Abstract
Thyroid hormone (TH) plays a critical role in development, growth, and metabolism by binding to nuclear TH receptors to modulate gene expression. In the absence of TH, TH receptors repress genes that are TH-activated by recruiting the nuclear receptor corepressor (NCoR), which exists in a tight complex with histone deacetylase 3 (HDAC3). Here we explored the actions of TH in the deacetylase activating domain mutant (DADm) mouse, whose NCoR-HDAC3 interaction is genetically disrupted. Several TH-activated genes were derepressed in the liver of euthyroid and hypothyroid DADm mice, consistent with the corepressor paradigm and a critical role of the NCoR-HDAC3 interaction in basal repression. The role of corepressors in genes that are down-regulated by TH is less well understood. Remarkably, circulating TSH levels were increased in euthyroid DADm mice, and the pituitary expression of TSHα, a classic TH-down-regulated gene, was modestly but significantly elevated regardless of TH status. Thus, the NCoR interaction with HDAC3 modulates expression of both positively- and negatively-regulated genes by TH in vivo.
Nuclear Receptor Corepressor (NCoR), in partnership with Histone Deacetylase 3 (HDAC3), physiologically modulates a subset of thyroid hormone responsive genes in liver and pituitary.
Thyroid hormone (TH) plays a pivotal role in development, growth, and cellular metabolism ( 1). TH exerts its actions on gene expression by activating TH receptors (TRs) ( 2). TRs belong to the nuclear receptor (NR) superfamily of ligand-regulated transcription. Unlike some other NRs, particularly steroid hormone receptors, TR is constitutively nuclear ( 3) and its TH ligand is not required for DNA binding by TR ( 4,5,6,7,8). In the absence of TH, TR interacts with corepressors, the best studied of which are the nuclear receptor corepressor (NCoR) and silencing mediator of retinoid and thyroid receptors (SMRT) ( 9,10,11,12). TR has higher affinity for NCoR than SMRT ( 13,14,15), and thus NCoR is believed to preferentially control repression by TR. Indeed, interference with NCoR levels or recruitment impairs basal repression by TR in mouse liver ( 2,16).
NCoR, as well as SMRT, contains multiple repression domains ( 17) that interact tightly with proteins that constitute a core corepressor complex, including transducin β-like protein 1, G-protein suppressor 2, and histone deacetylase 3 (HDAC3) ( 18,19,20). HDAC3 interacts with a distinct region containing a SANT (SWI3, ADA2, NCoR, TFIIB) motif similar to that found in other transcriptional regulators ( 21). Knockdown of HDAC3 markedly dampens the magnitude of repression by TR ( 22). Moreover, the SANT (SWI3, ADA2, NCoR, TFIIB) domain, together with an NCoR/SMRT-specific N-terminal extension, is required to activate the catalytic function of the HDAC3 enzyme, and thus referred to as the deacetylase activating domain (DAD) ( 23,24). Indeed, a mutation in the DAD domain of NCoR (Y478A) abrogates repression by TR ( 25).
We recently described an engineered C57Bl/6 mouse line harboring the Y478A mutation within the DAD domain of both NCoR alleles (DADm) ( 26). These DADm mice are viable, and their adult tissues contain normal levels of the mutant NCoR that does not bind HDAC3 ( 26). Here we report that several TH-responsive genes are modestly derepressed in the hypothyroid state in the pituitary and liver of DADm mice, demonstrating an important role of the NCoR-HDAC3 interaction in basal repression. Surprisingly, several genes that are normally repressed by TH are also modestly increased in the DADm mice. These results show that HDAC3 interaction with NCoR is critical for maintaining the basal levels of both positively and negatively regulated TR target genes in vivo.
Materials and Methods
Animals and treatments
All animal procedures followed the National Institutes of Health Guidelines for the Care and Use of Experimental Animals and were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC). Mice were housed in a temperature-controlled facility with 12-h light, 12-h dark cycle and had ad libitum access to food and water. Female wild type and DADm (19 wk old) were used for the experiment. Fresh drinking water was provided daily containing 1% perchlorate and 0.05% methimazole for 2 wk. Fourteen hours before they were killed, all animals were given a sc injection of vehicle (0.9% saline in 100 μl volume) for control and hypothyroid group or 40.0μg/100 g T4 with 4.0μg/100 g T3 for hyperthyroid group. Mice were killed by exposure to CO2 according to the IACUC-approved protocol. Tissues were immediately frozen in liquid nitrogen and stored at −80 C until the analyses were performed.
ELISA for serum total T4 and T3
Blood samples were taken by retro-orbital at d 0 and by cardiac puncture at the time the animals were killed. Serum was obtained by centrifugation, and ELISA was performed to measure serum total T4 (1100; Alpha Diagnostic, San Antonio, TX) and T3 (1700; Alpha Diagnostic) according the manufacturer’s instruction.
RIA for serum TSH
TSH was measured in 50 μl serum using a sensitive, heterologous, disequilibrium double-antibody precipitation RIA as previously described ( 60). Results are expressed in bioassayable TSH units.
Real-time quantitative RT-PCR
Total RNA from tissues was isolated using RNeasy kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. RNA integrity was determined with UV spectrophotometry. Reverse transcription was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. This cDNA served as the template for real-time quantitative PCR, using the ABI Prism 7900 sequence detection system (Applied Biosystems). The housekeeping gene Gapdh was used to normalize results to starting amounts of cDNA. All reactions were performed using SYBR Universal PCR Master Mix (Applied Biosystems) and were evaluated using the standard curve method. The QuantiTect Primer Assays (QIAGEN) is used for amplification.
