Abstract
In this study, the new drug FK-506 (FK) was tested in dogs for its ability to prevent rejection of kidneys when given alone, with small doses of cyclosporine or steroids, or both.
MATERIALS AND METHODS
Mongrel donors weighing 13 to 15 kg provided kidneys for beagle recipients of the same size under 25 mg/kg pentobarbital anesthesia intravenously. When needed, supplementary pentobarbital or ketamine, 2 mg/kg, or both, were given intravenously. The renal grafts were placed intraabdominally by connecting the renal artery and vein to the recipient iliac vessels and using uretero-neocystotomies. The recipient native kidneys were removed at the same operation. One gram of cephalosporin was given intravenously or intramuscularly during the operation and for three days afterwards. A diet was started the next day.
All immunosuppressive drugs were given orally starting on the morning after transplantation and continuing daily. To prevent vomiting caused by FK or other postoperative factors, 2 mg atropine sulphate was given intramuscularly twice a day for the first week and once a day for the second week. During the third week, the atropine dose was reduced to 1 mg/d.
The FK was provided in powder form by the Fujisawa Pharmaceutical Co, Ltd, Osaka, Japan, and placed in commercial capsules. The dogs were docile well-trained beagles who allowed deep digital placement of the capsules in the oropharynx. Prednisone (Pred) was given as a 5 mg tablet. Cyclosporine (CyA) in the commercial oil carrier used clinically was given orally by syringe.
FK Administration Alone
FK was the sole immunosuppressive treatment. The animals were placed in four groups: I, untreated control, n = 6; II, FK (0.5 mg/kg/d), n = 6; III, FK (1.0 mg/kg/d), n = 6; and IV, FK (1.5 mg/kg/d), n = 6.
FK in Combination
The smallest dose in the foregoing studies (group II, 0.5 mg/kg/d) or half this dose was combined with small doses of other agents as follows: group V, FK (0.5 mg/kg/d), CyA (5.0 mg/kg/d), Pred (5.0 mg/d), n = 6; group VI, FK (0.25 mg/kg/d), CyA (2.50 mg/kg/d), Pred (2.5 mg/d), n = 6; and group VII, CyA (5.0 mg/kg/d), Pred (5.0 mg/d), n = 6.
The results in group II were considered to be a single-agent control for those obtained with the triple-drug combination of group V. The animals of group VII who received CyA and Pred were considered controls for the triple-drug, FK-containing regimen of group V.
Biochemical and Pathological Studies
