Abstract
Runt-related transcription factor 2 (Runx2) and muscle segment homeobox homolog 2-interacting nuclear target (MINT) (Spen homolog) are transcriptional regulators critical for mammalian development. MINT enhances Runx2 activation of osteocalcin (OC) fibroblast growth factor (FGF) response element in an FGF2-dependent fashion in C3H10T1/2 cells. Although the MINT N-terminal RNA recognition motif domain contributes, the muscle segment homeobox homolog 2-interacting domain is sufficient for Runx2 activation. Intriguingly, Runx1 cannot replace Runx2 in this assay. To better understand this Runx2 signaling cascade, we performed structure-function analysis of the Runx2-MINT trans-activation relationship. Systematic truncation and domain swapping in Runx1:Runx2 chimeras identified that the unique Runx2 activation domain 3 (AD3), encompassed by residues 316–421, conveys MINT+FGF2 trans-activation in transfection assays. Ala mutagenesis of Runx2 Ser/Thr residues identified that S301 and T326 in AD3 are necessary for full MINT+FGF2 trans-activation. Conversely, phosphomimetic Asp substitution of these AD3 Ser/Thr residues enhanced activation by MINT. Adjacent Pro residues implicated regulation by a proline-directed protein kinase (PDPK). Systematic screening with PDPK inhibitors identified that the casein kinase-2/homeodomain-interacting protein kinase (HIPK)/dual specificity tyrosine phosphorylation regulated kinase inhibitor 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT), but not ERK, c-Jun N-terminal kinase, p38MAPK, or other casein kinase-2 inhibitors, abrogated Runx2-, MINT-, and FGF2-activation. Systematic small interfering RNA-mediated silencing of DMAT-inhibited PDPKs revealed that HIPK3 depletion reduced MINT+FGF2-dependent activation of Runx2. HIPK3 and Runx2 coprecipitate after in vitro transcription-translation, and recombinant HIPK3 recognizes Runx2 AD3 as kinase substrate. Furthermore, DMAT treatment and HIPK3 RNAi inhibited MINT+FGF2 activation of Runx2 AD3, and nuclear HIPK3 colocalized with MINT. HIPK3 antisense oligodeoxynucleotide selectively reduced Runx2 protein accumulation and OC gene expression in C3H10T1/2 cells. Thus, HIPK3 participates in MINT+FGF2 regulation of Runx2 AD3 activity and controls Runx2-dependent OC expression.
MINT and FGF2 stimulate Runx2 AD3 transactivation function. Signaling requires HIPK3, a proline-directed protein kinase that binds MINT, phosphorylates Runx2, and controls FGF2-regulated Runx2 accumulation.
Fibroblast growth factor (FGF)2 signaling plays a critical role in stage-specific control of osteoblast proliferation, gene expression, and bone mass accrual ( 1,2,3,4,5). Hurley and co-workers ( 6,7,8,9) were the first to establish the contributions of FGF2 to bone formation in vivo. The skeletal response is biphasic ( 10), but FGF2 is one paracrine mediator of anabolism elicited by the prototypic and clinically useful bone anabolic hormone, PTH ( 7,9). In our studies of osteoblast gene transcription, we identified that FGF2/FGF receptor signaling and muscle segment homeobox homolog 2 (Msx2)-dependent transcriptional suppression converge at the osteocalcin (OC) FGF response element (OCFRE) ( 11,12). This element, encompassed by nucleotides −154 to −113 relative to the rat OC transcriptional start site, binds a multiprotein complex containing runt-related transcription factor 2 (Runx2) and the nuclear matrix proteins Ku antigen and Msx2-interacting nuclear target (MINT) ( 13,14,15). Msx2 binds to MINT and Runx2, thus preventing stable association of Runx2 with the OC gene in native chromatin context ( 14). Systematic analyses revealed that it was the Runx2 component of this complex that possessed the FGF2 responsive trans-activation function (TAF) and that this FGF2-regulated TAF is augmented by MINT ( 14,15).
We wished to better understand the structural features of the Runx2 TAFs ( 16) that confer regulation by MINT+FGF2( 14). We identify that Runx2 activation domain (AD)3 ( 16) encompasses the TAF targeted for activation by the MINT Msx2-interacting domain (MID) domain in conjunction with FGF2 signaling ( 13,14). Intact Ser/Thr-Pro motifs in the N-terminal part of Runx2 AD3 are required for MINT+FGF2 activation. Signaling via homeodomain-interacting protein kinase (HIPK)3, a proline-directed protein kinase (PDPK) ( 17,18), is required for robust OCFRE activation by MINT+FGF2, and HIPK3 regulates Runx2 protein accumulation and OC gene expression in C3H10T1/2 multipotent osteoprogenitors ( 19).