Chromatin immunoprecipitation
Mouse liver was used for chromatin immunoprecipitation (ChIP). The assay was performed as previously described ( 26) with the following antibodies: anti-HDAC3 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-NCoR ( 61), and anti-SMRT (Affinity Bioreagents, Rockford, IL). “Cyp7a1 UTR” is located in the 3′UTR of Cyp7a1 gene that is more than 10 kb downstream of native Cyp7a1 TH response element (TRE) (−3 kb), and was used as a negative control. A coding sequence of Arbp was used as an internal control. The following primer pairs were used for analyze enrichments: mCyp7a1 TRE, 5′-AAAGAGACTGACCCACTAGAGATGC-3′, 5′-AACCCTGGGAACACAGTGTAAAAC-3′; mCyp7a1 UTR, 5′-AATACGACCGGTACCTTGAT-3′, 5′-CTTCCAGGACATATTGTAGCTCC-3′; mArbp, 5′-CTGGGACGATGAATGAGGAT-3′, 5′-AGCAGCTGGCACCTAAACAG-3′.
Microarray analysis
Total RNA was extracted from the pituitary using RNeasy tissue Mini kit (QIAGEN) according the manufacturer’s instructions. Preparation of RNA for hybridization to Affymetrix MoGene 1.0 ST (Affymetrix, Santa Clara, CA) and scanning of the arrays were performed by University of Pennsylvania Microarray Facility (http://www.bioinformatics.upenn.edu/index.html) according to the manufacturer’s instructions. Robust Multiarray Averaging (RMA) signal extraction, normalization, and filtering were performed by the University of Pennsylvania Microarray Facility bioinformatics group (http://core.pcbi.upenn.edu/) using Partek Genomics Suite (Partek, St. Louis, MO). Adjusted P value based on False Discovery Rate (FDR) was used for filtering.
Results
Manipulation of TH levels
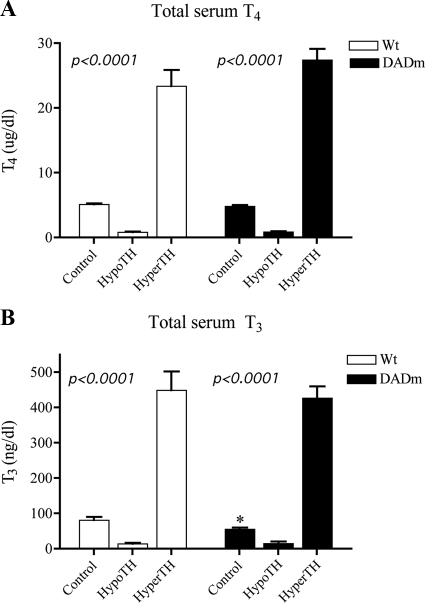
The effects of disruption of the NCoR-HDAC3 interaction on TH-responsive genes were studied by comparing wild-type and DADm C57Bl/6 mice in the euthyroid (Control), hypothyroid (HypoTH), and hyperthyroid (HyperTH) states. Mice were rendered hypothyroid by inclusion of antithyroid drugs perchlorate and methimazole in their drinking water for 2 wk. A hyperthyroid state was induced by sc injection of a physiological ratio of thyroid hormones (T4:T3 in a 10:1 ratio) 14 h before the mice were killed. This acute hyperthyroid paradigm was chosen to minimize secondary effects of long-term TH treatment and to maximize the likelihood that gene expression changes represented direct effects of TH. No difference in T4 levels was noted across genotypes in all treatment groups (Fig. 1A), and no difference in T3 levels was noted except for a slight reduction in the euthyroid DADm mice (Fig. 1B) that was not observed in all experiments (see below and Fig. 3A).
Figure 1.
Total serum T4 and T3 after TH manipulation. TH levels were manipulated as described in Materials and Methods. A, Total serum T4 levels. B, Total serum T3 levels. One-way ANOVA was performed for statistical analysis of treatment variation. Mean ± sem of the hormone levels are shown (n = 5). Wt, Wild type. *, P < 0.05.
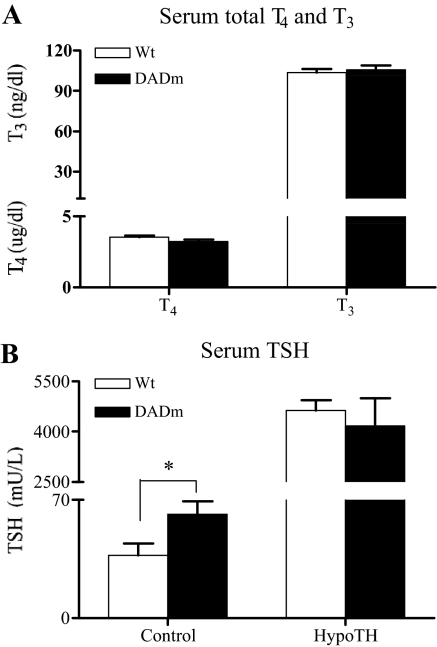
Figure 3.
Disruption of NCoR-HDAC3 interaction increases circulating TSH in DADm mice. A, Total serum T4 and T3. B, Serum TSH. Mean ± sem of the hormone levels are shown (n = 13–17). Unpaired t test was performed for statistical analysis of genotype variation. Wt, Wild type. *, P < 0.05.
