Abstract
TGF-β superfamily ligands are homo- or heterodimeric and recruit two type I and two type II Ser/Thr kinase receptors to initiate a transmembrane signaling cascade. Even with the known structure of the homodimer ligands in complex with extracellular domains of both receptor types, the sequential assembly of the signaling complex with its cognate receptors in the cell membrane remains elusive. We generated a bone morphogenetic protein-2/-6 heterodimer carrying two asymmetric interfaces for each receptor type. We demonstrate that the heterodimer possesses high affinity to both receptor types and increased Smad1-dependent signaling activity by both cell-based and chondrogenesis assays. Furthermore, we find that the minimal signaling complex consists of two type II receptors and one type I receptor per dimer. Our study reveals how the engineered heterodimers may use their independent binding interfaces to differentially recruit the different receptors for each receptor type to create new biological properties.
BMP-2/BMP-6 heterodimer is more potent in signaling than either BMP-2 or BMP-6 homodimer. The ligand-receptor assembly of BMP-2/BMP-6 heterodimer illustrates how it does.
The TGF-β superfamily contains nearly forty biologically diverse growth factors. Among several subfamilies, the bone morphogenetic protein/growth and differential factor (BMP/GDF) subfamily comprises the largest and most highly conserved group ( 1), consisting of BMP-2, -3, -4, -5, -6, -7, -8, -8B, -9, -10, and -15 and 10 additional GDF subfamily members. Despite initial isolation for the ability to induce ectopic bone and cartilage formation in vivo ( 2), the BMPs have subsequently been identified as critical regulators in many other cellular processes, from the development and patterning of many different organ systems ( 3) to the influence of cell fate and differentiation of both early embryonic cells and stem cell cultures ( 4,5). They function in these systems as extracellular signaling molecules through autocrine, paracrine, and juxtacrine manners ( 6).
BMP ligands are translated as large two-domain precursor proteins. The N-terminal prodomain is proteolytically cleaved in vivo yielding the C-terminal mature domain ( 6). The biologically active BMP ligands contain a highly conserved set of seven cysteine residues forming a structure known as cysteine knot in the mature domain, of which one residue forms a disulfide bridge between two monomer subunits resulting in a covalently linked dimer. Many studies use heterologous expression of homodimers by means of expression of a single BMP gene or recombinant homodimeric BMP ligand to elucidate the wide range of biological roles of homodimers. Heterodimeric ligands comprising two BMP genes simultaneously expressed in vivo ( 7) also exist, and such coexpression is required for normal embryonic development ( 8,9). Additionally, in vitro studies show that coexpression of BMP ligands can result in heterodimeric ligand formation ( 10,11,12,13). These results demonstrated not only that the heterodimer ligands can have increased activity compared with their homodimer counterparts during a variety of biological processes such as bone formation ( 10,11,12,13,14,15,16), but also that they may play some critical roles of embryonic development such as proper body axis determination ( 17).
To carry out the signaling across the cell membrane, the dimeric BMP ligands assemble concurrently with two kinds of receptors in the membrane, the type I and type II receptors. The receptors contain an extracellular ligand binding domain and a large intracellular serine/threonine kinase domain linked by a single transmembrane region. Each ligand dimer contains two binding sites for the type I receptors and two binding sites for type II receptors, resulting in the formation of a complex consisting of a dimer ligand and two type I receptors and two type II receptors ( 18,19,20). Formation of the six-chain ligand-receptor complex in the membrane permits the constitutively active type II receptor kinase to phosphorylate the type I receptor kinase, thus activating the type I receptor kinase activity. The activated type I receptors phosphorylate downstream signaling molecules, termed Smads, which translocate to the nucleus where Smads act as transcription factors to regulate the target genes ( 21,22).
The structural mode of assembly of the receptor extracellular domians (ECDs) and a ligand forming a six-chain signaling complex shows significant variations between the BMP and TGF-β subfamilies ( 23,24,25). Given that only seven type I receptors and five type II receptors exist for approximately 40 members of the TGF-β, activin, and BMP/GDF subfamilies, some receptors interact with several ligands of different subfamilies. For instance, ALK2 can bind BMP-7 and activin, whereas ActRII binds BMP-2 and BMP-7 as well as interacting with both activin and myostatin. This raises the question of how the overlapping receptor specificities of a given ligand govern the mode of assembly of the ligand-receptor complex. Furthermore, while the two type I and two type II receptor-binding interfaces of a homodimeric ligand are symmetrical, respectively, a heterodimeric ligand has built-in asymmetry between the pair of type I or II receptor-binding interfaces. Thus, up to four distinct receptors can be recruited by four structurally distinct receptor-binding interfaces of a heterodimeric ligand. Most previous experiments have been performed with homodimer ligands with all type I or type II receptor interfaces disrupted uniformly ( 26,27) or by knocking out expression of different type I or type II receptors ( 28). As a result, it has not been firmly established whether two of each receptor types differ and are physically necessary to carry out the signaling.
To address these questions, we have devised a new strategy to dissect the nature of the requirements of the ligand-receptor signaling complex. In this report, we have generated a BMP-2/BMP-6 heterodimeric ligand and demonstrate its unique receptor binding and signaling properties compared with the homodimeric ligands. With this heterodimeric ligand, we were able to individually and independently alter specific type I or type II receptor binding interfaces. These modified BMP-2/BMP-6 ligands enabled us to show that the stability of the ligand-receptor assembly, signaling activity, and biological activity of the signaling complex are highly correlated. We also report the unexpected observation that only one of the two type I receptor binding sites is sufficient for transmembrane signaling as long as the signaling complex is bound by two type II receptors.
