Liver transplantation has been increasingly used to treat end-stage liver diseases since the introduction of CyA in combination with other immunosuppressive agents.1 Although CyA has a powerful immunosuppressive effect on allograft rejection, many transplant recipients still experience rejection episodes that are often irreversible. The nephrotoxicity of CyA in some recipients has necessitated the reduction of CyA dosage below effective levels. With liver recipients refractory to conventional immunosuppression or with CyA-induced nephrotoxicity, a first-phase clinical trial of a new immunosuppressive drug, FK 506, has been initiated at our institution.2
FK 506 was shown by in vitro and in vivo studies to be considerably more immunosuppressive than CyA.3–7 The successful outcome of the initial clinical studies on 14 liver transplant recipients2 has led to a formal clinical trial of FK 506 as the primary immunosuppressive agent in combination with low doses of steroids.8
Appropriate dosages of FK 506 are being determined to provide optimal immunosuppression without significant adverse side effects. The monitoring of blood levels of FK 506 is essential in these studies. This monitoring has become possible with an enzyme immunoassay (ELISA) using anti-FK 506 MoAbs. This assay was developed by Tamura et al9 and modified by Cadoff et al.10
FK 506 is a highly lipophilic macrolide (molecular weight 822).11 Although little is known about the metabolites of FK 506, recent studies have implicated the liver as the primary site of FK 506 metabolism.12
The objective of this study was to determine the relationship between plasma levels of FK 506 as measured by ELISA and by a bioassay based on the proliferative response of an alloreactive T cell line.
MATERIALS AND METHODS
Plasma
Five milliliters of heparinized blood was collected from four liver transplant recipients during the first month following transplantation. Each sample was drawn just prior to FK 506 administration. All blood samples were incubated at 37°C for 1 hour prior to centrifugation. The plasma was analyzed for FK 506 trough levels using an indirect ELISA10 and a bioassay described in detail below.
Drug Source
FK 506 was supplied in crystalline form by the Fujisawa Pharmaceutical Co Ltd, Osaka, Japan. A stock solution of 100 μg/ml FK 506 was prepared in methanol and kept at −4°C.
Bioassay of FK 506 Levels in Plasma
Inhibition Effect of FK 506 on Lymphocyte Proliferation
This was determined in a secondary proliferation assay using a DQwl- specific alloreactive T cell clone DB2913 as responder cells. Frozen cloned cells were thawed and washed in RPMI 1640 (Gibco) and resuspended at 105 cells/ml in tissue culture medium (TCM) consisting of RPMI 1640 supplemented with 25 mmol/L Hepes buffer, 100 U/ml gentamicin, and 10% nontransfused human male serum. The inhibitory effect of FK 506 on the primed lymphocyte (PLT) response of DB29 cells was measured at different concentrations of the drug, ranging from 0.003–1 ng/ml. In these 3-day PLT assays, 104 responder cells were incubated in triplicate with 105 DQwl-positive stimulator cells irradiated (2,000 rad) in 200 μl of TCM. During the final 20 hours of incubation, each culture was labeled with 1 μCi of 3H-thymidine. The cultures were harvested and counted in a liquid scintillation counter (LKB). The inhibitory effect of FK 506 at various concentrations was determined with the following formula:
Effect of Plasma Samples on Lymphocyte Proliferation
Plasma samples were diluted (½ to 1/64) in TCM. The inhibitory effect of different plasma dilutions on the PLT response of DB29 cells was measured as described for FK 506. In these assays, 104 responder cells (50 μl) and 105 stimulator cells (50 μl) were incubated with 100 μl of plasma. A pretransplant plasma sample from each patient was used as a control. The results were calculated with the following formula:
Calculation of FK 506 Levels From Bioassay Results
The plasma concentrations of FK 506 were calculated from the inhibition curve observed with various plasma dilutions in comparison with a standard curve for FK 506. These calculations were made using a nonlinear sigmoidal model curve fitting program (Statistical Analysis System [SAS] Institute, release 5.18, Cary, NC). The IC50 from the standard curve was determined as the concentration of FK 506 (ng/ml) which caused 50% inhibition of the PLT response of DB29. From the plasma inhibition curve, the ID50 was determined as the dilution factor for which plasma induced 50% inhibition of PLT response of DB29. From these values, the plasma concentration of FK 506 was calculated as IC50 × ID50.
