FK 506 is a potent immunosuppressant agent which has undergone extensive in vitro and animal studies since its discovery in 1983.1–3 Since the first International Workshop on FK 506 in 1987, growing excitement has surrounded the development of an agent which is 100 times more potent than CyA.1 The major limitation of CyA has been the almost universal development of nephrotoxicity, leading to renal failure and the need for dialysis in some patients.4,5 The development of an agent which is more potent than CyA and which is less nephrotoxic would be a major advance in organ transplantation. We report our preliminary results on renal function and associated factors, using FK 506 as the major immunosuppressant in humans after liver transplantation.
PATIENTS AND METHODS
The first human experience with FK 506 was obtained in patients who will hereafter be referred to as the “rescue” group. These were adult patients with significant CyA toxicity, including chronic renal failure or hypertension. In addition to these complications, patients with coexisting pathologic evidence for rejection, which had been refractory to conventional methods, were included in this group. A second group of patients (“primary”) receiving orthotopic liver transplants were treated with FK 506 as the major immunosuppressant agent without prior exposure to CyA.
FK 506 was initially administered intravenously (0.15 mg/kg/d) for the first day, then switched to the oral form on the second day (0.3 mg/kg/d). FK 506 drug levels were monitored by immunoassay and CyA levels by radioimmunoassay.6 In most patients, CyA was discontinued on the first day of FK 506 therapy. Daily routine laboratory data included blood sugar, electrolytes, blood urea nitrogen, serum creatinine (SCr). complete blood cell count, and liver chemistries.
The initial 20 primary treatment patients were retrospectively matched with 20 CyA-treated patients by liver disease, age, sex, and UNOS classification. These subjects were not matched for pretransplant renal function. The CyA-treated patients served as control subjects in comparisons of renal function and other associated variables.
RESULTS
Primary Treatment Group
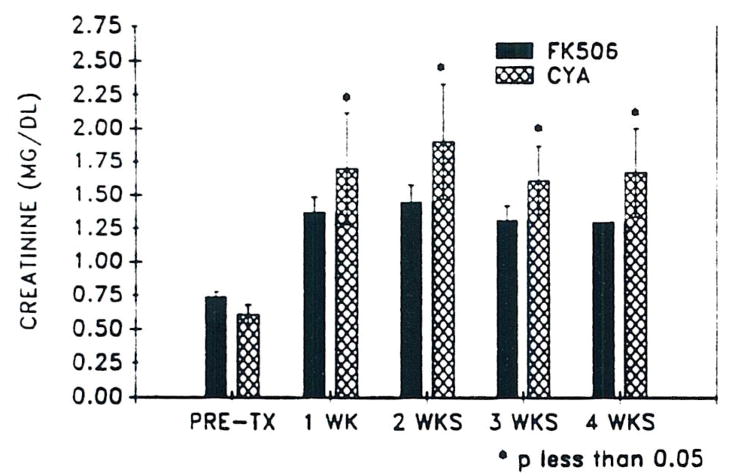
Since FK 506 and CyA-treated patients were not controlled for renal function, pretransplant SCr was statistically different when all patients were considered. Serum creatinine was significantly greater in the CyA group at all times when compared with primary FK 506-treated patients (Fig 1). Three of the CyA group required hemodialysis during the initial transplant admission, but none of the FK 506 group were dialyzed.
Fig 1.
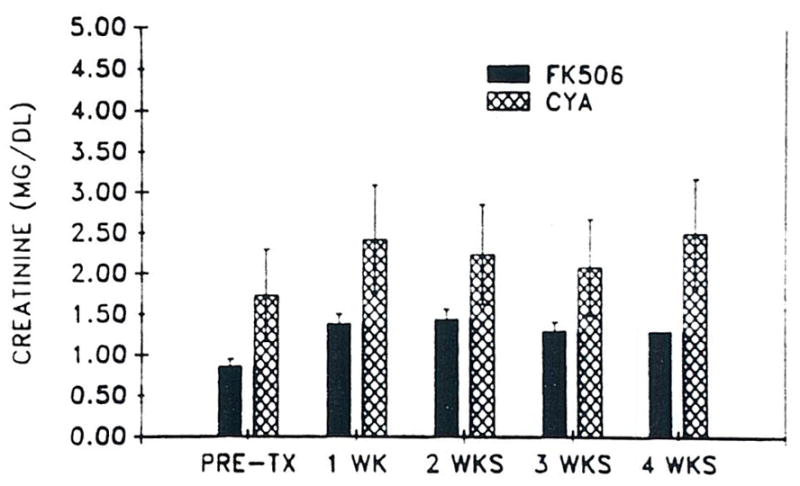
Mean SCr (± SD) versus time in 20 primary FK 506 patients and 20 CyA controls.
