Abstract
How cAMP-dependent protein kinases [protein kinase A (PKA)] transduce the mitogenic stimulus elicited by TSH in thyroid cells to late activation of cyclin D3-cyclin-dependent kinase 4 (CDK4) remains enigmatic. Here we show in PC Cl3 rat thyroid cells that TSH/cAMP, like insulin, activates the mammalian target of rapamycin (mTOR)-raptor complex (mTORC1) leading to phosphorylation of S6K1 and 4E-BP1. mTORC1-dependent S6K1 phosphorylation in response to both insulin and cAMP required amino acids, whereas inhibition of AMP-activated protein kinase and glycogen synthase kinase 3 enhanced insulin but not cAMP effects. Unlike insulin, TSH/cAMP did not activate protein kinase B or induce tuberous sclerosis complex 2 phosphorylation at T1462 and Y1571. However, like insulin, TSH/cAMP produced a stable increase in mTORC1 kinase activity that was associated with augmented 4E-BP1 binding to raptor. This could be caused in part by T246 phosphorylation of PRAS40, which was found as an in vitro substrate of PKA. Both in PC Cl3 cells and primary dog thyrocytes, rapamycin inhibited DNA synthesis and retinoblastoma protein phosphorylation induced by TSH and insulin. Although rapamycin reduced cyclin D3 accumulation, the abundance of cyclin D3-CDK4 complexes was not affected. However, rapamycin inhibited the activity of these complexes by decreasing the TSH and insulin-mediated stimulation of activating T172 phosphorylation of CDK4. We propose that mTORC1 activation by TSH, at least in part through PKA-dependent phosphorylation of PRAS40, crucially contributes to mediate cAMP-dependent mitogenesis by regulating CDK4 T172-phosphorylation.
PKA-dependent activation of mTOR contributes to mediate the activation of CDK4 in the proliferation of thyrocytes stimulated by TSH.
Thyroid function, differentiation expression, and proliferation are mainly regulated by pituitary TSH. Most, if not all, cell proliferation effects induced by either TSH, TSH receptor-stimulating autoantibodies, and activating mutations of TSH receptor or Gsα, are mediated by elevation of cellular cAMP levels ( 1), according to a paradigm first demonstrated using primary cultures of canine thyroid cells ( 2) and confirmed in primary cultures of normal human thyrocytes ( 3). The known pathogenesis of TSH-dependent goiter, Graves’ disease, autonomous hyperfunctional adenomas, or congenital hyperthyroidism demonstrates this conclusion ( 1).
To investigate cellular and molecular mechanisms of mitogenesis stimulated by TSH/cAMP, several cell systems have been used, including rat thyroid cell lines (FRTL-5, WRT, and PC Cl3 cells), and dog and human thyrocytes in primary culture, that allow comparison with growth factor-dependent mechanisms ( 4). In all these models, TSH/cAMP-dependent proliferation requires the comitogenic action of insulin and/or IGF-I mainly through activation of the PI3-kinase (PI3-K)/PKB cascade. In vivo models also point to a role for PI3-K/protein kinase B (PKB) signaling in the development of thyroid follicular neoplasms ( 5,6,7,8). Downstream of cAMP, the signaling involved in the TSH mitogenic action is still largely unknown, controversial, or different from one model to another ( 4). As in the action of other pituitary trophic hormones acting through cAMP on their target cells ( 9,10), positive cross-signaling of TSH/cAMP pathway with ERK and PI3-K cascades has been claimed to signal cAMP-dependent cell proliferation in FRTL-5 and WRT cells (reviewed in Refs. 4 and 11). However, we have shown in more physiologically relevant thyroid primary cultures that cAMP triggers and supports cell cycle progression without activating Ras ( 12), ERKs ( 13), and PI3-K/PKB pathways (14), without inducing D-type cyclin expression ( 15,16) and despite a paradoxical up-regulation of p27kip1 ( 16,17). In thyroid cells, cAMP inactivates the central oncosuppressor/cell cycle regulator retinoblastoma protein (Rb) by activating cyclin D3- cyclin-dependent kinase 4 (CDK4) complexes ( 15,16,18). In dog thyrocytes, cyclin D3-CDK4 activation by cAMP includes its increased assembly ( 15), stabilization in the nucleus through binding to nuclear p27kip1 ( 17), and activation through T172-phosphorylation of CDK4 depending on poorly understood regulatory mechanisms ( 16,19,20,21). In the various in vitro thyroid models, the only convergent early signaling event found in response to TSH/cAMP, insulin, and growth factors is the phosphorylation and activation of p70S6K1 ( 14,22,23), the kinase responsible for phosphorylating the 40S ribosomal protein S6 and other substrates involved in protein synthesis ( 24). Interestingly, cAMP-dependent activation of p70S6K1 is also observed in other models of cAMP-dependent mitogenesis ( 22,25,26,27), but not in cells in which cAMP does not act as a mitogen or inhibits proliferation ( 28,29,30).
p70S6K1 is generally activated by mTOR (mammalian target of rapamycin) complexed to raptor (mTORC1), although activation of S6K1 by cAMP stimuli without mTORC1-dependent T389 phosphorylation has been reported ( 26). By also controlling mRNA translation at the initiation step by phosphorylating 4E-BP1, mTORC1 is emerging as a key regulator of energy homeostasis, cell physiology, and growth in eukaryotes ( 31,32,33). There are two major mechanisms by which insulin and growth factors, and energy balance via AMP-activated protein kinase (AMPK), regulate mTORC1 activity. One involves the tuberous sclerosis complex 2 (TSC2) gene product (tuberin) and its association with TSC1 (hamartin), and the small G protein Rheb. Phosphorylation of TSC2 by PKB or the ERKs activates mTORC1 by derepressing the TSC2 GTPase-activating protein (GAP) activity toward Rheb ( 34,35,36,37,38). In addition, in response to insulin the phosphorylation by PKB of PRAS40 (proline-rich Akt substrate of 40 kDa) causes its dissociation from mTORC1, also contributing to mTORC1 activation ( 39,40,41,42). mTOR also exists as a second complex with rictor (mTORC2), which is resistant to acute inhibition by rapamycin and has been found to be responsible for S473 phosphorylation of PKB and essential phosphorylation of PKCs ( 43,44,45).
In dog thyrocytes in primary culture, our group has found that TSH through cAMP and cAMP-dependent protein kinases (PKA) ( 46) activates S6K1 without activating PI3-K and PKB, and that mTORC1 inhibitor rapamycin markedly inhibits the TSH/cAMP-dependent proliferation ( 14). Identical observations have been recently reported by Di Cristofano and associates ( 47) for TSH-dependent thyroid hyperplasia in mice in vivo, demonstrating their physiological relevance and underscoring the necessity to further investigate mechanisms of cAMP-dependent mTOR activation. Importantly, this study has also shown that mTOR inhibition abrogates the hyperplastic but not the hypertrophic responses to TSH, thus uncoupling both processes ( 47). In the present study, we address the mechanisms of S6K1-activating phosphorylation induced in the absence of PKB activation by TSH and cAMP, further demonstrate the involvement of mTOR by direct investigation of mTORC1 kinase activity, and define the mechanism(s) of its cAMP/PKA-dependent regulation. Moreover, we show that mTORC1 inhibition by rapamycin impairs thyroid cell cycle progression stimulated by TSH and insulin by inhibiting CDK4 phosphorylation and activation.
Results
TSH induces phosphorylation of p70S6K1 and 4E-BP1 but does not activate PKB
As observed in dog thyrocytes in primary culture ( 14) and in mouse thyroid in vivo ( 47), TSH and cAMP do not activate PI3-K and PKB in the differentiated PC Cl3 rat thyroid cell line ( 48), at variance with claims from other rat thyroid cell lines ( 11,49,50). Because dog thyroid tissue has become difficult to obtain, we used PC Cl3 cells to explore the mechanisms of cAMP-dependent activation of p70S6K1. As shown in Fig. 1, A and B, TSH and the adenylyl cyclase activator forskolin induced the phosphorylation of S6K1 in these cells seen by increased T389 phosphorylation and decreased migration by SDS-PAGE. This is similar to the effect of a high concentration of insulin that activates both insulin and IGF-I receptors. However, unlike insulin, TSH and forskolin did not induce the T308 phosphorylation and activation of PKB. Nevertheless, in several experiments (10 of 26), the S473 phosphorylation of PKB was modestly increased by forskolin (as seen in the experiments illustrated in Fig. 1) and/or TSH (not shown), as observed in other rat thyroid cell lines ( 50) and even in dog thyrocytes in primary culture (our unpublished results). This was clearly insufficient for PKB activation (Fig. 1B). The observation that wortmannin inhibited both S473 phosphorylation of PKB and S6K1 phosphorylation stimulated not only by insulin but also by forskolin (Fig. 1B) was explained by the fact that wortmannin is a potent inhibitor of mTORC1 and mTORC2 in addition to PI3-K ( 43,51).
Figure 1.
TSH and forskolin induce p70S6K1 phosphorylation but do not activate PKB. PC Cl3 cells were treated without (c) or with 5 μg/ml insulin (i) and/or 10 μm forskolin (F) or 1 mU/ml TSH (T) for 20 min. In some conditions (in panel B) cells were pretreated with 200 nm wortmannin (w) before stimulation. PKB activity was assayed after immunoprecipitation by incubation with a specific substrate and [γ32P] ATP. 32P incorporation in PKB substrate was measured by scintillation counting (CPM). In parallel, immunoblotting after SDS-PAGE from the same samples was performed for detection of PKB, pPKB (S473), pPKB (T308), S6K1 and pS6K1 (T389). A and B, Two independent experiments are illustrated.