Blood samples were taken every three mornings for the measurement of levels of blood urea nitrogen, creatinine, SGOT, and total bilirubin. Complete postmortem examination was performed immediately after the animals died. Tissues were fixed with formalin and stained with hematoxylin and eosin. Histopathologic changes were scored blindly according to a subjective scale from 1 to 4.
Statistics
The Wilcoxon rank sum test and Student’s t test were applied for the stitistical analysis.
RESULTS
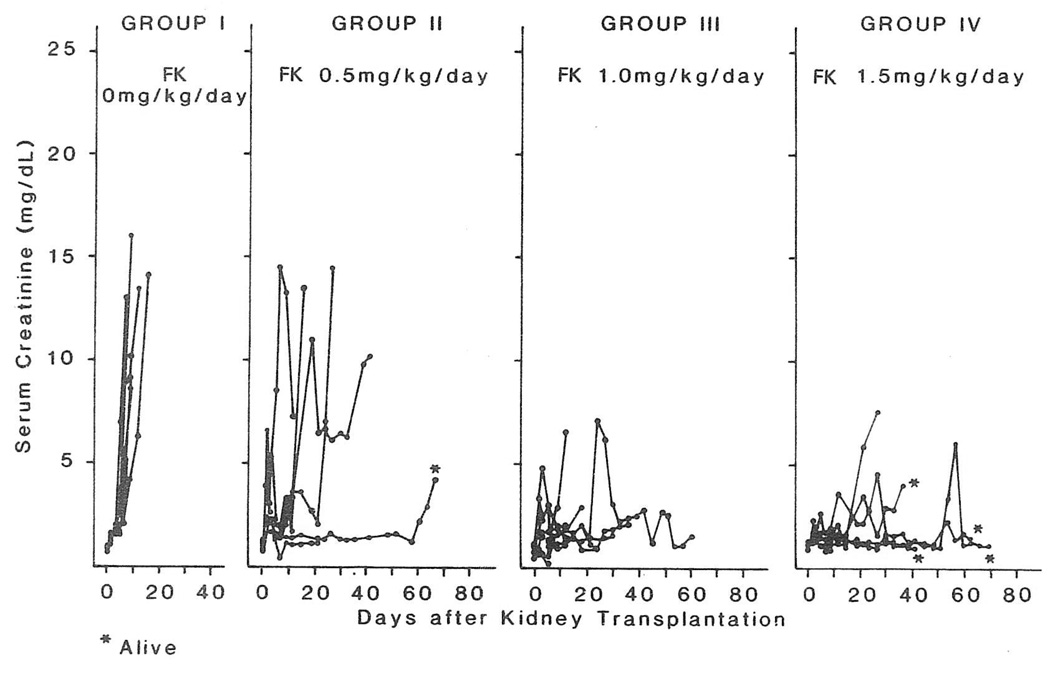
The outcome in each individual experiment is summarized in Table 1. The untreated animals died of rejection in eight to 19 days. Survival was significantly prolonged with all doses of FK, the best results being with 1.5 mg/kg. With this high dose, rejection as judged histopathologically was absent or mild in the two animals who provided tissue for histopathologic analysis. The other four are alive after 39 to 71 days. Increases in serum creatinine levels caused by rejection were minimized or avoided in animals receiving 1.0 and 1.5 mg/kg/d (Fig 1). With doses of 0.5 mg/kg/d, rejection was always found histo-pathologically (Table 1), and elevated creatinine levels were the rule (Fig 1).
Table 1.
Effect of FK Dose on Results of Canine Kidney Transplantation
| Groups | Oral Dosage (mg/kg/d) |
Animal No |
Survival (d) | Cause of Death |
Lastest Value |
Histological severity of Rejection |
|
|---|---|---|---|---|---|---|---|
| BUN (mg/dl) | Cr (mg/dl) | ||||||
| I | 0 | 3 | 19 | Rejection | 359 | 14.1 | Severe |
| 4 | 9 | Rejection | 154 | 8.2 | Severe | ||
| 5 | 12 | Rejection | 142 | 16.0 | Severe | ||
| 6 | 11 | Rejection | 271 | 13.5 | Severe | ||
| 7 | 8 | Rejection | 193 | 13.0 | Severe | ||
| 8 | 11 | Rejection | 135 | 8.7 | Severe | ||
| II | 0.5 | 102 | 16 | Rejection | 172 | 13.5 | Moderate |
| 104 | 24 | Intussusception | 44 | 1.1 | Mild | ||
| 109 | >70 | — | 77 | 4.3 | — | ||
| 112 | 44 | Rejection | 195 | 5.1 | Moderate | ||
| 121 | 13 | Rejection | 336 | 7.7 | Moderate | ||
| 122 | 28 | Rejection | 140 | 14.4 | Severe | ||
| III | 1.0 | 101 | 37 | Unknown | 154 | 2.5 | Moderate |
| 105 | 63 | Unknown | 24 | 1.0 | Severe | ||
| 108 | 16 | Rejection | 219 | 6.3 | Severe | ||
| 114 | 21 | Unknown | 59 | 2.9 | Severe | ||
| 123 | 14 | Unknown | 39 | 2.0 | None | ||
| 124 | 35 | Unknown | 41 | 1.5 | Moderate | ||
| IV | 1.5 | 103 | >71 | — | 20 | 1.0 | — |
| 107 | 28 | Pancreatitis (?) | 108 | 8.8 | Mild | ||
| 113 | >67 | — | 20 | 1.3 | — | ||
| 125 | 17 | Unknown | 42 | 1.2 | None | ||
| 127 | >44 | — | 19 | 0.9 | — | ||
| 133 | >39 | — | 95 | 4.0 | — | ||
Abbreviation: Cr, creatinine.
Fig. 1.
Influence of FK dose on serum creatinine level. The rises in the serum creatinine concentration in animals of all groups were usually caused by rejection.