An analysis of only patients with SCr < 1.5 mg/dl is illustrated in Fig 2. This analysis included 17 FK 506 patients and 12 CyA patients. Pretransplant SCr was less in CyA-treated patients (0.62 ± 0.24) compared with FK 506 patients (0.74 ± .04 mg/dl). P = 0.08. Thereafter, SCr was significantly greater in the CyA-treated patients. Further, all of the patients eventually requiring dialysis had SCr <1.5 mg/dl. Acute renal failure requiring dialysis was associated with ischemic acute tubular necrosis in all cases.
Fig 2.
Mean SCr (± SD) versus time in 20 primary FK 506 and control CyA patients with pretransplant SCr < 1.5 mg/dl.
There was a poor correlation between SCr and FK 506 levels (r = 0.45). FK 506 drug levels ranged from 0.2 to 8 ng/ml. Decreasing drug dose and the associated drug level did not decrease SCr consistently. Conversely, CyA drug level and SCr were closely associated (r = 0.88). The CyA drug levels ranged from 400 to 2.500 ng/ml.
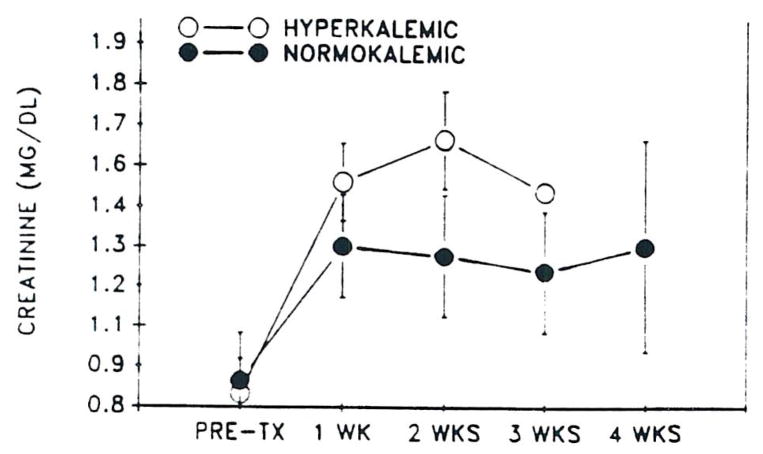
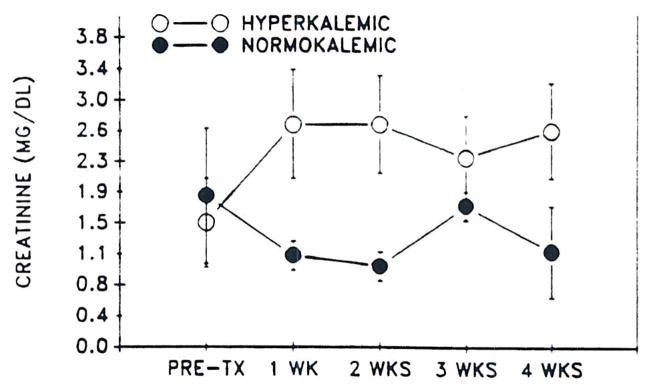
Hyperkalemia was a frequent occurrence in both CyA- and FK 506-treated patients. In the FK 506 group, hyperkalemia was associated with low or low normal renin and aldosterone levels in all cases. In addition, hyperkalemic patients in both groups had higher SCr than normokalemic patients (Figs 3 and 4). The hyperkalemia was not severe and was responsive to fludrocortisone acetate (0.1 to 0.2 mg twice/d) in all cases requiring treatment.
