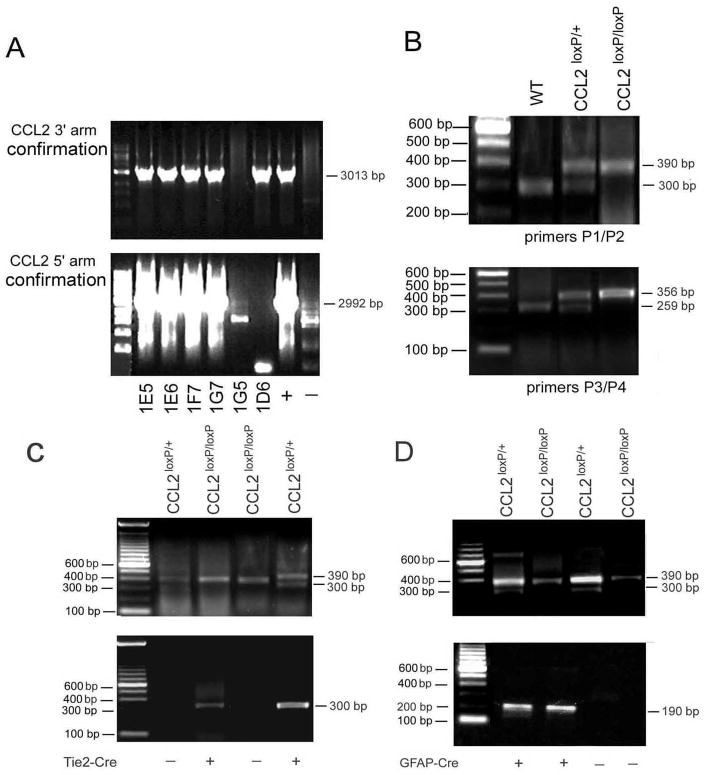
Fig. 2. Molecular characterization of cell-targeted CCL2 deletions.
(A) Identification of homologous recombination in ES cells. Correctly targeted ES cells were identified by nested PCR. The primers Scr1, Scr2, Scr3 and Scr4 for nested PCR of 3′ region confirmation amplify a 3013 bp fragment; the primers Scr5, Scr6, Scr7 and Scr8 for nested PCR for 5′ region pair amplify a 2992 bp fragment. The lane designations (+) and (−) on the far right indicate positive and negative controls, respectively. (B) Genotyping of CCL2loxP/+ and CCL2loxP/loxP mice Mouse DNA was analyzed by PCR: primers P1/P2 result in a 300 bp fragment for the CCL2 wild type allele: primers P3/P4 result in a 390 bp fragment for the CCL2 mutant allele; primers P5/P6 result in a 256 bp fragment for the CCL2 wild type allele; and primers P7/P8 result in a 356 bp fragment for the CCL2 mutant allele. (C and D) Genotyping of CCL2loxP/loxP/GFAP-Cre and CCL2loxP/loxP/Tie2-Cre mice. Mouse DNA was analyzed by PCR: primers P5/P6 result in a 300 bp fragment for the Tie2-Cre gene; primers P7/P8 result in a 190 bp fragment for the GFAP-Cre gene. The lane designations (+) and (−) reflect presence or absence, respectively, of the Cre gene.
Creation of separate, astrocyte- and endothelial cell-targeted CCL2 KO mouse lines
To target inactivation of the CCL2 gene to astrocytes or endothelial cells, mice containing a floxed CCL2 allele (CCL2loxP/+) were intercrossed with, respectively, GFAP-Cre transgenic mice expressing Cre under control of the astrocyte-specific GFAP promoter (Zhuo et al., 2001) or Tie2-Cre transgenic mice expressing Cre under control of the endothelial-specific Tie2 promoter (Koni et al., 2001). The resulting CCL2loxP/+/GFAP-Cre and CCL2loxP/+/Tie2-Cre animals were further mated with CCL2loxP/loxPmice to generate CCL2loxP/loxP/GFAP-Cre and CCL2loxP/loxP/Tie2-Cre mice, respectively (Fig. 1). Notably, as Tie2 expression has been evidenced by endothelial cells throughout the vascular tree (Thurston et al., 2000; Anghelina et al., 2005; Ohtsuki et al., 2005), CCL2 gene deletion should be efficient at all branches of the CNS vasculature.
Genotyping
For mating purposes, genomic DNA was isolated from the tails of 4–6 week old mice, following lysis of tail snips in buffer containing proteinase K, and used for PCR. The primers (5′ → 3′) for the Tie2-Cre transgene were P5 (GATATCTCACGTACTGACGG) and P6 (TGACCAGAGTCATCCTTAGC), which amplify a 300bp fragment (Fig. 2C), and those for the GFAP-Cre transgene were P7 (ACTCCTTCATAAAGCCCT) and P8 (ATCACTCGTTGCATCGACCG), which amplify a 190bp fragment (Fig. 2D). To confirm that the neo was correctly removed and loxPs existed in their recombination sites, two additional primer pairs were used: P1 (AGAGCTCAGACTAGGCCTTT) and P2 (TTCAGTTAGCACAGGAGGCA), which amplify a 390 bp fragment from the wild-type allele, and one of 300bp from the mutant allele; and P3 (AGAGTGAATGGCCCACTCTT) and P4 (GTCTGTTCTCCACTGTCTGA), which amplify a 259bp fragment from the wild-type allele, and one of 356bp from the mutant allele (Fig. 2B). To identify the homozygous CCL2loxP/loxP/Tie2-Cre and CCL2loxP/loxP/GFAP-Cre mice, tail DNA from offspring were analyzed by using varying combinations of primers. For CCL2loxP/loxP/Tie2-Cre mice, primers P1, P2, P3, P4, P5 and P6 were used to detect the loxP sites and Cre gene; and primers P1, P2, P3, P4, P7 and P8 were similarly employed for evaluation of CCL2loxP/loxP/GFAP-Cre mice. To confirm targeted KOs, DNA from cultured endothelial cells and astrocytes were examined by PCR, with P1 and P4 primers used to detect CCL2 gene deletion in each cell type (see below). This primer pair amplifies a 256 bp fragment in the mutant allele, with the wild-type allele being too large to be detected.
Isolation and culture of mouse astrocytes and brain microvascular endothelial cells (BMEC)
Genotypes were confirmed by toe snip analysis at postnatal day 4–5, and then cultures were established within 24 hr. Brain tissue from mice at postnatal day 5–6 was used as the sole source of astrocytes and BMEC cultures. This narrow postnatal age range was critical, as younger mice did not allow purification of intact microvessels, and older mice provided very poor astrocyte yield. Following decapitation, brains were removed and split mid-sagitally into two hemispheres, with the cerebral cortex from one hemisphere being used for the preparation of astrocytes, and the cortex of the other hemisphere for isolation of BMEC.
BMEC were isolated as previously detailed by this laboratory (Song and Pachter 2003; 2004). Primary cultures were typically grown for approximately five days prior to sub-culturing for experiments. At that time, purity was gauged to be ≥98% BMEC, according to diI-acetylated LDL uptake (Song and Pachter 2003). BMEC also exhibited common endothelial characteristics, e.g., CD31 and vWF immunostaining, plus displayed expression of the tight-junction associated proteins ZO-1 and occludin found enriched at the BBB.
