Cyclosporine (CsA) is extensively metabolized and primarily excreted in bile. Renal elimination of CsA is a minor pathway of elimination in humans and animals with only 6% of an administered dose of radioactive CsA recoverable from the urine.1 In contrast to its poor renal elimination, 59% of radioactivity after administration of tritiated CsA to rats was recovered in bile.1 The presence of CsA and its metabolites in bile is important for several reasons. The immunologic activity and toxicity of CsA metabolites have not been elucidated, and thus, a local effect on the biliary system may be present and would be particularly important in a liver transplant patient. If significant quantities of an active or toxic substance are present in bile, enterohepatic recirculation of those compounds might have a measurable pharmacological effect. Finally, other drugs and diseases that influence cyclosporine metabolism could preferentially produce pharmacologically inactive or toxic metabolites.
This article reviews our quantitative work with CsA in bile, describes our qualitative work with CsA metabolites in bile, and discusses other currently published information on CsA metabolites in human bile.
Patients and Methods
Quantitative Studies
A complete description of our patients and methods has been published previously.2 Bile was collected during 18 studies from 13 patients with a T-tube in their common bile ducts. Twelve patients were receiving CsA following orthotopic liver transplantation, and one patient with liver disease was given a single intravenous (IV) CsA dose. Bile was collected over an 8- to 24-hour interval and was analyzed for unchanged CsA by high-performance liquid chromatography (HPLC). A radioimmunoassay (RIA) procedure3 was used to detect total quantities of CsA plus metabolites reactive with the RIA (abbreviated as CsT for clarity). Patients were arbitrarily divided into those having normal hepatic function (total serum bilirubin less than 1 mg/dL) and those with poor function (total serum bilirubin greater than 1 mg/dL) for comparison.
Qualitative Studies
Pooled bile from a single liver transplant patient was collected over two days from a T-tube in the common bile duct. Three-milliliter aliquots of bile were extracted with diethyl ether according to the blood extraction procedure of Sawchuk and Cartier.4 The extracted material was reconstituted with mobile phase and injected onto the HPLC column. The HPLC system was similar to a previous description5 except that a 60-minute gradient of acetonitrile-water was used as the mobile phase.
Major peaks were collected, mobile phase was removed, and the residue obtained was analyzed by desorption chemical ionization mass spectrometry using an Extrel Model 400-2D mass spectrometer (Extrel Corporation. Pittsburgh).
Cyclosporin G (CsG), 50 μg, was added during one extraction procedure as an internal standard to provide an estimate of the amount of each metabolite in the pooled bile sample. The areas of the metabolite peaks were compared with the peak area of CsG to provide an estimate relative to the amount of CsG in the sample.
Results
A wide range of concentrations of CsA were observed in the bile of liver transplant patients. The CsA concentration in bile determined by HPLC ranged from 32 ng/mL in the patient with severe liver disease to 5,212 ng/mL in a transplant patient with normal hepatic function. Table 1 lists the mean concentrations of CsA in bile measured by HPLC for the normal and poor liver function patients. The CsT concentration in bile measured by RIA ranged from 18 to 36 times higher than the CsA concentration. In our subjects, the ratio of CsT:CsA ranged from 23:1 to 39:1 with a mean of 30:1. Table 2 presents the estimated percentage of an absorbed CsA dose excreted in bile and demonstrates that CsA represents less than 4% of the measured RIA-reactive compounds in bile. Since the total volume of bile does not drain from the T-tube, the predicted row in Table 2 adjusts the volume of bile to 1 L/day.
Table 1. CsA in Bile Measured by HPLC.
| Liver Function Group | Average Bile Volume (mL/h) | Average Bile HPLC Concentration (ng/mL) | Average Amount of CsA in Bile (μg/h) |
|---|---|---|---|
| Normal (n = 3) | 24.1 | 3288 | 79.2 |
| Poor (n = 15) | 5.5 | 1379 | 7.6 |
Table 2. CsA in Bile Measured by RIA and HPLC.
| Percentage of Absorbed Dose Excreted in Bile | ||
|---|---|---|
| Patients | HPLC | RIA |
| Poor liver function* | 0.12 | 3.6 |
| Normal liver function* | 0.85 | 25.5 |
| Predicted in normal liver function† | 1.46 | 43.8 |
Amount recovered from T-tube.
Amount predicted in 1 L/day of bile.
While the relationship between blood and bile CsA is not always consistent, CsA in bile peaks several hours following a CsA dose. Figure 1 demonstrates the blood and bile CsA concentrations following a CsA dose.
Fig 1.