S6K1 phosphorylation was detected slightly earlier in response to insulin than to TSH (Fig. 2A). For both hormones, the response reached a maximum in 30 min and only partially declined after 4 h. The combination of TSH and insulin induced a stronger and more sustained response, which was still observed after 20 h (Fig. 2A). Activation of S6K1 in response to insulin and growth factors is described as a sequential process that involves first phosphorylation at S/TP sites (including T421 and S424) in the autoinhibitory domain, then T389 phosphorylation in the hydrophobic motif by mTORC1, and finally phosphorylation of the activation loop at T229 by phosphoinositide-dependent protein kinase (PDK)1 ( 52,53). In PC Cl3 cells, phosphorylation of these different sites was induced by both insulin and TSH (Fig. 2B). Moreover, S6 was also strongly phosphorylated in response to both insulin and TSH, which confirmed the activation of S6K1 (Fig. 2B). In agreement with the accepted hierarchical sequence, as in the case of insulin, rapamycin inhibited the TSH-induced phosphorylation at T389 and subsequent phosphorylation at T229 (Fig. 2B), indicating that they depend directly or indirectly on mTORC1 activity. Interestingly, TSH and insulin more markedly synergized on phosphorylation of T421/S424, which was insensitive to rapamycin in response to both hormones (Fig. 2B). The resistance to rapamycin of the T421/S424 phosphorylation is not due to an insufficient concentration of the drug (40 nm) because 2 nm rapamycin sufficed to completely inhibit the T389 phosphorylation of S6K1 induced by the combination of insulin and TSH (data not shown).
Figure 2.
TSH and insulin induce phosphorylation of S6K1 and 4E-BP1. A, Kinetics of S6K1 phosphorylation stimulated by insulin and/or TSH. PC Cl3 cells were treated or not (−) with 5 μg/ml insulin (i) and/or 1 mU/ml TSH (T) for 5 min up to 20 h. After SDS-PAGE total S6K1 was detected by immunoblotting. Arrows and arrowheads indication the position of hypophosphorylated and hyperphosphorylated S6K1 forms, respectively. A (lower panel) shows the densitometry scanning quantitation of the T389 phosphorylation of S6K1 detected by immunoblotting from the same samples. Data are expressed as fold stimulation compared with untreated control cells. B–D, Rapamycin inhibits most but not all phosphorylation of S6K1 (B and C) and 4E-BP1 (D) induced by insulin and/or TSH. PC Cl3 cells were treated without (c) or with 5 μg/ml insulin (i) and/or 1 mU/ml TSH (T) for 20 min. Rapamycin, 40 nm (rapa or r) was added or not 2 h before treatment. B, S6K1, pS6K1 (T421/S424), pS6K1 (T389), pS6K1 (T229), and pS6 (S235/236) were immunodetected after SDS-PAGE of whole-cell lysates. C, S6K1 was immunoprecipitated (IP S6K1), the immunoprecipitate was separated by two-dimensional gel electrophoresis, and total S6K1 was detected by immunoblotting. The main nonmodified form of S6K1 is numbered as 0, and the presumably (multi-) phosphorylated forms are numbered from 1–13 according to their increasing isoelectric point shifts. D, 4E-BP1, p4E-BP1 (T37/S46), p4E-BP1 (S65), and pmTOR (S2448) were immunodetected after SDS-PAGE of whole-cell lysates.
Phosphorylation adds negative charges to proteins and thus predictably shifts their isolectric point toward more acidic pHs. To further compare phosphorylation of S6K1 induced by insulin and/or TSH, and their sensitivity to inhibition by rapamycin, S6K1 was immunoprecipitated, and its different forms were resolved by isoelectric focusing followed by SDS-PAGE and then detected by Western blotting (Fig. 2C). Whereas in unstimulated cells treated with rapamycin, S6K1 mostly appeared as a single major spot, in cells maximally stimulated with insulin and TSH (without rapamycin) S6K1 displayed an unexpectedly complex profile with the coexistence of multiple forms. If all the observed isoelectic point shifts reflect phosphorylation, maximally stimulated S6K1 appears to be a mixture of forms containing between 4 and up to 13 induced phosphorylation events (Fig. 2C). Addition of insulin and TSH separately similarly shifted the isoelectric profile of S6K1, indicating that S6K1 was entirely recruited by various combinations of multiple phosphorylation events induced by both hormones. Rapamycin inhibited most, but not all, the modification of S6K1 induced by insulin and/or TSH (Fig. 2C), consistent with the known resistance of T421/S424 phosphorylation to rapamycin inhibition (as seen in Fig. 2B). Again, insulin and TSH appeared to mainly synergize on the rapamycin-resistant phosphorylation sites (Fig. 2, B and C).
Another major substrate of mTORC1 is the translational repressor 4E-BP1 ( 54). As shown in Fig. 2D, both insulin and TSH induced 4E-BP1 phosphorylation at T37/46 and S65, which cause the disassociation of 4E-BP1 from eIF4E ( 54). Of note, although both phosphoacceptor sites are recognized substrates of mTORC1, only phosphorylation at S65 was inhibited by rapamycin as previously observed ( 55).
In these experiments, both insulin and TSH increased the S2448 phosphorylation of mTOR in its C-terminal repressor domain (Fig. 2D). This phosphorylation has often been regarded as a readout of mTOR activation, although its role in mTOR regulation remains elusive ( 56,57,58). Although it has been proposed to be mediated by S6K1 ( 58,59), because rapamycin did not affect the S2448-phosphorylation of mTOR either induced by insulin or TSH (Fig. 2D), S6K1 is unlikely to be the responsible kinase in these experiments.
Collectively, these results suggested that TSH activates mTORC1 without activating PKB. Other experiments also showed that ERK activation was not required for the cAMP-dependent activation of mTORC1, because PD98059 inhibited ERK phosphorylation without reducing phosphorylation of both S6K1 and 4E-BP1 (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
cAMP-dependent phosphorylation of S6K1 requires amino acids but is not affected by inhibition of AMPK and glycogen synthase kinase 3 (GSK3)
A hallmark of mTORC1 regulation by mitogenic hormones and growth factors is its dependence on nutrients, especially amino acids ( 61). Because mTORC1 activity is required for most of the phosphorylation events in S6K1 induced by both TSH and insulin, we compared the influence of amino acids on S6K1 phosphorylation stimulated in PC Cl3 cells by insulin or cAMP (forskolin). Amino acid deprivation impaired stimulation of S6K1 T389 phosphorylation by insulin and forskolin (Supplemental Fig. 2). Administration of amino acids alone only weakly stimulated this phosphorylation, but it permitted stimulation by both insulin and forskolin (Supplemental Fig. 2). mTORC1-dependent phosphorylation of S6K1 thus integrates stimulation by insulin and cAMP with the amino acid availability.
mTORC1 activation is also inhibited by energy depletion, which involves phosphorylation of TSC2 by AMPK and GSK3, thus enhancing TSC2 GAP activity toward Rheb ( 62). AMPK also inhibits mTORC1 activity by phosphorylating raptor at S792 ( 63). Because in some cells PKA mimics PKB by phosphorylating and inhibiting both AMPK (at S485/491) ( 64) and GSK3 (at S9/21) ( 65), we investigated the impact of an AMPK activator [5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR)] and inhibitors of AMPK (compound C) and GSK3 (LiCl) on the mTORC1-dependent phosphorylation of S6K1 and 4E-BP1 stimulated by either insulin or forskolin in PC Cl3 cells. These experiments are further detailed in the legends of Supplemental Figs. 1–3. AMPK activation inhibited mTORC1 activity in PC Cl3 cells, as AICAR induced the S792-phosphorylation of raptor and markedly attenuated the S6K1 T389 phosphorylation irrespective of its stimulation by insulin or forskolin (Supplemental Fig. 3A). Insulin, but not forskolin, induced the inhibitory phosphorylation of AMPK at S485/491 (Supplemental Fig. 3, A and B) and consistently reduced the basal AMPK-dependent inhibitory phosphorylation of raptor at S792 (Supplemental Fig. 3A). Moreover, compound C only increased the phosphorylation of 4E-BP1 and S6K1 induced by insulin without apparently affecting the effects of forskolin (Supplemental Fig. 3B). Similarly, insulin, but not forskolin, also increased the inhibitory phosphorylation of GSK3α and -β (Supplemental Fig. 3C), whereas LiCl markedly increased the stimulation of T389 phosphorylation of S6K1 by insulin without affecting the stimulation by forskolin (Supplemental Fig. 3C). Collectively, these experiments suggest that inhibition of the activity of both AMPK and GSK3 could be an important factor in the activation of mTORC1 by insulin, but not in mTORC1 activation by cAMP agonists.
TSH/cAMP increases PRAS40 phosphorylation and mTORC1 kinase activity
Activation of mTORC1 by insulin and growth factors depends on several steps including Rheb-GTP binding and activation of catalytic function and increased binding of substrates to raptor ( 60,66,67). Activation of mTORC1 by Rheb is controlled by the Rheb-GAP TSC2, which is inhibited by phosphorylation on different sites by PKB ( 62,68), ERK (38), and p90RSK (RSK, ribosomal S6 kinase) ( 69). Increased substrate binding to mTORC1 involves the T246 phosphorylation by PKB of PRAS40 ( 39,40,41), which functions as both a substrate and inhibitor of mTORC1 by competing with the binding of its other substrates ( 41,42,70,71). As shown in Fig. 3, insulin stimulated the PKB-dependent T246 phosphorylation of PRAS40 and T1462 phosphorylation of TSC2 in PC Cl3 cells. By contrast, TSH and forskolin mimicked the effect of insulin on PRAS40 phosphorylation but not at all on TSC2 T1462 phosphorylation (Fig. 3). Interestingly, insulin, but not TSH, also markedly induced the phosphorylation of TSC2 at Y1571 (Fig. 3), a rarely studied residue phosphorylated in response to vanadate and serum and mutation of which in hamartomas impairs TSC2-TSC1 interaction ( 72).
