Abstract
The gonadal steroid, 17β-estradiol (E2), acts as a protective hormone preventing β-cell apoptosis in vivo in mice of both sexes and in cultured mouse and human islets. E2 signals via the classical estrogen receptor (ER)α and ERβ, an extranuclear form of ERα and the G protein-coupled estrogen receptor (GPER). In a recent study, we determined the contribution of these receptors to β-cell survival, using a combination of genetic and pharmacological tools in mice and cultured mouse and human islets. We showed that E2 favors islet survival by preventing apoptosis via ERα and ERβ through ERE-independent, extra-nuclear mechanisms and with a predominant ERα effect. We also revealed that E2 prevents apoptosis via GPER-dependent mechanisms. Here, we show that E2 prevents apoptosis independently of gene transcription or de novo protein synthesis suggesting that E2 cytoprotection happens independently of nuclear events. Furthermore, we report that E2 islet cytoprotection can be mimicked by the nonfeminizing E2 stereoisomer, 17α-estradiol, suggesting that it is partially non-estrogen receptor mediated. These studies identify novel estrogen pathways and targets to protect islet survival.
Keywords: 17α-estradiol, estrogen receptor, nongenomic, islet, beta-cell, apoptosis, type 1 diabetes
The gonadal steroid, 17β-estradiol (E2), is primarily known to be a female sex hormone, but the actions of E2 are not limited to reproductive tissues. E2 influences the development, differentiation, growth, and function of various tissues. E2 is also an important survival factor. For example, E2 is a neuroprotective hormone against multiple oxidative and pro-apoptotic injuries. In addition, E2 protects islet survival from pro-apoptotic insults (1). Using a mouse model of estrogen deficiency by deletion of the aromatase gene, we initially showed that circulating E2 acts as a protective hormone preventing pancreatic β-cell apoptosis in vivo in both sexes (2). Male and female mice develop a vulnerability to streptozotocin (STZ)-induced β-cell damage and insulin deficiency when estradiol production is genetically suppressed (2). We showed that E2 prevents STZ-induced β-cell apoptosis at least partially via the estrogen receptor (ER)α (2). E2 signals via classical ER-α, ERβ, and an extranuclear form of ERα. In a recent study, we determined the contribution of these receptors to β-cell survival (3). In the classical ER signaling pathway, E2-activated ERα binds as a homodimer to either an ERE or a non-ERE tethered promoter to initiate gene transcription (4). To investigate whether an ERα-ERE or non-ERE signaling mechanism protects β-cell survival in vivo, we used an ERα knock-in mouse with a mutation of the DNA-binding domain of ERα that eliminates ERα binding to the ERE. We observed that ERα cytoprotection of islets in vivo is ERE independent. We also observed that both ERα and ERβ showed predominant cytosolic localization in mouse and human β-cells. We showed that E2 prevents islet apoptosis via extra-nuclear and membrane ERs with a predominant ERα effect in mouse and human islets. The existence of both ERα and ERβ in β-cells did not demonstrate evidence of ERβ antagonism of ERα action, since pharmacological inhibition or genetic elimination of ERβ in islets did not enhance E2 anti-apoptotic action via ERα. In addition, the combined elimination of these receptors did not synergize to abolish E2 cytoprotection following exposure of islets to oxidative stress. We conclude that ERα and ERβ favor islet survival using non-redundant and distinct cellular pathways. These pathways are under investigation.
The G protein-coupled estrogen receptor (GPER), also known as GPR30, is a membrane receptor for estrogens that mediates rapid non-genomic signals (5, 6). Ten years ago, Angel Nadal reported the existence of a membrane GPCR in β-cells, unrelated to ERα and ERβ, which may be GPER (7, 8). A more recent study showed that GPER-deficient mice have impaired E2-stimulated insulin release from isolated islets and impaired glucose-stimulated insulin secretion in vivo, suggesting that GPER is involved in islet biology (9). We found that GPER protein is expressed in mouse and human islets and that elimination of GPER predisposes to STZ-induced islet apoptosis in female mice (2). In addition, we showed that pharmacological activation of GPER using the agonist G1, which selectively activates GPER in a cellular environment containing ERα and ERβ (10), is efficient in protecting H2O2-induced apoptosis in cultured mouse and human islets. However, E2 cytoprotection from apoptosis was retained in cultured GPER-deficient islets demonstrating that ERα can compensate for GPER deficiency. Thus, E2 protects islet survival by preventing apoptosis via ERα and ERβ and GPER pathways with a predominant ERα effect.
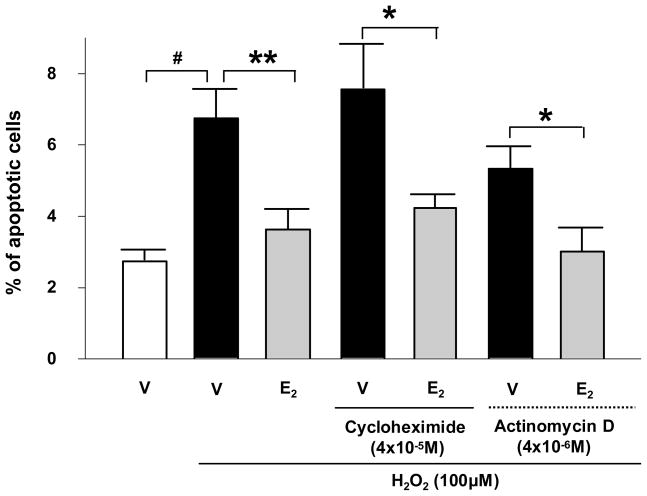
It is interesting to observe that during the initial five most critical hours of the induction of apoptosis (length of islet exposure to H2O2), the E2-mediated pro-survival effect does not necessitate the presence of an ER in the nucleus. Furthermore, we provide additional evidence that E2 signals favor survival independently of nuclear events since E2 anti-apoptotic action is not impaired by inhibition of gene transcription or de novo protein synthesis (Fig. 1). This suggests that ER- and GPER-mediated antiapoptotic actions involve rapid events such as the alteration in the phosphorylation of proteins or the function of ion channels. These pathways are under investigation.