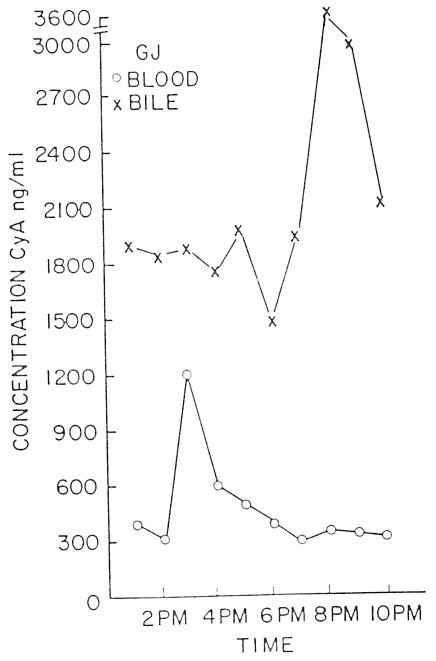
Cyclosporine blood and bile concentration v time in an orthotopic liver transplant patient.
Diethyl ether extraction of bile produced an HPLC chromatogram with seven major peaks having retention times shorter than CsA (Figure 2). Cyclosporinc and its metabolites produced a characteristic mass spectral fragmentation pattern under methane chemical ionization conditions. The metabolites that were analyzed and CsA produced a quasimolecular ion and an M + 29 adduct ion. The mass spectra also demonstrated a loss of a seven-carbon fragment with an atomic mass unit of 112, except for peaks 1, 4, and 6, which lost a mass unit of 128.
Fig 2.
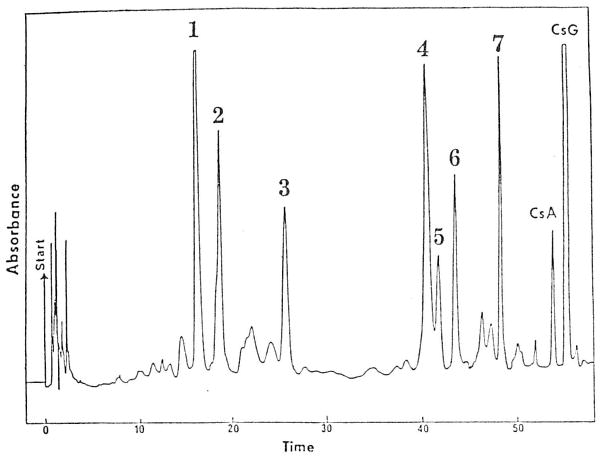
HPLC chromatogram for an ether extract of bile. The patient received CsA; CsG was added to the bile as an internal standard. Peaks 4, 5, 6, and 7 correspond to M17, M1, M18, and M21 isolated by Maurer et al.6
Peak 1 produced an M + 1 ion at 1,235, indicating two hydroxyl substitutions and considered to be the equivalent of M8 described by Maurer et al.6 Peak 3 produced an M + 1 ion at 1,205, indicating metabolic loss of a methyl group and the addition of a hydroxyl substitution. Peak 3 has the equivalent mass of Maurer et al's M13.6
Additional evidence of peak identity was provided by injection of previously isolated and identified metabolites (kindly provided by G. Maurer). Injection of Maurer et al's M17, M1, and M21 produced identical retention times to peaks 4, 5, and 7, respectively. Peaks 4, 5, 6, and 7 produced identical retention times to M17, M1, M18, and M21 on a separate chromatographic system (T. Rosano, personal communication).
By comparison of peak areas in the pooled bile sample, the seven major peaks were 1.5 to 4.0 times the peak area of CsA.
Discussion
Biliary excretion of CsA and its metabolites is the major route of drug elimination. While substances in bile can be reabsorbed and recirculated, our study shows that less than 2% of a dose of CsA appears in the bile even in the presence of normal liver function. The reabsorption of the parent compound is therefore inconsequential in discussions of a second peak concentration of CsT measured by RIA7 or late peaks of immunosuppressive activity.8
Cyclosporine metabolites are present in bile in large concentrations. Even though CsA metabolites only selectively cross-react with the RIA,9 concentrations of CsT approaching 100,000 ng/mL have been measured in bile.3 Table 2 would indicate that only about one half of CsA-related compounds can be measured by the RIA. These CsA metabolites would also have the opportunity to be reabsorbed and are therefore a potential explanation for late increases in serum immunosuppressive activity or for the disparity occasionally observed between CsA blood concentrations and clinical effect.
Two major hydroxylated metabolites, M17 and M18, appear to express less immunosuppressive activity than CsA.10 More recent studies have suggested that the concentrations of M17 that are achieved in the blood of renal transplant patients may be as active in mixed lymphocyte cultures as is CsA.11 Both M17 and M18, identified as peaks 4 and 6 in our studies, are found in bile and will most likely be present in concentrations exceeding blood concentrations. Their immunosuppressive effects locally on the biliary tract would be additive to that of CsA. While CsA appears in bile in high concentrations in patients with normal liver function, the additive local immunosuppressant properties of the metabolites may be critical in patients with impaired liver function (who have the lowest CsA bile concentrations) due to rejection or technical complications following liver transplantation.