For the preparation of astrocytes, a modified version of the protocol described by Ge and Pachter (2004) was used. Cerebral cortices were first cut into small pieces (~ 1 mm), and the minced tissue then incubated in dissecting medium (Hank’s Balanced Salt Solution [Gibco/BRL, Rockville, MD], containing 0.5% glucose, 0.7% sucrose, 20 mM Hepes pH 7.4.) with 0.03% trypsin at 37°C for 20–30 min. Next, the tissue extract was centrifuged at 1000 × g for 5 min and the pellet was washed and resuspended in plating medium (Earl’s Modified Eagle Medium [Gibco/BRL] containing 10% fetal bovine serum, 10% horse serum, 2 mM glutamine, 20 mM D-glucose, 4 mM sodium bicarbonate, 100 μg/ml penicillin and 100 μg/ml streptomycin). The tissue was mildly triturated to produce a single cell suspension, and the dissociated cells plated onto tissue culture flasks (T-12.5 cm2) coated with poly-lysine (BD Biosciences, Bedford, MA). Cultures were maintained up to 1 week in plating medium in a humidified atmosphere (5% CO2) at 37°C. After this time, cultures were shaken at 200 rpm for 2 hr at 4°C to dislodge weakly adherent microglia. Cultures were then shaken for an additional 18 hr at 37°C to remove the neuronal population. The enriched astrocyte population was further depopulated of remaining microglia by L-leucine methyl ester (LME; Sigma-Aldrich, St. Louis, MO) treatment (Hamby et al 2006). LME was dissolved in plating medium prior to being added to cultures at concentrations of 50 mM, and the cells then incubated at 37°C for 90 min. All LME solutions were adjusted to pH 7.4 and filtered prior to addition to cells. Following LME treatment, monolayers were washed thoroughly with culture medium. Cell purity was determined by immunocytochemistry using a monoclonal anti-human GFAP (Sigma St. Louis, MO) antibody, and cultures assessed to be ≥ 98% astrocytes (GFAP+).
Measurement of CCL2 protein
For the purpose of measuring CCL2 protein released into the media, astrocytes were exposed to 10μg/ml lipopolysaccharide (LPS, from Escherichia coli Serotype 026:B6 Sigma, Louis, MO) for 6 h at 37 °C, while BMEC were unstimulated under these same ambient conditions. After this time, culture media was removed, cells were washed, and further incubated in fresh media without LPS for another 18 hr. Culture supernatants were then collected and stored at −80 °C until analyzed. CCL2 protein released into the supernatants was determined by a sandwich-type immunoassay kit (BioSource International Inc., Camarillo, CA). Following removal of supernatant, cells were washed with cold PBS, then scraped into lysis buffer containing 10 mM Tris, pH 7.6, 0.14 M NaCl, 1% Triton X-100, 1% deoxycholate, 0.1 % SDS, and protease inhibitors (20 μg/ml aprotinin, 20 μg/ml leupeptin, and 1 mM phenylmethylsulfony fluoride). Lysates were cleared by centrifugation at 9,000 × g for 10 min, and resulting supernatants assayed for protein concentration by Micro BCA protein assay kit (Pierce, Rockford, IL), using bovine serum albumin as a standard.
Determination of CCL2 mRNA (RT-PCR)
Cells were treated as for protein measurement. Total RNA was extracted from cells using the RNeasy kit (QIAGEN Inc. Valencia, CA) according to the manufacturer’s instructions. Quantification and purity assessment of the extracted RNA was performed by UV spectrophotometry at 260 and 280 nm. Reverse transcription (RT) and polymerase chain reaction (PCR) were performed with the RT-PCR OneStep kit (QIAGEN Inc.). Primers for CCL2 and β-actin were purchased from BioSource International Inc. (Carlsbad, CA). The RT reaction was performed at 50°C for 30 min, and finally at 95 °C for 15 min to inactivate the reverse transcriptase and initiate PCR activation. The PCR amplification was carried out for 28 cycles with the following specifications: denaturation at 94°C for 30 s, annealing at 58°C for 45 s, and extension at 72 °C for 1 min, with only the last cycle including an extension step at 72°C for 10 min. The PCR products were analyzed by agarose gel (1.5%) electrophoresis in the presence of 0.5 μg/ml ethidium bromide, and visualized and photographed under UV illumination.
Immunohistochemistry-guided Laser Capture Microdissection (immuno-LCM)
Mice of age 4–6 weeks were injected with LPS (i.p. 4mg/kg weight). At 4 hr post-injection, animals were euthanized, the entire brain was removed, snap-frozen in liquid N2, and stored at −80 °C. Frozen brain tissue was embedded in OCT compound (Miles, IN) and coronal sections were cut at 7 μm thickness. Tissues sections were double-immunostained by immunofluorescence and immunohistochemistry to identify astrocytes and BMEC, respectively, and LCM performed on a PixCell IIe microscope (Molecular Devices, Sunnyvale, CA) to selectively retrieve each of these cell types, as recently elaborated by this laboratory (Kinnecom and Pachter 2005, Macdonald et al, 2008). Tissue was captured onto HS caps (Molecular Devices), and solubilized in 100 μl TRIzol® (Invitrogen; Carlsbad, CA). RNA was isolated from TRIzol® extracts following the manufacturer’s instructions, and processed for qRT-PCR to obtain relative CCL2 mRNA expression level (Macdonald et al., 2008).
qRT-PCR
RNA isolated from the TRIzol®-solubilized tissue extract, or prepared from cell cultures by RNeasy kit, was treated with Turbo DNAse (Ambion; Austin, TX) according to the protocol provided by the manufacturer. Next, mRNA was then reverse transcribed using the SuperScript III (Invitrogen) standard protocol, slightly modified by changing the extension temperature to 42 °C (instead of 50 °C) for 60 min. The resulting cDNA was stored at −20 °C until used for further analysis.
Measurements of cDNA levels were performed by qRT-PCR using an ABI PRISM 7500 Sequence Detection System Version 1.3, and SYBR green (ABI) fluorescence used to quantify relative amplicon amount. Separate controls included a no template control and no reverse transcriptase control, and standard curves were constructed for all primers used. Relative amplicon quantification was performed using the formula: (1+Eref)Ct (ref)/(1+Etarget)Ct (target)×100%, with ref: CD31, GFAP or RPL19; target: gene of interest; E: primer pair efficiency; and Ct: threshold cycle. Duplicates for each gene tested were averaged, after normalization to the mean CD-31, GFAP and RPL-19 expression values.