Disruption of the NCoR-HDAC3 interaction selectively derepresses TH-responsive genes in liver
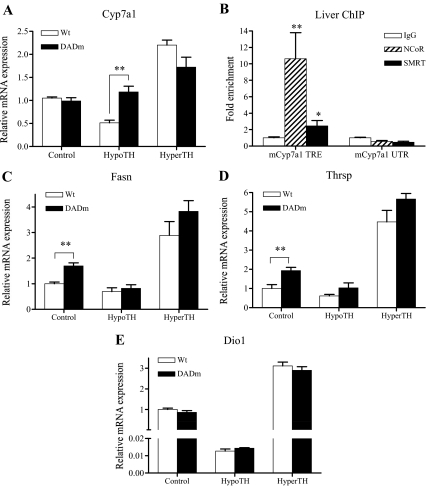
Unliganded TR is generally believed to repress genes in the absence of TH by recruiting NCoR ( 13,14,15). Because HDAC3 is a stoichiometric component of the NCoR complex and required for TH repression of model reporter genes, we expected to see derepression of positive TH targets in the liver of hypothyroid DADm mice. Indeed, expression of the Cyp7a1 gene, which was induced by TH treatment, as has been previously shown ( 27,28,29,30), was more than 2-fold higher in the liver of the hypothyroid DADm mice than control hypothyroid mice (Fig. 2A). This difference disappeared after induction of Cyp7a1 expression by TH (Fig. 2A), consistent with the molecular model implicating NCoR particularly in hypothyroid states. Note that no significant difference in Cyp7a1 was observed in DADm mice under euthyroid conditions. This suggested that euthyroid TH levels were sufficient to reverse the NCoR-dependent derepression of this gene. ChIP confirmed the presence of NCoR at the TRE of the Cyp7a1 gene, but not at a site 10 kb downstream of the gene (Fig. 2B). The related corepressor SMRT (also called NCOR2), was also detected, albeit less robustly than NCoR (Fig. 2B). Interestingly, the expression of two other TH-responsive genes, Fasn ( 31,32) and Thrsp ( 33,34,35), expression was significantly elevated in euthyroid states, 1.7 fold and 1.9 fold, respectively (Fig. 2, C and D). This indicates that the NCoR-HDAC3 interaction is not only involved in gene repression in the absence of TH but also plays a critical role in maintaining basal levels of TH-responsive gene expression in the euthyroid state. Both of these genes also showed a trend toward increased expression in the DADm mice in the hypothyroid as well as the hyperthyroid state, although this was not statistically significant (Fig. 2, C and D). Another gene that is strongly induced in liver by TH, the Dio1 gene ( 36), was unaffected by genotype regardless of thyroid status (Fig. 2E). Thus, the role of the NCoR-HDAD3 interaction in regulating TH-responsive genes is gene specific.
Figure 2.
Disruption of NCoR-HDAC3 interaction derepresses TH-responsive genes in liver. A, Cyp7a1 levels. B, ChIP of NCoR and SMRT in liver tissue. C, Fasn levels. D, Thrsp levels. E, Dio1 levels. Mean ± sem of the expression levels are shown (n = 4–5). For all four genes shown, expression was significantly reduced in HypoTH and increased in HyperTH in both wild-type and DADm mice (one-way ANOVA). Unpaired t test was performed for statistical analysis of genotype variation in each treatment. **, P < 0.01. Unpaired t test was performed for statistical analysis of relative fold enrichment of mCyp7a1 TRE. Mean ± sem of the fold enrichment are shown (n = 7–8). Wt , Wild type. **, P < 0.01; *, P < 0.05.
Disruption of the NCoR-HDAC3 interaction selectively alters TH-responsive gene expression in pituitary
TH also has important effects in the pituitary, where it normally negatively regulates the expression and secretion of TSH. In a cohort of mice whose thyroid axis was manipulated as described earlier, T4 and T3 levels were not different between wild-type and DADm mice (Fig. 3A). Note that this experiment included 13–17 mice in each group, whereas the earlier experiment that showed a very modest change in T3 (Fig. 1B) examined only five mice per group. However, TSH levels were significantly greater (1.7-fold) in the euthyroid DADm mice (Fig. 3B).
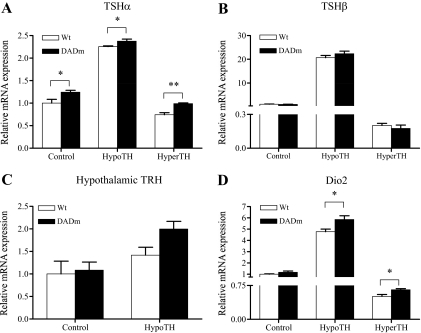
The serum TSH level is under feedback control by TH, such that euthyroid TH levels are the primary determinant of “normal” TSH levels. In the pituitary, TH represses gene expression of both TSH subunits, TSHα ( 37) and TSHβ ( 38). Pituitary TSHα gene expression was modestly increased in the DADm mice, both in the euthyroid (20%) and hypothyroid (10%) states (Fig. 4A), suggesting that disruption of the NCoR-HDAC3 interaction had a positive effect on TSHα gene expression. No significant difference in pituitary TSHβ mRNA levels was observed between wild type and DADm (Fig. 4B). TSH is also regulated by hypothalamic TRH, whose gene expression is also negatively regulated by TH ( 39). Hypothalamic expression of TRH was increased under hypothyroid conditions, with a nonstatistically significant trend toward greater increase in the DADm mice, which could contribute to increased serum TSH (Fig. 4C). Dio2, another gene down-regulated by TH in pituitary ( 40), was expressed at higher levels in hypothyroid but not in euthyroid DADm pituitary relative to wild-type mice (Fig. 4D), indicating a selective role of the NCoR interaction with HDAC3 on genes that are negatively regulated by TH.
Figure 4.
Disruption of NCoR-HDAC3 interaction enhances TSHα gene expression in pituitary. A, TSHα levels. B, TSHβ levels. C, Hypothalamic TRH levels. D, Dio2 levels. Mean ± sem of the expression levels are shown (n = 5). For all pituitary genes shown, expression was significantly increased in HypoTH and reduced in HyperTH in both wild-type and DADm mice (one-way ANOVA). Unpaired t test was performed for statistical analysis of genotype variation. Wt, Wild type. **, P < 0.01; *, P < 0.05.