Results
Synthesis of the BMP heterodimer ligand
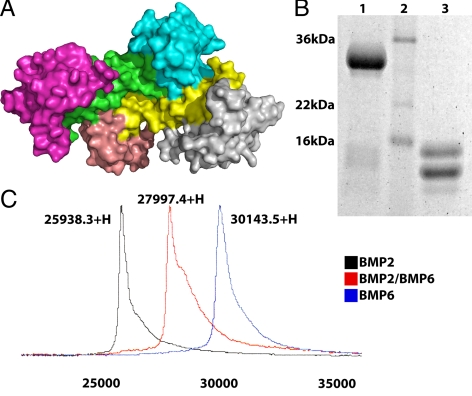
The crystal structure of BMP-2/BMPRIa-ECD/ActRII(b)-ECD has shown that each receptor ECD does not associate with each other extracellularly ( 23,25). This mode of assembly is in contrast to that of TGF-β subfamily ligand for which the receptor ECDs make physical interaction ( 24). Unlike a ligand homodimer, a heterodimer has two structurally distinct type I interfaces and two structurally distinct type II interfaces (Fig. 1A). To address the ligand-receptor assembly and its functional significance of a heterodimeric ligand, we have synthesized recombinant BMP-2/BMP-6 heterodimers using a method modified from the expression, refolding, and purification of BMP homologues ( 29). Briefly, the BMP-2 and BMP-6 subunits were purified from inclusion bodies separately and combined in an equimolar mixture during the refolding step (see Materials and Methods) to create the heterodimer ligand. The BMP-2/BMP-6 heterodimer was isolated from the respective homodimers based on each ligand’s heparin sulfate binding properties. The purity of the heterodimer was verified by SDS-PAGE. Figure 1B illustrates the migration of the BMP-2/BMP-6 heterodimer as a single band under nonreducing conditions (lane 1) and as two distinct bands under reducing conditions (lane 3) on SDS-PAGE. The two distinct bands correlate to the two different monomer species (13 and 15 kDa, for BMP-2 and BMP-6 subunits, respectively). The purity of BMP-2/BMP-6 is further evidenced with surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) data. Three purified samples, BMP-2 and BMP-6 homodimers and the BMP-2/BMP-6 heterodimer, were separated and individually assayed on SELDI-TOF-MS. The BMP-2 and BMP-6 homodimers are single peaks each corresponding to their predicted molecular masses of 26 and 30 kDa, respectively (Fig. 1C). The BMP-2/BMP-6 heterodimer is a single peak found between the homodimer peaks corresponding to a molecular mass of 28 kDa (Fig. 1C). The results confirm the predicted sized of the BMP-2/BMP-6 heterodimer and the absence of BMP-2 or BMP-6 homodimers.
Figure 1.
Generation of BMP-2/BMP-6 heterodimer. A, Four independent ligand-receptor interfaces represented by BMP-2 homodimer (yellow and green) in complex with BMPRIa-ECD (light blue and salmon) and ActRII-ECD (gray and magenta). Protein Data Bank: 2GOO. B, BMP-2/BMP-6 sample on SDS-PAGE. The BMP-2/BMP-6 sample nonreduced in lane 1, molecular weight marker in lane 2, and the BMP-2/BMP-6 sample reduced in lane 3. C, SELDI-TOF-MS spectra overlaid. Each spectrum is from separate samples of BMP-2, BMP-6, and BMP-2/BMP-6 ligands.
In vitro activity of BMP heterodimer
To assay the interactions between the BMP-2/BMP-6 and receptor ECDs, surface plasmon resonance (SPR) was used to measure the in vitro affinity. The ECDs of the type I receptor BMPRIa and the type II receptor ActRIIb were immobilized to a SPR chip, and the ligands were flowed over the chip surface while monitoring the interactions. Table 1 summarizes the association and dissociation rates and the varying affinities the ligands possess for the receptor ECDs. BMP-2 has a KD of 1.31 nm for BMPRIa and a weaker KD of 38.5 nm for ActRIIb (Table 1). Conversely, BMP-6 displays a relatively weak affinity for BMPRIa with a KD of 62.8 nm and a higher affinity for ActRIIb with a KD of 6.68 nm (Table 1). These values are in agreement with previously reported affinities of BMP-2 and BMP-6 for these receptors ( 29). Unlike the homodimers, the BMP-2/BMP-6 heterodimer exhibits a relatively high affinity for each receptor type. BMP-2/BMP-6 has a KD of 1.02 nm for BMPRIa, which is comparable to that of BMP-2 (KD of 1.31 nm) and a KD of 6.52 nm for ActRIIb, which is comparable to that of BMP-6 (KD of 6.68 nm) (Table 1). The data indicate that the high affinity of BMP-2/BMP-6 for the type II receptor ECD is primarily contributed by the BMP-6 subunit, while the high affinity for the type I receptor ECD is primarily contributed by the BMP-2 subunit. These results support that binding affinity of each of the four receptor ECDs is not influenced by binding at the three other binding interfaces, and thus the overall binding affinity of the entire complex is achieved by the higher-affinity binding interface of each of type I and II receptor ECDs individually.
Table 1.
Ligand affinity data from SPR experiments
| Ligand | Receptor BMPRIa
|
Receptor ActRIIb
|
||
|---|---|---|---|---|
| koff (1/sec)/kon (1/M · sec) | KD (nm) | koff (1/sec)/kon (1/M · sec) | KD (nm) | |
| BMP-2 | 1.11 × 10−3/8.52 × 105 | 1.31 | 2.57 × 10−2/6.68 × 105 | 38.47 |
| BMP-6 | 9.37 × 10−3/1.50 × 105 | 62.46 | 1.82 × 10−3/2.73 × 105 | 6.68 |
| BMP-2/BMP-6 | 1.05 × 10−3/1.03 × 106 | 1.02 | 8.61 × 10−3/1.32 × 106 | 6.52 |
The SPR data are shown as the dissociation rate, koff, and the association rate, kon, based on a global fit using the kinetic model 1:1 Langmuir binding with mass transfer. The binding constant KD is calculated as koff/kon. The receptors were immobilized to the chip surface, with the ligands flowed over the surface (see Materials and Methods).