RESULTS
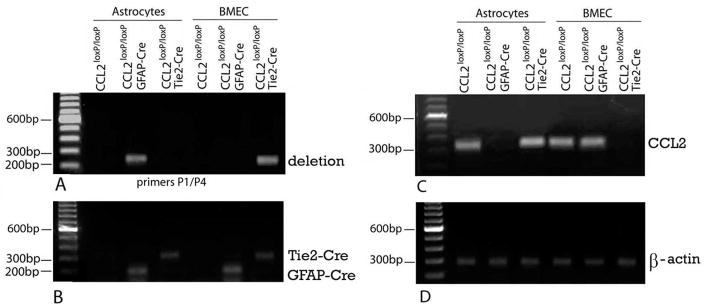
To initially determine whether there was targeted excision of the CCL2 gene in astrocytes and BMEC, analyses were performed on separate cultures of these cell types derived from mice of the following three genotypes: CCL2loxP/loxP/GFAP-Cre (astrocyte KO); CCL2loxP/loxP/Tie2-Cre (endothelial KO); and CCL2loxP/loxP littermates, serving as W.T. controls for each KO. PCR analysis of genomic DNA of cultured astrocytes and BMEC derived from these respective lines revealed that the deletion band (256 bp) representing the excised CCL2 gene was present only in astrocytes from astrocyte KO mice and BMEC from endothelial KO mice (Fig. 3A). Furthermore, presence of the Cre gene was revealed in both KO strains, highlighting that the absence of the deletion band in astrocytes from endothelial KO mice, and BMEC from astrocyte KO mice, was not due to absence of Cre (Fig. 3B). These results underscore that GFAP promoter-driven Cre recombinase activity was present in astrocytes but not in BMEC, while Tie2 promoter-driven Cre recombinase activity was evident in BMEC but not in astrocytes – thus allowing for separate, cell-targeted ablation of the CCL2 gene in the two cell types.
Fig. 3. Targeted CCL2 gene deletion in astrocyte and BMEC cultures from conditional CCL2 KO mice.
Separate astrocyte and BMEC cultures were derived from opposite cerebral hemispheres of the following groups of mice at postnatal day 5–6: CCL2loxP/loxP/GFAP-Cre (astrocyte KO); CCL2loxP/loxP/Tie2-Cre (endothelial KO); and CCL2loxP/loxP (W.T. littermates of the respective KOs). (A, B) PCR analysis of genomic DNA (100 ng) from astrocytes and BMEC, indicating (A) the deletion band (256 bp) representing the excised CCL2 gene, and (B) Presence of the Cre gene. (C, D) RT-PCR analysis (28 cycles) of RNA (200 ng) from astrocytes (+LPS) and BMEC, showing results for (C) CCL2 mRNA and (D) β-actin mRNA (indicating loading efficiency).
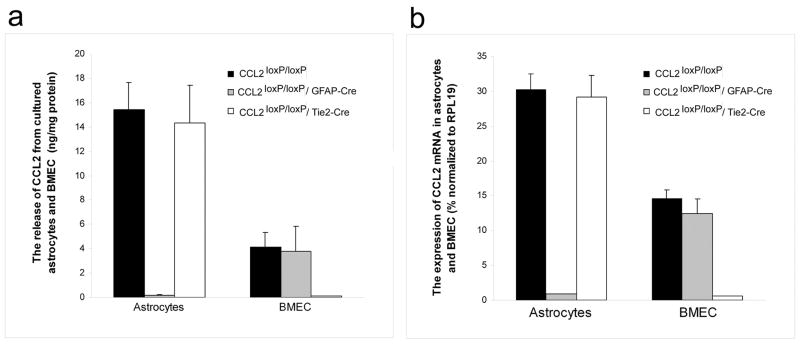
To substantiate that these targeted deletions resulted in CCL2 null phenotypes in both cultured astrocytes and BMEC, CCL2 RNA and protein expression was also analyzed. Under normal conditions, cultured murine astrocytes constitutively express only low levels of CCL2 but respond to LPS stimulation with greatly increased expression of this chemokine (Hayashi et al., 1995). In contrast, cultured murine BMEC show comparatively high-level CCL2 expression even in the absence of LPS (Ge et al., 2008). The effects of cell-targeted CCL2 gene deletion on CCL2 phenotype were thus gauged in cultured astrocytes in response to LPS stimulation, and in BMEC under resting conditions. RT-PCR showed lack of detectable CCL2 RNA in LPS-stimulated astrocytes from astrocyte KO mice, while astrocytes from both endothelial KO and W.T. mice clearly displayed CCL2 expression (Fig. 3C, D). On the other hand, no detectable CCL2 RNA signal was observed in resting BMEC from endothelial KO, but was demonstrably present in BMEC from astrocyte KO and W.T. mice. qRT-PCR and ELISA additionally provided quantitative confirmation of these results for CCL2 RNA and protein, respectively (Fig. 4A, B). That astrocytes from CCL2loxP/loxP/W.T. mice showed identical CCL2 RNA level to astrocytes from endothelial KO mice, and BMEC from CCL2loxP/loxP/W.T. mice exhibited a CCL2 RNA level mirroring that of BMEC from astrocyte KO mice, underscores that mere floxing of the CCL2 gene was not the cause of the targeted loss of CCL2 expression in both cell types. Together, the DNA, RNA and protein data underscore that targeted CCL2 gene deletion to astrocytes or BMEC demonstrated efficiency and specificity.
Fig. 4. Quantification of CCL2 protein and RNA expression in astrocyte and BMEC cultures from conditional CCL2 KO mice.
Cultures were established as in Fig. 4 from CCL2loxP/loxP/GFAP-Cre (astrocyte KO), CCL2loxP/loxP/Tie2-Cre (endothelial KO) and CCL2loxP/loxP (W.T. littermate)mice. Astrocytes were stimulated with LPS (10 μg/ml, 6 hr), while BMEC were unchallenged. (A) Supernatants were collected from cultures and analyzed for CCL2 protein by ELISA. (B) Following removal of supernatants, cells were processed for RNA, and analysis performed by qRT-PCR (40 cycles). The relative difference in CCL2 RNA levels between KOs and controls was > 32-fold in all cases (some CCL2 RNA coming from minor contaminating cells).
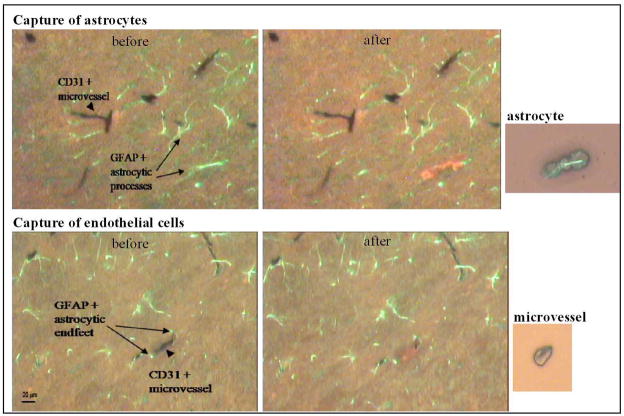
Astrocyte KO and endothelial KO mice were further evaluated for expression of CCL2 in situ. CNS expression of CCL2 was induced by peripheral LPS injection (i.p.), which has been shown to cause elevated CCL2 expression in the brain within hours (Thibeault et al., 2001; Sun et al., 2008; Qin et al., 2008; Thomspon et al., 2008). At 4 hr following LPS injection, astrocytes or BMEC were selectively retrieved from their in situ locales by immuno-LCM and then subject to qRT-PCR analysis of CCL2 RNA levels. The high degree of resolution afforded by immuno-LCM in separately retrieving these two cell types is shown in Fig. 5, underscoring that while astrocytes and BMEC are extremely closely apposed, each type of cell can be removed without detectable contamination by the other. Of further importance, BMEC were captured from parenchymal microvessels of varied size, thereby fostering representation of the different branches of the microvascular tree.