Figure 3.
TSH and cAMP increase the phosphorylation of PRAS40 but not of TSC2. PC Cl3 cells were treated without (c) or with 5 μg/ml insulin (i) and/or 10 μm forskolin (F) or 1 mU/ml TSH (T) for 20 min. pS6K1 (T389), p4E-BP1 (T37/46), pTSC2 (T1462), pTSC2 (Y1571), TSC2 and pPRAS40 (T246), were immunodetected after SDS-PAGE of whole-cell lysates.
To directly demonstrate that increased PRAS40 phosphorylation is associated with a stable (i.e. resistant to extraction) activation of mTORC1 by TSH and cAMP, we assessed mTORC1 kinase activity in immune complexes ( 60). It is important to consider that, in such assays, regulations may be lost, in part or totally, because even the gentlest purification schemes do not preserve the interaction between mTORC1 and Rheb ( 39). mTORC1 was immunoprecipitated by an antibody directed against the C-terminal region of raptor (CRap) ( 60). The integrity of the core mTORC1 complex was shown by the coimmunoprecipitation of mTOR, raptor, and LST8 (Fig. 4, A and D). To determine their activity, the immune complexes were incubated with [γ-32P]ATP and 4E-BP1. Treating PCCl3 cells with TSH, forskolin, or insulin increased the in vitro phosphorylation of 4E-BP1 by mTORC1 (Fig. 4, A and D). No activity was observed in similar immunoprecipitations performed using a nonimmune IgG (Fig. 4D). The addition of inhibitors of mTOR, LY294002 and rapamycin in the presence of its binding protein FKBP12, during the in vitro incubation of immune complex with 4E-BP1, totally inhibited the activity of mTORC1 enhanced by cell treatment with forskolin, thus confirming the specificity of the assay (Fig. 4A).
Figure 4.
cAMP agonists as well as insulin activate mTORC1 by stimulating the binding of 4E-BP1 to raptor and the phosphorylation of PRAS40. In panels A–C, PC Cl3 cells were treated without (−) or with (+) 10 μm forskolin (F) for 20 min. In panels D and E, they were treated without (c) or with 5 μg/ml insulin (i) and/or 10 μm forskolin (F) or 1 mU/ml TSH (T) for 20 min. A, Forskolin activates mTORC1. mTOR, raptor, and pS6K1 (T389) were immunodetected from whole-cell lysates (lys) (upper left panel). Endogenous mTOR complexes were immunoprecipitated (IP) with a raptor antibody (CRap) and a sample was taken for immunoblotting detection of mTOR and raptor (upper right panel). The immune complexes were mixed with [γ-32P]ATP and recombinant 4E-BP1 fragment, and the in vitro kinase reaction (K) was performed without (c) or with mTOR inhibitors, 10 μm LY294002 (LY), 5 μm FKBP12 (FKB), or FKBP12 + 5 μm rapamycin (FKB + r), and phosphorylation of 4E-BP1 was visualized by immunoblotting of p4E-BP1 (T37/46) or phosphorimaging (32P incorporation)(lower panel). B, Forskolin increases the T246 phosphorylation of PRAS40 and reduces the association of PRAS40 with mTORC1. mTOR, raptor, pS6K1 (T389), and pPRAS40 (T246) were immunodetected from whole-cell lysates (lys) (left panel). mTOR complexes were immunoprecipitated with the raptor antibody (CRap), and coimmunoprecipitated mTOR and PRAS40 were immunodetected (right panel). C, High-salt washing of mTORC1 increases its basal activity. mTOR complexes were immunoprecipitated with the raptor antibody (CRap) and washed with the same buffer containing 500 mm NaCl or 150 mm NaCl. Coimmunoprecipitated mTOR and raptor were immunodetected and the kinase activity (K) of these complexes was assayed as in panel A. D, Insulin, forskolin, and TSH activate mTORC1. mTORC1 complexes were immunoprecipitated with the raptor antibody (CRap) or nonimmune IgG (NI) as a control, and a sample was taken for immunoblotting detection of coimmunoprecipitated mTOR, raptor, and LST8. The kinase activity (K) of these complexes was assayed as in panel A. E, Insulin, forskolin, and TSH stimulate the binding of raptor to immobilized 4E-BP1. The same cell extracts as in panel D were incubated with 4E-BP1 or its mutant F113A (F/A) coupled to agarose beads. 4E-BP1-bound raptor was immunoblotted.
mTORC1 activation by PRAS40 phosphorylation results from decreased affinity of PRAS40 to mTORC1 ( 39,40,41). We indeed observed that increased T246 phosphorylation of PRAS40 in forskolin-treated PC Cl3 cells was associated with a reduced association of PRAS40 with mTORC1 (Fig. 4B). Interestingly, PRAS40 association with mTORC1 has been shown to be salt sensitive. Thus, washing of mTORC1 immunoprecipitates with buffers containing high-salt concentrations increases their activity if it is restrained by PRAS40 binding ( 39). Indeed, we observed that washing of immunoprecipitated mTORC1 from PC Cl3 cells with a buffer containing 500 mm instead of 150 mm of NaCl increased the in vitro activity of mTORC1 from unstimulated cells but not from forskolin-stimulated cells (Fig. 4C). Finally, we have previously shown that decreased affinity of T246-phosphorylated PRAS40 to mTORC1 allows an increased binding to raptor of mTORC1 substrates through their TOR signaling (TOS) motifs ( 60,71). As shown in Fig. 4, D and E, activation of mTORC1 by insulin, TSH, and forskolin in PC Cl3 cells was associated with increased binding of raptor to immobilized 4E-BP1 but not to 4E-BP1 mutated in its TOS motif (F113A), exactly as we have observed it in insulin-stimulated adipocytes ( 60). Collectively, these data indicate that TSH and cAMP produce a stable activation of mTORC1, at least in part, through enhanced T246 phosphorylation of PRAS40, leading to increased binding of substrates to the raptor subunit.
Because the T246 residue of PRAS40 is a well-demonstrated substrate of PKB, it was unexpected that it could be phosphorylated in response to TSH and forskolin, which do not activate PKB. We thus evaluated whether PRAS40 could be directly phosphorylated by PKA. As shown in Fig. 5A, PKA phosphorylated PRAS40 at T246 in vitro. Interestingly, T246 was not the only site phosphorylated by PKA, as shown by the similar 32P incorporation in PRAS40-T246A (Fig. 5A). In fact, the two-dimensional phosphopeptide mapping indicated that another site was more strongly phosphorylated by PKA in the T246A mutant (Fig. 5A). Both in PC Cl3 cells and in primary cultures of dog thyrocytes, the selective PKA activator N6-monobutyryl-cAMP ( 46) also increased T246 phosphorylation of PRAS40 and S6K1 phosphorylation, (Fig. 5B). PKA activation might thus be directly implicated in TSH/cAMP-dependent phosphorylation of PRAS40.
Figure 5.
PRAS40 can be phosphorylated by PKA. A, Recombinant PRAS40 wild type (wt) and mutant T246A proteins (0.5 μg) were incubated with PKA catalytic subunit (0.1 μg), which was pretreated without or with H-89 (10 μm) or PKA inhibitor fragment 14–22 (10 μm) (PKI). Phosphorylation of PRAS40 was visualized by phosphoimager (32P incorporation) and T246 phosphorylation [pPRAS40(T246)] was immunodetected with a phosphospecific antibody. Substrates were stained with 0.5% ponceau S (PRAS40). 32P-labeled PRAS40 wt and T246A were digested by trypsin and resolved by two-dimensional phosphopeptide mapping with electrophoresis at pH 1.9 as the first dimension and chromatography as the second dimension. The peptide containing the T246 phosphorylation is shown (T246). B, PKA activation stimulates the phosphorylation of PRAS40, S6K1, and S6 in PC Cl3 cells and dog thyrocytes in primary culture. The cells were treated without (c) or with 5 μg/ml insulin (i) or the selective PKA activator N6-monobutyryl-cAMP (MB; 500 μm) for 20 min. pS6K1 (T389), S6K1, pS6 (S235/236), pPRAS40 (T246), and PRAS40 were immunodetected after SDS-PAGE of whole-cell lysates.
mTORC1 activity is involved in cAMP-dependent cell cycle progression and CDK4 activation
In PC Cl3 cells as in other rat thyroid cell lines and canine and human thyroid primary cultures, TSH and insulin synergize to induce DNA synthesis and cell cycle progression ( 4,73). As shown in Fig. 6A, rapamycin inhibited, in large part but not totally, DNA synthesis induced by insulin, TSH, and the combination of both hormones. Rapamycin similarly inhibited Rb phosphorylation, including at T826, which is specifically targeted by CDK4 (Fig. 6B). We thus analyzed the expression and activity of CDK4 and its regulatory partners, cyclin D3 and p27kip1, which were reported as key cell cycle-regulatory targets of rapamycin in other cell types ( 74,75,76). As in canine (15) and human ( 16) thyrocytes, cyclin D3 is the crucial D-type cyclin involved in TSH-dependent mitogenesis in PC Cl3 cells ( 18). CDK4 presence was constitutive and unaffected by the different treatments in PC Cl3 cells (Fig. 6B). Rapamycin partially inhibited the accumulation of cyclin D3 increased by insulin and TSH (Fig. 6B). On the other hand, it did not appreciably affect the accumulation of p27, which was markedly down-regulated by insulin but not by TSH (Fig. 6B).
