Initial human trials with FK 506 conducted at the University of Pittsburgh have demonstrated a remarkable immunosuppressive effect of the drug on liver, kidney, and pancreatic transplants.1-3 It is expected that the drug may be used more extensively in the field of transplantation over the next few years. Since some of the early experiments suggested that FK 506 might be diabetogenic,4 oral glucose tolerance tests were performed in 12 liver transplant recipients who received FK 506, searching for possible derangements in glucose metabolism.
MATERIALS AND METHODS
The 12 patients whose immunosuppression consisted of FK 506 and low doses of prednisone were all adult individuals with no acute illness, no previous history of diabetes mellitus, and normal fasting blood sugar levels before transplantation. Table 1 shows the age, sex, steroid dose, and time after transplant of these 12 patients.
Table 1.
Features of the 12 Liver Transplant Recipients Tested
| Patient No. |
Age (yr) |
Sex | Days After Transplant |
Prednisone Dose (mg/d) |
Fasting Blood Sugar Level (mg/dl) |
Peak Insulin Level μU/ml) |
|---|---|---|---|---|---|---|
| 1 | 41 | F | 25 | 5 | 82 | 30 |
| 2 | 31 | M | 15 | 10 | 95 | 42 |
| 3 | 18 | M | 25 | 5 | 100 | 66 |
| 4 | 38 | M | 19 | 10 | 154 | 22 |
| 5 | 42 | M | 23 | 0 | 99 | 41 |
| 6 | 39 | M | 34 | 5 | 90 | 23 |
| 7 | 49 | M | 24 | 0 | 85 | 35 |
| 8 | 28 | M | 21 | 20 | 115 | 26 |
| 9 | 55 | M | 35 | 10 | 103 | 37 |
| 10 | 36 | F | 24 | 10 | 99 | 128 |
| 11 | 33 | F | 27 | 5 | 97 | 34 |
| 12 | 36 | M | 49 | 5 | 80 | 27 |
| Mean ± SD | 37 ± 9 | 27 ± 9 | 7 ± 5 | 99 ± 18 | 48 ± 28 |
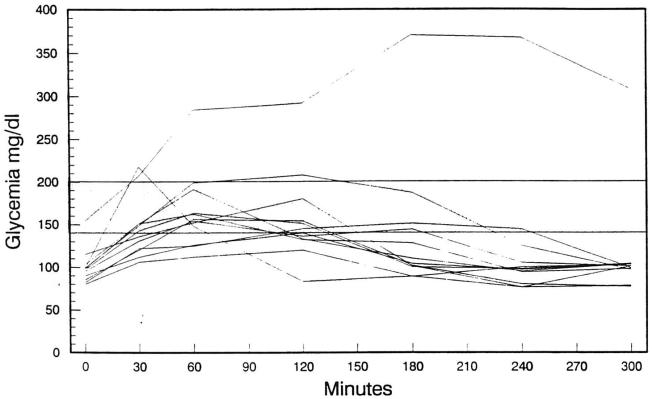
The test was conducted in the following fashion: after a 10-hour fast, a sample was obtained, and 75 g of glucose was administered orally. Samples for venous serum sugar levels were obtained at 30 minutes and 1, 2, 3, 4, and 5 hours. Simultaneous insulin levels were done at fasting and at hourly intervals for 5 hours. The glucose determinations were made using the Kodak Ektachem Colorimetric method (Eastman Kodak Company, Rochester. NY), while the insulin measurements were done by the radioimmunoassay method (1251 Insulin Radioimmunoassay Kit, Cambridge Medical Diagnostics). The results of this test are shown in Figs 1 and 2.
Fig 1.
Oral glucose tolerance test in 12 liver transplant recipients receiving FK 506.
Fig 2.
Corresponding insulin curve in the 12 liver transplant recipients receiving FK 506.
RESULTS
None of the patients were on insulin therapy. The fasting blood sugar levels were normal in 11 patients and abnormal in one (patient no. 4). Their mean values were 99 ± 18 (SD) mg/dl (Table 1). In six patients, the oral glucose tolerance test was considered normal (patients no. 2, 3, 6, 9, 10, and 12). In two patients (nos. 4 and 5), the results were consistent with incipient diabetes mellitus, while in the remaining four patients (nos. 1, 7, 8, and 11), although not entirely normal (a 2-hour sample <200 and >140 mg/dl with intermediate values of <200 mg/dl), the tests could not be classified as being indicative of diabetes or of impaired glucose tolerance tests. The fasting insulin levels were <20 μU/ml in all patients, with mean peak insulin levels of 42 ± 28 μU/ml (Table 1). The insulin curves are shown in Fig 2, and most follow a normal pattern. Again, patient no. 4 presented with the lowest peak insulin levels and a flat insulin curve. This patient had a family history of diabetes mellitus and a DR4 on human leukocyte antigen typing, which suggested that heredity was a major contributor to this patient's abnormal glucose tolerance test. Although asymptomatic, the patient has continued to have mildly elevated fasting blood sugar levels. The other patient with a diabetic-type curve (patient no. 5) has remained asymptomatic, with normal fasting blood sugar levels. This last patient has been completely weaned from steroids. DR typing for this patient was not available.
DISCUSSION
Posttransplant hyperglycemia is a well-recognized hazard of organ transplantation. The reason may be multifactorial. although drug toxicity may play a major role, since most standard immunosuppressive regimens contain prednisone and/or CyA, which are known to be diabetogenic in humans.5-8 Since similar effects had been ascribed to FK 506 in experimental animals,4 we were prompted to perform the present study. A significant diabetogenic effect of FK 506 on liver transplant recipients, at least during the first weeks following transplantation, was not found to be different than in patients under conventional immunosuppression. Longer-term studies are needed to determine whether the minimal abnormalities seen in some of these patients will eventually disappear or whether they will evolve into a diabetic state.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Starzl TE, Todo S, Fung J, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todo S. Transplant Proc. this issue. [Google Scholar]
- 3.Fung J, Todo S, Jain A, et al. Transplant Proc. this issue. [Google Scholar]
- 4.Collier D J, Calne R, Thiru S, et al. Transplant Proc. 1987;19:3975. [PubMed] [Google Scholar]
- 5.Starzl TE. Experience in Renal Transplantation. WB Saunders Company; Philadelphia, PA: 1964. p. 117. 161, 221. [Google Scholar]
- 6.Ruiz JO, Simmons RL, Callender CO, et al. Surgery. 1973;73:759. [PubMed] [Google Scholar]
- 7.Roth M, Milgrom M, Esquenai V, et al. Transplantation. 1989;47:278. [PubMed] [Google Scholar]
- 8.Ost L, Tyden G, Fehrman I. Transplantation. 1989;46:370. doi: 10.1097/00007890-198809000-00007. [DOI] [PubMed] [Google Scholar]