RESULTS
Initial studies showed highly consistent dose response curves of PLT inhibition of DB29 cells by FK 506. Fig 1 shows representative results of three separate experiments. These data yielded a mean IC50 value of 0.063 ± 0.001 ng/ml.
Fig 1.
Standard curves of the FK 506 bioassay. The concentration of FK 506 is plotted versus percent Inhibition of PLT response of DB29 cells. The IC50 was calculated using a nonlinear sigmoidal model fitted to the points.
Examples of PLT inhibition of DB29 by patient plasma are shown in Table 1. These bioassays were done on a liver allograft recipient at 7, 12, and 14 days posttransplant. The plasma FK 506 levels were calculated by multiplying the ID50 values with the IC50 value of 0.063 ng/ml.
Table 1.
Bioassay of FK 506 Levels in Plasma From a Liver Transplant Recipient
| Dilutions (% Inhibition) |
||||||
|---|---|---|---|---|---|---|
| Days Posttransplant | 1:4 | 1:8 | 1:16 | 1:32 | ID50* | FK 506† (ng/ml) |
| 7 | 99 | 89 | 48 | 0 | 15 | 0.94 |
| 12 | 93 | 40 | 0 | 0 | 7 | 0.44 |
| 14 | 99 | 98 | 76 | 16 | 21 | 1.32 |
ID50 represents the plasma dilution factor causing 50% inhibition of PLT response of DB29 cells.
Calculated by multiplying ID50 by the IC50 of 0.063 ng/ml.
Comparison Between FK 506 Levels Measured by ELISA and Bioassay
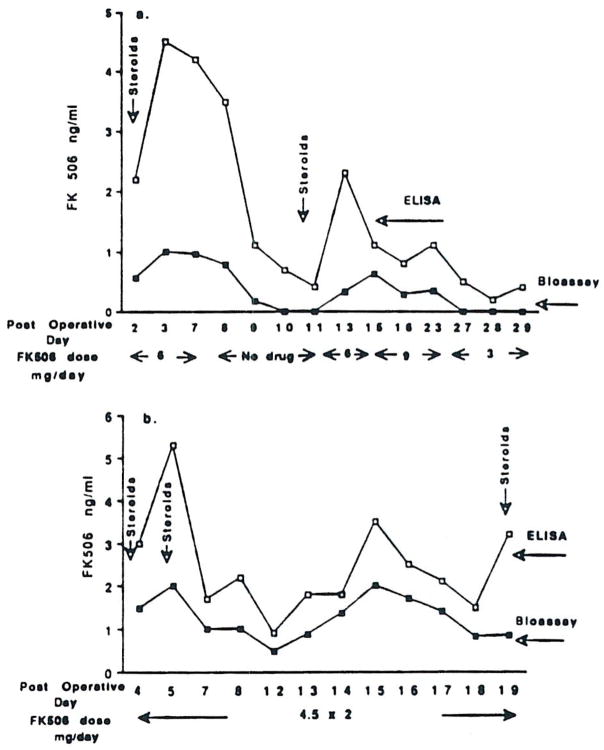
In all plasma samples tested, the values for FK 506 levels obtained by ELISA were generally much higher than those determined in the bioassay. Fig 2 compares FK 506 levels determined by ELISA and bioassay for two liver transplant recipients.
Fig 2.
FK 506 levels in plasma measured by the bioassay and by the ELISA for liver transplant patients, (a) P2 and (b) P14.
Patient P2 was originally diagnosed with sclerosing cholangitis and had lost five previous liver allografts while on CyA treatment. Following the sixth transplant, 6 mg/d of FK 506 was administered intravenously for 7 days. Methylprednisolone (1 g) was given on the second day posttransplant and was tapered daily to a maintenance dose of 10–20 mg/d. Although FK 506 levels determined by bioassay were always lower than those measured in ELISA (0.2–2 ng/ml versus 0.5–4.5 ng/ml), they showed a significant correlation (P < 0.001, r2 = 0.77).
Following the first bolus of methylprednisolone (day 2 postsurgery), the FK 506 concentrations measured by ELISA were four- to fivefold higher than the bioassay levels. FK 506 was discontinued for 4 days; the ELISA concentrations of FK 506 dropped sharply to 0.5–1 ng/ml, and no detectable immunosuppressive activity was found in the bioassay in those plasma samples. On day 11 postsurgery, the patient showed clinical symptoms of graft dysfunction as determined by changes in liver enzymes. On the same day, FK 506 (6 mg/d) and a bolus of methylprednisolone (1 g) were administered. Consequently, there was a rapid increase in the ELISA plasma concentrations of FK 506 and a more gradual rise in the bioassay-determined FK 506 levels (Fig 2a).