Fig. 5. In situ retrieval of astrocytes and BMEC by immuno-LCM.
Arrowheads point to CD31+ BMEC in microvessels, and arrows demarcate GFAP+ astrocytic processes. Top figure shows selective capture of astrocyte, while bottom figure shows retrieval of a single microvessel. before, shows tissue section before LCM; after, shows same section following cellular retrieval. The small images to the side show the astrocyte and microvessel, respectively, each deposited onto a cap, from which the tissue is extracted and RNA isolated. Notably, the LCM process is highly selective in retrieving the targeted cell types; even the closely apposed astrocyte foot processes are not disturbed upon BMEC/microvessel retrieval (bottom figure).
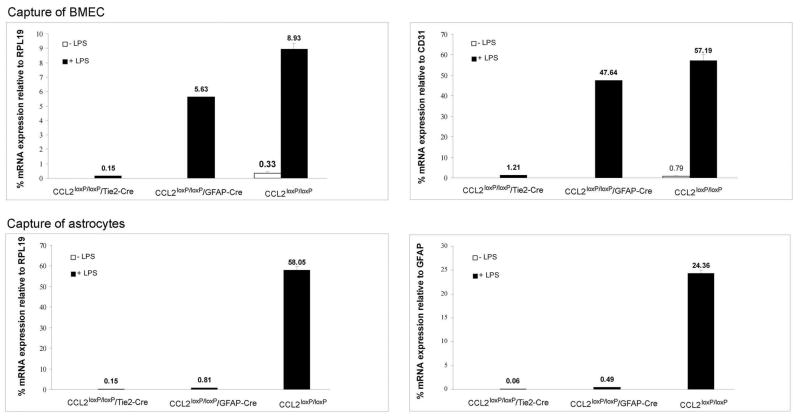
LCM/qRT-PCR revealed that BMEC from W.T. and astrocyte KO mice demonstrated strong CCL2 expression in situ in response to LPS, while showing near undetectable constitutive expression (i.e., no LPS) in these animals (Fig. 6). In comparison, BMEC of endothelial KO mice in our study showed a barely detectable CCL2 RNA level at any time, and that following LPS injection was < 3% that of either astrocyte KO or W.T. mice. Thus, just as in culture, BMEC in situ demonstrated a null CCL2 phenotype only in endothelial KO mice, reflecting efficiency and specificity of the conditional, gene targeting approach.
Fig. 6. In vivo confirmation of cell conditional CCL2 KOs.
CCL2loxP/loxP/GFAP-Cre (astrocyte KO) and CCL2loxP/loxP/Tie2-Cre (endothelial KO) mice, along with their corresponding CCL2loxP/loxP (W.T.) littermates, were injected with LPS (i.p., 4 mg/kg). After 4 hr, brain tissue was subject to immuno-LCM to selectively retrieve BMEC (top) or astrocytes (bottom). RNA was isolated from the respective astrocyte and BMEC samples and subject to qRT-PCR to quantify relative CCL2 levels. For BMEC, CCL2 mRNA level was expressed relative to either the housekeeping gene RPL19 (left) or the endothelial marker CD31 (right). For astrocytes, CCL2 mRNA levels were normalized to either RPL19 (left) or the astrocyte marker GFAP (right).
Regarding expression of CCL2 by astrocytes in situ, these cells too manifested a vivid LPS induction in W.T. littermates – with no constitutive level of CCL2 RNA being detectable in these animals (Fig. 6). And analogously to what was seen with BMEC in situ in endothelial KO mice, astrocytes from astrocyte KO mice exhibited a scarcely discernable CCL2 RNA level even following LPS injection. Remarkably, however, astrocytes from endothelial KO mice also failed to show induction of CCL2 RNA, displaying a level that was just minimally measurable in response to LPS. This scenario differs significantly from that observed in culture where, as expected, astrocytes from both W.T. and endothelial KO mice expressed equally high levels of CCL2 as a result of LPS stimulation, and only astrocytes from astrocyte KO mice showed a null CCL2 phenotype. In light of these surprising results, we examined whether other brain responses to LPS stimulation were differentially affected in the KO mice. Specifically, induced expression of E-selectin and P-selectin was evaluated, as both these adhesion molecules have been shown to be up-regulated in brain following peripheral LPS administration and to mediate early inflammatory sequelae (Piccio et al., 2002). Fig. 7 indicates that astrocyte KO mice, endothelial KO mice, and the corresponding W.T. littermates, all vigorously responded to LPS injection by up-regulating brain E- and P-selectin expression. Thus, endothelial KO mice did not show a peculiar global global insensitivity to LPS.
Fig. 7. E- and P-selectin expression in response to LPS.
Following LPS injection of CCL2loxP/loxP/GFAP-Cre (astrocyte KO), CCL2loxP/loxP/Tie2-Cre (endothelial KO), and CCL2loxP/loxP (W.T.) littermate mice (as described in Fig. 6), brain tissue was frozen and cut at 7 μm. Individual brain sections (‘tissue scrapes’) representing each of these groups was then subject to TRIzol®-mediated RNA extraction/isolation and qRT-PCR analysis for E- and P-selectin mRNA.
DISCUSSION
To the best of our knowledge, these results offer the first description of cell-targeted chemokine knockout mice. Specifically, evidence from cell culture experiments was presented showing targeted CCL2 gene elimination in vivo to astrocytes or endothelial cells, two cell types critical in the pathogenesis and elaboration of a host of neuroinflammatory conditions. And both cell culture and in vivo studies further showed that astrocytes from astrocyte KO mice, and BMEC from endothelial KO mice, were unable to up-regulate expression of CCL2 following an inflammatory challenge with LPS a reflection of these cells manifesting a null CCL2 phenotype.
That cultured astrocytes from astrocyte KO, but not BMEC from the contralateral cerebral cortex of these animals, showed CCL2 gene excision and a null CCL2 phenotype, unequivocally demonstrated that the approach for targeting deletion of the CCL2 gene to astrocytes was both effective and specific. And this was equally the case with endothelial targeting, as cultured BMEC from endothelial KO mice, but not astrocytes derived from these same animals, exhibited effects of CCL2 gene deletion. Inasmuch as the culturing process was likely to have culled astrocytes and BMEC from various populations and regions throughout the cortical mantle, these results importantly underscore that most, if not all, variants of these two cell types, both of which display considerable functional heterogeneity (Bachoo et al., 2004; Ge et al., 2005; Lee et al., 2006; Bechmann et al., 2007; Kipp et al., 2008), suffered successful elimination of the CCL2 gene.