Global effects of NCoR-HDAC3 interaction in the mouse pituitary
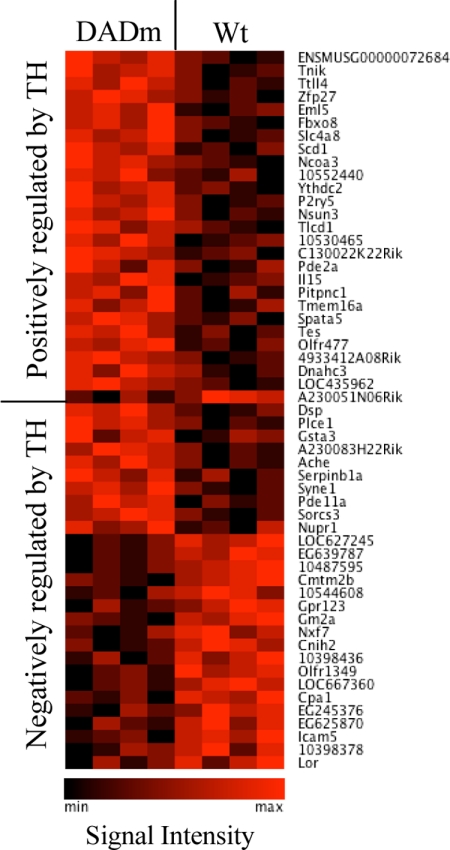
Because the derepression of genes normally repressed by TH was not seen in all of the pituitary genes examined, the role of the NCoR-HDAC3 interaction was examined on a more global scale using gene expression arrays of pituitaries from hypothyroid and hyperthyroid mice of both genotypes. This analysis identified 803 genes that were up-regulated and 703 genes that were down-regulated by TH in wild-type mice. Expression of 55 of these genes was altered by disruption of the NCoR-HDAC3 interaction (Fig. 5). These included 28 of the genes that were negatively regulated by TH [i.e. up-regulated in hypothyroidism (Table 1)]. Ten of these were derepressed, similar to TSHα and Dio2, in the hypothyroid DADm mice (Table 1), whereas the others were oppositely regulated in the hypothyroid DADm mice.
Figure 5.
Pituitary whole gene expression array identified subset of genes that are sensitive to loss of HDAC3 interaction with NCoR in transcription regulation by TH. The heat map (Genesis, http://genome.tugraz.at/) shows different expression levels of TH-responsive genes. Each column represents probe signal intensity for each mouse (n = 4 per group). Wt, Wild type; min, lowest expression; max, highest expression.
Table 1.
Genes negatively regulated by TH and sensitive to disruption of NCoR-HDAC3 interaction in pituitary
| Probeset ID | Gene symbol | RefSeq | Fold change
|
|
|---|---|---|---|---|
| HypoTH DADm vs. wild type | HyperTH DADm vs. wild type | |||
| 10404649 | Dsp | BC033467 | 1.26 | 1.59 |
| 10462922 | Plce1 | NM_019588 | 1.30 | 1.52 |
| 10345065 | Gsta3 | NM_001077353 | 1.22 | 1.23 |
| 10461878 | A230083H22Rik | BC094224 | 1.20 | 1.14 |
| 10526559 | Ache | NM_009599 | 1.23 | NCa |
| 10408557 | Serpinb1a | NM_025429 | 1.61 | NCa |
| 10361381 | Syne1 | NM_001079686 | 1.21 | NCa |
| 10483828 | Pde11a | NM_001081033 | 1.35 | NCa |
| 10463875 | Sorcs3 | NM_025696 | 1.28 | NCa |
| 10567995 | Nupr1 | NM_019738 | 1.25 | −1.18 |
| 10399228 | LOC627245 | XR_034503 | −1.35 | −1.26 |
| 10358754 | EG639787 | XR_034437 | −1.40 | −1.19 |
| 10487595 | −1.21 | −1.13 | ||
| 10574464 | Cmtm2b | NM_028524 | −1.26 | −1.13 |
| 10544608 | −1.21 | −1.13 | ||
| 10558535 | Gpr123 | NM_177469 | −1.22 | −1.19 |
| 10376208 | Gm2a | NM_010299 | −1.30 | −1.06 |
| 10606792 | Nxf7 | NM_130888 | −1.26 | NCa |
| 10464917 | Cnih2 | NM_009920 | −1.34 | NCa |
| 10398436 | −1.27 | NCa | ||
| 10559788 | NM_207136 | −1.24 | NCa | |
| 10458938 | LOC667360 | XR_033768 | −1.26 | NCa |
| 10537051 | Cpa1 | NM_025350 | −1.24 | NCa |
| 10603909 | EG245376 | NM_001081666 | −1.21 | NCa |
| 10521979 | Olfr1349 | XM_914025 | −1.23 | NCa |
| 10583535 | Icam5 | NM_008319 | −1.30 | NCa |
| 10398378 | −1.33 | NCa | ||
| 10499870 | Lor | NM_008508 | −1.20 | 1.16 |
NC indicates no change.
Of the genes induced by TH (i.e. down-regulated in hypothyroidism), 27 genes were differentially regulated in the DADm mice (Table 2). Consistent with the corepressor paradigm of repression in the unliganded states, all but one of these genes were expressed at higher levels in the hypothyroid DADm pituitaries than in hypothyroid control pituitaries (Table 2). These data indicate that the NCoR-HDAC3 interaction is important not only for genes that are positively regulated by TH but also for genes that are negatively regulated by TH in the pituitary. Note that although the disruption of the NCoR-HDAC3 interaction primarily affected gene expression in the hypothyroid state, a subset of these genes was also differentially regulated in hyperthyroid DADm mice (Tables 1 and 2). In these cases, the direction of the change was most often in the same direction as in the hypothyroid setting; for example, genes derepressed in hypothyroidism were super-activated in hyperthyroidism (Table 2).