Fig 3.
Mean SCr (± SEM) versus time in hyperkalemic and normokalemic primary FK 506-treated patients.
Fig 4.
Mean SCr (± SD) versus time in hyperkalemic and normokalemic CyA-treated controls.
Hypertension developed postoperatively in only one FK 506-treated patient with Alagilles’ syndrome and presumed renal artery stenosis. None of the remaining FK 506-treated patients required antihypertensive therapy at any time.
FK 506 as Primary Therapy For Liver Transplantation
FK 506 as initial therapy for liver transplantation has revealed unprecedented results. The incidence of rejection has been reduced to very low levels, and prednisone doses have been drastically reduced. This will be discussed in detail by Dr Todo in his report on FK 506 as primary therapy for organ transplantation (in this issue).
FK 506-Treated Rescue Group
By definition, all of the rescue patients had CyA nephrotoxicity prior to starting FK 506 therapy. Although mean SCr prior to FK 506 in liver transplant patients was 1.55 ± 1.3 mg/dl, the range was 0.7 to 5.4 mg/dl. Further, the mean glomerular filtration rate (GFR) as measured by iothalamate clearance was 35 ± 16.9 cc/min, suggesting severely impaired renal function. None of these patients, however, was dialysis-dependent prior to FK 506 therapy.
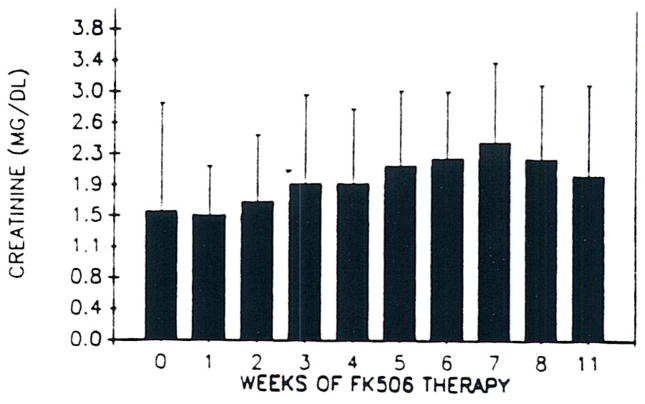
Renal function deteriorated in all rescue patients after starting FK 506 (Fig 5). This was associated with elevated CyA levels initially in most cases, but SCr remained elevated after CyA in the plasma became undetectable. Serum creatinine fell in many patients after 6 to 8 weeks of therapy. In many recently treated rescue patients not included in this analysis. FK 506 was initially given orally without prior intravenous therapy. This practice has attenuated the later increase in SCr in most patients.
Fig 5.
Mean SCr (± SEM) versus time in rescue patients.
Hyperkalemia occurred in all rescue patients at some time during therapy. However, all except two had been hyperkalemic during the 6 months prior to FK 506 therapy and while receiving CyA. Similar to the primary group, renin and aldosterone levels were low or low normal while these patients were hyperkalemic. Likewise, hyperkalemia was associated with increased SCr and was responsive to replacement therapy with fludrocortisone acetate (Florinef). Florinef, in doses less than 0.2 mg twice/d, was usually ineffective in controlling the hyperkalemia.
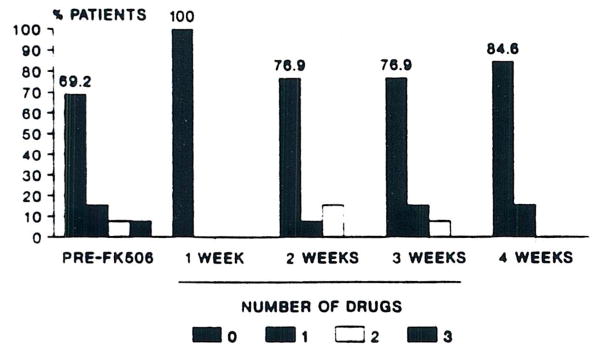
Hypertension requiring antihypertensive therapy was present in most of patients prior to FK 506 therapy (Fig 6). Some patients required as many as three antihypertensive agents to control blood pressure. In the 20 rescue patients, all antihypertensive agents were discontinued in the first week of FK 506 therapy due to an improvement in blood pressure. Three patients requiring three agents stopped all antihypertensives in the first week of FK 506 treatment. During weeks 2 to 3, the percentage of drug-free patients decreased; this was associated with increasing SCr. By week 4, the percentage of drug-free patients had increased to approximately 85%. During weeks 2 to 3, one patient required an antihypertensive for the first time, but this was discontinued during week 4. There were no instances of new onset severe hypertension in any of the rescue patients. Subsequent experience with patients not included in this study has generally confirmed these observations in liver transplant patients.