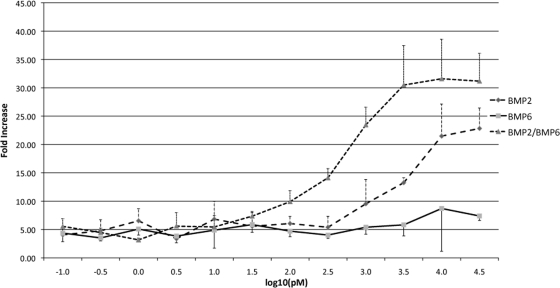
To examine how receptor ECD affinity of BMP-2/BMP-6 correlates with signaling activity, we have used a luciferase reporter assay. Using the C2C12 mouse myoblast cell line, the BMP ligands were quantitatively tested for the ability to activate a Smad1-dependent reporter. BMP-2, BMP-6, and BMP-2/BMP-6 all showed dose-dependent reporter activation (Fig. 2). As in our previous study, BMP-2 has a significantly higher activity than BMP-6 ( 29). Interestingly, the BMP-2/BMP-6 showed a further increase in activation of the reporter than the BMP-2 or BMP-6 homodimers at the same ligand concentration (Fig. 2). While both BMP-2 and BMP-6 remain at background levels at 300 pm, BMP-2/BMP-6 showed a 10-fold activation over background. This increase in activation is similar to previous observations in other experiments ( 10,11,12,13,14,15,16). Our data support that high affinities of BMP-2/BMP-6 heterodimer to both type I and type II receptor ECDs result in substantially increased level of intracellular signaling activity than its homodimer counterparts.
Figure 2.
BMP-2/BMP-6 heterodimer exhibits higher signaling activity in Smad1-reporter assay than homodimers. C2C12 whole cell Smad1-dependent reporter assay with BMP-2 (long dashed line), BMP-6 (solid line), and BMP-2/BMP-6 (short dashed line) ligands. Error bars are indicated.
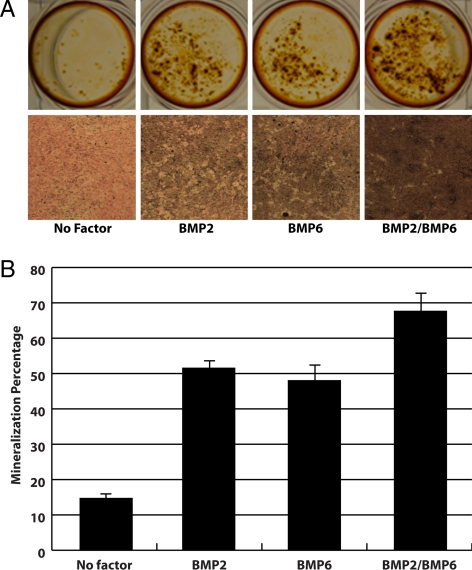
To confirm the increased receptor binding affinity and Smad1 activation of the BMP-2/BMP-6 heterodimer translates into increased functionality, we also tested the ability of the ligands to induce mineralization in the mouse MC3T3-E1 preosteoblast cell line. The addition of BMP ligands has been shown to induce the formation of calcium deposits in MC3T3-E1 cells ( 30). Because this system does not require the introduction of any exogenous reporters, it should reflect the signaling activity of BMP-2, BMP-6, and BMP-2/BMP-6 through endogenous pathways. The cells were treated with 10 ng/ml ligands for 4 d, after which they were stained with both Alizarin Red S and von Kossa. Alizarin Red staining visualizes calcium deposits in the cells in response to exogenously added BMP ligands. Similar to the luciferase reporter assay, the BMP-2/BMP-6 heterodimer clearly shows higher levels of calcium deposition compared with either BMP-2 or BMP-6 (Fig. 3A). von Kossa staining allows for not only visualization but also a quantitative measurement of cell mineralization. Both BMP-2 and BMP-6 show similar levels of mineralization at 51.5% and 48%, respectively (Fig. 3B). Interestingly, unlike in the luciferase reporter assay, BMP-6 appears to have comparable activity to BMP-2 in the mineralization assay. Importantly, similar to the luciferase reporter assay, BMP-2/BMP-6 shows a dramatic increase in the percent mineralization with levels at approximately 67.7% (Fig. 3B). This difference in percent mineralization between BMP-2 or BMP-6 and BMP-2/BMP-6 is statistically significant with P values of 0.0064 and 0.0067, respectively. The data further support that BMP-2/BMP-6 has a greater functional response than either of the homodimers.
Figure 3.
BMP-2/BMP-6 heterodimer exhibits higher mineralization of preosteoblast cells than homodimers. A, Visualization of calcium deposits in MC3T3-E1 cells stained with Alizarin Red S (top) and mineralization of MC3T3-E1 cells shown by von Kossa staining (bottom). The absence of growth factor and the addition of BMP-2, BMP-6, and BMP-2/BMP-6 at 10 ng/ml are indicated. B, Quantification of calcium mineralization in MC3T3-E1 cells after von Kossa staining. Error bars are indicated.