Table 2.
Genes positively regulated by TH and sensitive to disruption of NCoR-HDAC3 interaction in pituitary
| Probeset ID | Gene symbol | RefSeq | Fold change
|
|
|---|---|---|---|---|
| HypoTH DADm vs. wild type | HyperTH DADm vs. wild type | |||
| 10413220 | ENSMUSG00000072684 | ENSMUST00000100823 | 1.28 | 1.21 |
| 10491136 | Tnik | AK122306 | 1.27 | 1.38 |
| 10347460 | Ttll4 | NM_001014974 | 1.27 | 1.26 |
| 10561787 | Zfp27 | NM_001037707 | 1.20 | 1.14 |
| 10402020 | Eml5 | NM_001081191 | 1.20 | 1.22 |
| 10571849 | Fbxo8 | NM_015791 | 1.21 | 1.12 |
| 10426924 | Slc4a8 | NM_021530 | 1.25 | 1.10 |
| 10467979 | Scd1 | NM_009127 | 1.47 | 1.39 |
| 10478718 | Ncoa3 | NM_008679 | 1.20 | 1.13 |
| 10552440 | 1.20 | 1.14 | ||
| 10455483 | Ythdc2 | ENSMUST00000037763 | 1.21 | 1.10 |
| 10416371 | P2ry5 | NM_175116 | 1.27 | NCa |
| 10440238 | Nsun3 | NM_178925 | 1.29 | NCa |
| 10379034 | Tlcd1 | NM_026708 | 1.25 | NCa |
| 10530465 | 1.25 | NCa | ||
| 10540141 | C130022K22Rik | NM_172730 | 1.20 | NCa |
| 10555510 | Pde2a | NM_001008548 | 1.24 | NCa |
| 10579958 | Il15 | NM_008357 | 1.25 | NCa |
| 10392347 | Pitpnc1 | NM_145823 | 1.26 | NCa |
| 10569618 | Tmem16a | NM_178642 | 1.32 | NCa |
| 10491704 | Spata5 | NM_021343 | 1.23 | NCa |
| 10536483 | Tes | NM_207176 | 1.25 | NCa |
| 10556123 | Olfr477 | NM_146926 | 1.20 | −1.10 |
| 10464407 | 4933412A08Rik | XM_001480761 | 1.36 | −1.19 |
| 10567428 | Dnahc3 | XM_355934 | 1.21 | −1.11 |
| 10552110 | LOC435962 | XM_001481136 | 1.30 | −1.21 |
| 10446066 | A230051N06Rik | ENSMUST00000070225 | −1.26 | −1.16 |
NC, No change.
Discussion
TH, in the form of L-T4, is the third most commonly prescribed drug in the United States (http://www.thyroid.org/professionals/publications/statements/01_06_04_hormone.html). Although much has been learned about TH action from the cloning of the TR ( 41,42) and the discovery of multiple TRs and their mechanisms of action ( 43,44,45,46), TH action in vivo is poorly understood. Here we have demonstrated that the interaction between the TR corepressor NCoR and the epigenetic modulating enzyme HDAC3 is required for maintaining the basal expression of both positively and negatively regulated TR target genes in vivo.
Both NCoR and HDAC3 are required for embryogenesis ( 47,48). Inhibition of NCoR function in liver by overexpression of a mutant that competes for NR binding or by genetic mutation of the NR interaction motifs in NCoR has demonstrated a role of NCoR in repression of TR-responsive genes ( 49,50). However, these models interrupt NCoR interaction with other NRs and also do not address the role of HDAC3. Indeed, there is little or no understanding of the role of HDAC3 in TR function in the adult.
In the liver, expression of the TH-responsive Cyp7a1 gene was significantly elevated in hypothyroid DADm mice relative to hypothyroid control mice, indicating that the NCoR-HDAC3 interaction is necessary for basal repression of this gene by TR. Interestingly, Fasn and Thrsp were elevated under euthyroid conditions, indicating a role for NCoR, and specifically its interaction with HDAC3, in the regulation of TR target genes at physiological levels of TH. These data are consistent with previous findings of a role for NCoR in the basal repression of TH-responsive hepatic gene expression ( 49,50) and extend that conclusion by demonstrating an important role for NCoR recruitment of HDAC3. Although it is possible that some of these gene changes could be indirect, treatment with TH was performed 14 h before the mice were killed to focus on acute gene changes and minimize the likelihood of indirect effects of interrupting the NCoR-HDAC3 interaction.
In both liver and pituitary, we found that the NCoR-HDAC3 interaction was important for the basal repression of some, but not all, genes that are positively regulated by TH. Thus the phenomenon of gene selectivity is not tissue-specific and likely related to regulation by other gene-specific factors. For example, it is possible that basal repression of some genes is mediated by NCoR repression domains other than the DAD ( 51). It is also likely that the repression of some genes by TR is mediated by SMRT, either redundantly with NCoR or even exclusively. Although TR may have higher affinity for NCoR than SMRT, SMRT clearly interacts with TR ( 52,53) and, indeed, we found it enriched at the Cyp7a1 TRE. Understanding the full extent of NCoR and SMRT redundancy will require genome-wide location analysis for each of these corepressors. The gene-selectivity of NCoR-HDAC3 could also reflect a role for other transcription factors, coregulators (coactivators as well as corepressors), and/or other epigenetic regulators.