However, animals treated with the higher doses of FK had significant evidence on histo-pathologic examination of toxicity involving the liver, heart, and pancreas (Table 2). The findings were similar to those reported elsewhere in this symposium in dogs not subjected to transplantation.
Table 2.
Histological Changes in Extrarenal Organs After Kidney Transplantation Under FK
| Group | Dosage (mg/kg/d) |
Liver |
Heart |
Pancreas: Acinar cell Degeneration (Focal Necrosis) |
||
|---|---|---|---|---|---|---|
| Swelling of Hepatocytes |
Fatty Change of Hepatocytes |
Arteritis | Infarction (Focal Large) |
|||
| I | 0 | 0/6 (0%) | 1/6 (16%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| II | 0.5 | 1/5 (20%) | 1/5 (20%) | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) |
| III | 1.0 | 1/6 (16%) | 0/6 (0%) | 2/6 (33%) | 2/6 (33%) | 3/6 (50%) |
| IV | 1.5 | 1/2 (50%) | 0/2 (0%) | 1/2 (50%) | 1/2 (50%) | 2/2 (100%) |
Vomiting and weight loss were common in all of the treatment groups. Severe emaciation with loss of more than 30% of the body weight was seen in four of the 18 FK-treated animals. Two animals each in groups II and III died with severe rejection plus lethal emaciation.
FK in Combination
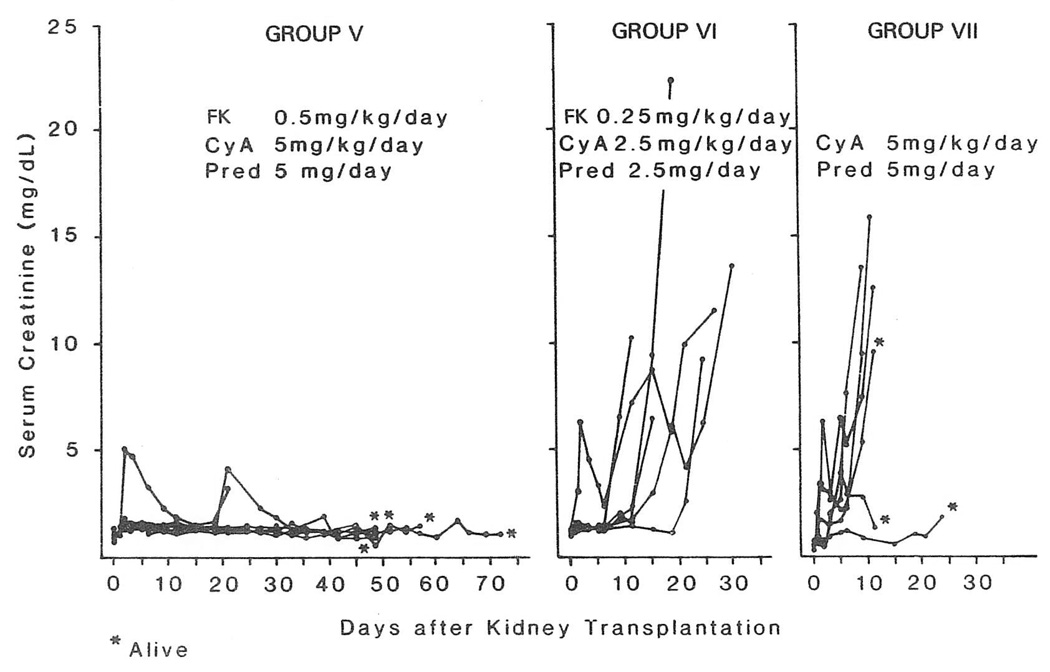
Five of six dogs in group V that were treated with 0.5 mg/kg FK, 5 mg/kg CyA, and 5 mg Pred are alive with normal renal function after 51 to 71 days. The survival results (Table 3) were better than those in group II in which 0.5 mg/kg FK was used alone (P < .01) and better than those in group VII in which 5 mg/kg CyA and Pred were given (P < .01). None of the animals in group V lost body weight.
Table 3.