Ex vivo activity of BMP heterodimer
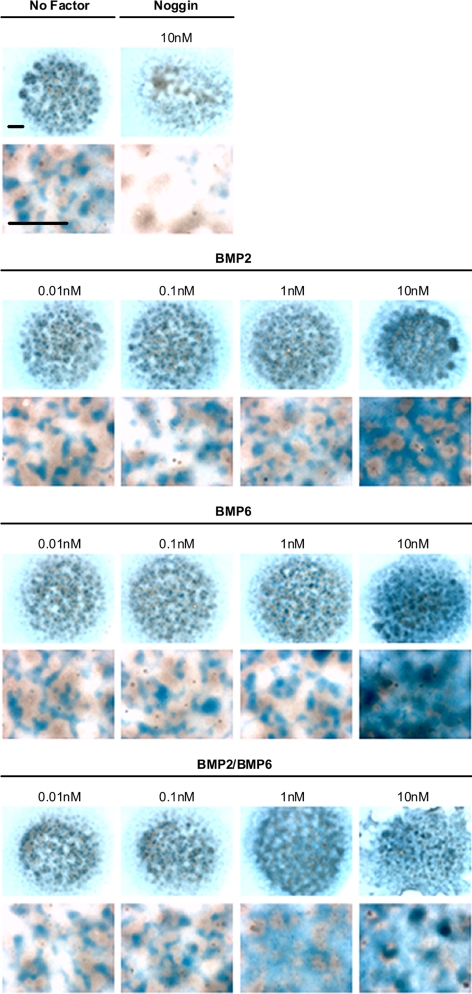
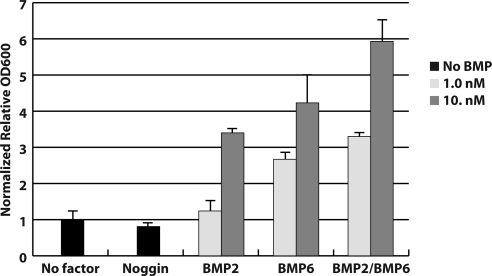
The chondrogenesis of primary cultured limb bud mesenchymal cells has been shown to be induced by human TGF-β and BMP ligands ( 31). Therefore, to further characterize the activities of the homo- and heterodimer ligands in their natural milieu, we used an ex vivo developmental assay of chick limb bud mesenchyme cells in micromass culture. Figure 4 displays micrograph images of stained chondrogenic nodules whose formation is stimulated by BMPs. The extent of chondrogenesis is evaluated by measuring dye bound to the chondrogenic nodule (Fig. 5). Similar to the reporter assay, we observed a dose-dependent chondrogenesis of the limb bud mesenchymal cells by the different BMP ligands. Consistent with the in vitro data, we observed a higher chondrogenic response from the addition of BMP-2/BMP-6 compared with either homodimer, at both 1 and 10 nm concentrations (Figs. 4 and 5). This is confirmed by statistical examinations; at 1 nm, BMP-2/BMP-6 induced a higher maximum response than BMP-6 and BMP-2 with P values of 0.0011 and <0.0001 respectively, and similarly, with P values of 0.0134 and 0.0002, respectively at 10 nm concentration. Combined, our results highlight a direct correlation between higher ligand-receptor affinity and increased cellular signaling activity as the basis for the increased chondrogenesis activity of the BMP-2/BMP-6 heterodimer.
Figure 4.
BMP-2/BMP-6 heterodimer exhibits higher activity to induce chondrogenesis than homodimers. Visualization of chick limb bud mesenchyme cell micromass culture chrondogenesis assays after 5 d. The top panel shows the culture with no factor and the extracellular antagonist Noggin. The second through fourth panels show the culture with ligands BMP-2, BMP-6, and BMP-2/BMP-6, respectively. Bars, 1 mm.
Figure 5.
Quantitative evaluation of higher chondrogenesis inducing ability of BMP-2/BMP-6 heterodimer than homodimers. Quantification of chick limb bud mesenchyme cell micromass culture chrondogenesis assays after 3 d. The absence of growth factor and the addition of Noggin, BMP-2, BMP-6, and BMP-2/BMP-6 at different concentrations and error bars are indicated.
Minimal assembly of TGF-β signaling complex required for signaling
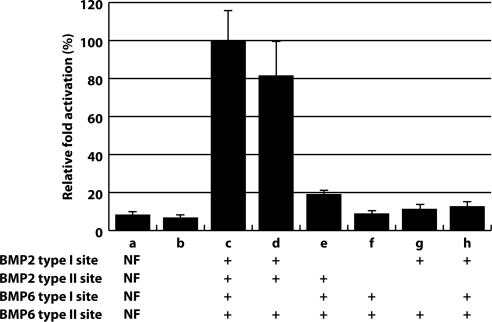
Given that BMP-2/BMP-6 contains the high affinity receptor interfaces for each receptor type resulting in increased cellular signaling and ex vivo activity inducing chondrogenesis, we analyzed whether all four receptor-binding interfaces need to be occupied to form a competent signaling complex. The asymmetric nature of BMP-2/BMP-6 is ideally suited to address the minimum assembly of the TGF-β signaling complex because each TGF-β receptor site can be individually and independently manipulated. Figure 6 displays the specific mutagenesis of the BMP-2/BMP-6 ligand and the quantification of the different mutant variants to activate a Smad1-dependent reporter. The values represent the relative activities of the mutant ligands, tested at 1 nm concentration, compared with BMP-2/BMP-6 as normalized to 100% activation of the reporter. The BMP-2/BMP-6 mutants carrying one defective type I receptor interface but with two intact type II receptor interfaces (Fig. 6, samples d and e) activate the reporter to levels at 20 or 80% compared with BMP-2/BMP-6. Interestingly, disruption of the type I binding site from BMP-2, which has approximately 50-fold higher binding affinity to the type I receptor than BMP-6, results in the more significant reduction in the overall affinity to 20% level of BMP-2/BMP-6. In contrast, the BMP-2/BMP-6 mutant carrying one defective type II receptor interface but with two intact type I interfaces (Fig. 6, sample h) cannot activate the reporter above background levels. Further, the BMP-2/BMP-6 mutants disrupting one type I and type II receptor interfaces simultaneously (Fig. 6, samples f and g) do not activate the reporter above background. These results demonstrate that two type II sites are required for signaling activity, while only one type I site is sufficient for signaling.
