More than two decades ago, Laurell and Erickson1 recognized the association between familial pulmonary emphysema and alpha-1-antitrypsin deficiency (A1AD). Subsequently, Freier et al2 and Sharp et al3 described the first children with A1AD and liver disease. This association has also been described in adults.4,5
No specific medical therapy exists for A1AD patients who develop liver disease.6,7 Furthermore, although portal-systemic shunt procedures have been performed in such patients in an attempt to prevent the development of progressive liver disease,8 such procedures have not improved liver function or quality of life and have not enhanced the long-term survival when cirrhosis is already established. Currently orthotopic liver transplantation (OLT) appears to be the best option available to these patients. The purpose of this report is to present our experience with OLT in patients with A1AD and advanced liver disease.
PATIENTS AND METHODS
Patient Population
Five hundred and sixty-two patients underwent hepatic transplantation between March 1, 1980 and February 28, 1986 at the University of Pittsburgh Health Center. Three hundred and thirty-nine patients were adults, and 223 were children (<18 years of age). Thirty nine of these (29 children, 10 adults) had end-stage liver disease due to A1AD. In each case the diagnosis of A1AD was established by measurement of the A1A level in serum and determination of the specific Pi type of each. Moreover, histologic confirmation of classic A1AD liver disease was available from liver biopsy samples obtained in 27 patients preoperatively and from the excised liver specimen in all 39. The pediatric patients ranged in age from 8 months to 13 years, with a mean of 5 years. The adult patients ranged in age from 18 to 48 years, with a mean of 34 years.
The basic immunosuppressive regimen used consisted of cyclosporine (Cs) and prednisone.9 Since December 1983 OKT3 (Orthoclone; ORTHO Pharmaceuticals, Raritan, NJ) has been used for the treatment of acute rejection episodes resistant to conventional immunosuppression therapy.10
All data are presented as mean values and range. Patient survival was calculated by the life table method (MOP, statistical software, Los Angeles, CA).
RESULTS
Pediatric Patients
The clinical features present in the 29 children herein reported led to the diagnosis of A1AD with the presence of neonatal cholestasis in 23, ascites in three, and portal hypertension with gastrointestinal (GI) bleeding in three patients. The mean patient age at the time of diagnosis was 11 months, with a range of 1 month to 6 years. A summary of the A1A phenotype data and serum levels of A1A in these individuals is presented in Table 1. At the time of the initial diagnosis, liver biopsies obtained from these children demonstrated diastase-resistant intracytoplasmic eosinophilic bodies that reacted with periodic acid-Schiff (PAS) stains in 22 patients, cirrhosis in 17 patients, and hepatic fibrosis in four patients. Four patients who had PiZZ phenotype had emphysema. Two of these children had been treated with a creation of portal systemic shunts, and one child with associated extrahepatic biliary atresia had had two portoenterostomy procedures (Kasai) prior to liver transplantation. The dominant clinical features at the time of OLT in these 29 children are shown in Table 2.
Table 1.
Preoperative Phenotype and A1A Enzyme Activity of 39 Liver Transplant Recipients
| Children |
Adults |
||||||
|---|---|---|---|---|---|---|---|
| No. of | Serum A1A mg/dL* |
Serum A1A mg/dL* |
|||||
| Patients | Phenotype | Mean | Range | Patients | Phenotype | Mean | Range |
| 22 (76%) | PiZZ | 43.3 | (0.4–80) | 8 (80%) | PiZZ | 28.3 | (12–45) |
| 2 (7%) | PiSZ | 121.5 | (38.2–285) | ||||
| 2 (7%) | PiMZ | 121.5 | (38.2–285) | 2 (20%) | PiMZ | 90.0 | (70–115) |
| 3 (10%) | Unknown | Unknown | |||||
| n = 29 | n = 10 | ||||||
Normal = 85.0 to 213 mg/dL.
Table 2.