Figure 6.
mTORC1 activity is involved in cell cycle progression, Rb phosphorylation, and cyclin D3-CDK4 activation stimulated by both insulin and TSH in PC Cl3 cells. Cells were stimulated without (c) or with 5 μg/ml insulin (i) and/or 1 mU/ml TSH (T) for 48 h (panel A) or 20 h (panels B–D) in the presence or not of 40 nm rapamycin (r). A, Rapamycin inhibits insulin- and TSH-stimulated DNA synthesis. BrdU was present during the last 24 h. The percentage of BrdU-positive nuclei was counted at the microscope. Results are mean + sd from four independent experiments. B, Rapamycin inhibits insulin- and TSH-stimulated Rb phosphorylation and cyclin D3 accumulation. Rb, pRb (T826), CDK4, cyclin D3, and p27 were immunodetected from whole-cell lysates (lys). The arrowhead indicates phosphorylated forms of Rb. C, Rapamycin inhibits insulin- and TSH-stimulated cyclin D3-CDK4 activity. Cell lysates were immunoprecipitated (IP) with anticyclin D3 (D3) or anti-p27 (p27) antibodies, assayed for Rb-kinase activity, separated by SDS-PAGE, and immunoblotted. Cyclin D3, CDK4, p27, and the in vitro T826 phosphorylation of the Rb fragment [pRb (T826)] were detected using specific antibodies. D, Rapamycin inhibits insulin- and TSH-stimulated activating phosphorylation of CDK4. Cyclin D3-CDK4 complexes were immunoprecipitated (IP) with cyclin D3 antibodies (D3). The immune complexes were separated by two-dimensional gel electrophoresis, and CDK4 was immunodetected. The percentage of the T172-phosphorylated form of CDK4 (arrows) vs. total CDK4 is indicated (% P-CDK4).
We next analyzed the impact of rapamycin on the formation and Rb-kinase activity of CDK4 complexes coimmunoprecipitated using cyclin D3 and p27 antibodies. Insulin induced the Rb-kinase activity associated with both cyclin D3 and p27 immune complexes (Fig. 6C). By contrast, TSH even more potently induced the activity associated with cyclin D3, whereas p27-bound CDK4 was inactive in TSH-treated cells (Fig. 6C). This paradoxical situation is likely explained by the much higher levels of p27 in TSH-treated PC Cl3 cells than in the insulin-treated ones, resulting in an increased ratio of p27 in cyclin D3 immune complexes from TSH-treated cells when compared to insulin-treated cells (Fig. 6C). This is consistent with a stoichiometric model of inactivation of cyclin D-CDK4-p27 complexes by additional p27 molecule(s) ( 19). Rapamycin markedly inhibited the Rb- kinase activity of cyclin D3-CDK4 complexes in PC Cl3 cells stimulated by insulin, TSH, or both (Fig. 6C). Although lysates from cells treated with rapamycin had slightly less cyclin D3 levels, this did not appear to affect cyclin D3-CDK4 complex formation (Fig. 6C). p27 was unlikely involved in the inhibition of CDK4 activity by rapamycin (Fig. 6C) because 1) its concentration was not increased by rapamycin treatment; 2) p27-binding to CDK4 was not detectably increased by rapamycin; 3) p27 did not inhibit CDK4 activity in insulin-stimulated cells; 4) in TSH-stimulated PC Cl3 cells active cyclin D3-CDK4 was not associated with p27 and thus could not be regulated by the recently evidenced mTORC1-dependent phosphorylation of p27 ( 77).
In several systems including normal thyrocytes stimulated by TSH or forskolin, we have identified the activating T172 phosphorylation of CDK4 as a crucial target for regulation of the activity of cyclin D1/3-CDK4 complexes ( 19,20,30,78). The relative presence of phosphorylated and nonphosphorylated CDK4 forms in coimmunoprecipitated complexes was assessed ( 19) using two-dimensional gel electrophoresis. We have previously identified the most negatively charged form as the T172-phosphorylated CDK4 using 32P-phosphate incorporation, a new phosphospecific antibody, in vitro phosphorylation by recombinant CDK-activating kinase (CAK), and analysis of T172A-mutated CDK4 ( 19). As shown in Fig. 6D, insulin and TSH increased the appearance of CDK4 phosphorylated at T172 in cyclin D3 complexes of PC Cl3 cells, and the combined hormones synergized this effect. Rapamycin treatment reduced the proportion of the phosphorylated form in CDK4 bound to cyclin D3 under all the conditions tested (Fig. 6D), thus explaining the inhibition of Rb-kinase activity (Fig. 6C).
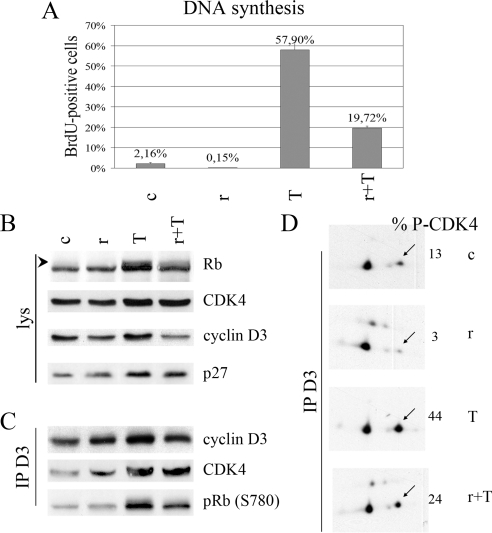
These different observations were confirmed in primary cultures of normal dog thyrocytes (Fig. 7). Rapamycin inhibited both DNA synthesis and the phosphorylation of Rb induced by TSH in the presence of insulin (Fig. 7, A and B) ( 14). The induction of p27 by TSH was not affected by rapamycin (Fig. 7B). Rapamycin partially inhibited the accumulation of cyclin D3, but this effect was insufficient to appreciably reduce the assembly of cyclin D3-CDK4 complexes (Fig. 7, B and C). However, the TSH-dependent Rb-kinase activity of these complexes was markedly inhibited by rapamycin (Fig. 7C), which correlated with a proportionate decrease in the phosphorylation of cyclin D3-bound CDK4 stimulated by TSH (Fig. 7D). Therefore, both in PC Cl3 cells and in normal dog thyrocytes, rapamycin inhibits the TSH/insulin-mediated accumulation of cyclin D3 and the activating phosphorylation of CDK4. This suggests that activation of mTORC1 by insulin and TSH is instrumental in both processes.
Figure 7.
mTORC1 activity is involved in cAMP-dependent cell cycle progression and CDK4 activation in dog thyrocytes in primary culture. Dog thyrocytes cultured with 1 μg/ml insulin were stimulated without (c) or with 1 mU/ml TSH (T) for 48 h (panel A) or 20 h (panels B–D) in the presence or not of 40 nm rapamycin (r). A, Rapamycin inhibits TSH-induced DNA synthesis. BrdU was present during the last 24 h. The percentage of BrdU-positive nuclei was counted at the microscope (mean + range from duplicate dishes). B, Rapamycin inhibits TSH-stimulated Rb phosphorylation. Rb, CDK4, cyclin D3, and p27 were immunodetected from whole-cell lysates (lys). The arrowhead indicates phosphorylated forms of Rb. C, Rapamycin inhibits TSH-stimulated cyclin D3-CDK4 activity. Cell lysates were immunoprecipitated (IP) with anticyclin D3 (D3) antibodies, assayed for Rb-kinase activity, separated by SDS-PAGE, and immunoblotted. Cyclin D3, CDK4, and the in vitro S780 phosphorylation of the Rb fragment [pRb (S780)] were detected using specific antibodies. D, Rapamycin inhibits basal and TSH-stimulated activating phosphorylation of CDK4. Cyclin D3-CDK4 complexes were immunoprecipitated (IP) with cyclin D3 antibodies (D3). The immune complexes were separated by 2-dimensional gel electrophoresis, and CDK4 was immunodetected. The percentage of the T172-phosphorylated form of CDK4 (arrows) vs. total CDK4 is indicated (% P-CDK4).
Discussion
In primary culture systems of canine and human thyrocytes, the mechanisms by which TSH/cAMP induces mitogenesis, particularly between the early activation of PKA ( 46) and the late activation of cyclin D3-CDK4 ( 15,16,20), are largely unknown. In these model systems, cAMP-dependent cell cycle progression in thyrocytes is not signaled through activation of canonical Ras-Raf-ERK and PI3-K/PKB pathways ( 12,13,14) and appears largely posttranslational because many early mitogenic transcription factors and D-type cyclins are not induced during the process ( 4,15,16). Here we provide further evidence that the cAMP-dependent mitogenesis involves the stimulation of mTOR-raptor (mTORC1) kinase activity and identifying its cell cycle regulatory targets. Indeed, we demonstrate: 1) that the previously shown cAMP/PKA-dependent activation of S6K1 ( 14,22,23,46,47) reflects the activation of mTORC1, through mechanisms that are independent of PKB or ERK stimulation but involves the stimulation of PRAS40 phosphorylation, likely by PKA, and increased substrate interaction with raptor; 2) that mTORC1 activation by both TSH/cAMP and insulin control cyclin D3-CDK4 activity by impacting both the accumulation of cyclin D3 and the activating T172 phosphorylation of CDK4; 3) that in thyrocytes as in other cell types ( 32,33,61), mTORC1 activation by both TSH/cAMP and insulin depends on both amino acids and the cellular energy (AMPK) status. As a master integrating node, mTORC1 activity thus also functions to couple cAMP/insulin-dependent stimulation of thyrocyte proliferation with their nutrient and energy availability.