LPS injection of W.T. animals resulted in stimulated expression of CCL2 by both astrocytes and BMEC, in accord with previous reports (Thibeault et al., 2001; Sun et al., 2008). However, our observations that astrocytes in situ from endothelial KO mice showed only minimal CCL2 expression following LPS injection was surprising, as these cells in culture did not display the CCL2 gene deletion band and exhibited a robust CCL2 response on par with astrocytes cultured from their W.T. littermates. That endothelial KO mice might have suffered a unique, widespread lack of CNS responsiveness to LPS is an unlikely scenario, as astrocyte KO, endothelial KO and W.T. mice all displayed similar E- and P-selectin induction in brain following LPS injection. Another possible explanation may lie in the significant cross-talk between astrocytes and BMEC in vivo (Abbott, 2000; Prat et al., 2001; Pachter et al., 2003; Jovanova-Nesic and Shoenfeld, 2007; Choi and Kim, 2008), and the prospect that CCL2 might function as a mediator in this interchange. Recent evidence that global deletion of the CCL2 gene causes diminished expression of several chemokines in the brain following acute LPS endotoxemia (Thompson et al., 2008) is consistent with our results and a role for CCL2 in relaying proinflammatory signals in the CNS – possibly from BMEC to astrocytes. And further supporting CCL2 acting as such a relay are reports from this and other laboratories that astrocytes are functionally responsive to CCL2 stimulation (Heesen et al., 1996; Andjelkovic et al., 2002; Zuurman et al., 2003; Ge and Pachter, 2004). Related to this issue, another factor in our findings may be the route of LPS administration. LPS delivered by i.p. injection arguably reaches the BMEC first, before having any opportunity to interact with the parenchyma. At relatively early times after such delivery (e.g., at 4 hr post i.p injection), LPS might only directly access the BMEC – any outright contact with astrocytes being largely restricted due to an initially intact BBB (Singh and Jiang, 2004). Because the Toll-like receptor (TLR) 4 receptor for LPS is expressed by BMEC (Singh and Jiang, 2004; Zhou et al., 2006), LPS-stimulated release of CCL2 from BMEC might serve as the primary or exclusive conduit to induce CCL2 expression by astrocytes soon after i.p. injection. However, this situation might change at later times, if and when the BBB becomes sufficiently disrupted (Xiao et al., 2001) to allow circulating LPS to enter the parenchyma and directly stimulate TLR4-bearing astrocytes (Krasowska-Zoladek et al., 2007) to express CCL2 (Hayshi et al., 1995; Carpentier et al., 2005). One might expect that in this case astrocytes obtained by LCM in situ from endothelial KO mice would show elevated expression of CCL2 following i.p. LPS injection – and not the reduced expression seen here at an early time-point. This possibility needs to be explored further.
Ongoing studies in this laboratory have indicated that these conditional KO mice will be highly useful in resolving pathogenic mechanisms. Specifically, both astrocyte and endothelial KO mice were observed to be resistant to EAE induced by active immunization with myelin oligodendrocyte glycoprotein – yet each line showed a different disease phenotype at the clinical and histological level (manuscript in preparation). Implicit in these findings are that cell-targeted deletion of CCL2 interfered with separate, critical steps in disease pathogenesis.
These cell-conditional CCL2 KO lines will thus provide essential tools for elaborating where, how and when CCL2 executes its function in the CNS during disease. As specifically regards where, excluding CNS expression of CCL2 from selective domains will clarify the sources of this chemokine that feature critically in pathogenesis. For example, despite evidence from adoptive T cell transfer and bone marrow chimera studies that CCL2 originating - somewhere - within the CNS regulates murine EAE (Huang et al., 2001; Elhofy et al., 2008), the precise pathogenic origins of the chemokine remain unidentified. Systematic elimination of CCL2 from defined CNS sites, as provided by these and future cell-targeted animals, will help resolve this issue.
Concerning the how and when of CCL2 function in the CNS, systematic elimination of defined CCL2 pools will assist in mapping the course of CCL2 distribution throughout disease and, thereby, illuminate this chemokine’s fate and mechanism of action. As a case in point, immunohistochemical localization of CCL2 protein in both astrocytes and BMEC during EAE (Berman et al., 1996) does not allow for resolution as to whether this chemokine originates from the former and passes to the latter, as would occur if CCL2 liberated from astrocyte foot processes was taken up and trancytosed by BMEC (Ge et al., 2008). Clearly, by removing one confounding source of CCL2 at a time, comparative immunohistochemical analysis of W.T. with astrocyte KO and endothelial KO mice would aid in resolving this matter. Carrying out such analysis over time would further enable the change in CCL2 distribution pattern to be correlated with various stages of disease – providing a window on the means by which both CCL2 and leukocytes communicate and the progression of neuroinflammation is orchestrated.
Besides offering insight into the pathogenic mechanisms of CCL2 during neuroinflammatory disease, studies with astrocyte KO and endothelial KO mice will provide information vital for the optimal design of anti-CCL2 drugs that target CCL2 signaling during neuroinflammation (Hamann et al., 2008; Infante-Duarte, 2008; Kalinowska and Losy, 2008). Should sequestration of CCL2 by glycosaminoglycans (GAGs) in the sub-endothelial matrix critically regulate CNS leukocyte accumulation, as is the case in the kidney (Celie et al., 2007), then it would be required that any anti-CCL2 therapeutic effectively breach the BBB in order to disrupt CCL2 signaling. Confined by GAGs to the perivascular domain, CCL2 could stimulate disruption of the BBB’s junctional integrity upon binding to the abluminal microvascular surface (Song and Pachter, 2004; Song et al., 2007) and/or establish a haptotactic gradient for directed leukocyte migration (Preischl et al., 1995; Quandt and Dorivini-Zis, 2004), as a means of promoting neuroinflammation. Such a scenario is conceivable if the main pathogenic source of CCL2 is that pool released from astrocyte foot processes, which are intimately associated with the GAG-containing basement membrane complexes surrounding brain microvessels (Sixt et al., 2001; van Horssen et al., 2005). The astrocyte KO mice would assist in illuminating this possibility.
On the other hand, another mode of action of CCL2 might be its presentation on the luminal endothelial surface, embedded within the GAG-rich glycocalyx, wherein it could stimulate firm leukocyte adhesion as a prelude to diapedesis. Indeed, both in vitro and in vivo studies support such an ‘arrest’ function for CCL2 (Gerszten et al., 1999; dos Santos et al., 2005). While there may be no need for an anti-CCL2 therapeutic to cross the BBB in this situation, another consideration might be the need to overcome the significant interactions between CCL2 and the luminal GAGs (Lau et al., 2004). In fact, disruption of these interactions has recently been shown to be critical for reducing clinical EAE disease (Handel et al., 2008), underscoring their role as a legitimate target in neuroinflammation. As expression and luminal release of CCL2 by BMEC could potentially set up such CCL2:GAG interactions, as well as could endothelial transcytosis of CCL2 derived from astrocytes (Ge et al., 2008), both endothelial KO and astrocyte KO mice would be instrumental in clarifying this prospect.
Endothelial KO and astrocyte KO mice will thus afford significant opportunity to dissect the pathway(s) by which CCL2, released from within CNS sites, leads to accumulation of mononuclear leukocytes in the parenchyma and, ultimately, to neuroinflammatory disease. In turn, this will highlight means by which to best target anti-CCL2-based therapeutics to those sites critical to disease pathogenesis. And as multi-focal release of CCL2 by astrocytic, endothelial and/or other cell types may direct the incidence and progression of a host of diseases – both in and outside the CNS (Dawson et al., 2003; Zernecke and Weber, 2005; Shober et al., 2008) – these cell-conditional KO lines are anticipated to be broadly utilitarian for investigating physiological and pathophysiological processes.