Figure 6.
Requirement of ligand-receptor interface in the asymmetric BMP-2/BMP-6 ligand for its signaling activity. BMP-2/BMP-6 mutants displaying activation of Smad1 reporter gene. Sample a contains no factor (background); Sample b contains a BMP-2 homodimer with no active receptor sites. Samples c–h contain different components of the BMP-2/BMP-6 heterodimers. All quantities are normalized to Sample c BMP-2/BMP-6 heterodimer with all receptor sites intact (normalized to 100% for activation of reporter gene). Error bars are indicated.
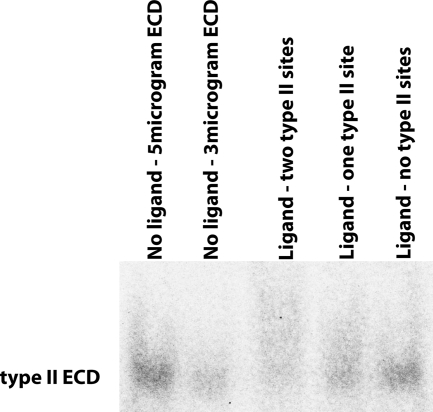
Although the BMP-2/BMP-6 mutant carrying only one intact type II receptor site is unable to signal (Fig. 6, sample h), it is still able to bind type II receptor ECD. Figure 7 illustrates an ActRII-ECD saturation assay in which the binding to ActRII-ECD is assayed on native-PAGE. In this assay, we quantitatively measured the total amount of unbound type II receptor ECD in the presence or absence of various BMP-2/BMP-6 ligands. As a calibration reference, the two left lanes show five or three micrograms of ActRII-ECD each. In the next lane, the ligand containing two intact type II receptor-binding sites (Fig. 6, sample c) diminishes the intensity of the free ActRII-ECD band to about 11% of the level of the five micrograms of ActRII-ECD. We interpret this result as an indication that the majority of the receptor ECD has been incorporated into the ligand-receptor complex. In the next lane, the ligand with only one active type II receptor site (Fig. 6, sample h) shows an increase of free ActRII-ECD to about 36% of the level of the five micrograms. And in the final lane, a ligand with no active type II receptor sites (Fig. 6, sample b) further increases free ActRII-ECD to about 66% of the level of the five micrograms. Assuming the remaining approximately 34% as the background loss of ActRII-ECD in this assay, the reduction of AcRII-ECD intensity from 66% to 36% to 11% by the ligand carrying no, one, or two intact type II receptor interfaces, respectively, suggests that the intact type II receptor interface present in the nonsignaling BMP-2/BMP-6 mutant (Fig. 6, sample h) is able to bind the type II receptor ECD. Therefore, the inability to signal most likely lies at the level of structural orientation and conformational changes required to carry out downstream signaling steps, not at the level of recruitment of receptors and assembly of the ligand-receptor complex at the cell surface.
Figure 7.
Independent binding of each of two type II receptor interface to receptor ECD. Native-PAGE illustrating the amount of type II receptor ECD remaining after being saturated into a receptor-ligand complex with the indicated BMP-2/BMP-6 mutants. The first two lanes are calibration references that contain 5 and 3 μg of the type II receptor ECD without ligand.
Discussion
Analysis of the BMP heterodimer ligand
While previous work has relied on methods such as recombinant baculovirus-mediated expression systems for recombinant BMP heterodimers ( 12), we have demonstrated that purified recombinant BMP heterodimers can be expressed in E. coli as inclusion bodies, chemically refolded, and purified (Fig. 1). The data obtained from the surface plasmon resonance affinity studies (Table 1) show that the BMP-2/BMP-6 ligand adopts the higher affinity receptor sites from its two subunits BMP-2 and BMP-6, which provides a mechanistic basis for the higher potency of heterodimers compared with their homodimer counterparts. With high affinity for both type I and type II receptor ECDs, the BMP-2/BMP-6 ligand exhibits increased signaling ability in the whole cell reporter (Fig. 2) and cell mineralization (Fig. 3) assays compared with the corresponding homodimers. Additionally, the ex vivo data from the mesenchyme cell assays (Figs. 4 and 5) demonstrate the ability of the BMP-2/BMP-6 to induce a response at a lower concentration and to a higher maximum than its homodimer counterparts. Combined, these data directly support the correlation between stability of the ligand-receptor complex, increased signaling activity, and higher biological outputs.