Because the role of NCoR in liver had been examined previously in other models, we analyzed pituitary gene expression in the DADm in more detail. Gene Ontology of all genes significantly affected by the DADm mutation (Tables 1 and 2) using the Functional Annotation Tool (David Bioinformatics Resources, http://david.abcc. ncifcrf.gov/home.jsp) indicated that the pathway most significantly regulated in pituitary by the NCoR-HDAC3 interaction was “Lipid catabolic process” (P = 5.5E-2). However, the significance of this result is uncertain because the number of genes affected by the DADm mutation was modest.
In addition to positively regulated genes, which we expected to see derepressed in the DADm mice, we were surprised to find enhancement of gene expression of several genes that are negatively regulated by TH. These included two well-known and important TH-regulated genes, TSHα and Dio2. Although the expression changes are modest, this is the first direct evidence that NCoR and its interaction with HDAC3 play a role in repressing genes that are normally inhibited by TH. Of note, TSHα gene expression was reported not to be up-regulated in pituitaries of mice harboring a mutation in SMRT ( 54), suggesting that this is a unique role of NCoR. In contrast to TSHα, TSHβ mRNA was not increased in the DADm pituitary, suggesting that the NCoR-HDAC3 interaction does not regulate this gene. In this regard, it should be noted that, although NCoR and HDAC3 are expressed ubiquitously and thus is it likely that both are present in TSHβ-expressing thyrotrophs, this has not yet been formally demonstrated.
Consistent with enhancement of pituitary TSHα expression in the DADm mice, we found that serum TSH level was also modestly, but significantly elevated in euthyroid DADm mice. Because TSHβ gene expression was not up-regulated in the DADm pituitary, it is possible that the increased serum TSH is related to other factors. Expression of hypothalamic TRH, which promotes TSH secretion and is down-regulated by TH, was modestly increased in the DADm mice, although the difference from wild type did not reach statistical significance. Also, although not assessed in these studies, it is formally possible that the NCoR-HDAC3 interaction somehow controls TSH bioactivity, which could affect the interpretation of these results. As with the role of NCoR-HDAC3 in regulation of positive targets of TH, only a subset of negatively regulated genes were influenced by NCoR interaction with HDAC3. This is consistent with the finding of Astapova et al. ( 49) that an NCoR mutation did not alter the expression of a negatively regulated hepatic gene.
Although current models of hormone action suggest that corepressors should not play a role in gene regulation at saturating TH concentrations, the expression of some pituitary genes was differentially regulated in hyperthyroid DADm mice. This may reflect the fact that corepressors and coactivators interact with overlapping surfaces of TR ( 10) and thus TH does not completely abrogate affinity for corepressor. Alternatively, TH may actually stabilize binding of NCoR to TR in some cases, analogous to the reported stabilization of PPARγ-NCoR interactions in the context of transrepression by PPARγ ligands ( 55).
Recently, using a cell culture transfection system, Wang et al. ( 50) concluded that HDAC3 positively contributes to TSHα expression in vitro in the absence of TH. This result contrasts with our finding that loss of HDAC3 binding to NCoR increases TSHα expression in vivo. There are several potential explanations for this apparent discrepancy. For example, HDAC3 may regulate TSHα independently of its binding to NCoR. The different conclusions may also derive from the use of different experimental systems, with Wang et al. ( 50) ectopically manipulating cultured cells while the present studies are in a more physiological context in intact tissues. Altogether our data support the concept that the NCoR interaction with HDAC3 is critical for maintaining expression of both positively- and negatively-regulated genes by TH in vivo.
Coregulators are critical determinants of nuclear receptor function ( 56). The present work has important implications for the role of coregulators in TH action. Coactivators, in particular the Steroid Receptor Coactivator 1, has been shown to regulate TH action as well as the hypothalamus-pituitary-thyroid (HPT) axis ( 57,58). Although gene expression changes were gene-selective and modest in magnitude, the present work is the first to directly demonstrate corepressor regulation of the HPT axis. The role of corepressors in regulation of the HPT axis in vivo has been suggested by the phenotypes of mice and humans with resistance to TH due to germline dominant negative mutations of TRβ ( 9,59). Our finding that the corepressor NCoR regulates the HPT axis via its association with HDAC3 suggests a potential therapeutic role for inhibition of HDAC3 activity in difficult cases of resistance to TH.
Acknowledgments
We thank Greg Brent (UCLA) for helpful discussions.
Footnotes
These studies were supported by National Institutes of Health Grant R37 DK43806 (to M.A.L.) and by the Tzedakah Foundation.
First Published Online April 28, 2010
Abbreviations: ChIP, Chromatin immunoprecipitation; DAD, deacetylase activating domain; DADm, DAD mutant; HDAC3, histone deacetylase 3; HPT, hypothalamus-pituitary-thyroid; HyperTH, hyperthyroid; HypoTH, hypothyroid; NCoR, nuclear receptor corepressor; NR, nuclear receptor; SMRT, silencing mediator of retinoid and thyroid receptors; TH, thyroid hormone; TR, TH receptor; TRE, TH response element.