In different cell types, the impact of cAMP on mTORC1 activity (as deduced from S6K1 and 4E-BP1 phosphorylation) often correlates with, and thus potentially contributes to, the opposite mitogenic or antimitogenic effects that cAMP exerts ( 79). It is generally expected to result from the divergent cross-signaling of cAMP with growth factor-elicited pathways ( 9,10,80). For instance, cAMP-mediated inhibition of the phosphorylation of S6K1 and 4E-BP1 in the thyroid papillary carcinoma cell line TPC-1 is explained by the strong inhibition by PKA of the c-Raf/ERK cascade ( 30). Conversely, in rat ovarian granulosa cells stimulated by FSH, cAMP/PKA-dependent activation of mTORC1 has been ascribed to cAMP-dependent activation of either PKB ( 81) or ERK ( 27). In the present study, consistent with previous observations from canine thyroid primary cultures ( 14) and mouse thyroid in vivo ( 47), we show that the activation of mTORC1 by TSH and cAMP in rat thyroid PC Cl3 cells is not secondary to activation of ERK1/2 or PKB. Indeed, TSH and cAMP did not induce the PI3-K/PDK1-dependent T308 phosphorylation of PKB, PKB activity, and PKB-dependent events such as T1462 phosphorylation of TSC2. Interestingly, TSH and cAMP modestly increased the S473 phosphorylation of PKB in several experiments. Although PKB remained inactivated without T308 phosphorylation, consistent with previous reports, ( 82) the increased S473 phosphorylation suggests that TSH and cAMP may also activate mTORC2, which is responsible for this phosphorylation ( 43,44). Previous reports that TSH would activate the PI3-K/PKB axis, as evidenced only by increased S473 phosphorylation, ( 49,50,83) might thus have to be reconsidered.
Mechanisms of mTORC1 and S6K1 activation by cAMP
mTORC1 is regulated at several steps, including a Rheb-GTP-induced activation of mTOR catalytic function, and second, a Rheb-independent reconfiguration of the intact complex that enhances access to and/or affinity for substrates ( 66,67). Rheb-dependent activation of mTORC1 is regulated by the Rheb-GAP TSC2, which, through its multiple activating and inhibitory phosphorylation, integrates the stimulating PI3-K/PKB ( 35,36,68) and ERK/p90RSK cascades ( 38,69) activated by insulin and growth factors, as well as mTORC1 inhibition by AMPK and GSK3 ( 62). In the present study, we found no evidence that TSH and cAMP can mimic insulin actions on TSC2. Indeed, unlike insulin, TSH and forskolin did not induce the TSC2 phosphorylation at T1462 and Y1571, again differing from cAMP action in other systems ( 27,81,84). Moreover, lithium and compound C, presumably by inhibiting GSK3 and AMPK and thus their stimulation of TSC2-GAP activity ( 62), enhanced mTORC1 activation by insulin but less so, or not at all, by forskolin. This suggests that inhibition of GSK3 and AMPK by PKB-dependent phosphorylation could be an important factor in the activation of mTORC1 by insulin but not in its activation by cAMP. However, phosphospecific antibodies were not available to investigate all the described phosphorylation sites of TSC2 in response to cAMP, and other regulatory phosphorylation events might also remain to be uncovered. On the other hand, it has recently been found that amino acids control the activity of the mTORC1 pathway by regulating the movement of mTORC1 to an intracellular compartment that contains Rheb ( 85). Our data suggest that this amino acid-regulated colocalization of mTORC1 with Rheb is necessary for both insulin-induced and cAMP-induced phosphorylation of S6K1.
In the present study, we show, for the first time, that TSH/cAMP, like insulin in adipocytes ( 60), induces 4E-BP1 phosphorylation and directly produces a stable increase in the kinase activity of mTORC1. Such a persistent activation of mTORC1 in vitro occurs in the absence of GTP-Rheb association and likely involves stable modifications of mTOR, Raptor, or PRAS40. TSH/cAMP, like insulin, increased both the S2448 phosphorylation of mTOR and the T246 phosphorylation of PRAS40. We also show, for the first time, that PKA can directly phosphorylate PRAS40 on several sites including T246, which most likely explains how TSH and a selective PKA activator could induce this phosphorylation without activating PKB. Whereas the role of mTOR S2448 phosphorylation remains elusive ( 56,57,58), T246 phosphorylation of PRAS40 facilitates its disassociation from mTORC1 ( 39,40), thus increasing the binding of the other TOS motif substrates ( 41,71). TSH and cAMP increased the binding to raptor of 4E-BP1, which was abolished by mutation of its TOS motif, exactly as we have previously shown in insulin-stimulated adipocytes ( 41). Our data thus provide a consistent mechanism of mTORC1 activation by the TSH/cAMP/PKA pathway. This does not exclude additional mechanisms. For instance, p90RSK-mediated phosphorylation of raptor has recently been found to be important for mTORC1 activation by the Ras/ERK pathway ( 86). Whether these or similar phosphorylation sites could also be targeted by PKA remains to be investigated.
The stimulation of S6K1 activity by both insulin and TSH/cAMP thus depends on mTORC1 activation. As shown by two-dimensional gel electrophoresis, the phosphorylation pattern of S6K1 appears even more complex than so far described, being compatible with up to 13 stimulated phosphorylation events, which clearly exceeds the eight identified ones ( 53). Most of them are inhibited by rapamycin and thus depend, directly or indirectly, on mTORC1 activation. Interestingly, phosphorylation of T421/S424 of S6K1 is synergistically induced by insulin and TSH/cAMP and is resistant to rapamycin. These phosphorylation sites in the autoinhibitory domain contain a proline residue at +1 position and a hydrophobic residue at −2 position. The putative proline-directed kinase(s) responsible for the stimulated phosphorylation of these sites remain unknown ( 52,53). In the sequential phosphorylation model of S6K1 phosphorylation, T389 phosphorylation by mTORC1 is facilitated by T421/S424 phosphorylation, which in turn allows the T229 phosphorylation by PDK1. The stimulation of T229 phosphorylation by TSH and cAMP, without activation of PI3-K ( 14,48), is likely explained by the fact that phosphorylation of S6K1 by PDK1 does not require its binding to phosphatidylinositol(3,4,5)P3 ( 87).
Mechanisms of mTORC1 implication in TSH/insulin-dependent mitogenesis
We show that rapamycin largely inhibits the stimulation of DNA synthesis by TSH and insulin used alone, as well as the synergistic effect of their combination in PC Cl3 cells. These effects thus recapitulate the impact of mTORC1 inhibition in mice in vivo on thyroid hyperplasia elicited either by strong PI3-K stimulation or by TSH ( 7,47). Of note, the inhibition was not complete indicating that mTORC1-independent mechanisms are also involved. mTORC1 has been mostly considered to impact cell proliferation through its various targets involved in the protein synthesis machinery and its regulation, including S6K1 and thus ribosomal S6 protein, eIF4E, 4E-BP1, eIF3, and others ( 67,88,89). However, rapamycin often only modestly reduces overall protein synthesis ( 90), which we also observed in cultured thyrocytes (our unpublished data), and mTORC1 inhibition does not prevent the hypertrophic response of thyrocytes to PI3-K and TSH in vivo ( 7,47). mTORC1 is thus viewed as mostly regulating the translation of specific mRNAs, but the earlier idea that the role of S6 kinases and S6 phosphorylation was to control the translation of 5′-TOP mRNAs has not been substantiated by data from S6K knockout mice ( 91) or from knock-in mice with alanine substitution of all S6 phosphorylatable serines ( 24). In addition, it should be pointed out that cell cycle progression is not compromised in these mice ( 24,91,92). On the other hand, mTORC1-dependent phosphorylation of 4E-BP1 could be more crucial for cell cycle progression because a constitutively active nonphosphorylatable 4E-BP1 mutant inhibits proliferation in a manner that mimics rapamycin ( 93). 4E-BP1 phosphorylation releases the inhibition of eIF4E, which has been described as a protooncogene, putatively because it preferentially enhances the translation of highly structured mRNAs involved in growth control, such as those of c-myc and cyclin D1 ( 88,89,94).
In PC Cl3 cells and dog and human thyrocytes, cyclin D3 is the critical D-type cyclin in TSH/insulin-dependent proliferation ( 15,16,18). As reported in mouse thyrocytes in vivo ( 7), we observe that rapamycin treatment reduced cyclin D3 levels. This rather general effect of rapamycin ( 74,75,78) has been ascribed to either an inhibition of cyclin D3 mRNA translation ( 74) or a destabilization of the protein ( 75). In the present experiments in both PC Cl3 cells and dog thyrocytes, this partial inhibition of cyclin D3 accumulation was insufficient, nevertheless, to induce a marked reduction of cyclin D3-CDK4 complexes. Instead we ascribe the inhibition of Rb phosphorylation to a rapamycin-dependent inhibition of the activity of these complexes but not of their assembly. This inhibition could not be explained by an impact of rapamycin on levels of p27 or its association to cyclin D3-CDK4, but it closely correlated with inhibition of the activating T172 phosphorylation of CDK4 within cyclin D3 complexes. We have also recently observed a partial inhibition by rapamycin of CDK4 phosphorylation in serum-stimulated glioblastoma cell lines ( 78). Therefore, independently of the association of CDK4 with D-type cyclins or p27, we identify the activating T-loop phosphorylation of CDK4 as a new cell cycle-regulatory target of the mTORC1 pathway. How CDK4 phosphorylation could be linked to mTORC1 is totally unclear. The only identified CDK4-activating kinase is the cyclin H-CDK7-Mat1 complex (CAK), which is also responsible for the activating phosphorylation of the other cell cycle CDKs ( 95,96). However, its presence and activity are not generally regulated ( 21,95), which we have confirmed in several systems ( 19,30,78). Several arguments, including the observation that mutation of a unique proline at +1 position of the phosphoacceptor site abrogates CDK4 T172 phosphorylation and activation in various cells but not its activation by CAK in vitro, have led us to conclude that the implication of CAK in regulated CDK4 phosphorylation should be reinvestigated ( 19,21,97). Identification of mechanisms responsible for the regulated phosphorylation of CDK4 will thus be required for the full understanding of how mTORC1 controls Rb phosphorylation and cell cycle progression.