Signaling complex requirements
Having a clean preparation of BMP-2/BMP-6 enabled us to address whether recruitment of one type II and one type I receptor is sufficient to initiate cross-phosphorylation and subsequent signaling of the complex. Using a subset of BMP-2/BMP-6 mutants carrying different degrees of defective receptor-binding interface, we show that the minimal assembly required for BMP-2/BMP-6 to activate the reporter gene is one type I receptor site and two type II receptor sites (Fig. 6, samples d and e). Interestingly, the degree of signaling activity of BMP-2/BMP-6 with defective BMP-2 type I (20%, Fig. 6, sample e) site and that with defective BMP-6 type I site (80%, Fig. 6, sample d) site add to that of the intact BMP-2/BMP-6 (normalized 100%; Fig. 6, sample c). This implicates that the two type I receptor kinase molecules in the ligand-receptor complex operate largely independently for downstream signaling. On the contrary, BMP-2/BMP-6 variant with only one type II receptor site does not show incremental change, but completely fails to activate the reporter gene (Fig. 6, samples f–h). Because the type II receptor ECD can physically bind to the BMP-2/BMP-6 with only one active type II receptor interface (Fig. 7), the inability to signal is not at the level of signaling complex assembly between ligand and receptor but likely at the level of how binding to two type II receptors is necessary to initiate transmembrane signaling. One possible mechanistic explanation is that two type II receptor kinase domains must autophosphorylate each other before they can cross-phosphorylate the type I receptor kinase domain in the same signaling complex. It is consistent with the views with other autophosphorylating kinase domains ( 32,33) indicating that dimerization of the type II receptors is a prerequisite for autophosphorylation. The other viable structural explanation is that cell signaling does not occur because type I receptors cannot be recruited for binding to their binding site present at the subunit junction of the ligand ( 23) until two subunits of the ligand spread out as a result of binding to two type II receptors present in the cell membrane ( 34). This wing-spread hypothesis predicts that the type I receptor-binding interface does not exist in the ligand surface until the two wings of the ligand extend out fully by being bound to two cell membrane-embedded type II receptors, but this hypothesis needs experimental verification.
Independent binding model of signaling complex assembly
A production of pure and recombinant BMP-2/BMP-6 by chemical refolding enabled us to demonstrate the direct correlation between various ligand-receptor assemblies with respect to their ligand-receptor affinity, signaling activity, and biological activity. Our study with BMP heterodimer variants further illustrated how each ligand-receptor interaction contributes to the overall activity and how the asymmetric heterodimers may add further complexity in overall signaling outcomes. By this analysis, we demonstrated that the minimal assembly required as a signaling-competent complex is two type II receptors and one type I receptor even if each receptor can be physically assembled individually. Together with our recent work demonstrating a single amino acid change in BMP-3 that alters its signaling activity dramatically ( 29), it is quite conceivable to expand the signaling repertoire of TGF-β superfamily by designing novel TGF-β ligand variants to endow them potentially with new biological activity. Such molecules can be screened for their clinical potential, including therapeutic applications such as bone remodeling, osteoarthritis therapy, and as molecules for stem cell maintenance and differentiation guidance.
Materials and Methods
Protein expression and purification
The mature domains of human BMP-2 (residues 1–110) and human BMP-6 (residues 1–132) were expressed in Escherichia coli as inclusion bodies. Mutations to the wild-type ligand sequences were based on previously published findings which disrupt the ligand-receptor interfaces ( 26,27). BMP-2 subunits carry changes of Leu51 to Pro and the double mutation of Ala34 to Asp and Leu90 to Ala to disrupt type I and II receptor binding interfaces, respectively. BMP-6 subunits carry changes of Leu68 to Pro to disrupt type I receptor binding interface. The BMP-2 and BMP-6 subunits were expressed separately as inclusion bodies, isolated, and purified before being refolded using a modified protocol ( 35). The BMP-2 and BMP-6 subunits were combined in an equimolar mixture during the refolding step to create the heterodimer ligand. The refolded BMP-2 and BMP-6 homodimers, and BMP-2/BMP-6 heterodimer, were concentrated in the refolding solution and diluted into a urea buffer. The monomer and aggregate were separated from the dimer product using a HiTrap heparin column (GE Healthcare, Uppsala, Sweden). The BMP-2/BMP-6 heterodimer was isolated from the expected respective homodimers through differences in each ligand’s heparin sulfate binding properties. A final purification was performed by reversed phase chromatography (GraceVydac, Deerfield, IL). The ligands were lyophilized and re-suspended in 10 mm sodium acetate (pH 4.0). The ECDs of human BMPRIa (residues 1–129) and mouse ActRIIb (residues 1–98) were expressed in E. coli as thioredoxin fusion proteins using a modification of published procedures ( 36). Mouse ActRII-ECD (residues 1–102) was expressed and purified from a Pichia pastoris expression system as described ( 37).
Surface plasmon resonance affinity studies
Using Biacore 3000 (GE Healthcare), the binding affinity to BMPRIa, ActRII, and ACTRIIb was measured and analyzed by using BIAevaluation software version 4.1 (GE Healthcare). The receptor ECDs were immobilized on a CM5 chip by primary amine coupling. Each receptor ECD was immobilized independently on flow cells 2–4 for 10 min at a flow rate of 5 μl/min and a concentration of 20 μm in 10 mm sodium acetate (pH 4.0). Flow cell 1 was left blank with no immobilized protein as a negative control. The experiments were performed by flowing various ligands at a flow rate of 50 μl/min in 20 mm Tris-HCl (pH 7.9), 250 mm NaCl, 0.36% 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), and 0.005% Tween 20. At least five concentrations, plus a zero concentration, were run per sample for kinetic analysis. The data were fit by using a global 1:1 Langmuir binding with mass transfer.
Luciferase reporter assays
Smad1-dependent luciferase assays were performed as previously described ( 29). In brief, C2C12 cells are cultured in DMEM + 5% FBS supplemented with l-glutamine and antibiotics. For luciferase reporter assays, cells were trypsinized, washed twice with PBS, and plated into 48-well plates with DMEM + 0.1% FBS. Twenty-four hours later, cells were transfected with −1147Id1-luciferase construct containing the Smad binding sites (Id1-Luc) ( 38), a Smad-1 expression construct, and a CAGGS-LacZ plasmid using Fugene6 (Roche, Basel, Switzerland) according to the manufacturer’s instruction. Luciferase activity was measured 24 h after stimulation with ligands, and the values were normalized for transfection efficiency using β-galactosidase activity. The activity of the luciferase reporter is expressed in fold induction relative to control values that are obtained using −927Id1-luciferase that lacks Smad1 binding domains (Id1-Luc mut).