Results
MINT augments FGF2-stimulated Runx2 trans-activation via the MID domain
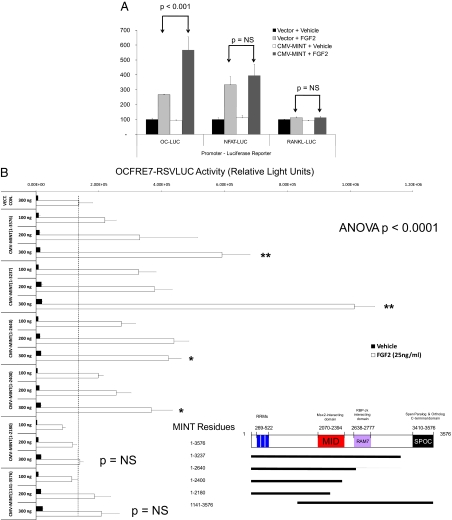
Our early studies of MINT/Spen demonstrated that the N-terminal RNA recognition motif (RRM) domain could bind a G/T-rich element in the OC promoter ( 13); expression of the N-terminal 90-kDa fragment encompassing the three RRM domains suppressed OCFRE activity ( 13). However, subsequent studies with full-length MINT(1-3576) demonstrated enhancement of OCFRE-dependent transcription, with MINT activation dependent upon the Runx2 osteoblast-specific element 2 cognate present in the OCFRE ( 14). As with the concatemerized OCFRE, in the presence of FGF2, full-length MINT stimulates transcription driven by the OCFRE in the native context of the 0.2-kb OC promoter (the 0.2-kb OC promoter fragment encompasses the Runx2/OC-specific element 2 and the G/T-rich RRM binding motif) (Fig. 1A) ( 14). Activation by MINT was promoter specific, because receptor activator of nuclear factor (NF)-κB ligand (RANKL) and nuclear factor of activated T cells (NFAT)-promoter-luciferase reporter gene (LUC) constructs were not stimulated (Fig. 1A). We wished to further refine the domains of MINT necessary for stimulation of OC gene expression. Therefore, we generated a systematic series of MINT truncation mutants and tested transcriptional activation of the OCFRE using C3H10T1/2 cells, a murine osteoprogenitor line ( 19). For these studies, we took advantage of OCFRE7-Rous sarcoma virus (RSV)LUC, a luciferase reporter construct possessing seven copies of the OCFRE cloned upstream of the RSV minimal promoter. Deletion of the MINT Spen paralog and ortholog C-terminal (SPOC) domain ( 20) altered the dose response but did not eliminate activation of the OCFRE (Fig. 1B). Further C-terminal truncation that removes the Notch-regulating recombination signal-binding protein suppressor of hairless (RBP-J) binding domain (RAM7) domain of MINT ( 21,22), as in MINT(1-2640), had little additional impact (Fig. 1B). However, MINT(1-2180), lacking an intact MID at residues 2070-2394, completely lost the capacity to trans-activate the OCFRE (Fig. 1B). Western blot analyses for hemagglutinin (HA)-tagged MINT fusion proteins demonstrated expression of all HA-MINT variants (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Of note, all MINT variants studied retained at least two nuclear localization signals and localized to the nucleus (data not shown). Thus, OCFRE activation by MINT requires an intact motif present in residues 2180-2400, separate from the RAM7 and C-terminal SPOC domains (Fig. 1C).
Figure 1.
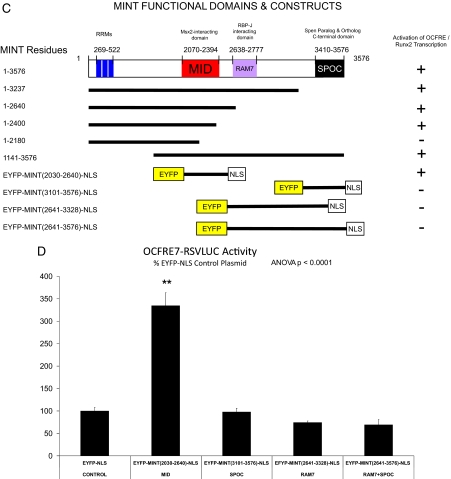
MINT stimulates Runx2- and FGF2-dependent transcriptional activation of the OCFRE via the MID domain. A, C3H10T1/2 cells were transiently transfected OC promoter, RANKL promoter, and NFAT response element-LUC reporter vectors. Plasmids for CMV vector or CMV-MINT expression (full length) were cotransfected as indicated, and cultures were treated with either vehicle or FGF2 (50 ng/ml) as described in Materials and Methods. Runx2 expression plasmid was included in all transfections. B, The OCFRE reporter OCFRE7-RSVLUC was used as a reporter to assess a systematic series of CMV-MINT variants in transient transfection assay. All transfections included Runx2 expression plasmid. As compared with FGF2-treated vector control (dotted vertical line) all C-truncated MINT expression constructs except for CMV-MINT(1–2180) significantly stimulated OCFRE activity. Comparisons were made vs. FGF2-treated vector control. One-way ANOVA with post hoc testing was carried out as described in Materials and Methods. Inset, A depiction of expressed MINT protein fragments and domains. C, A schematic representation of MINT C-truncated, N-truncated, and EYFP-fusion protein variants with OCFRE/Runx2 activation summary. D, MINT transcriptional regulatory domains were expressed as EYFP fusion proteins, with a uniform C-terminally located NLS and assayed in cotransfection assays with OCFRE7-RSVLUC. Only the MINT MID domain significantly stimulated OCFRE activity (asterisks). E, Coexpression of full-length MINT augments the TAF of the G4-Runx2, but not G4-USF1, in the mammalian one-hybrid assay. Inset, A schematic representation of G4-Runx2 fusion protein. See text for details. *, P < 0.05; **, P < 0.01; ***, P < 0.001. NS, Not significant; G4, Gal4 DNA binding domain. Figure continued on next two pages.