Acknowledgments
This work was supported by grants PP-1215 from the National Multiple Sclerosis Society and R21-NS057241 from the National Institutes of Health to J.S.P. We thank Dr. Cai-Ying Guo for assistance with generating the ES lines and Ms. Jennifer Macdonald for helpful discussions.
References
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Adamus G, Machnicki M, Amundson D, Adlard K, Offner H. Similar pattern of CCL2 expression in spinal cords and eyes of Lewis rats with experimental autoimmune encephalomyelitis associated anterior uveitis. J Neurosci Res. 1997;50:531–538. doi: 10.1002/(SICI)1097-4547(19971115)50:4<531::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Andjelkovic AV, Song L, Dzenko KA, Cong H, Pachter JS. Functional expression of CCR2 by human fetal astrocytes. J Neurosci Res. 2002;70:219–231. doi: 10.1002/jnr.10372. [DOI] [PubMed] [Google Scholar]
- Andjelkovic AV, Pachter JS. Characterization of MCP-1 and MIP-1α binding sites along human brain microvessels. J Neurochem. 2000;75:1898–1906. doi: 10.1046/j.1471-4159.2000.0751898.x. [DOI] [PubMed] [Google Scholar]
- Anghelina M, Moldovan L, Moldovan NI. Preferential activity of Tie2 promoter in arteriolar endothelium. J Cell Mol Med. 2005;9:113–121. doi: 10.1111/j.1582-4934.2005.tb00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoo RM, Kim RS, Ligon KL, Maher EA, Brennan C, Billings N, Chan S, Li C, Rowitch DH, Wong WH, DePinho RA. Molecular diversity of astrocytes with implications for neurological diseases. Proc Natl Acad Sci USA. 2004;101:8384–8389. doi: 10.1073/pnas.0402140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Elhofy A, Canto MC, Tani M, Ransohoff RM, Karpus WJ. CCL2 transgene expression in the central nervous system directs diffuse infiltration of CD45(hi)CD11b(+) monocytes and enhanced Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Neurovirol. 2003;9:623–636. doi: 10.1080/13550280390247551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JW, Guida MP, Warren J, Amat J, Brosnan CF. Localization of monocyte chemoattractant protein-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J Immunol. 1996;156:3017–3023. [PubMed] [Google Scholar]
- Blakeley J. Drug delivery to brain tumors. Curr Neurol Neurosci Rep. 2008;8:235–241. doi: 10.1007/s11910-008-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier PA, Begolka WS, Olson JE, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:36–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Celie JWAM, Rutjkes NWP, Keuning ED, Soininen R, Heljasvaara R, Pihlajaniemi T, Drager A, Zweegman S, Kessler FL, Beelen HJ, Florquin S, Aten J, van den Born J. Subendothelial heparan sulfate proteoglycans become major L-selectin and monocyte chemoattractant protein-1 ligands upon renal ischemia/reperfusion. Am J Pathol. 2007;170:1865–1878. doi: 10.2353/ajpath.2007.070061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YK, Kim KW. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB reports. 2008;41:345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- Crane IJ, Wallace CA, McKillop-Smith S, Forrester JV. Control of chemokine production at the blood-brain barrier. Immunology. 2000;101:426–433. doi: 10.1046/j.1365-2567.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J, Miltz W, Mir AK, Wiessner C. Targeting monocyte chemoattractant protein-1 signaling in disease. Expert Opin Ther Targets. 2003;7:35–48. doi: 10.1517/14728222.7.1.35. [DOI] [PubMed] [Google Scholar]
- De Boer AG, Gaillard PJ. Strategies to improve drug delivery across the blood-brain barrier. Clin Pharmacokinet. 2007;46:533–576. doi: 10.2165/00003088-200746070-00002. [DOI] [PubMed] [Google Scholar]
- dos Santos AC, Barsante MM, Arantes RME, Bernard CCA, Teixeira MM, Carvalho-Tavares J. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis – an intravital microscopy study. J Neuroimmunol. 2005;162:122–129. doi: 10.1016/j.jneuroim.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Elhofy A, Wang J, Tani M, Fife BT, Kennedy KJ, Bennet J, Huang D, Ransohoff RM, Karpus WJ. Transgenic expression of CCL2 in the central nervous system prevents experimental autoimmune encephalomyelitis. J Leuk Biol. 2005;77:229–237. doi: 10.1189/jlb.0804465. [DOI] [PubMed] [Google Scholar]
- Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- Fujioka T, Purev E, Rostami A. Chemokine mRNA expression in the cauda equina of Lewis rats with experimental allergic neuritis. J Neuroimmunol. 1999;97:51–59. doi: 10.1016/s0165-5728(99)00048-x. [DOI] [PubMed] [Google Scholar]
- Ge S, Pachter JS. Caveolin-1 knockdown by small interfering RNA suppresses responses to the chemokine MCP-1 by human astrocytes. J Biol Chem. 2004;279:6688–6695. doi: 10.1074/jbc.M311769200. [DOI] [PubMed] [Google Scholar]
- Ge S, Song L, Pachter Where is the blood-brain barrier…really? J Neurosci Res. 2005;79:421–427. doi: 10.1002/jnr.20313. [DOI] [PubMed] [Google Scholar]
- Ge S, Song L, Serwanski D, Kuziel WA, Pachter JS. Transcellular transport of CCL2 across brain microvascular endothelial cells. J Neurochem. 2008;104:1219–1232. doi: 10.1111/j.1471-4159.2007.05056.x. [DOI] [PubMed] [Google Scholar]
- Gerszten RE, Garcia-Zapeda EA, Lim YC, Yoshida M, Ding H, Gimbrone MA, Luster AD, Luscinskas FW, Rosensweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Balasingam V, Tani M, Kunkel SL, Streiter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury the brain. J Immunol. 1996;156:4363–4368. [PubMed] [Google Scholar]
- Glabinski AR, Tani M, Tuohy VK, Tuthill RJ, Ransohoff RM. Central nervous system chemokine mRNA accumulation follows initial leukocyte entry at the onset of acute murine experimental autoimmune encephalomyelitis. Brain Behav Immunol. 1995;9:315–330. doi: 10.1006/brbi.1995.1030. [DOI] [PubMed] [Google Scholar]
- Hamann I, Zipp F, Infante-Duarte C. Therapeutic targeting of chemokine signaling in multiple sclerosis. J Neurol Sci. 2008 doi: 10.1016/j.jns.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2004;150:128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Handel TM, Johnson Z, Rodrigues DH, dos Santos AC, Cirillo R, Muzio V, Riva S, Mack M, Derauz M, Borlat F, Vitte PA, Wells TNC, Teixeira MM, Proudfoot AEI. An engineered monomer of CCL2 has anti-inflammatory properties emphasizing the importance of oligomerization for chemokine activity in vivo. J Leuk Biol. 2008;84:1101–1108. doi: 10.1189/jlb.0108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KA, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN. Cytokine regulation of MCP-1 expression in brain and retinal microvascular endothelial cells. J Neuroimmunol. 2003;142:1–9. doi: 10.1016/s0165-5728(03)00251-0. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Luo Y, Laning J, Streiter RM, Dorf ME. Production and function of monocyte chemoattractant protein and other beta-chemokines in murine glial cells. J Neuroimmunol. 1995;60:143–150. doi: 10.1016/0165-5728(95)00064-9. [DOI] [PubMed] [Google Scholar]