Two separate groups of ether-extractable CsA metabolites are present in bile: those that are very nonpolar (peaks 4 to 7) and those that are slightly more polar (peaks 1 to 3). No single metabolite appears to predominate, and other more-polar metabolites might be identified using different extraction technqiues. For example, a carboxylic acid metabolite has been isolated from an ethyl acetate extraction of rabbit and human bile.12 The more-polar metabolites are less likely to cross lipoprotein membranes and express activity. For example, while there is general agreement that Maurer et al's M17 possesses some minor pharmacologic activity, the addition of another polar group (hyroxyl) to form M8 makes the compound inactive.11
Proper quantitation of CsA metabolites in blood or bile is important in understanding their potential pharmacologic or toxicologic effects. Hartman et al12 claim that the carboxylic acid metabolite is the major metabolite without quantitating other nonpolar CsA metabolites. Another recent report makes some interesting observations on the immunological activity of diethyl ether–soluble isolates from blood, bile, and urine13 but provides only an imprecise metabolite quantitation of the RIA. Precise quantitation of metabolites in blood, bile, and urine by HPLC must be available for a meaningful assessment of the pharmacologic role of CsA metabolites. In this manner, quantitation of the impact of drug-metabolite interactions, such as has already been reported for phenytoin,14 can be predicted.
Summary
Quantitative and qualitative studies of cyclosporine and its metabolites were performed on human bile from liver transplant and liver disease patients. The concentration of CsA in bile is higher in patients with normal liver function than in those with poor liver function but in neither case could account for more than 2% of an absorbed dose of CsA. Although concentrations of CsA plus metabolites in bile measured by RIA were 18 to 36 times higher than HPLC concentrations, they accounted for less than 50% of an absorbed CsA dose. By means of mass spectrometry and HPLC retention times of known metabolites, peaks equivalent to the previously isolated CsA M8, M13, M17, M1, M18, and M21 of Maurer et al were found in the ether extracts of bile. Future studies should not only concentrate on the pharmacologic and toxicologic effects of the metabolites but should also accurately quantitate these compounds in blood, plasma, urine, and bile.
Acknowledgments
Supported in part by Grant 5R01 AM34475, National Institute of Arthritis, Diabetes, Digestive, and Kidney Diseases, and by Sandoz Pharmaceuticals, East Hanover, NJ.
References
- 1.Wood AJ, Maurer G, Niederberger W, et al. Transplant Proc. 1983;15:193. [Google Scholar]
- 2.Venkataramanan R, Starzl TE, Yang S, et al. Transplant Proc. 1985;17:286. [PMC free article] [PubMed] [Google Scholar]
- 3.Scanlon L, Baloh R, Gridelli B, et al. Transplantation. 1986;41:657. doi: 10.1097/00007890-198605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawchuk RT, Cartier LL. Clin Chem. 1981;27:1368. [PubMed] [Google Scholar]
- 5.Ptachcinski RJ, Venkataramanan R, Rosenthal JT, et al. Clin Pharmacol Ther. 1985;38:296. doi: 10.1038/clpt.1985.174. [DOI] [PubMed] [Google Scholar]
- 6.Maurer G, Lossli HR, Shreier E, et al. Drug Metab Disp. 1984;12:120. [PubMed] [Google Scholar]
- 7.Kahan BD, Reid M, Newberger J. Transplant Proc. 1983;15:446. [Google Scholar]
- 8.Kahan B. Transplantation. 1985;40:457. doi: 10.1097/00007890-198511000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Abisch E, Beveridge T, Gratwohl A, et al. Pharmacol Week. 1982;4:84. doi: 10.1007/BF01962250. [DOI] [PubMed] [Google Scholar]
- 10.Maurer G. Transplant Proc. 1985;17:19. [PubMed] [Google Scholar]
- 11.Rosano TG, Freed BM, Cerilli J, et al. Transplantation. 1986;42:262. doi: 10.1097/00007890-198609000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hartman NR, Trimble LA, Vederas JC, et al. Biochem Biophys Res Commun. 1985;133:964. doi: 10.1016/0006-291x(85)91230-6. [DOI] [PubMed] [Google Scholar]
- 13.Wong PY, Mason N, Cheung F, et al. Transplantation. in press. [Google Scholar]
- 14.Freeman DJ, Laupacis A, Keown PA, et al. Br J Clin Pharmacol. 1984;18:887. doi: 10.1111/j.1365-2125.1984.tb02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