We next examined the impact of N-terminal truncation on MINT activation of the OCFRE ( 13,23). Deletion of the RRM domain ( 13,23) encoded by MINT residues 269-522 significantly reduced but did not completely ablate induction (Fig. 1B). This suggested that activation might be independent of intrinsic MINT nucleic acid binding activity. To directly test this notion and to further map this MINT activation function, we constructed a series of enhanced yellow fluorescence protein (EYFP)-MINT-nuclear localization sequence (NLS) fusion proteins that provide a uniform NLS in addition to an EYFP tag (Fig. 1C, lower panel). As shown in Fig. 1D, in the presence of Runx2, the expression of the MID domain fusion protein EYFP-MINT(2030-2640)-NLS enhanced OCFRE7-RSVLUC activity 3-fold as compared with the EFYP-NLS control. By contrast, EFYP-NLS fusion proteins containing the RAM7 and SPOC domains, either alone or in combination, failed to stimulate OCFRE-dependent transcription (Fig. 1D). Western blot analysis for the EYFP tag confirmed expression of all EFYP-MINT fusion proteins (Supplemental Fig. 2). Thus, MINT MID domain (2030-2640) confers OCFRE activation.
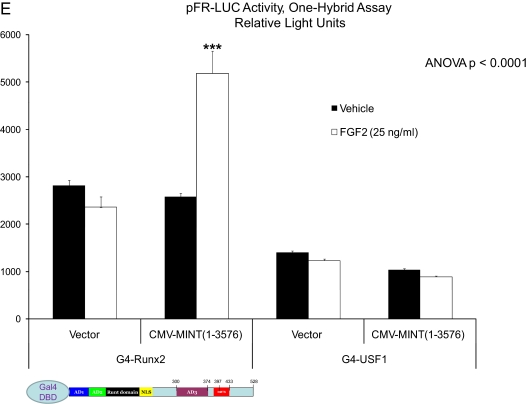
The results obtained using EYFP-MINT(2030-2640)-NLS indicated that OCFRE activation could be mediated independent of the intrinsic MINT nucleic acid binding activity but dependent upon Runx2 ( 13,23). To provide additional evidence to confirm this notion, we implemented the one-hybrid assay. In this assay, transcription is driven by Runx2 TAF present in chimeric Gal4 DNA binding domain (G4)-Runx2 fusion protein. However, the TAF is recruited to a heterologous pFR-LUC via the G4 DNA recognition cognate multimerized upstream of the LUC transcription initiation site ( 14). As shown in Fig. 1E, full-length MINT(1-3576) augmented transcription driven by G4-Runx2 in FGF2-treated cultures. Activation was transcription factor specific, because MINT did not augment activation of G4-upstream stimulatory factor (USF)1 in one hybrid assay (Fig. 1E). MINT(1141-3576) also significantly enhanced FGF2-stimulated G4-Runx2 transcription and was dependent upon Runx2 recruitment via the Gal4DBD (Supplemental Fig. 3). Thus, MINT augments Runx2-dependent transcription in concert with FGF2 signaling. Intrinsic MINT nucleic acid binding activity is not required for stimulating Runx2 TAF. MINT core activation functions mapped between residues 2030–2400, encompassing the MINT MID.
Runx2 residues 316–421, encoding AD3, mediate MINT-dependent trans-activation
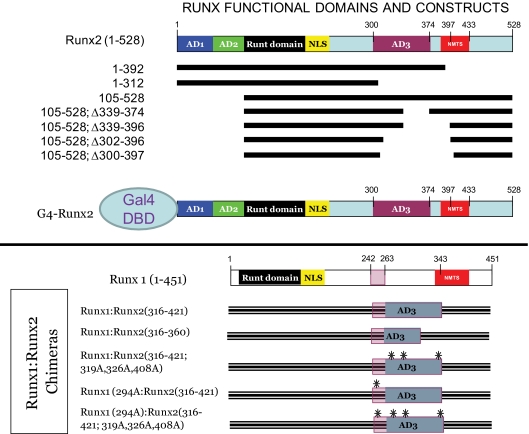
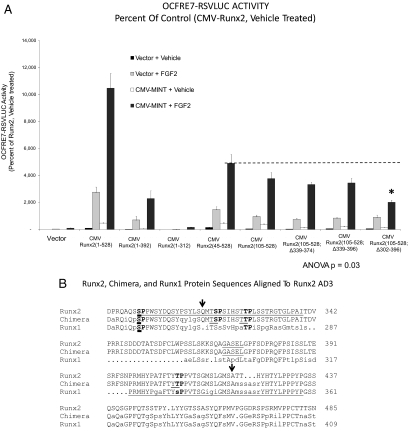
We wished to better understand the structural features of the Runx2 TAFs ( 16) regulated by MINT. To map domains that respond to MINT+FGF2, we generated Runx2 variants lacking known regulatory domains (Fig. 2, upper panel) and then tested trans-activation of OCFRE7-RSVLUC in transient transfection assays. Although much less active than full-length Runx2(1-528), Runx2(1-392) retained significant responsiveness to full-length MINT+FGF2 in C3H10T1/2 cells [Runx2(1-392) retains nuclear localization cues but lacks the nuclear matrix targeting signal (NMTS)] (Fig. 3A) ( 24,25). By contrast, Runx2(1-312) was completely inactive, revealing that the DNA binding Runt domain and N-terminal AD1 and AD2 domains were insufficient to convey MINT+FGF2 responsiveness. Moreover, Runx2(45-528) and Runx2(105-528), lacking Runx2 AD1 and AD1+AD2, respectively, were still capable of conveying significant responsiveness to MINT in the presence of FGF2 stimulation (Fig. 3A). Thus, AD1, AD2, and the NMTS were not required for Runx2 responses to MINT+FGF2.