Clinical Features at the Time of OLT
| Pediatric Group (n = 29) No. of Patients |
Adult Group (n = 10) No. of Patients |
|
|---|---|---|
| Ascites | 23 (79%) | 8 (80%) |
| GI bleeding | 17 (59%) | 8 (80%) |
| Jaundice | 11 (38%) | 2 (20%) |
| Encephalopathy | 3 (10%) | 4 (40%) |
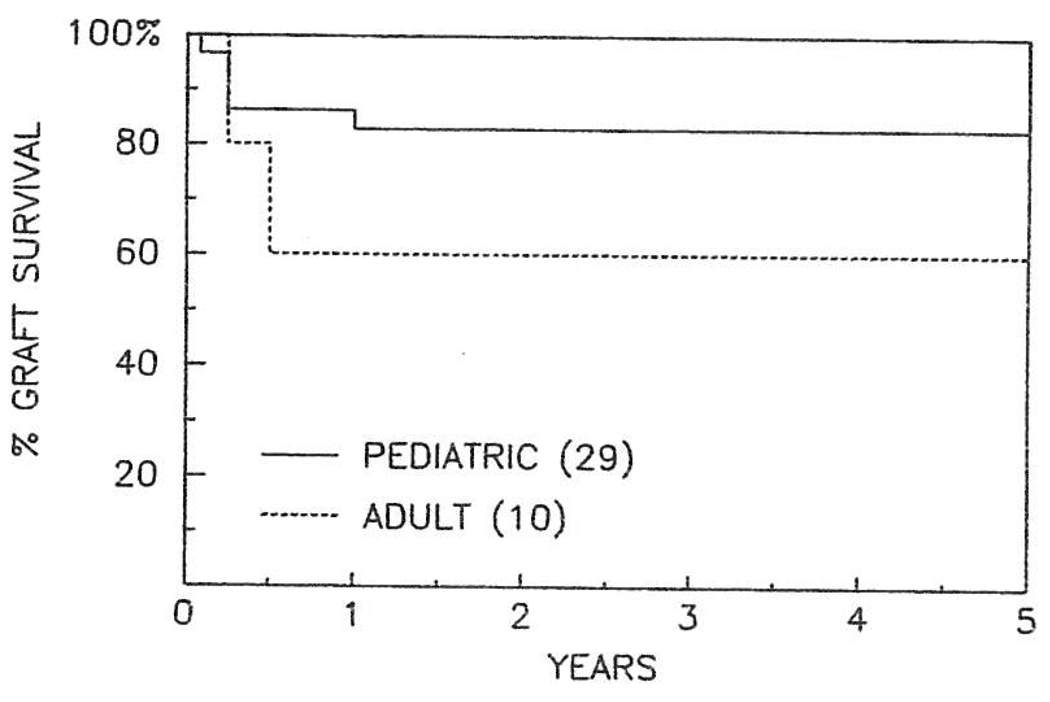
At the time of data collection 24 out of 29 pediatric patients were discharged from the hospital and were alive, with a follow-up ranging from 8 to 60 months (mean 26 months). The actuarial survival at 5 years is 83% (Fig 1). Six patients (20%) required a second hepatic allograft, and the indications for the second transplant procedures are listed in Table 3. Five patients (17%) died during the first 3 postoperative months. Vascular complications were responsible for the death of two, and each death actually occurred after retransplantation. One of these two patients had a ruptured mycotic aneurysm involving a donor iliac artery graft that had been used for the arterial reconstruction. The causes of death in the other three patients were primary graft nonfunction, varicella pneumonitis, and shock due to small bowel obstruction. Biliary complications occurred in five. Three developed biliary leaks, and in two of these three the cause of the biliary leak was thought to be thrombosis of the hepatic artery with duct ischemic injury. Two patients developed biliary strictures thought to be the result of thrombosis of the hepatic artery in one and rejection in the other. Other major complications present in these patients are listed in Table 3.
Fig 1.
Actuarial survival curve in adult and pediatric liver recipients after hepatic transplantation for A1AD.
Table 3.
Major Complications and Indications for Liver Retransplantation in A1AD Pediatric Recipients
| Patients | Re Tx | Deaths | |
|---|---|---|---|
| Vascular Complications | |||
| Hepatic artery thrombosis | 3 | 2 | 2 |
| Portal vein and vena cava thrombosis |
1 | 1 | 0 |
| Thrombosis of hepatic artery and vena cava |
1 | 1 | 0 |
| Biliary Complications | |||
| Biliary leak | 3 | 1 * | 0 |
| Biliary stricture | 2 | 0 | |
| Miscellaneous | |||
| Primary allograft nonfunction | 1 | 1 | 1 |
| Varicella pneumonitis | 1 | 0 | 1 |
| Chronic rejection | 1 | 1 | 0 |
| Intraabdominal abscess | 1 | 0 | 0 |
| Postoperative hemorrhage | 1 | 0 | 0 |
| Small bowel obstruction | 1 | 0 | 1 |
This patient had thrombosis of the hepatic artery; therefore it is also included under vascular complications.