Combination Therapy With Low Doses of FK, CyA, and Pred After Canine Kidney Transplantation
| Groups | Treatement Dose (Oral) |
Animal No. |
Survival (d) | Cause of Death |
Latest Value |
|
|---|---|---|---|---|---|---|
| BUN (mg/dL) | Cr (mg/dL) | |||||
| V | FK, 0.5 mg/kg/d | 106 | >71 | — | 16 | 1.0 |
| CyA, 5/0 mg/kg/d | 115 | 23 | Unknown | 159 | 3.2 | |
| Pred, 5.0 mg/d | 116 | >60 | — | 24 | 1.3 | |
| 118 | >53 | — | 16 | 1.2 | ||
| 119 | >51 | — | 29 | 1.0 | ||
| 120 | >51 | — | 24 | 1.2 | ||
| VI | FK, 0.25 mg/kg/d | 129 | 13 | Rejection | 94 | 6.6 |
| CyA, 2.5 mg/kg/d | 130 | 19 | Rejection | 113 | 22.4 | |
| Pred, 2.5 mg/d | 132 | 31 | Rejection | 107 | 6.2 | |
| 134 | 30 | Rejection | 176 | 11.6 | ||
| 135 | 19 | Rejection | 123 | 6.5 | ||
| 136 | 26 | Rejection | 105 | 9.3 | ||
| VII | CyA, 5.0 mg/kg/d | 137 | >24 | — | 30 | 1.9 |
| Pred, 5.0 mg/d | 138 | 12 | Rejection | 288 | 13.3 | |
| 139 | 12 | Rejection | 181 | 8.9 | ||
| 140 | 12 | Rejection | 147 | 15.5 | ||
| 141 | >12 | — | 120 | 12.5 | ||
| 142 | >12 | — | 52 | 2.8 | ||
The superiority of the triple-drug combination of group V was also evident from the serum creatinine levels (Fig 2). However, the three drugs when given at half dosage (group VI) were no longer effective (Table 3 and Fig 2).
Fig. 2.
Serum creatinine levels in dogs treated with drug combinations. Note the superior results in group V.
DISCUSSION
The principal studies of FK-506 in dogs by Ochiai et al1 and those by us2 have not yet been published. Ochiai’s Chiba University group had almost universal survival after canine renal transplantation without much evidence of toxicity.1 Our findings were less dramatic or favorable. Rejection often was not controlled, even at doses that led to profound illness or sometimes fatal emaciation. In addition, there was histopathologic evidence of toxicity in multiple extrarenal organs.
The toxicity of dogs may be a species-specific phenomenon. Rats have tolerated high-dose FK therapy well.3,4 The effect of FK on subhuman primates is under study at several institutions, but no results are available at this time. However, even if FK proves to have substantial dose-related toxicity in all species including humans, it could still be a valuable agent for clinical use. The synergism of small doses of FK with suboptimal doses of CyA and steroids was striking in our canine experiments herein reported. The results were consonant with those using the same drug combination in rats submitted to heterotopic heart transplantation.5 With in vitro studies of human lymphocytes, Zeevi et al6 also have obtained convincing evidence for a true synergism as opposed to an additive effect of FK and CyA.
More and more, polypharmaceutical therapy has been used to allow potent immunosuppressive agents to be used in doses small enough so that side effects inherent with high doses of single agents can be avoided.
SUMMARY
The immunosuppressive agent FK permitted increased kidney transplant survival in dogs over a wide dose range, but with weight loss and manifold evidence of toxicity. The best use of FK at low doses was in combination with CyA and Pred.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Ochini T, Nagata M, Nakajima K, et al. Transplantation. 1987 doi: 10.1097/00007890-198712000-00002. (in press) [DOI] [PubMed] [Google Scholar]
- 2.Todo S, Makowka L, Starzl TE. Surg Res Comm. 1987 (in press) [PMC free article] [PubMed] [Google Scholar]
- 3.Ochiai T, Nakajima K, Nagata M, et al. Transplant Proc. 1987;19:1284. [PubMed] [Google Scholar]
- 4.Lee P, Murase N, Todo S, et al. Surg Res Comm. 1987 (in press) [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Lee P, Lei H, et al. Transplant Proc. (this issue) [Google Scholar]
- 6.Zeevi A, Duquesnoy R, Eiras G, et al. Transplant Proc. (this issue) [Google Scholar]