Figure 2.
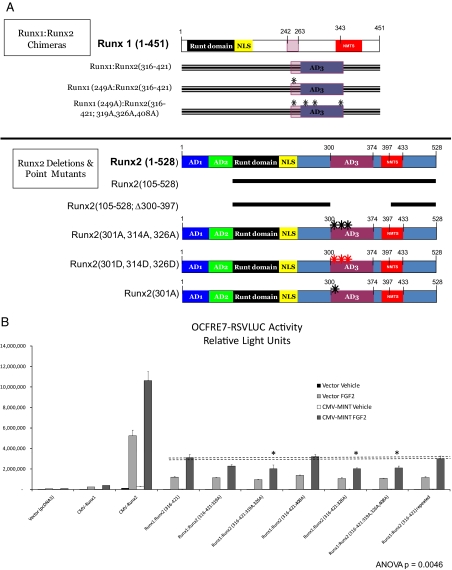
Schematic representation of Runx2 deletion variants, G4-Runx2 fusion protein, and Runx1:Runx2 chimeras. Upper panel, A systematic series of Runx2 truncation mutants and internal deletion variants were generated for functional analyses in addition to the G4-Runx2 fusion for one-hybrid analysis. Lower panel, Runx1:Runx2 chimeric proteins were generated, including variants lacking key proline-directed phosphor-acceptor sites (S/T → A mutants; black asterisks).
Figure 3.
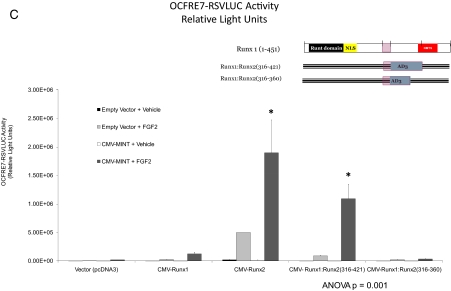
Runx2 AD3 conveys activation by MINT+FGF2. A, The OCFRE reporter OCFRE7-RSVLUC was used as a reporter to assess a systematic series of CMV-Runx2 variants in transient transfection assays. Data are presented as percent of activity observed under control conditions vs. CMV-Runx2-treated with vehicle and vector. Runx2(105–528) encompasses the MINT+FGF2 responsive AD, dependent upon the intact AD3 lesioned by deletion of residues 302–396 (asterisk). B, The sequence alignment of Runx2 (top line), Runx1 (bottom line), and the Runx1:Runx2 chimera Runx1:Runx2(316-421). Arrows point to the positioning of the chimeric Runx2 domain insertion into Runx1. The C-terminal end of AD3( 16) (“…GASEL”) is underlined in Runx2. The shared NMTS ( 24,25) is underlined in Runx1 beginning at “PRMHY…” The Ser/Thr proline-directed kinase residues of AD3 are bolded, including Ser301 in Runx2 that corresponds to Ser249 in Runx1 (bold underline). C, Activity of Runx1:Runx2 AD3 chimeras. The Runx2 domain 316-421 conveys MINT+FGF2 response onto the very poorly responsive Runx1. Inset, Schematic representation of Runx1;Runx2 chimeras analyzed. *, P < 0.05 vs. MINT+FGF2-treated Runx1. Figure continued on next page.