Figure 1. E2 prevents β-cell apoptosis independently of gene transcription and de novo protein synthesis.
Percentage of apoptotic MIN6 cells treated with Cycloheximide or Actinomycin D. Apoptosis was measured by nuclear morphology as described (3). V: vehicle. # P<0.001, * P<0.05, ** P<0.01
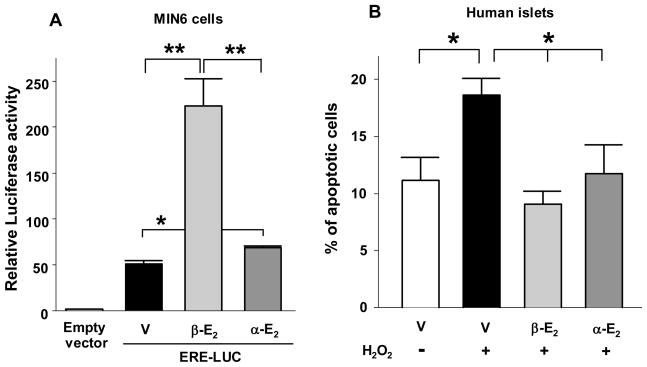
Evidence indicates that estrogens provide cytoprotection via mechanisms independent of ERs (11). For example, the neuroprotective effect of E2 can be reproduced by the nonfeminizing nonestrogenic E2 stereoisomer 17α-estradiol (12). It is not clear whether 17α-estradiol selectively binds a specific extranuclear pool of ERα and ERβ providing selective non-genomic effects with minimal transcription, or binds to a membrane receptor independent from ERα and ERβ (13). It is clear, however, that 17α-estradiol is not capable of activating GPER (10). 17α-Estradiol has also been reported to intercalate into cell membranes, where it terminates lipid peroxidation, thereby preserving membrane integrity in an ER-independent manner (14). In MIN6 β-cells, we observe that 17α-estradiol shows weak transcriptional activity compared to E2 on a reporter construct containing an ERE (Fig. 2A). However, we find that exposure of human islets to E2 or 17 α-estradiol produced a similar and robust protection against H2O2-induced apoptosis (Fig. 2B). Thus, estrogens protect islet survival via extranuclear, membrane, and perhaps non-receptor mediated events. Importantly, 17α-estradiol may be a candidate for gender-neutral antiapoptotic therapy in diabetes, because it has few of the biological effects associated with the female hormone activity.
Figure 2. 17α-estradiol protects islet survival.
(A) Relative luciferase activity in MIN6 cells transfected with an ERE reporter construct and treated with E2 (β-E2) or 17α-estradiol (α-E2) (10−8M). (B) Percentage of apoptotic cells in cultured human islets. Islets were treated with E2 (β-E2) or 17α-estradiol (α-E2) (10−8M) for 48 h, followed by exposure to H2O2 (100μM) for the last 5 hours. Apoptosis was assessed by nuclear morphology as described (3). Values represent the mean ± SE of five independent experiments. * P<0.05, ** P<0.01.
Acknowledgments
We acknowledge the Islet Cell Resource Consortium, funded by the National Institutes of Health (NIH) and administered by the ABCC for providing human islets. This work was supported by grants from NIH RO1 DK074970, the Juvenile Diabetes Research Foundation 1-2006-837 and the March of Dimes 6-FY7-312.
Footnotes
Addenda to: Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. Importance of extranuclear estrogen receptor-{alpha} and membrane G protein-coupled estrogen receptor in pancreatic islet survival.
References
- 1.Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. 17beta-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation. 2002;74:1252–1259. doi: 10.1097/00007890-200211150-00010. [DOI] [PubMed] [Google Scholar]
- 2.Le May C, Chu K, Hu M, Ortega C, Simpson E, Korach K, Tsai M, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. Importance of extranuclear estrogen receptor-{alpha} and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009 doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 5.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 6.Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- 7.Nadal A, Rovira JM, Laribi O, Leon-quinto T, Andreu E, Ripoll C, Soria B. Rapid insulinotropic effect of 17beta-estradiol via a plasma membrane receptor. Faseb J. 1998;12:1341–1348. doi: 10.1096/fasebj.12.13.1341. [DOI] [PubMed] [Google Scholar]
- 8.Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci U S A. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 10.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 11.Behl C. Oestrogen as a neuroprotective hormone. Nat Rev Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- 12.Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- 13.Toran-Allerand CD. Estrogen and the Brain: Beyond ER-{alpha}, ER-{beta}, and 17{beta}-Estradiol. Ann N Y Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 14.Dykens JA, Moos WH, Howell N. Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann N Y Acad Sci. 2005;1052:116–135. doi: 10.1196/annals.1347.008. [DOI] [PubMed] [Google Scholar]