To conclude, our study demonstrates mTORC1 activation as an important mediator of thyroid cell regulation by TSH through the cAMP/PKA cascade. It appears as a crucial node integrating the control by TSH with the action of general mitogenic hormones and their nutriment and energy status to influence cell fate decisions such as cell cycle commitment. The mechanism of the cAMP- dependent activation of mTORC1 involves the phosphorylation of PRAS40, likely by PKA, which does not exclude additional mechanisms, and a possible activation of mTORC2 remains to be explored. Further studies should define how mTORC1 activity could be wired to cell cycle regulation through CDK4 phosphorylation, possibly in addition to its well-characterized impact on protein synthesis.
Materials and Methods
Cell cultures
Rat thyroid PC Cl3 cells were cultured as described ( 73) in Coon’s modified F12 Medium (Invitrogen, Carlsbad, CA), supplemented with 5% heat-inactivated fetal bovine serum, 1 μg/ml bovine insulin (Sigma, St. Louis, MO), 5 μg/ml transferrin (Sigma), 1 mU/ml bovine TSH (Sigma) (3H-medium), and antibiotics. Cells were made quiescent by switching to medium containing transferrin and BSA (500 μg/ml) for 3 d. According to the experiment, the cells were preincubated for 2 h with inhibitors [rapamycin (Calbiochem), wortmannin (Sigma), Compound C (Calbiochem), LiCl (Merck, Whitehouse Station, NJ)] or vehicle and stimulated for various durations with 5 μg/ml insulin, 1 mU/ml TSH, 10 μm forskolin (Calbiochem) and/or 2 mm AICAR (Cell Signaling Technology, Beverly, MA). For primary cultures of dog thyroid (obtained according to a protocol approved by the Institutional Animal Use Committee), thyroid follicles obtained by collagenase digestion were seeded (∼2 × 104 cells/cm2) and cultured in monolayer in a medium that included DMEM, Ham’s F12 medium, and MCDB 104 medium (2:1:1, vol/vol) supplemented by ascorbic acid (40 μg/ml), and antibiotics ( 2,46). As indicated, insulin (5 μg/ml) was added or not to this medium from the seeding. Quiescent cells were stimulated at d 4 or d 5.
DNA synthesis
Cells in 3-cm Petri dishes were stimulated for 48 h, and bromodeoxyuridine (BrdU) was added for the last 24 h. The incorporation of BrdU was detected by immunofluorescence, and BrdU-labeled nuclei (1000/dish) were counted as described ( 46).
Western blotting detections of proteins
Cells were rinsed once with chilled PBS and resuspended in immunoprecipitation (IP) base buffer, pH 7.4 (1 mm EGTA, 1 mm EDTA, 50 mm β-glycerophosphate, 10 mm Na2HPO4, 50 mm NaF) supplemented with 1 mm dithiothreitol (DTT), 0.1% Tween 20, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml pepstatin, and 0.5 μm microcystin. The suspension was homogenized with a 20G1½ syringe and centrifuged at 10,000 rpm for 10 min. Sodium dodecyl sulfate (SDS) sample buffer 5× (3 m Tris-HCl, pH 6.8; 20% SDS; 1% glycerol) was added to the supernatants. Alternatively, for analysis of cell cycle-regulatory proteins in long-term stimulation experiments, cells were lysed in 2% SDS lysis buffer as described elsewhere ( 15). Equal amounts of cell extracts (10–20 μg of proteins) were separated by SDS-PAGE, and the proteins of interest or their phosphorylation state was immunodetected after Western blotting. The following antibodies were used: phospho-mTOR (S2448), phospho-Akt (T308), phospho-Akt (S473), phospho-raptor (S792), raptor, TSC2, phospho-TSC2 (T1462), phospho-TSC2 (Y1571), phospho-β-catenin (S33/37/T41), phospho-GSK3 α/β (S21/9), phospho-ERK (T202/Y204), phospho-AMPK (S485), phospho-p70S6K (T389), phospho-p70S6K (T421/424), phospho-S6 (S235/236), phospho-4E-BP1 (T37/46), phospho-4E-BP1 (S65), 4E-BP1, phospho-Rb (S780), DCS-156 monoclonal anti-CDK4, anti-PRAS40 rabbit monoclonal antibody (all from Cell Signaling Technology); p70S6K, C-22 polyclonal anti-CDK4, C-19 polyclonal anti-p27 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); phospho-p70S6K (T229), phospho-PRAS40 (T246), phospho-Rb (T826) (Biosource, San Jose, CA); DCS-22 monoclonal anti-cyclin D3, DCS-72 anti-p27 (NeoMarkers, Fremont, CA), anti-Rb catalog no. 554136 (Pharmingen, San Diego, CA). Antibodies against mTOR ( 51), raptor (C-Rap) ( 60), and mLST8 (98) were described previously.
PKB activity assay
This was performed using the Akt1/PKBα Immunoprecipitation-Kinase Assay Kit (Upstate Biotechnology, Inc., Lake Placid, NY; catalog no. 17-188) according to manufacturer instructions.
Immunoprecipitation of mTORC1
Cells were rinsed once with chilled PBS and resuspended in IP base buffer, pH 7.4, supplemented with 1 mm DTT, 0.1% Tween 20, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml pepstatin, and 0.5 μm microcystin. The suspension was homogenized with a 20G1½ syringe and centrifuged at 10,000 rpm for 10 min. The raptor subunit of mTORC1 was targeted by using C-Rap antibody ( 60). Cell extracts (600 μl) were incubated with protein A-agarose beads (15 μl) bound to C-Rap antibody (2 μg) or nonimmune IgG at 4 C for 2 h with constant mixing. The beads were then washed twice with 1 ml of Buffer W2 (IP base buffer, supplemented with 0.5 or 0.15 m NaCl, 0.2% Tween 20, 1 mm DTT), once with 1 ml of Buffer W1 (IP base buffer, supplemented with 1 mm DTT), and then once with 1 ml of Buffer W4 (10 mm HEPES-NaOH, pH 7.4; 50 mm NaCl; 50 mm β-glycerophosphate, pH 7.4; 0.1 mm EGTA; 1 mm DTT; 0.5 μm microcystin). After removing an aliquot for detection of mTOR, raptor, and mLST8 by immunoblotting, the remainder of the immune complexes were used for the mTOR activity assay.
Immune complex assay of mTORC1 activity
Washed complexes were resuspended in 20 μl of Buffer W4. The kinase reactions were initiated by adding 5 μl of Buffer W4 supplemented with 2.5 μCi [γ-32P]ATP (PerkinElmer Life Sciences, Boston, MA), 50 mm MnCl2, 500 μm ATP, and 1 μg of His-tagged 4E-BP1 ( 60). Reactions were terminated after 30 min at 30 C by adding 5× SDS sample buffer. In vitro phosphorylation of 4E-BP1 was determined by Western blotting with a phosphospecific antibody [4EBP1 (T37/46)] or by phosphorimaging after SDS-PAGE. Measurements under these conditions reflect the initial rate of phosphorylation because less than 5% of the available substrates were phosphorylated, and the reactions proceed linearly for 60 min.
Binding of raptor to 4E-BP1 affinity resins
4E-BP1 affinity resin was prepared as described previously ( 60). Cell extracts (800 μl) were incubated with 15 μl of beads coupled with His-tagged 4EBP1 protein at 4 C for 2 h with constant mixing and washed as described for immunoprecipitations. The relative amounts of raptor retained by the beads were determined by immunoblotting.
In vitro phosphorylation of PRAS40 by PKA
Recombinant PRAS40 wild-type and mutant T246A proteins prepared as described ( 71) were mixed with PKA catalytic subunit (New England Biolabs, Boston, MA) in 20 μl kinase buffer (10 mm MgCl2, 50 mm NaCl, 0.1 mm EGTA, 1 mm DTT, 0.5 μm microcystin LR, 10 mm HEPES, and 50 mm β-glycerophosphate, pH 7.4), with or without H89 or PKA inhibitor fragment 14–22 (Sigma). The kinase reactions were initiated by adding 5 μl of kinase buffer supplemented with 0.5 mm [γ-32P]ATP (PerkinElmer Life Sciences, 1000 mCi/mmol). Reactions were terminated after 30 min at 30 C by adding SDS sample buffer. Phosphorylation of PRAS40 was determined by phosphor imager and immunoblotting with PRAS40-T246 phosphospecific antibodies after SDS-PAGE. Two-dimensional phosphopeptide mapping was performed as described elsewhere ( 71). Briefly, 32P-labeled PRAS40 wild-type and mutant proteins phosphorylated in vitro by PKA were excised from polyvinylidene difluoride membrane and digested with trypsin. The trypsin-digested peptides were resolved by electrophoresis at pH 1.9 as the first dimension and chromatography as the second dimension. 32P-labeled peptides were visualized by phosphorimaging chromatography plates.
Immunoprecipitation of p70S6K1
Cells were rinsed once with chilled PBS and resuspended in extraction buffer (50 mm Tris-HCl, pH 8; 120 mm NaCl; 15 mm Na4P2O7; 1% Nonidet P-40; 1 mm EDTA; 1 mm EGTA; 1 mm NaF; 60 μg/ml pefabloc; 1 mm benzamide; 30 mm 4-nitrophenylphosphate), homogenized (glass/glass), agitated for 10 min at 4 C and centrifuged for 10 min at 4 C at 13,000 rpm. The supernatant was incubated at 4 C for 3 h with protein A-Sepharose that had been preincubated overnight with 2 μg of anti-p70S6K1 (C-18 from Santa Cruz). The beads were then washed three times with extraction buffer.
Immunoprecipitation of cyclin D3-CDK4 complexes and Rb-kinase assay
This was performed exactly as described ( 20). Coimmunoprecipitations were performed using the DCS-28 monoclonal antibody against cyclin D3 (Neomarkers). Immune complexes were incubated with ATP and a recombinant Rb fragment (QED Bioscience, San Diego, CA), before SDS-PAGE separation of the incubation mixture and Western blotting detection of the S780 or T826 phosphorylation of the Rb fragment, cyclin D3, and CDK4.