Fig 6.
Percentage of rescue patients requiring antihypertensive agents versus time.
“Compassionate Rescue Patients”
Four patients with refractory hepatic rejection who were dialysis-dependent, infected, required ventilatory support, and in intensive care units were treated with FK 506. These patients were termed “compassionate rescues” since many of them were not expected to survive given the severity and number of complications. FK 506 was initiated with 0.15 mg/kg/d intravenously, and 0.3 mg/kg/d orally when the patients tolerated oral feedings. In one patient, urine output increased 24 hours after starting FK 506 and discontinuing CyA. Renal function improved in the other patients within 2 weeks of starting therapy with FK 506. Hepatic rejection was completely reversed in all patients, and none of these previously critically ill patients died.
One patient with CyA-induced hemolytic uremic syndrome was also treated with FK 506. This patient experienced a prompt correction in hematologic parameters and improvement in renal function. This patient will be described in detail in another communication.
Efficacy in Treating Refractory Hepatic Rejection
FK 506 reversed ongoing refractory hepatic rejection in the majority of cases. Many of these patients would have required retransplantation prior to FK 506 therapy. These remarkable results were accomplished without the use of high-dose steroids or OKT3. This will be discussed in detail by Dr Fung in his analysis of the results of FK 506 as rescue therapy.
DISCUSSION
The first human trial of FK 506 has proven that it is a potent immunosuppressant with few side effects. This is contrary to early reports in animals, which suggested that it was a toxic agent causing vasculitis and failure to thrive in the majority of subjects.7 Subsequent human studies have not reproduced the severe toxicity noted in these studies.8
Based on our experience with human subjects, it appears that (1) FK 506 is not a classic nephrotoxin leading to renal cell death and acute renal failure; (2) FK 506 does not have the pattern of nephrotoxicity found with CyA, but may have mild nephrotoxicity; and (3) prior exposure to CyA results in a consistent deterioration of renal function which may improve after weeks of therapy.
In the first 20 patients treated with FK 506 alone, no cases of acute renal failure occurred during the first 4 weeks or subsequently. This suggests that FK 506 is not an obvious renal cytotoxic agent, similar to aminoglycoside antibiotics or other nephrotoxins. Further, multiple urinalyses in both primary and rescue patients have not revealed findings suggesting subclinical acute tubular necrosis. Given the wide range of FK 506 drug levels, such a cytotoxic effect should have been uncovered at the higher drug levels. The modest rise in SCr noted in this study was not due to renal cell death.
Previous reports of CyA nephrotoxicity have disclosed a close association between drug level and SCr.5 The changes in CyA dose were reflected in SCr within 24 hours. With FK 506, a poor correlation exists between drug level and renal function, while CyA-induced acute renal failure has been described, usually associated with toxic drug levels. We have not noted this phenomenon with FK 506. FK 506 drug levels varied from 0.2 to 8 ng/ml, without associated changes in SCr. It is unlikely that changes in SCr will be a reliable marker of FK 506 toxicity.
Previous studies have suggested that CyA, by increasing renal vascular tone, predisposes to toxic or ischemic acute renal failure.5 The relatively high occurrence of acute renal failure in our CyA control group and a previous study at this center support this observation.4 Conversely, the absence of acute renal failure in FK 506 patients suggests that it does not predispose to other causes of acute renal failure. In an earlier study, we noted a 90% mortality in liver transplant patients requiring dialysis. Reducing the incidence of acute renal failure and infection should reduce mortality in patients treated with FK 506.