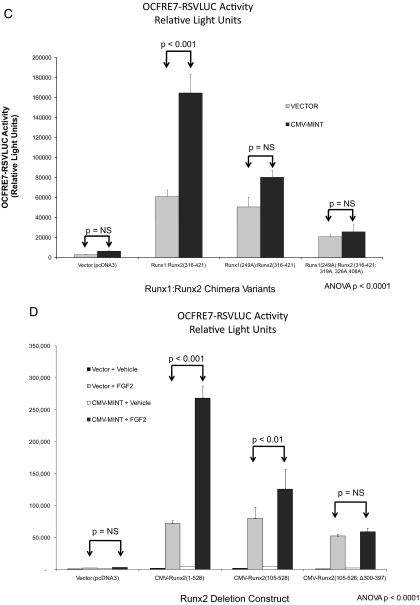
We next generated a series of internal deletion constructs to remove AD3 and its adjacent segments in the context of Runx2(105-528). Runx2(105-528; Δ339-374) and Runx2(105-528; Δ339-396) conveyed MINT responsiveness to an extent similar to that of Runx2(105-528) (Fig. 3A). However, Runx2(105-528; Δ302-396), lacking residues 302-339 in AD3 ( 16), exhibited significantly reduced activity vs. Runx2(105-528) (for Runx2(105-528; Δ302-396), see Fig. 3A, asterisk; residues 302-339 underlined in Fig. 3B). We wished to further refine the features of Runx2 AD3 that convey MINT+FGF2 responsiveness. Therefore, we took advantage of our observation that the related family member, Runx1(1-451), conveys at best only modest induction of the OCFRE7-RSVLUC, with little if any response to full-length MINT (see below). We generated and functionally evaluated a series of Runx1:Runx2 chimeric proteins, including the placement of Runx2 AD3 and surrounding elements in the equivalent region of Runx1 (see Materials and Methods and Fig. 3B). Runx1 residues Ser264 through Ala342 were replaced in contiguous open reading frame by Runx2 residues Gln316 through Ala421, generating the chimeric protein Runx1(Met1-Ser263):Runx2(Gln316-Ala421):Runx1 (Met343-Tyr451). For brevity, this chimera is denoted as Runx1:Runx2(316-421). As shown in Fig. 3C, Runx2 residues 316-421 conveyed MINT+FGF2 responsiveness onto Runx1 [compare Runx1:Runx2(316-421) with Runx1(1-451)]. However, the chimera Runx1:Runx2(316-360) that lacked the C terminus of Runx2 AD3( 16) was inactive (Fig. 3C). Thus, unique structural motifs present in Runx2(316-421), a 106 amino acid sequence encompassing the AD3 core ( 16), encodes information necessary and sufficient to convey MINT responsiveness onto the unresponsive heterologous family member, Runx1.
Proline-directed kinase motifs in Runx2(316-421) are important for MINT-dependent stimulation
We wished to further define the region of Runx2(316-421) conveying MINT+FGF2 responsiveness. Runx2 residues 316-421 encompass three Ser and Thr residues immediately upstream and adjacent to Pro residues (Fig. 3B). PDPKs recognize such motifs as substrates, and the FGF2/FGF receptor utilizes a number of cellular PDPKs to mediate signal transduction ( 26). To identify whether the putative PDPK motifs in Runx2(316-421) participate in activation by MINT+FGF2, we first performed Ala mutagenesis in the Runx1:Runx2 chimera (for a schematic representation, see Fig. 4A); we replaced codons for Thr318-Ser319, Thr325-Thr326, and Thr407-Thr408 with Ala-Ala codons to disrupt PDPK cognates in the N-terminal aspect of the Runx2 AD3 domain (for convenience, only the amino acid immediately preceding the Pro residue is denoted). As shown in Fig. 4B, Runx1:Runx2(316-421:319A;326A) and Runx1:Runx2(316-421:326A) exhibited significantly reduced responsiveness to MINT as compared with Runx1:Runx2 (316-421). By contrast, Runx1:Runx2(316-421:408A) was unaffected. Mutation of the three central PDPK sites (Fig. 4B), as in Runx1:Runx2(316-421:319A,326A,408A), did not further diminish the response to MINT but retained the reduced activity level of Runx1:Runx2(316-421:326A) (Fig. 4B, asterisks). Thus, all mutants pointed to the functional importance of the PDPK cognate at Thr326. Of note, protein kinase C and casein kinase-2 (CK2) cognates as Ser330, Ser364, Ser347, and Ser388 were also mutated to Ala, but none of these individually contributed to MINT/FGF2-dependent Runx2 trans-activation (data not shown).
Figure 4.
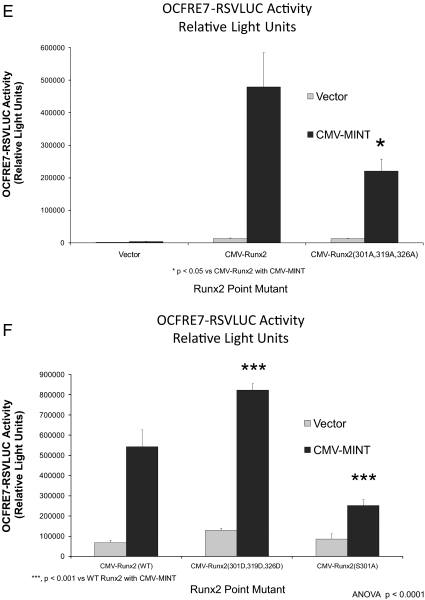
Activation of Runx2 AD3 by MINT+FGF2 requires intact proline-directed kinase cognates. The OCFRE reporter OCFRE7-RSVLUC was used as a reporter to assess a systematic series of CMV-Runx1:Runx2 chimeras and CMV-Runx2 point mutants in transient transfection assays. A, Schematic representation of Runx1:Runx2 chimeras (upper panel) and Runx2 deletion and point mutants (lower panel) analyzed. Black asterisks, S/T → A point mutations that disrupt potential Pro-directed phospho-acceptor sites. Red asterisks, S/T → D point mutations that introduce phosphomimetic changes at potential Pro-directed phospho-acceptor sites. B, AL mutagenesis of the Runx1:Runx2(316-421) chimera revealed contribution of Thr326 in AD3. *, P < 0.05 vs. nonmutated chimera control treated with MINT+FGF2. C, Additional Ala mutagenesis of Runx1 Ser249, equivalent to Runx2 Ser301, in Runx1:Runx2(316-421) chimera abrogated MINT+FGF2 activation. D, Deletion of Runx2 residues 300–397 in the context of Runx2 abrogated MINT+FGF2 induction. E, Point mutations that destroy Pro-directed phosphor-acceptor sites at residues Ser-301, Ser-319, and Thr-326 in native full-length Runx2 significantly reduce MINT-dependent trans-activation without affecting basal activity. F, Conversely, the Runx2(301D, 319D, and 326D) phosphomimetic mutations increase activation by MINT. Mutation of Ser-301 to Ala in context of full-length Runx2 reduces basal activity as observed in Runx1:Runx2 chimeras. NS, Not significant.