- Heesen M, Tanabe S, Berman MA, Yoshizawa I, Luo Y, Kim RJ, Post TW, Gerard C, Dorf ME. Mouse astrocytes respond to the chemokines MCP-1 and KC, but reverse transcriptase-polymerase chain reaction does not detect mRNA for the KC or new MCP-1 receptor. J Neurosci Res. 1996;45:382–391. doi: 10.1002/(SICI)1097-4547(19960815)45:4<382::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Huang D, Tani M, Wang J, Han Y, TT, Weaver J, Charo IF, Tuohy VK, Rollins BJ, Ransohoff RM. Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice. J Neurosci. 2002;22:10633–10642. doi: 10.1523/JNEUROSCI.22-24-10633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein-1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–725. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Wujek J, Kidd G, He TT, Cardona A, Sasse ME, Stein EJ, Kish J, Tani M, Charo IF, Proudfoot AE, Rollins BJ, Handel T, Ransohoff RM. Chronic expression of monocyte chemoattractant protein-1 in the central nervous system causes delayed encephalopathy and impaired microglial function in mice. FASEB J. 2005;19:761–772. doi: 10.1096/fj.04-3104com. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Alegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22:308–317. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Hulkower K, Brosnan CF, Acquino DA, Cammer W, Kulshrestha S, Guida MP, Rapoport DA, Berman JW. Expression of CSF-1, c-fms, and MCP-1 in the central nervous system of rats with experimental allergic encephalomyelitis. J Immunol. 1993;150:2525–2533. [PubMed] [Google Scholar]
- Infante-Duarte C, Waiczies S, Weurfel J, Zipp F. New developments in understanding and treating neuroinflammation. J Mol Med. 2008;86:975–985. doi: 10.1007/s00109-007-0292-0. [DOI] [PubMed] [Google Scholar]
- Joshi S, Ornstein E, Bruce JN. Targeting the brain: rationalizing the novel methods of drug delivery to the central nervous system. Neurocrit Care. 2007;6:200–212. doi: 10.1007/s12028-007-0034-8. [DOI] [PubMed] [Google Scholar]
- Jovanova-Nesic K, Shoenfeld Y. Autoimmunity in the brain: the pathogenesis insight from cell biology. Ann NY Acad Sci. 2007;1107:142–154. doi: 10.1196/annals.1381.016. [DOI] [PubMed] [Google Scholar]
- Kalinowska A, Losy J. Investigational C-C chemokine receptor 2 antagonists for the treatment of autoimmune diseases. Expert Opin Investig Drugs. 2008;17:1267–1279. doi: 10.1517/13543784.17.9.1267. [DOI] [PubMed] [Google Scholar]
- Karpus WB, Fife BT, Kennedy KJ. Immunoneutralization of chemokines for the prevention and treatment of central nervous system autoimmune disease. Methods. 2003;29:362–368. doi: 10.1016/s1046-2023(02)00360-2. [DOI] [PubMed] [Google Scholar]
- Kim KS. Cytokines and adhesion molecules in stroke and related diseases. J Neurol Sci. 1996;137:69–78. doi: 10.1016/0022-510x(95)00338-3. [DOI] [PubMed] [Google Scholar]
- Kipp M, Norkute A, Johann S, Lorenz L, Braun S, Hieble A, Gingele S, Pott F, Richter J, Beyer C. Brain-region-specific astroglial responses in vitro after LPS exposure. J Mol Neurosc. 2008;35:235–243. doi: 10.1007/s12031-008-9057-7. [DOI] [PubMed] [Google Scholar]
- Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule-1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnowska-Zoladek A, Banaszewska M, Kraszpulski M, Konat GW. Kinetics of inflammatory response of astrocytes induced by TLR3 and TLR4 ligation. J Neurosci Res. 2007;85:205–212. doi: 10.1002/jnr.21088. [DOI] [PubMed] [Google Scholar]
- Kunkel SL, Standiford T, Kasahara K, Streiter RM. Stimulus specific induction of monocyte chemoattractant protein-1 (MCP-1) gene expression. Adv Exp Med Biol. 1991;305:65–71. doi: 10.1007/978-1-4684-6009-4_8. [DOI] [PubMed] [Google Scholar]
- Larhtz F, Piali L, Spanaus KS, Seebach J, Fontana A. Chemokines and chemotaxis of leukocytes in infectious meningitis. J Neuroimmunol. 1998;85:33–43. doi: 10.1016/s0165-5728(97)00267-1. [DOI] [PubMed] [Google Scholar]
- Lau EK, Paavola CD, Johnson Z, Gaudry JP, Geretti E, Borlat F, Kungl AJ, Proudfoot A, Handel TM. Identification of the glycosaminoglycan binding site of the CC chemokine, CCL2: implications for structure and function in vivo. J Biol Chem. 2004;279:22294–222305. doi: 10.1074/jbc.M311224200. [DOI] [PubMed] [Google Scholar]
- Lee Y, Su M, Messing A, Brenner M. Astrocyte heterogeneity revealed by expression of a FAFP-LacZ transgene. Glia. 2006;53:677–687. doi: 10.1002/glia.20320. [DOI] [PubMed] [Google Scholar]
- Leonard E, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Mentlein R. Glial cross-talk by transmembrane chemokines CX3CL1 and CXCL16. J Neuroimmunol. 2008;198:92–97. doi: 10.1016/j.jneuroim.2008.04.024. [DOI] [PubMed] [Google Scholar]
- McCarty JH. Cell biology of the neurovascular unit: implications for drug delivery across the blood-brain barrier. Assay Drug Dev Technol. 2005;3:89–95. doi: 10.1089/adt.2005.3.89. [DOI] [PubMed] [Google Scholar]
- McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF. CCL2, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J Neuroimmunol. 1998;86:20–29. doi: 10.1016/s0165-5728(98)00002-2. [DOI] [PubMed] [Google Scholar]
- Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astocytes display nocioceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Kamiya N, Hori S, Terasaki T. Vascular endothelium-selective gene induction by Tie2 promoter/enhancer in the brain and retina of a transgenic rat. Pharm Res. 2005;22:852–857. doi: 10.1007/s11095-005-4579-y. [DOI] [PubMed] [Google Scholar]
- Omari KM, Chui R, Dorivini-Zis K. Induction of beta-chemokine secretion by human brain microvessel endothelial cells via CD40/CD40L interactions. J Neuroimmunol. 2004;146:203–208. doi: 10.1016/j.jneuroim.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Pachter JS, de Vries HE, Fabry The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62:593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier and neurotherapeutics. NeuroRx. 2005;2:1–2. doi: 10.1602/neurorx.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson KE, Erret JS, Wei T, Dimcheff DE, Ransohoff R, Kuziel WA, Evans L, Chesebro B. MCP-1 and CCR2 contribute to non-lymphocyte-mediated brain disease induced by Fr98 polytropic retrovirus infection in mice: role for astrocytes in retroviral neuropathogenesis. J Virol. 2004;78:6449–6458. doi: 10.1128/JVI.78.12.6449-6458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio L, Rossi B, Scarpini E, Laudanna C, Ciagulli C, Issekutz AC, Vestweber D, Butcher EC, Constantine G. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric Gi-linked receptors. J Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- Prat A, Biernacki K, Lavoie JF, Poirier J, Duquette P, Antel JP. Migration of multiple sclerosis lymphocytes through brain endothelium. Arch Neurol. 2002;59:391–397. doi: 10.1001/archneur.59.3.391. [DOI] [PubMed] [Google Scholar]