The effects of FK 506 on renal function are strikingly different in primary subjects when compared with rescue patients. Prior exposure to CyA and its resulting nephrotoxicity has led to a predictable deterioration in renal function which improves after a period of weeks. The likely reason for this is the combined severe nephrotoxicity of CyA with the likely mild nephrotoxicity of FK 506. It is possible that renal tissue CyA persists after plasma drug levels have diminished. Nonetheless, the combined use of FK 506 and CyA will be complicated by worsening renal function and should be avoided. In patients not suffering from chronic CyA nephrotoxicity, renal function has been relatively well-preserved. When comparing primary therapy patients to their CyA controls, FK 506 appears to be significantly less nephrotoxic. Whether it causes less intrarenal vasoconstriction cannot be answered in this preliminary report, but ongoing studies in humans at this center will address this in later communications. In a previous study of dogs and rats conducted by Todo et al using an ischemic renal model. FK 506 was also found to be less nephrotoxic than CyA.9 Likewise, a study by Nelesnik et al (in this issue), has confirmed these findings.
Hyperkalemia, which is usually mild, is a common occurrence with both CyA and FK 506. Previous studies in CyA-treated patients have usually revealed hyporeninemic hypoaldosteronism.10,11 This finding has also been noted in the FK 506-treated patients. The excellent response to mineralocorticoid replacement strongly supports this. In both CyA- and FK 506-treated patients, an increase in SCr was also associated with hyperkalemia, suggesting that reduced excretion of potassium is due, in part, to a reduction in GFR.
An unexpected finding in this study was the beneficial effect of FK 506 on CyA-induced hypertension. This suggests that FK 506 reduces the peripheral vascular resistance caused by CyA. Although a reduction in antihypertensive medications was not universal, a dramatic elimination of hypertension occurred in some patients. This effect was unfortunately attenuated in the rescue patients by worsening renal function and presumed volume retention. It is likely that blood pressure control will also improve as renal function ameliorates with time.
Although we have found no strong evidence that FK 506 is as nephrotoxic as CyA, it is likely that FK 506 has a milder nephrotoxic effect, both early and after chronic exposure. More detailed studies of renal function are currently under way at the University of Pittsburgh, which will answer these questions in the future.
In summary, FK 506 is a remarkably potent immunosuppressant agent which, at the very least, has less nephrotoxicity than CyA after liver transplantation. Its ability to prevent rejection and to treat refractory rejection makes this an exciting and potentially revolutionary drug. More detailed studies, both in animals and humans, will more clearly define the influence of FK 506 on renal function.
Acknowledgments
Supported by Research Grant No. DK29961 from the National Institutes of Health, Bethesda, MD, and the Veterans Administration.
References
- 1.Starzl TE, Makowka L, Todo S. Transplant Proc. 1987;19(suppl 5) [PMC free article] [PubMed] [Google Scholar]
- 2.Thomson AW. Immunol Today. 1989;10:6. doi: 10.1016/0167-5699(89)90057-1. [DOI] [PubMed] [Google Scholar]
- 3.Morris RE, Hoyt EG, Murphy MP, et al. Tranplant Proc. 1989;21(suppl 1):1042. [PubMed] [Google Scholar]
- 4.McCauley J, Van Thiel D, Starzl TE, et al. Nephron. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyers BD. Kidney Int. 1986;30:964. doi: 10.1038/ki.1986.280. [DOI] [PubMed] [Google Scholar]
- 6.Tamura K, Kobayashi M, Hashimoto K, et al. Transplant Proc. 1987;19(suppl 6) [PubMed] [Google Scholar]
- 7.Thiru S, Collier St J, Calne R. Transplant Proc. 1987;19(suppl 6):98. [PubMed] [Google Scholar]
- 8.Starzl TE, Todo S, Fung J, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todo S, Murase N, Ueda Y, et al. Transplant Proc. 1988;20:215. [PMC free article] [PubMed] [Google Scholar]
- 10.Stanek B, Kovarik J, et al. Clin Nephrol. 1987;28:186. [PubMed] [Google Scholar]
- 11.Stanek B, et al. Nephron. 1985;41:124. doi: 10.1159/000183562. [DOI] [PubMed] [Google Scholar]