Mineralization assays
Preosteoblast cell line, originated from new-born mouse calvaria, MC3T3-E1 cells were grown in α-MEM containing 10% fetal bovine serum, antibiotics with 5 mm β-glycerol phosphate and 50 μg/ml of ascorbic acid for inducing osteogenic differentiation. Cells were grown under standard culture conditions (5% CO2 at 37 C), and the media were refreshed every 3 d. MC3T3-E1 cells were plated at >1 × 105 cells per well on 24-well plates and treated with 10 ng/ml of BMP-2, BMP-6, or BMP-2/BMP-6. For Alizarin red staining, the cells were cultured for 4 d, fixed with cold 70% ethanol, and stained with Alizarin red S (2% in H2O, pH adjusted to 4.2 with ammonium hydroxide) for 10 min with multiple times of washing. For von Kossa staining, the cells were cultured for 3 d, fixed in 4% paraformaldehyde buffer (Biosesang, Korea), stained with silver nitrate (Sigma, St. Louis, MO) for 60 min under the UV light, and washed three times with distilled water. The reaction was stopped with 5% sodium thiosulfate for 5 min and washed with water. Nuclear fast red was used for counter staining, and plates were dehydrated with ethanol. The percentage of mineralization results were scored by Motic image plus 2.0. Statistical analysis was performed with Student’s t test.
Chick limb bud micromass assays
Chick embryos at Hamburger-Hamilton stage 23–24 ( 39) were collected in Hanks’ solution containing Ca2+ and Mg2+, and distal 1/3 part of limb buds were dissected out. Ectodermal sheets were removed by trypsinization (0.5% in Hanks’ solution with Ca2+ and Mg2+) on ice for 30 min, and then mesenchymal tissues were recovered and incubated in Ca2+- and Mg2+-free Hanks’ solution at 37 C for 15 min. The mesenchyme cells were dissociated into single cells by pipetting in OptiMEM medium (Invitrogen, Carlsbad, CA) containing 1% FBS. Cultures were seeded into 96-well plates at 4 × 105 cells per well. After 1 h, media containing each ligand were added. Fresh media with ligands were changed daily, and cells were analyzed for chondrogenesis by Alcian blue staining to visualize cartilage nodule and quantification of chondrogenesis as described ( 40). Statistical analysis was performed with Student’s t test.
Native-PAGE ligand-receptor ECD complex formation
Five micrograms of purified ActRII-ECD alone and with 10 μg of BMP-2/BMP-6, BMP-2/BMP-6 with a single active type II receptor interface, or BMP-2 with no active type II receptor interfaces was loaded onto a native-PAGE gel in 50 mm Tris-HCl (pH 7.9), 700 mm NaCl, and 1.8% CHAPS. The Coomassie Brilliant Blue (Bio-Rad, Hercules, CA) stained gel was analyzed using the “Integrated Density” function with National Institutes of Health ImageJ software ( 41).
Acknowledgments
We thank Inno Maslennikov, Witek Kwiatkowski, and Mark Vega for technical assistance and numerous discussions, Robert Benezra for the Id1-luciferase reporters, and Elizabeth Komives for SPR data collection.
Footnotes
This research was supported by grants from the National Institutes of Health (HD013527, to S.C.), California Institute for Regenerative Medicine (S.C.), Incheon Free Economic Zone, and World Class University, Korea (S.C.).
Present address for Y.K.: Department of Genetics, Cell Biology, and Development, and Stem Cell Institute, McGuire Translational Research Facility, University of Minnesota, 2001 6th Street Southeast, Minneapolis, Minnesota 55455.
Disclosure Summary: The authors have nothing to declare.
First Published Online May 19, 2010
Abbreviations: BMP, Bone morphogenetic protein; ECD, extracellular domain; GDF, growth and differential factor; SELDI-TOF-MS, surface-enhanced laser desorption/ionization time-of-flight mass spectrometry; SPR, surface plasmon resonance.
References
- Kingsley DM 1994 The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 8:133–146 [DOI] [PubMed] [Google Scholar]
- Urist MR 1965 Bone: formation by autoinduction. Science 150:893–899 [DOI] [PubMed] [Google Scholar]
- Hogan BLM 1996 Bone morphogenetic proteins in development. Curr Opin Genet Dev 6:432–438 [DOI] [PubMed] [Google Scholar]
- Wagner TU 2007 Bone morphogenetic protein signaling in stem cells—one signal, many consequences. FEBS J 274:2968–2976 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li L 2005 BMP signaling and stem cell regulation. Dev Biol 284:1–11 [DOI] [PubMed] [Google Scholar]
- Massagué J 1990 The transforming growth factor-β family. Annu Rev Cell Biol 6:597–641 [DOI] [PubMed] [Google Scholar]
- Lyons KM, Hogan BLM, Robertson EJ 1995 Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev 50:71–83 [DOI] [PubMed] [Google Scholar]
- Katagiri T, Boorla S, Frendo JL, Hogan BLM, Karsenty G 1998 Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev Genet 22:340–348 [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ 1999 Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development 126:1753–1768 [DOI] [PubMed] [Google Scholar]
- Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM 1996 Heterdimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 13:291–300 [DOI] [PubMed] [Google Scholar]
- Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y 1995 Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Comm 210:670–677 [DOI] [PubMed] [Google Scholar]
- Hazama M, Aono A, Ueno N, Fujisawa Y 1995 Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem Biophys Res Comm 209:859–866 [DOI] [PubMed] [Google Scholar]
- Butler SJ, Dodd J 2003 A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron 38:389–401 [DOI] [PubMed] [Google Scholar]