- Prieschl EE, Kulmburg PA, Baumrucker T. The nomenclature of chemokines. Int Arch Allergy and Imunol. 1995;107:475–483. doi: 10.1159/000237089. [DOI] [PubMed] [Google Scholar]
- Qin H, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflamm. 2008;5:10–26. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt J, Dorovini-Zis K. The beta chemokines CCL4 and CCL5 enhance adhesion of specific CD4+ T cell subsets to human brain endothelial cells. J Neuropathol Exp Neurol. 2004;63:350–362. doi: 10.1093/jnen/63.4.350. [DOI] [PubMed] [Google Scholar]
- Rankine EL, Hughes PM, Botham MS, Perry VH, Felton LM. Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leukocyte recruitment. Eur J Neurosci. 2006;24:77–86. doi: 10.1111/j.1460-9568.2006.04891.x. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. JE/MCP-1: an early-response gene encodes a monocyte-specific response. Cancer Cells. 1991;3:517–524. [PubMed] [Google Scholar]
- Rollins BJ, Walz A, Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991;78:1112–1116. [PubMed] [Google Scholar]
- Sakurai-Yamashita Y, Shigematsu K, Yamashita K, Niwa M. Expression of MCP-1 in the hippocampus of SHRSP with ischemia-related delayed neuronal death. Cell Mol Neurobiol. 2006;26:823–831. doi: 10.1007/s10571-006-9077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequin R, Biernacki K, Rotondo RL, Prat A, Antel JP. Regulation and functional effects of monocyte migration across human brain-derived endothelial cells. J Neuropathol Exp Neurol. 2003;62:412–419. doi: 10.1093/jnen/62.4.412. [DOI] [PubMed] [Google Scholar]
- Schober A. Chemokines in vascular dysfunction and remodeling. Aterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Newcombe J, Cuzner ML, Woodruffe MN. Expression of monocyte chemoattractant protein and other beta chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol. 1998;84:238–249. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Sixt M, Engelhardt B, Pausch F, Hallman R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalitis. J Cell Biol. 2001;153:933–945. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So EY, Kang MH, Kim BS. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler’s murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia. 2006;53:858–867. doi: 10.1002/glia.20346. [DOI] [PubMed] [Google Scholar]
- Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in microvascular endothelial cells. Blood. 2007;109:1515–1523. doi: 10.1182/blood-2006-07-034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Pachter JS. Culture of murine brain microvascular endothelial cells that maintain expression and cytoskeletal association of tight junction-associated proteins. In Vitro Cell Dev Biol Anim. 2003;39:313–320. doi: 10.1290/1543-706X(2003)039<0313:COMBME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Song L, Pachter JS. Monocyte chemoattractant protein-1 alters expression of tight junction-associated proteins in brain microvascular endothelial cells. Microvasc Res. 2004;67:78–89. doi: 10.1016/j.mvr.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Sun J, Zheng JH, Zhao M, Lee S, Goldstein H. Increased in vivo activation of microglia and astrocytes in the brains of mice transgenic for an infectious R54 human immunodeficiency type 1 provirus and for CD4-specific expression of human cyclin T1 in response to stimulation by lipopolysaccharides. J Virol. 2008;82:5562–5572. doi: 10.1128/JVI.02618-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibeault I, Laflamme N, Rivest S. Regulation of the gene encoding the monocyte chemoattractant protein 1 (MCP-1) in the mouse and rat brain in response to LPS and proinflammatory cytokines. J Comp Neurol. 2001;434:461–477. doi: 10.1002/cne.1187. [DOI] [PubMed] [Google Scholar]
- Thompson WL, Karpus WJ, Van Eldik LJ. MCP-1-deficient mice show reduced neuroinflammatory responses and increased peripheral inflammatory responses to peripheral endotoxin insult. J Neuroinflamm. 2008;5:35–48. doi: 10.1186/1742-2094-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Baluk P, McDonald DM. Determinants of endothelial cell phenotype in venules. Microcirculation. 2000;7:67–80. [PubMed] [Google Scholar]
- Toborek M, Lee YW, Pu H, Malecki A, Flora G, Garrido R, Hennig B, Bauer HC, Nath V. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J Neurochem. 2003;84:169–179. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- Van der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van DV, De GC. Expression of CCL2 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154:45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen J, Bo L, Vos CMP, Virtanen I, de Vries HE. Basement membrane proteins in multiple sclerosis-associated inflammatory cuffs: potential role in influx and transport of leukocytes. J Neuropathol Exp Neurol. 2005;64(7):22–729. doi: 10.1097/01.jnen.0000173894.09553.13. [DOI] [PubMed] [Google Scholar]
- Wang X, Feuerstein GZ. Induced expression of adhesion molecules following focal brain ischemia. J Neurotrauma. 1995;12:825–832. doi: 10.1089/neu.1995.12.825. [DOI] [PubMed] [Google Scholar]
- White FA, Wilson NM. Chemokines as pain mediators and modulators. Curr Opin Anaesthesiol. 2008;21:580–585. doi: 10.1097/ACO.0b013e32830eb69d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res. 2001;896:36–42. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Horiba M, Buescher JL, Huang D, Gendelman HE, Ransohoff RM, Ikezu Y. Overexpression of monocyte chemoattractant protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse amyloid deposition. Am J Pathol. 2005;166:1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Takeya M, Takahashi K, Kuratsu J, Leonard EJ. Production and characterization of mouse monoclonal antibodies against human monocyte chemoattractant protein-1. J Immunol. 1991;147:2229–2233. [PubMed] [Google Scholar]
- Zernecke A, Weber C. Inflammatory mediators in atherosclerotic vascular disease. Basic Res Cardiol. 2005;100:93–101. doi: 10.1007/s00395-005-0511-6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Smith C, Howlett C, Stanimirovic D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1beta. J Cereb Blood Flow Metab. 2000;20:967–978. doi: 10.1097/00004647-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177:8103–8110. doi: 10.4049/jimmunol.177.11.8103. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- Zuurman MW, Heeroma J, Brouer N, Boddeke HW, Biber K. LPS-induced expression of a novel chemokine receptor (L-CCR) in mouse glial cells in vitro and in vivo. Glia. 2003;41:327–336. doi: 10.1002/glia.10156. [DOI] [PubMed] [Google Scholar]