References
- Brent GA 1994 The molecular basis of thyroid hormone action. N Engl J Med 331:847–853 [DOI] [PubMed] [Google Scholar]
- Forrest D, Vennström B 2000 Functions of thyroid hormone receptors in mice. Thyroid 10:41–52 [DOI] [PubMed] [Google Scholar]
- Samuels HH, Tsai JS, Casanova J, Stanley F 1974 Thyroid hormone action: in vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. J Clin Invest 54:853–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA 1993 Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193 [DOI] [PubMed] [Google Scholar]
- Evans RM 1988 The steroid and thyroid hormone receptor superfamily. Science 240:889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995 The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RC, Kushner PJ, Baxter JD 1995 The nuclear hormone receptor gene superfamily. Annu Rev Med 46:443–453 [DOI] [PubMed] [Google Scholar]
- You SH, Gauger KJ, Bansal R, Zoeller RT 2006 4-Hydroxy-PCB106 acts as a direct thyroid hormone receptor agonist in rat GH3 cells. Mol Cell Endocrinol 257–258:26–34 [DOI] [PubMed] [Google Scholar]
- Yoh SM, Chatterjee VK, Privalsky ML 1997 Thyroid hormone resistance syndrome manifests as an aberrant interaction between mutant T3 receptors and transcriptional corepressors. Mol Endocrinol 11:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Lazar MA 1999 The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93–96 [DOI] [PubMed] [Google Scholar]
- Lin BC, Hong SH, Krig S, Yoh SM, Privalsky ML 1997 A conformational switch in nuclear hormone receptors is involved in coupling hormone binding to corepressor release. Mol Cell Biol 17:6131–6138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoh SM, Privalsky ML 2001 Transcriptional repression by thyroid hormone receptors. A role for receptor homodimers in the recruitment of SMRT corepressor. J Biol Chem 276:16857–16867 [DOI] [PubMed] [Google Scholar]
- Hu X, Li Y, Lazar MA 2001 Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol Cell Biol 21:1747–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibusawa N, Hollenberg AN, Wondisford FE 2003 Thyroid hormone receptor DNA binding is required for both positive and negative gene regulation. J Biol Chem 278:732–738 [DOI] [PubMed] [Google Scholar]
- Webb P, Anderson CM, Valentine C, Nguyen P, Marimuthu A, West BL, Baxter JD, Kushner PJ 2000 The nuclear receptor corepressor (N-CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs). Mol Endocrinol 14:1976–1985 [DOI] [PubMed] [Google Scholar]
- Cohen RN, Brzostek S, Kim B, Chorev M, Wondisford FE, Hollenberg AN 2001 The specificity of interactions between nuclear hormone receptors and corepressors is mediated by distinct amino acid sequences within the interacting domains. Mol Endocrinol 15:1049–1061 [DOI] [PubMed] [Google Scholar]
- Perissi V, Staszewski LM, McInerney EM, Kurokawa R, Krones A, Rose DW, Lambert MH, Milburn MV, Glass CK, Rosenfeld MG 1999 Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev 13:3198–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R 2000 A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev 14:1048–1057 [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J 2000 Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 19:4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kalkum M, Chait BT, Roeder RG 2002 The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell 9:611–623 [DOI] [PubMed] [Google Scholar]
- Aasland R, Stewart AF, Gibson T 1996 The SANT domain: a putative DNA-binding domain in the SWI-SNF and ADA complexes, the transcriptional co-repressor N-CoR and TFIIIB. Trends Biochem Sci 21:87–88 [PubMed] [Google Scholar]
- Ishizuka T, Lazar MA 2003 The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol 23:5122–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Qin AX, McCue SA, Baldwin KM 1998 Thyroid receptor plasticity in striated muscle types: effects of altered thyroid state. Am J Physiol Endocr Metab 274:E1018–E1026 [DOI] [PubMed] [Google Scholar]
- Guenther MG, Barak O, Lazar MA 2001 The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol 21:6091–6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T, Lazar MA 2005 The nuclear receptor corepressor deacetylase activating domain is essential for repression by thyroid hormone receptor. Mol Endocrinol 19:1443–1451 [DOI] [PubMed] [Google Scholar]
- Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, Avila J, Bućan M, Ahima RS, Kaestner KH, Lazar MA 2008 Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 456:997–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon PB, Gurley EC, Stravitz RT, Litz JS, Pandak WM, Chiang JY, Vlahcevic ZR 1992 Hormonal regulation of cholesterol 7α-hydroxylase mRNA levels and transcriptional activity in primary rat hepatocyte cultures. J Biol Chem 267:16866–16871 [PubMed] [Google Scholar]
- Ness GC, Pendelton LC, Zhao Z 1994 Thyroid hormone rapidly increases cholesterol 7α-hydroxylase mRNA levels in hypophysectomized rats. Biochim Biophys Acta 1214:229–233 [DOI] [PubMed] [Google Scholar]
- Ness GC, Pendleton LC, Li YC, Chiang JY 1990 Effect of thyroid hormone on hepatic cholesterol 7α-hydroxylase, LDL receptor, HMG-CoA reductase, farnesyl pyrophosphate synthetase and apolipoprotein A-I mRNA levels in hypophysectomized rats. Biochem Biophys Res Commun 172:1150–1156 [DOI] [PubMed] [Google Scholar]
- Pandak WM, Heuman DM, Redford K, Stravitz RT, Chiang JY, Hylemon PB, Vlahcevic ZR 1997 Hormonal regulation of cholesterol 7α-hydroxylase specific activity, mRNA levels, and transcriptional activity in vivo in the rat. J Lipid Res 38:2483–2491 [PubMed] [Google Scholar]
- Roncari DA, Murthy VK 1975 Effects of thyroid hormones on enzymes involved in fatty acid and glycerolipid synthesis. J Biol Chem 250:4134–4138 [PubMed] [Google Scholar]