Similar findings are shown for patient P14, who was diagnosed with hepatitis B and juvenile diabetes mellitus. He received kidney, pancreas, and liver allografts. FK 506 (4.5 mg) was administered intravenously two times per day, and this dose was maintained for 19 days posttransplantation. Steroids were given on days 2, 5, and 19 postsurgery.
A significant correlation (P < 0.001, r2 = 0.75) was found between the two assays for plasma levels of FK 506 (Fig 2b).
Following steroid administration, FK 506 levels determined by bioassay were still much lower than ELISA plasma concentrations of FK 506.
Relationship Between FK 506 Dose and the Plasma Concentration
There was no apparent correlation between the dose of FK 506 given and the concentration of FK 506 in the patient’s plasma. Although patients P14, P16, and P32 received the same dose of FK 506, the plasma levels of FK 506 (ELISA and bioassay) were much higher in patients P14 and P16 than in the patient P32 (Fig 2 and Table 2). Furthermore, in three of four plasma samples tested from patient P32, the bioassay levels of FK 506 were <0.1 ng/ml, whereas the ELISA values ranged from 0.3–1.6 ng/ml. This patient had a histologically confirmed cellular rejection. Patient P16, on a similar dose of FK 506, had a stable posttransplant course. His plasma concentrations of FK 506 as determined by ELISA and bioassay were 1.2–6 ng/ml and 1–4 ng/ml, respectively.
Table 2.
Correlation Between Bioassay and ELISA
| Days Posttransplant | Drug Dose (mg/day) | Bioassay (ng/ml) | ELISA (ng/ml) |
|---|---|---|---|
| Patient P32 | |||
| 2 | 4.5 × 2 IV* | <0.1 | 1.6 |
| 5 | 4.5 × 2 IV* | 1.7 | 6.4 |
| 8 | 9 × 2PO† | < 0.06 | 0.9 |
| 12 | 9 × 2PO† | 0.06 | 0.3 |
| Patient P16 | |||
| 2 | 9 × 2PO† | 2.4 | 2.6 |
| 3 | 9 × 2PO† | 1.7 | 2.3 |
| 4 | 9 × 2PO† | 1.0 | 1.2 |
| 5 | 9 × 2PO† | 1.3 | 1.8 |
| 6 | 9 × 2PO† | 4 | 6 |
| 7 | 9 × 2PO† | 2.5 | 3 |
Intravenous dose of 4.5 mg FK 506 twice a day.
Oral dose of 9 mg/d twice a day.
DISCUSSION
This study was designed to assess the correlation between FK 506 levels measured by bioassay and ELISA.
The bioassay was based on the inhibition of PLT reactivity of an alloreactive T cell clone. Low concentrations of FK 506 significantly inhibited the proliferative responses of mixed lymphocyte reaction (MLR)-derived alloreactive T cell clones,4 and this inhibition was reproducible when the same T cell line was used (Fig 1). The sensitivity of the ELISA for FK 506 was 0.1 ng/ml10 whereas in the bioassay, drug levels of 0.063 ± 0.001 ng/ml had a significant inhibitory effect on the responder cells.
The levels of FK 506 determined in bioassay were consistently lower than those measured by ELISA. This suggests that the blood may contain inactive metabolites of FK 506 which could be detected by the ELISA. Since the ELISA is an immunoassay, it may measure biologically active and non-active metabolites that might cross-react with the anti-FK 506 antibody. Similarly, CyA metabolites are measured by monoclonal and polyclonal radioimmunoassay (RIA )used for monitoring trough levels of CyA in transplant recipients.14
FK 506-treated patients received a bolus of high-dose steroids the second day posttransplant and when acute allograft rejection was suspected. Steroids could affect the determination of FK 506 levels by the bioassay since this assay does not discriminate between various immunosuppressive drugs. Nevertheless, the FK 506 levels measured by bioassay poststeroid treatment were not much affected in contrast to the marked increases in immunoassayed FK 506. It is unclear how steroids influence ELISA assay. Corticosteroids have been shown to be inducers of hepatic drug metabolism.12 Experience with CyA immunosuppression has demonstrated that RIA levels of CyA increased after steroid treatment while high-pressure liquid chromatography-measured CyA levels appeared unaffected.15 Further studies are needed to elucidate the potential effect of steroids on FK 506 metabolism.