- Zhu W, Kim J, Cheng C, Rawlins BA, Boachie-Adjei O, Crystal RG, Hidaka C 2006 Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone 39:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Rawlins BA, Boachie-Adjei O, Myers ER, Arimizu J, Choi E, Lieberman JR, Crystal RG, Hidaka C 2004 Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J Bone Miner Res 19:2021–2032 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Kaneko E, Maeda J, Ueno N 1997 Mesoderm induction by BMP-4 and -7 heterodimers. Biochem Biophys Res Commun 232:153–156 [DOI] [PubMed] [Google Scholar]
- Tanaka C, Sakuma R, Nakamura T, Hamada H, Saijoh Y 2007 Long-range action of Nodal requires interaction with GDF1. Genes Dev 21:3272–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Karsenty G 2000 The family of bone morphogenetic proteins. Kidney Int 57:2207–2214 [DOI] [PubMed] [Google Scholar]
- Miyazono K, ten Dijke P, Heldin CH 2000 TGF-β signaling by Smad proteins. Adv Immunol 75:115–157 [DOI] [PubMed] [Google Scholar]
- Greenwald J, Le V, Corrigan A, Fischer W, Komives E, Vale W, Choe S 1998 Characterization of the extracellular ligand-binding domain of the type II activin receptor. Biochemistry 37:16711–16718 [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Inoue H, Hanai J, Nishihara A, Hanyu Aki, Takase M, Ishidou Y, Udagawa Y, Oeda E, Goto D, Yagi K, Kato M, Miyazono K 1999 Intracellular signaling of the TGF-β superfamily by Smad proteins. Ann NY Acad Sci 886:73–82 [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K 1998 Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev 9:49–61 [DOI] [PubMed] [Google Scholar]
- Allendorph GP, Vale WW, Choe S 2006 Structure of the ternary signaling complex of a TGF-β superfamily member. Proc Natl Acad Sci USA 103:7643–7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe J, Hinck CS, Samavarchi-Tehrani P, Zubieta C, Schuermann JP, Taylor AB, Schwarz PM, Wrana JL, Hinck AP 2008 Cooperative assembly of TGF-β superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell 29:157–168 [DOI] [PubMed] [Google Scholar]
- Weber D, Kotzsch A, Nickel J, Harth S, Seher A, Mueller U, Sebald W, Mueller TD 2007 A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct Biol 7:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Nickel J, Zhang JL, Sebald W, Mueller TD 2004 Molecular recognition of BMP-2 and BMP receptor IA. Nat Struct Mol Biol 11:481–488 [DOI] [PubMed] [Google Scholar]
- Kirsch T, Nickel J, Sebald W 2000 BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-II. EMBO J 19:3314–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M, Weis MB, Massagué J 1990 Concomitant loss of transforming growth factor (TGF)-β receptor types I and II in TGF-β-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem 265:18518–18524 [PubMed] [Google Scholar]
- Allendorph GP, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S 2007 BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry 46:12238–12247 [DOI] [PubMed] [Google Scholar]
- Suzawa M, Takeuchi Y, Fukumoto S, Kato S, Ueno N, Miyazono K, Matsumoto T, Fujita T 1999 Extracellular matrix-associated bone morphogenetic proteins are essential for differentiation of murine osteoblastic cells in vitro. Endocrinology 140:2125–2133 [DOI] [PubMed] [Google Scholar]
- Merino R, Gañan Y, Macias D, Economides AN, Sampath KT, Hurle JM 1998 Morphogenesis of digits in the avian limb is controlled by FGFs, TGFβs, and Noggin through BMP signaling. Dev Biol 200:35–45 [DOI] [PubMed] [Google Scholar]
- Pike AC, Rellos P, Niesen FH, Turnbull A, Oliver AW, Parker SA, Turk BE, Pearl LH, Knapp S 2008 Activation segment dimerization: a mechanism for kinase autophosphorylation of non-consensus sites. EMBO J 27:704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver AW, Paul A, Boxall KJ, Barrie SE, Aherne GW, Garrett MD, Mittnacht S, Pearl LH 2006 Trans-activation of the DNA-damage signalling protein kinase Chk2 by T-loop exchange. EMBO J 25:3179–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald J, Vega ME, Allendorph GP, Fischer WH, Vale W, Choe S 2004 A flexible activin explains the membrane-dependent cooperative assembly of TGF-β family receptors. Mol Cell 15:485–489 [DOI] [PubMed] [Google Scholar]
- Groppe J, Rumpel K, Economides AN, Stahl N, Sebald W, Affolter M 1998 Biochemical and biophysical characterization of refolded Drosophila DPP, a homolog of bone morphogenetic proteins 2 and 4. J Biol Chem 273:29052–29065 [DOI] [PubMed] [Google Scholar]
- Kirsch T, Nickel J, Sebald W 2000 Isolation of recombinant BMP receptor IA ectodomain and its 2:1 complex with BMP-2. FEBS Lett 468:215–219 [DOI] [PubMed] [Google Scholar]
- Greenwald J, Fischer WH, Vale WW, Choe S 1999 Three-finger toxin fold for the extracellular ligand-binding domain of the type II activin receptor serine kinase. Nat Struct Mol Biol 6:18–22 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Takizawa T, Ochiai W, Yanagisawa M, Hisatsune T, Nakafuku M, Miyazono K, Kishimoto T, Kageyama R, Taga T 2001 BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc Natl Acad Sci USA 98:5868–5873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL 1951 A series of normal stages in the development of the chick embryo. J Morph 88:49–92 [DOI] [PubMed] [Google Scholar]
- Wada N, Tanaka H, Ide H, Nohno T 2003 Ephrin-A2 regulates position-specific cell affinity and is involved in cartilage morphogenesis in the chick limb bud. Dev Biol 264:550–563 [DOI] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ 2004 Image processing with ImageJ. Biophotonics Int 11:36–42 [Google Scholar]