At the N-terminal junction of the Runx1-Runx2 chimera, we noted a 4th putative PDPK cognate, a Ser-Pro motif conserved in both Runx1 and Runx2 (Fig. 3B); Runx1 Ser-249 is a PDPK cognate equivalent to Runx2 Ser-301 (Fig. 3B, bold underline). Furthermore, we realized that our previous Runx2 AD3 internal deletions had left Ser-301 intact with an adjacent Pro residue (Fig. 3, A and B). Thus, this 4th PDPK cognate was mutated in the context of the Runx1:Runx2(316-421) chimera (Fig. 4A, upper panel). As shown in Fig. 4C, mutation of that single cognate, as in Runx1(249A):Runx2(316-421), significantly reduced MINT+FGF2 stimulation. Mutation of all four of these AD3 PDPK cognates in the chimera had a profound effect; unlike Runx1:Runx2(316-421), Runx1(249A):Runx2(316-421:319A,326A,408A) chimera was completely unresponsive to MINT+FGF2 (Fig. 4C). Finally, deletion of Runx2 residues 300-397 in the native Runx2(105-528) context, thus removing all of AD3, including Ser301, abrogated MINT+FGF2 activation with little effect on basal activity [see Runx2(105-528;Δ300-394) in Fig. 4D].
To confirm these observations, we generated a series of Ala mutations and Asp “phosphomimetic” alterations in Runx2 AD3 within the context of full-length native Runx2 (for a schematic representation, see Fig. 4A, lower panel). As shown in Fig. 4E, mutation of the PDPK cognates in residues Ser-301, Ser-319, and Thr-326 significantly reduced MINT activation of Runx2 without altering basal transcription. By contrast, introduction of the negatively-charged, phosphomimetic Asp side chain at residues 301, 319, and 326 in Runx2 AD3 significantly enhanced activation by MINT (Fig. 4F). As in the Runx1:Runx2 chimera, Ser-301 was critical for MINT regulation in the context of full-length Runx2 (Fig. 4F). Of note, Western blot analyses confirmed equivalent levels of these Runx2 protein variants (Supplemental Fig. 4). Thus, PDPK cognates at Ser301 and Thr326 in Runx2 AD3 contribute to Runx2 trans-activation by MINT+FGF2.
A 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT)-sensitive kinase participates in AD3 activation by MINT+FGF2
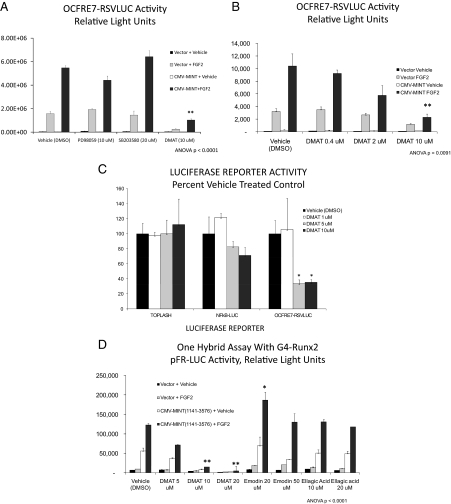
Because our structure-function studies indicated that a member of the PDPK family was necessary for MINT+FGF2 activation of Runx2, we screened a series of pharmacological inhibitors of known PDPKs. Additionally, we included analysis of CK2, a kinase with cognates in Runx2(316-421) that did not contribute to MINT+FGF2 activation (data not shown). C3H10T1/2 cells were transiently transfected with OCFRE7-RSVLUC reporter, full-length Runx2, and/or MINT expression plasmids. Twenty-four hours later, transfected cultures were treated with vehicle (dimethylsulfoxide 0.1%) or inhibitors at doses equal or higher than their IC50 for 1 h before and during an 18-hr treatment with FGF2 or its vehicle (PBS/BSA). As shown in Fig. 5A, the ERK1/ERK2 inhibitor PD98059 did not significantly diminish MINT+FGF2 transcriptional activation, whereas SB203580 (p38MAPK antagonist) had no effect. The c-Jun N-terminal kinase inhibitor SP600125 was also ineffective (10 μm) (data not shown). By contrast, and to our initial surprise, DMAT, a unique benzimidazole that inhibits dual specificity tyrosine phosphorylation regulated kinase (Dyrk)/HIPK proline-directed kinases, as well as CK2, profoundly reduced MINT+FGF2 activation of OCFRE7-RSVLUC (Fig. 5A). However, the action of DMAT was not mediated via inhibition of CK2, because other specific CK2 inhibitors, such as emodin and ellagic acid, did not antagonize MINT+FGF2 activation (Supplemental Fig. 5). Inhibition by DMAT was dose responsive, exhibiting an IC50 value less than 5 μm (Fig. 5, B and C). Moreover, DMAT inhibitory effects were promoter specific, because NF-κB-LUC and TOPFLASH reporter activities were not reduced by DMAT in transfected cells (Fig. 5C). To confirm that a DMAT-sensitive activity was necessary for functional Runx2 trans-activation, we tested the impact of DMAT in the one-hybrid system described above. As shown in Fig. 5D, 10 μm DMAT significantly reduced MINT(1141-3576) trans-activation of G4-Runx2. The alternative CK2 inhibitors emodin and ellagic acid failed to inhibit MINT+FGF2 activation of G4-Runx2 (activation actually enhanced by 20 μm emodin) (Fig. 5D). Thus, a DMAT-sensitive kinase that is not CK2 is required for MINT+FGF2 activation of Runx2 AD3.