Twenty-two of the 24 surviving children have normal hepatic function. One child developed lymphoproliferative disease involving the cervical nodes 1 year posttransplantation and was managed successfully by reducing the immunosuppression. At the present time this child is free of recurrence 20 months later. The child who had associated extrahepatic biliary atresia currently has an obstruction of the common bile duct at the confluence of the hepatic ducts, which occurred as a result of thrombosis of the hepatic artery. His biliary tree is being drained via a percutaneous catheter. Although his transaminase levels are twice the upper limit of normal, he remains free of biochemical jaundice. Moreover, he is growing well and is free of sepsis. Another child has advanced chronic rejection and currently is awaiting retransplantation. All children of school age are attending school.
Adult Patients
The presenting symptoms at the time of initial diagnosis of A1AD in the ten patients reported were GI bleeding in four, ascites in four, and jaundice in two patients. The phenotype and the A1A activity of these ten individuals are shown in Table 1. Liver histology at the time of initial diagnosis was cirrhosis in five. Diastase-resistant–PAS-positive intracytoplasmic eosinophilic bodies were identified in the liver tissue of all patients. Two of these ten adults were diagnosed as having A1AD early in childhood. Omitting these two patients, the mean time interval between the onset of symptoms of liver disease and OLT was 3 years, with a range of 1 to 9 years. Only one of these adult patients who was phenotyped as PiZZ had chronic obstructive pulmonary disease. Three of these ten adult patients had portosystemic shunts performed prior to transplantation. The clinical features at the time of transplantation are presented in Table 2.
Six of the ten adult patients are alive, with a projected 5-year survival of 60%. The four patients who died did so within 6 months of OLT. The causes of death in these four patients were listeria and pseudomonas septicemia (one case), intracerebral hemorrhage (one case), pneumocystis pneumonitis (one case), and viral and fungal sepsis occurring in a setting of hepatic artery thrombosis (one case). Two of these adult patients required retransplantation for hepatic artery thrombosis and for primary graft nonfunction and rejection (two grafts) in the other patient. Nine major complications occurred in five patients, and only one of these patients survived (Table 4). None of the other patients had postoperative complications.
Table 4.
Major Postoperative Complications in Five A1AD Liver Recipient Patients
| Listeria and pseudomonas septicemia |
| Bleeding duodenal ulcer |
| Thrombosis of the hepatic artery |
| Primary graft nonfunction |
| Chronic rejection |
| Cytomegalovirus hepatitis |
| Intracerebral hemorrhage |
| Intraabdominal hemorrhage |
| Biliary leak |
All six surviving adult patients currently have normal graft function. They are all working full time, and one recently delivered a healthy child. It should be noted that one of the adults had an incidental hepatoma at the time of OLT and is still alive and well with no evidence of recurrence 5 years after OLT.
DISCUSSION
Ten percent to 20% of the PiZZ children develop advanced histologic liver disease if neonatal hepatitis is present in the first months of life.11,12 Some children with A1AD have no clinical evidence of the liver disease except for abnormal liver injury tests early in life.11 A few adults (12%) with severe A1AD PiZZ also develop liver cirrhosis, and the risk increases in patients over the age of 50.13
It has been stated that approximately 40% of a normal A1A level is necessary to avoid liver damage14; however, in the present study two heterozygous adults (PiSZ, PiMZ) had normal values of the A1AD. These two individuals had histopathologic confirmation of their A1AD (presence of the intracytoplasmic eosinophilic bodies that were PAS positive and diastase resistant). These would appear to support the theory that the liver disease of A1AD is related to the retention of intracellular PAS-positive–diastase-resistant globules of A1A protein within hepatocytes rather than the A1AD per se.