Biologic assays have previously been used for the measurement of CyA in patient’s plasma based on the inhibition by the plasma of a third-party MLR. No attempt was made, however, to express the inhibitory activity as a concentration of biologically active CyA. Both Kahan14 and Reisman et al16 suggested that biologic assay has clinical relevance: patients with continually low plasma immunosuppressive activity are prone to rejection of their kidney allografts. Our preliminary observations also indicated that the absence of immunosuppressive activity in patient’s plasma was associated with liver allograft rejection (patient P32).
Recently, Jain et al17 have shown that moderate to severe hepatic dysfunction induced an increase in trough levels of FK 506 measured by ELISA. Also, the CyA RIA levels were higher than the specific measurements of CyA during rejection episodes following liver transplantation.18 Studies are currently in progress to analyze the relationship between ELISA and bioassay levels during acute allograft rejection.
In conclusion, a bioassay based on the inhibition of an alloreactive T cell clone proliferation was used to determine FK 506 levels in patient’s plasma. A positive correlation was demonstrated between drug levels measured by the bioassay and ELISA. However, the FK 506 levels were lower for the bioassay than for ELISA. Since FK 506 cannot yet be assayed accurately in plasma using specific chromatographic techniques, the bioassay is important for assisting in the interpretation of an immunoassay (ELISA) with unknown specificity for biologically non-active forms of FK 506. Furthermore, biologic assay may be useful for patient management if active metabolites are induced, or to detect the interaction between FK 506 and other drugs (high-dose steroids) in the immunosuppression of allograft rejection.
Acknowledgments
Supported by Grants No. HL36416 and AI23467 from the National Institutes of Health, Bethesda, MD.
References
- 1.Starzl TE. Transplant Proc. 1988;20(suppl 3):356. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Todo S, Fung J, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochiai T, Nakajima K, Nagata M, et al. Transplant Proc. 1987;19:1284. [PubMed] [Google Scholar]
- 4.Zeevi A, Duquesnoy RJ, Eiras G, et al. Transplant Proc. 1987;19(suppl 6):40. [PMC free article] [PubMed] [Google Scholar]
- 5.Sawada S, Suzuki G, Kawase Y, et al. J Immunol. 1897;138:1797. [PubMed] [Google Scholar]
- 6.Todo S, Ueda Y, Demetris JA, et al. Surgery. 1988;104:239. [PMC free article] [PubMed] [Google Scholar]
- 7.Todo S, Demetris JA, Ueda Y, et al. Surgery. 1989;106:444. [PubMed] [Google Scholar]
- 8.Fung J, Todo S, Demetris JA, et al. Transplant Proc. (this issue) [Google Scholar]
- 9.Tamura K, Kobayaski M, Hashimoto K, et al. Transplant Proc. 1987;19(suppl 6):23. [PubMed] [Google Scholar]
- 10.Cadoff EM, Venkataramanan R, Krajack A, et al. Transplant Proc. (this issue) [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka H, Kuroda A, Marusawa H, et al. J Am Chem Soc. 1987;109:5031. [Google Scholar]
- 12.Venkataramanan R, Jain A, Cadoff E, et al. Transplant Proc. (this issue) [Google Scholar]
- 13.Zeevi A, Duquesnoy RJ. J Immunogenet. 1985;12:17. doi: 10.1111/j.1744-313x.1985.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 14.Kahan DB. Transplantation. 1985;40:457. doi: 10.1097/00007890-198511000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Ptachcinski RJ, Venkataramanan R, Burckart GJ, et al. Transplant Proc. 1987;19:1728. [PubMed] [Google Scholar]
- 16.Reisman L, Lieberman KV, Martinelli G, et al. Am J Kidney Dis. 1988;12:104. doi: 10.1016/s0272-6386(88)80003-9. [DOI] [PubMed] [Google Scholar]
- 17.Jain AB, Venkataramanan R, Cadoff E, et al. Transplant Proc. (this issue) [PMC free article] [PubMed] [Google Scholar]
- 18.Burckart GJ, Ptachcinski RJ, Venkataramanan R, et al. Transplant Proc. 1986;18(suppl 5):188. [PMC free article] [PubMed] [Google Scholar]