Figure 5.
Activation of Runx2 by MINT+FGF2 is mediated via a DMAT-sensitive kinase. A, The OCFRE7-RSVLUC was used as a reporter to screen for the effects of proline-directed kinase inhibitors on MINT+FGF2 activation. DMAT, a mixed CK2 and Dyrk/HIPK inhibitor, significantly reduced activity. B, DMAT inhibition was dose dependent. C, Inhibition by DMAT was promoter specific. D, DMAT also inhibited MINT+FGF2 activation of Runx2 in one-hybrid assays. DMSO, Dimethylsulfoxide. *, P < 0.05; **, P < 0.01 vs. vehicle.
HIPK3, a DMAT-sensitive PDPK, supports MINT+FGF2 activation of Runx2
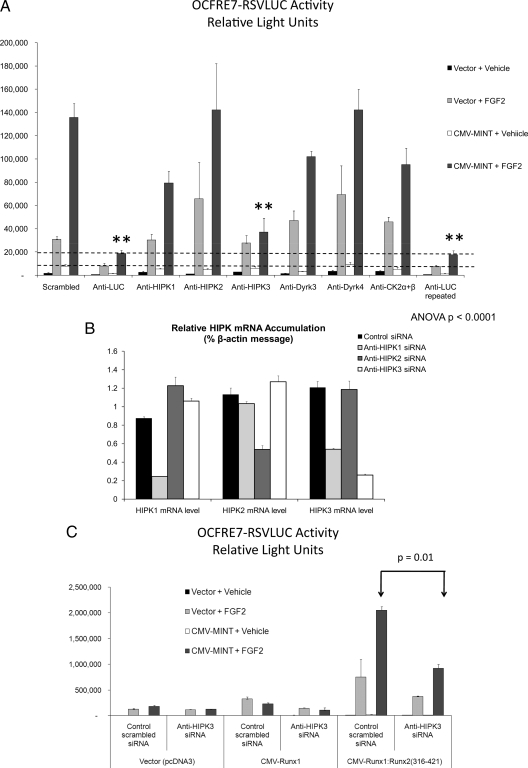
DMAT has been shown to potently inhibit four types of kinases: CK2, proviral integration site for murine leukemia virus kinases, PKD1, and the Dyrk/HIPK family of PDPKs ( 27). Because other well-characterized CK2 inhibitors had no effect, we discounted CK2 as a mediator. Furthermore, emodin, a potent CK2 inhibitor that also inhibits proviral integration site for murine leukemia virus kinase ( 27), did not inhibit Runx2 activation by MINT+FGF2 but actually enhanced responsiveness. Although PKD1 does participate in Runx2 regulation ( 28), it is not a PDPK, and PKD1 regulation maps to the Runt domain ( 28). However, Dyrk/HIPK family members are DMAT-sensitive PDPKs ( 17,29,30). Therefore, we studied the impact of depleting each Dyrk/HIPK family member upon MINT+FGF2 activation of the OCFRE. The eight family members evaluated (Dyrk1a, Dyrk1b, Dyrk2, Dyrk3, Dyrk4, HIPK1, HIPK2, and HIPK3) were all targeted by small interfering RNA (siRNA) screening, with CK2 subunit targeting included as one type of negative control (Fig. 6A) (data not shown). A scrambled siRNA served as a second negative control, and anti-LUC siRNA duplex served as a positive control for RNA interference within this transient transfection/LUC reporter assay system. Of all the Dyrk/HIPKs targeted by RNAi, only siRNA directed toward HIPK3 significantly inhibited MINT+FGF2 activation of the OCFRE7-RSVLUC reporter (Fig. 6A) (data not shown). One siRNA directed toward HIPK1 also consistently resulted in a nonsignificant trend for reducing promoter activation (Fig. 5A). However, RT-quantitative PCR (qPCR) analysis demonstrated that this HIPK1 siRNA partially down-regulated the related HIPK3 mRNA in addition to HIPK1, whereas HIPK3 RNAi was specific for HIPK3 message (Fig. 6B). Moreover, the HIPK3 siRNA efficiently down-regulated enhanced green fluorescent protein (EGFP)-HIPK3 protein accumulation (Supplemental Fig. 6). Consistent with our pharmacologic data, despite a reduction of 80% in endogenous CK2α levels (data not shown), no changes were observed for MINT+FGF2 activation of OCFRE reporter (Fig. 6A). To confirm these data, the impact of HIPK3 RNAi was tested on activation via Runx2 AD3 using the Runx1:Runx2(316-421) chimera. Once again, HIPK3 RNAi significantly reduced MINT+FGF2 activation as compared with the scrambled control siRNA (Fig. 6C). Thus, HIPK3 participates in MINT+FGF2 activation of Runx2 AD3 in C3H10T1/2 mesenchymal cells.