Two-dimensional gel electrophoresis
As described previously ( 19), immunoprecipitated proteins were denatured in a buffer containing 7 m urea and 2 m thiourea. Proteins were separated by isoelectric focusing on immobilized linear pH gradient strips (pH 3–10 for CDK4; pH 4–7 for p70S6K1). After SDS-PAGE separation and blotting, CDK4 and S6K1 were detected with the C-22 CDK4 polyclonal antibody or a p70S6K1 antibody from Santa Cruz Biotechnology.
Supplementary Material
Acknowledgments
This collaborative study is dedicated to the memory of Dr. John C. Lawrence, Jr., with whom it was initiated and fully discussed.
Footnotes
This work was supported by grants from the Belgian Fonds de la Recherche Scientifique, Fonds de la Recherche Scientifique Médicale, Opération Télévie, Actions de Recherche Concertées de la Communauté Française de Belgique, and National Institutes of Health grants DK28312 and DK52753 (to J.C.L.). S.B. was supported by Télévie fellowships and a grant from the Fondation Rose et Jean Hoguet. K.C., S.P., and P.P.R. are, respectively, Scientific Worker, Postdoctoral Researcher, and Senior Research Associate of the Belgian Fonds de la Recherche Scientifique.
Disclosure Summary: The authors have nothing to declare.
First Published Online May 19, 2010
Abbreviations: AICAR, 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside; AMPK, AMP-activated protein kinase; BrdU, bromodeoxyuridine; CAK, CDK-activating kinase; CDK4, cyclin-dependent kinase 4; DTT, dithiothreitol; GAP, GTPase activating protein; GSK3, glycogen synthase kinase 3; IP bse buffer, immunoprecipitation base buffer; mTOR, mammalian target of rapamycin; mTORC1, mTOR-raptor complex; PDK, phosphoinositide-dependent protein kinase; PKA, protein kinase A; PKB, protein kinase B; Rb, retinoblastoma protein; RSK, ribosomal S6 kinase; SDS, sodium dodecyl sulfate; TOS, TOR signaling; TSC, tuberous sclerosis complex.
References
- Dumont JE, Maenhaut C, Christophe D, Vassart G, Roger PP 2005 Thyroid regulatory factors. In: DeGroot LJ, Jameson JL, eds. Endocrinology. New York: Elsevier Saunders; 1837–1860 [Google Scholar]
- Roger PP, Servais P, Dumont JE 1983 Stimulation by thyrotropin and cyclic AMP of the proliferation of quiescent canine thyroid cells cultured in a defined medium containing insulin. FEBS Lett 157:323–329 [DOI] [PubMed] [Google Scholar]
- Roger P, Taton M, Van Sande J, Dumont JE 1988 Mitogenic effects of thyrotropin and adenosine 3′,5′-monophosphate in differentiated normal human thyroid cells in vitro. J Clin Endocrinol Metab 66:1158–1165 [DOI] [PubMed] [Google Scholar]
- Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP 2001 Regulation of thyroid cell proliferation by thyrotropin and other factors : a critical evaluation of in vitro models. Endocr Rev 22:631–656 [DOI] [PubMed] [Google Scholar]
- Furuya F, Lu C, Willingham MC, Cheng SY 2007 Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis 28:2451–2458 [DOI] [PubMed] [Google Scholar]
- Miller KA, Yeager N, Baker K, Liao XH, Refetoff S, Di Cristofano A 2009 Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res 69:3689–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager N, Brewer C, Cai KQ, Xu XX, Di Cristofano A 2008 Mammalian target of rapamycin is the key effector of phosphatidylinositol-3-OH-initiated proliferative signals in the thyroid follicular epithelium. Cancer Res 68:444–449 [DOI] [PubMed] [Google Scholar]
- Guigon CJ, Zhao L, Willingham MC, Cheng SY 2009 PTEN deficiency accelerates tumour progression in a mouse model of thyroid cancer. Oncogene 28:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS 2001 New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218 [DOI] [PubMed] [Google Scholar]
- Stork PJ, Schmitt JM 2002 Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266 [DOI] [PubMed] [Google Scholar]
- Rivas M, Santisteban P 2003 TSH-activated signaling pathways in thyroid tumorigenesis. Mol Cell Endocrinol 213:31–45 [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Roger PP, Dumont JE, Dremier S 2000 TSH and cAMP do not signal mitogenesis through Ras activation. Biochem Biophys Res Commun 273:154–158 [DOI] [PubMed] [Google Scholar]
- Vandeput F, Perpete S, Coulonval K, Lamy F, Dumont JE 2003 Role of the different mitogen-activated protein kinase subfamilies in the stimulation of dog and human thyroid epithelial cell proliferation by cyclic adenosine 5′-monophosphate and growth factors. Endocrinology 144:1341–1349 [DOI] [PubMed] [Google Scholar]
- Coulonval K, Vandeput F, Stein RC, Kozma SC, Lamy F, Dumont JE 2000 Phosphatidylinositol 3-kinase, protein kinase B and ribosomal S6 kinases in the stimulation of thyroid epithelial cell proliferation by cAMP and growth factors in the presence of insulin. Biochem J 348:351–358 [PMC free article] [PubMed] [Google Scholar]
- Depoortere F, Van Keymeulen A, Lukas J, Costagliola S, Bartkova J, Dumont JE, Bartek J, Roger PP, Dremier S 1998 A requirement for cyclin D3-cyclin-dependent kinase (cdk)-4 assembly in the cyclic adenosine monophosphate-dependent proliferation of thyrocytes. J Cell Biol 140:1427–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternot S, Dumont JE, Roger PP 2006 Differential utilization of cyclin D1 and cyclin D3 in the distinct mitogenic stimulations of human thyrocytes by growth factors and TSH. Mol Endocrinol 20:3279–3292 [DOI] [PubMed] [Google Scholar]
- Depoortere F, Pirson I, Bartek J, Dumont JE, Roger PP 2000 Transforming growth factor β(1) selectively inhibits the cyclic AMP-dependent proliferation of primary thyroid epithelial cells by preventing the association of cyclin D3-cdk4 with nuclear p27(kip1). Mol Biol Cell 11:1061–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motti ML, Boccia A, Belletti B, Bruni P, Troncone G, Cito L, Monaco M, Chiappetta G, Baldassarre G, Palombini L, Fusco A, Viglietto G 2003 Critical role of cyclin D3 in TSH-dependent growth of thyrocytes and in hyperproliferative diseases of the thyroid gland. Oncogene 22:7576–7586 [DOI] [PubMed] [Google Scholar]
- Bockstaele L, Kooken H, Libert F, Paternot S, Dumont JE, de Launoit Y, Roger PP, Coulonval K 2006 Regulated activating Thr172 phosphorylation of cyclin-dependent kinase 4 (CDK4): its relationship with cyclins and CDK “inhibitors.” Mol Cell Biol 26:5070–5085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternot S, Coulonval K, Dumont JE, Roger PP 2003 Cyclic AMP-dependent phosphorylation of cyclin D3-bound CDK4 determines the passage through the cell cycle restriction point in thyroid epithelial cells. J Biol Chem 278:26533–26540 [DOI] [PubMed] [Google Scholar]
- Paternot S, Bockstaele L, Bisteau X, Kooken H, Coulonval K, Roger PP 2010 Rb inactivation in cell cycle and cancer: the puzzle of highly regulated activating phosphorylation of CDK4 versus constitutively active CDK-activating kinase. Cell Cycle 9:689–699 [DOI] [PubMed] [Google Scholar]
- Cass LA, Meinkoth JL 1998 Differential effects of cyclic adenosine 3′,5′-monophosphate on p70 ribosomal S6 kinase. Endocrinology 139:1991–1998 [DOI] [PubMed] [Google Scholar]
- Suh JM, Song JH, Kim DW, Kim H, Chung HK, Hwang JH, Kim JM, Hwang ES, Chung J, Han JH, Cho BY, Ro HK, Shong M 2003 Regulation of the phosphatidylinositol 3-kinase, Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland. J Biol Chem 278:21960–21971 [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O 2006 Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31:342–348 [DOI] [PubMed] [Google Scholar]
- Withers DJ, Bloom SR, Rozengurt E 1995 Dissociation of cAMP-stimulated mitogenesis from activation of the mitogen-activated protein kinase cascade in Swiss 3T3 cells. J Biol Chem 270:21411–21419 [DOI] [PubMed] [Google Scholar]
- Lécureuil C, Tesseraud S, Kara E, Martinat N, Sow A, Fontaine I, Gauthier C, Reiter E, Guillou F, Crépieux P 2005 Follicle-stimulating hormone activates p70 ribosomal protein S6 kinase by protein kinase A-mediated dephosphorylation of Thr 421/Ser 424 in primary Sertoli cells. Mol Endocrinol 19:1812–1820 [DOI] [PubMed] [Google Scholar]
- Kayampilly PP, Menon KM 2007 Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology 148:3950–3957 [DOI] [PubMed] [Google Scholar]
- Graves LM, Bornfeldt KE, Argast GM, Krebs EG, Kong X, Lin TA, Lawrence Jr JC 1995 cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc Natl Acad Sci USA 92:7222–7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TA, Lawrence Jr JC 1996 Control of the translational regulators PHAS-I and PHAS-II by insulin and cAMP in 3T3-L1 adipocytes. J Biol Chem 271:30199–30204 [DOI] [PubMed] [Google Scholar]
- Rocha AS, Paternot S, Coulonval K, Dumont JE, Soares P, Roger PP 2008 Cyclic AMP inhibits the proliferation of thyroid carcinoma cell lines through regulation of CDK4 phosphorylation. Mol Biol Cell 19:4814–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TE, Lawrence Jr JC 2003 TOR signaling. Sci STKE 2003:re15 [DOI] [PubMed] [Google Scholar]
- Sabatini DM 2006 mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 6:729–734 [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN 2006 TOR signaling in growth and metabolism. Cell 124:471–484 [DOI] [PubMed] [Google Scholar]
- Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G 2003 Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 11:1457–1466 [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL 2003 Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17:1829–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC 2002 Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10:151–162 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D 2003 Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 5:578–581 [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Bonner P, Bernardi R, Franz DN, Witte D, Cordon-Cardo C, Pandolfi PP 2007 Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res 67:7106–7112 [DOI] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM 2007 PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25:903–915 [DOI] [PubMed] [Google Scholar]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH 2007 Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9:316–323 [DOI] [PubMed] [Google Scholar]
- Wang L, Harris TE, Roth RA, Lawrence Jr JC 2007 PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem 282:20036–20044 [DOI] [PubMed] [Google Scholar]
- Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, Kikkawa U, Yonezawa K 2007 The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem 282:20329–20339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM 2005 Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101 [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM 2006 Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev Cell 11:859–871 [DOI] [PubMed] [Google Scholar]
- Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL 2008 Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J 27:1919–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dremier S, Milenkovic M, Blancquaert S, Dumont JE, Døskeland SO, Maenhaut C, Roger PP 2007 Cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent protein kinases, but not exchange proteins directly activated by cAMP (Epac), mediate thyrotropin/cAMP-dependent regulation of thyroid cells. Endocrinology 148:4612–4622 [DOI] [PubMed] [Google Scholar]
- Brewer C, Yeager N, Di Cristofano A 2007 Thyroid-stimulating hormone initiated proliferative signals converge in vivo on the mTOR kinase without activating AKT. Cancer Res 67:8002–8006 [DOI] [PubMed] [Google Scholar]
- Lou L, Urbani J, Ribeiro-Neto F, Altschuler DL 2002 cAMP inhibition of Akt is mediated by activated and phosphorylated Rap1b. J Biol Chem 277:32799–32806 [DOI] [PubMed] [Google Scholar]
- Cass LA, Summers SA, Prendergast GV, Backer JM, Birnbaum MJ, Meinkoth JL 1999 Protein kinase A-dependent and -independent signaling pathways contribute to cyclic AMP-stimulated proliferation. Mol Cell Biol 19:5882–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsygankova OM, Saavedra A, Rebhun JF, Quilliam LA, Meinkoth JL 2001 Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol Cell Biol 21:1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence Jr JC, Abraham RT 1996 Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J 15:5256–5267 [PMC free article] [PubMed] [Google Scholar]
- Dennis PB, Pullen N, Pearson RB, Kozma SC, Thomas G 1998 Phosphorylation sites in the autoinhibitory domain participate in p70(s6k) activation loop phosphorylation. J Biol Chem 273:14845–14852 [DOI] [PubMed] [Google Scholar]
- Jacinto E, Lorberg A 2008 TOR regulation of AGC kinases in yeast and mammals. Biochem J 410:19–37 [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Hudson CC, Sekulić A, Williams JM, Hosoi H, Houghton PJ, Lawrence Jr JC, Abraham RT 1997 Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277:99–101 [DOI] [PubMed] [Google Scholar]
- McMahon LP, Choi KM, Lin TA, Abraham RT, Lawrence Jr JC 2002 The rapamycin-binding domain governs substrate selectivity by the mammalian target of rapamycin. Mol Cell Biol 22:7428–7438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence Jr JC 1998 Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA 95:7772–7777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe-Satney I, Gautier N, Hinault C, Lawrence Jr JC, Van Obberghen E 2004 In rat hepatocytes glucagon increases mammalian target of rapamycin phosphorylation on serine 2448 but antagonizes the phosphorylation of its downstream targets induced by insulin and amino acids. J Biol Chem 279:42628–42637 [DOI] [PubMed] [Google Scholar]
- Chiang GG, Abraham RT 2005 Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 280:25485–25490 [DOI] [PubMed] [Google Scholar]
- Holz MK, Blenis J 2005 Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem 280:26089–26093 [DOI] [PubMed] [Google Scholar]
- Wang L, Rhodes CJ, Lawrence Jr JC 2006 Activation of mammalian target of rapamycin (mTOR) by insulin is associated with stimulation of 4EBP1 binding to dimeric mTOR complex 1. J Biol Chem 281:24293–24303 [DOI] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G 2007 mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med 13:252–259 [DOI] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL 2006 TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955–968 [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ 2008 AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Barré LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA 2006 Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281:36662–36672 [DOI] [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast Jr RC, Woodgett JR, Mills GB 2000 Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA 97:11960–11965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J, Long X, Lin Y, Ortiz-Vega S, Rapley J, Papageorgiou A, Oshiro N, Kikkawa U 2009 Activation of mTORC1 in two steps: Rheb-GTP activation of catalytic function and increased binding of substrates to raptor. Biochem Soc Trans 37:223–226 [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J 2009 Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10:307–318 [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL 2002 TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4:648–657 [DOI] [PubMed] [Google Scholar]
- Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J 2004 Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA 101:13489–13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG 2007 PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem 282:24514–24524 [DOI] [PubMed] [Google Scholar]
- Wang L, Harris TE, Lawrence Jr JC 2008 Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem 283:15619–15627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher LD, Campbell JS, Yeung RS 2001 Tuberin phosphorylation regulates its interaction with hamartin. Two proteins involved in tuberous sclerosis. J Biol Chem 276:21017–21021 [DOI] [PubMed] [Google Scholar]
- Kimura T, Dumont JE, Fusco A, Golstein J 1999 Insulin and TSH promote growth in size of PC Cl3 rat thyroid cells, possibly via a pathway different from DNA synthesis: comparison with FRTL-5 cells. Eur J Endocrinol 140:94–103 [DOI] [PubMed] [Google Scholar]
- Hleb M, Murphy S, Wagner EF, Hanna NN, Sharma N, Park J, Li XC, Strom TB, Padbury JF, Tseng YT, Sharma S 2004 Evidence for cyclin D3 as a novel target of rapamycin in human T lymphocytes. J Biol Chem 279:31948–31955 [DOI] [PubMed] [Google Scholar]
- García-Morales P, Hernando E, Carrasco-Garcia E, Menéndez- Gutierrez MP, Saceda M, Martínez-Lacaci I 2006 Cyclin D3 is down-regulated by rapamycin in HER-2-overexpressing breast cancer cells. Mol Cancer Ther 5:2172–2181 [DOI] [PubMed] [Google Scholar]
- Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM 1994 Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 372:570–573 [DOI] [PubMed] [Google Scholar]
- Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM 2008 mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell 30:701–711 [DOI] [PubMed] [Google Scholar]
- Paternot S, Roger PP 2009 Combined inhibition of MEK and mammalian target of rapamycin abolishes phosphorylation of cyclin-dependent kinase 4 in glioblastoma cell lines and prevents their proliferation. Cancer Res 69:4577–4581 [DOI] [PubMed] [Google Scholar]
- Roger PP, Reuse S, Maenhaut C, Dumont JE 1995 Multiple facets of the modulation of growth by cAMP. Vitam Horm 51:59–191 [DOI] [PubMed] [Google Scholar]
- Dumaz N, Marais R 2005 Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. FEBS J 272:3491–3504 [DOI] [PubMed] [Google Scholar]
- Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M 2004 Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem 279: 19431–19440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Cohen P 1998 Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev 8:55–62 [DOI] [PubMed] [Google Scholar]
- Zaballos MA, Garcia B, Santisteban P 2008 Gβγ dimers released in response to thyrotropin activate phosphoinositide 3-kinase and regulate gene expression in thyroid cells. Mol Endocrinol 22:1183–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Battersby S, Williams AR, Anderson RA, Jabbour HN 2004 Prostaglandin E2 mediates phosphorylation and down-regulation of the tuberous sclerosis-2 tumor suppressor (tuberin) in human endometrial adenocarcinoma cells via the Akt signaling pathway. J Clin Endocrinol Metab 89:6112–6118 [DOI] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM 2008 The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320:1496– 1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP 2008 Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol 18:1269–1277 [DOI] [PubMed] [Google Scholar]
- McManus EJ, Collins BJ, Ashby PR, Prescott AR, Murray-Tait V, Armit LJ, Arthur JS, Alessi DR 2004 The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J 23:2071–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N 2006 mTOR, translation initiation and cancer. Oncogene 25:6416– 6422 [DOI] [PubMed] [Google Scholar]
- Averous J, Proud CG 2006 When translation meets transformation: the mTOR story. Oncogene 25:6423–6435 [DOI] [PubMed] [Google Scholar]
- Jefferies HB, Reinhard C, Kozma SC, Thomas G 1994 Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci USA 91:4441–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G 2004 S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol 24:3112–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M 2005 Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol 7:286–294 [DOI] [PubMed] [Google Scholar]
- Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J 2004 mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 24:200–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averous J, Fonseca BD, Proud CG 2008 Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene 27:1106–1113 [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Kato JY, Fisher RP, Morgan DO, Sherr CJ 1994 Activation of cyclin-dependent kinase 4 (cdk4) by mouse MO15-associated kinase. Mol Cell Biol 14:7265–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RP 2005 Secrets of a double agent: CDK7 in cell-cycle control and transcription. J Cell Sci 118:5171–5180 [DOI] [PubMed] [Google Scholar]
- Bockstaele L, Bisteau X, Paternot S, Roger PP 2009 Differential regulation of cyclin-dependent kinase 4 (CDK4) and CDK6, evidence that CDK4 might not be activated by CDK7, and design of a CDK6 activating mutation. Mol Cell Biol 29:4188–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LP, Yue W, Santen RJ, Lawrence Jr JC 2005 Farnesylthiosalicylic acid inhibits mammalian target of rapamycin (mTOR) activity both in cells and in vitro by promoting dissociation of the mTOR-raptor complex. Mol Endocrinol 19:175–183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.