- Wilson SB, Back DW, Morris SM Jr, Swierczynski J, Goodridge AG 1986 Hormonal regulation of lipogenic enzymes in chick embryo hepatocytes in culture. Expression of the fatty acid synthase gene is regulated at both translational and pretranslational steps. J Biol Chem 261:15179–15182 [PubMed] [Google Scholar]
- Jump DB 1989 Rapid induction of rat liver S14 gene transcription by thyroid hormone. J Biol Chem 264:4698–4703 [PubMed] [Google Scholar]
- Kinlaw WB, Schwartz HL, Hamblin PS, Mariash CN, Oppenheimer JH 1988 Triiodothyronine rapidly reverses inhibition of S14 gene transcription by glucagon. Endocrinology 123:2255–2260 [DOI] [PubMed] [Google Scholar]
- Lepar GJ, Jump DB 1989 Hormonal regulation of the S14 gene in 3T3-F442A cells. Mol Endocrinol 3:1207–1214 [DOI] [PubMed] [Google Scholar]
- Berry MJ, Kates AL, Larsen PR 1990 Thyroid hormone regulates type I deiodinase messenger RNA in rat liver. Mol Endocrinol 4:743–748 [DOI] [PubMed] [Google Scholar]
- Chatterjee VK, Lee JK, Rentoumis A, Jameson JL 1989 Negative regulation of the thyroid-stimulating hormone α gene by thyroid hormone: receptor interaction adjacent to the TATA box. Proc Natl Acad Sci USA 86:9114–9118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WM, Kao MY, Gordon DF, Ridgway EC 1989 Thyroid hormone regulates the mouse thyrotropin β-subunit gene promoter in transfected primary thyrotropes. J Biol Chem 264:14840–14847 [PubMed] [Google Scholar]
- Lechan RM, Kakucska I 1992 Feedback regulation of thyrotropin-releasing hormone gene expression by thyroid hormone in the hypothalamic paraventricular nucleus. In: Chadwick DJ, Marsh J, eds. Functional anatomy of the neuroendocrine hypothalamus. Budapest, Hungary: John Wiley and Sons; 144–164 [DOI] [PubMed] [Google Scholar]
- Koenig RJ, Leonard JL, Senator D, Rappaport N, Watson AY, Larsen PR 1984 Regulation of thyroxine 5′-deiodinase activity by 3,5,3′-triiodothyronine in cultured rat anterior pituitary cells. Endocrinology 115:324–329 [DOI] [PubMed] [Google Scholar]
- Sap J, Muñoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennström B 1986 The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 324:635–640 [DOI] [PubMed] [Google Scholar]
- Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM 1986 The c-erb-A gene encodes a thyroid hormone receptor. Nature 324:641–646 [DOI] [PubMed] [Google Scholar]
- Harvey CB, Williams GR 2002 Mechanism of thyroid hormone action. Thyroid 12:441–446 [DOI] [PubMed] [Google Scholar]
- Lazar MA 2003 Thyroid hormone action: a binding contract. J Clin Invest 112:497–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondisford FE 2003 Thyroid hormone action: insight from transgenic mouse models. J Investig Med 51:215–220 [DOI] [PubMed] [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Hermanson O, Jepsen K, Rosenfeld MG 2002 N-CoR controls differentiation of neural stem cells into astrocytes. Nature 419:934–939 [DOI] [PubMed] [Google Scholar]
- Knutson SK, Chyla BJ, Amann JM, Bhaskara S, Huppert SS, Hiebert SW 2008 Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. EMBO J 27:1017–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astapova I, Lee LJ, Morales C, Tauber S, Bilban M, Hollenberg AN 2008 The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc Natl Acad Sci USA 105:19544–19549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Xia X, Liu Y, Oetting A, Walker RL, Zhu Y, Meltzer P, Cole PA, Shi YB, Yen PM 2009 Negative regulation of TSHα target gene by thyroid hormone involves histone acetylation and corepressor complex dissociation. Mol Endocrinol 23:600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurowkawa R, Ryan A, Kamei Y, Söderström M, Glass CK, Rosenfeld MG 1995 Ligand-independent repression by the thyroid hromone receptor mediated by a nuclear co-repressor. Nature 377:397–404 [DOI] [PubMed] [Google Scholar]
- Chen JD, Evans RM 1995 A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454–457 [DOI] [PubMed] [Google Scholar]
- Hong SH, Wong CW, Privalsky ML 1998 Signaling by tyrosine kinases negatively regulates the interaction between transcription factors and SMRT (silencing mediator of retinoic acid and thyroid hormone receptor) corepressor. Mol Endocrinol 12:1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofsinger RR, Li P, Hong SH, Jonker JW, Barish GD, Ying H, Cheng SY, Leblanc M, Xu W, Pei L, Kang YJ, Nelson M, Downes M, Yu RT, Olefsky JM, Lee CH, Evans RM 2008 SMRT repression of nuclear receptors controls the adipogenic set point and metabolic homeostasis. Proc Natl Acad Sci USA 105:20021–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK 2005 A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature 437:759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, O'Malley BW 2007 Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700 [DOI] [PubMed] [Google Scholar]
- Sadow PM, Koo E, Chassande O, Gauthier K, Samarut J, Xu J, O'Malley BW, Seo H, Murata Y, Weiss RE 2003 Thyroid hormone receptor-specific interactions with steroid receptor coactivator-1 in the pituitary. Mol Endocrinol 17:882–894 [DOI] [PubMed] [Google Scholar]
- Weiss RE, Xu J, Ning G, Pohlenz J, O'Malley BW, Refetoff S 1999 Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J 18:1900–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman BD, Lee EJ, Park Y, Jameson JL 2005 A dominant negative peroxisome proliferator-activated receptor-γ knock-in mouse exhibits features of the metabolic syndrome. J Biol Chem 280:17118–17125 [DOI] [PubMed] [Google Scholar]
- Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S 1999 Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9:1265–1271 [DOI] [PubMed] [Google Scholar]
- Huang EY, Zhang J, Miska EA, Guenther MG, Kouzarides T, Lazar MA 2000 Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev 14:45–54 [PMC free article] [PubMed] [Google Scholar]