Liver cirrhosis associated to A1AD carries a fatal prognosis. The evolution is typical, with progressive deterioration leading to death due to progressive hepatocellular failure or hemorrhage occurring as a result of severe portal hypertension.15,16 Liver transplantation provides metabolic cure manifested by (1) acquisition of the phenotype of the donor, (2) normalization in the serum A1A level, (3) elimination of the liver disease, and, presumably, (4) prevention of the complications associated with PiZZ, such as pulmonary disease.17,18 The appropriate timing for transplantation in these patients is when signs of hepatic failure or decompensation begin to appear.
Long-term survival should not be the only parameter used to assess the results of liver transplantation. The quality of life of transplant recipients is also important and is probably the most important of the two parameters used to justify OLT. In the present series the majority of the patients transplanted enjoy a lifestyle identical to that of normal people. Liver replacement has given these patients a definitive metabolic cure with a chance for an excellent rehabilitation and long-term survival.
SUMMARY
Thirty-nine patients (29 children and ten adults) underwent OLT for liver disease associated with A1AD from March 1980 to March 1986. Thirty of thirty-six patients (83%) with available data were homozygous phenotype PiZZ. The other six were Pi heterozygotes, being either PiMZ or PiSZ. The mean A1A activity in homozygous and heterozygous patients was 38.8 mg/dL and 114.3 mg/dL respectively. Eight patients died during the first 3 months after OLT (20%). The 5-year actuarial survival is 83% and 60% in pediatric and adult recipients respectively. Today 30 (76%) of the recipients are alive, with follow-ups of 8 to 64 months (average 27 months). The quality of life in the surviving patients is excellent.
ACKNOWLEDGMENT
We thank Vicky Fioravanti, RN, for her contribution to this investigation and Nell Lang for secretarial assistance.
Supported by research grants from the Veterans Administration and Project Grant No. AM29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Laurell CB, Erickson S. Scand J Clin Lab Invest. 1963;15:132. [PubMed] [Google Scholar]
- 2.Freier EF, Sharp H, Bridges RA. Clin Chem. 1968;14:782. [Google Scholar]
- 3.Sharp HL, Bridges RA, Krivit W, et al. J Lab Clin Med. 1969;73:934. [PubMed] [Google Scholar]
- 4.Berg NO, Ericksson S. N Engl J Med. 1972;287:1264. doi: 10.1056/NEJM197212212872502. [DOI] [PubMed] [Google Scholar]
- 5.Palmer PG, Wolfe HJ, Gherardi GJ. Gastroenterology. 1973;65:284. [PubMed] [Google Scholar]
- 6.Gade JE, Fulmer JD, Gelfand JA, et al. J Clin Invest. 1980;66:82. doi: 10.1172/JCI109838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alagille D. Hepatology. 1984;4:115. [Google Scholar]
- 8.Starzl TE, Porter KA, Francavilla A, et al. Lancet. 1983;2:724. doi: 10.1016/s0140-6736(83)90390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Iwatsuki S, Van Thiel D, et al. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Fung JJ. Transplant Proc. 1986;18:937. [PMC free article] [PubMed] [Google Scholar]
- 11.Sveger T. N Engl J Med. 1976;294:1316. doi: 10.1056/NEJM197606102942404. [DOI] [PubMed] [Google Scholar]
- 12.Aegenaes O, Matlary A, Elgio K, et al. Acta Paediatr Scan. 1972;61:632. doi: 10.1111/j.1651-2227.1972.tb15960.x. [DOI] [PubMed] [Google Scholar]
- 13.Larsson C. Acta Med Scand. 1978;204:345. doi: 10.1111/j.0954-6820.1978.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 14.Svegev T. Pediatrics. 1978;62:22. [Google Scholar]
- 15.Burke JA, Kiesel JL, Blair JD. Am J Dis Child. 1976;130:621. doi: 10.1001/archpedi.1976.02120070047010. [DOI] [PubMed] [Google Scholar]
- 16.Wilson D, Smyth S. Am J Med. 1983;74:221. doi: 10.1016/0002-9343(83)90615-0. [DOI] [PubMed] [Google Scholar]
- 17.Hood JM, Koep LJ, Peteus RL, et al. N Engl J Med. 1980;302:272. doi: 10.1056/NEJM198001313020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putnam CH, Porter KA, Peters RL, et al. Surgery. 1977;81:258. [PMC free article] [PubMed] [Google Scholar]