Figure 6.
Activation of Runx2 AD3 by MINT+FGF2 requires HIPK3. A, The OCFRE7-RSVLUC was used as a reporter to screen for the effects of siRNAs directed against Dyrk/HIPK family members on MINT+FGF2 activation. RNAi directed against the LUC reporter was included as a positive control, whereas siRNAs directed against CK2 and scrambled sequences were included as negative controls. ANOVA was carried out for activity of MINT+FGF2-treated samples for each siRNA. Besides anti-LUC, only siRNA directed against HIPK3 significantly reduced MINT+FGF2 activation. **, P < 0.01. B, RT-qPCR analysis indicated that HIPK2 and HIPK3 siRNA were specific for knock-down of targeted HIPK family members, whereas HIPK1 siRNA also partially reduced HIPK3 mRNA in addition to HIPK1. HIPK2 siRNA was specific for HIPK2. C, HIPK3 RNAi significantly reduced MINT+FGF2 activation of Runx1:Runx2(316-421) containing Runx2 AD3.
HIPK3 binds Runx2 and MINT and colocalizes with MINT in the nuclear matrix
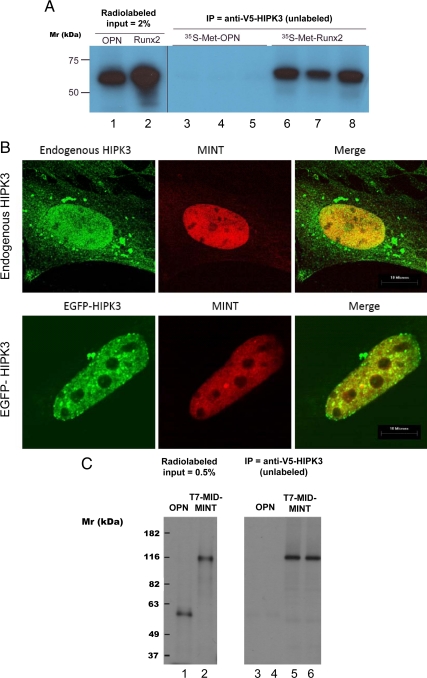
The functional requirement of HIPK3 for MINT- and FGF2-dependent activation of the Runx2-responsive OC promoter prompted us to look for evidence of physical interaction and intracellular proximity. Thus, coprecipitation studies were performed, initially using unlabeled V5-epitope-tagged HIPK3 “bait” protein to interrogate potential prey proteins radioactively labeled with 35S-methionine (all proteins generated by coupled in vitro transcription-translation). Osteopontin (OPN) was chosen as a conveniently available negative control. Unlabeled V5-tagged HIPK3 coprecipitates radiolabeled Runx2 but not radiolabeled OPN (Fig. 7A). Conversely, unlabeled Runx2 coprecipitates radiolabeled HIPK3 but not OPN (Supplemental Fig. 7). We next examined the subcellular localization of HIPK3 with respect to the nuclear matrix protein MINT ( 13,14). Although immunovisualization of endogenous HIPK3 with a polyclonal antibody exhibited some cytosolic staining, the strongest signal was nuclear (Fig. 7B, upper panel), colocalizing with MINT in the nuclear matrix ( 13). To confirm nuclear colocalization, we assembled a construct coding for HIPK3 fused to the C terminus of the GFP (EGFP-HIPK3). As observed with endogenous HIPK3, EGFP-HIPK3 also colocalized with MINT in C3H10T1/2 cells (Fig. 7B, lower panel). Moreover, in coprecipitation assays, HIPK3 interacts with the MINT MID domain but not with OPN (Fig. 7C). Thus, HIPK3 participates in protein-protein interactions with both Runx2 and MINT and colocalizes with MINT in the nuclear matrix ( 13).
Figure 7.
HIPK3 interacts with Runx2 and with MINT MID and colocalizes with MINT in the nucleus. A, In vitro synthesized and unlabeled V5-tagged-HIPK3 was incubated with radioactively labeled OPN or Runx2 and immunoprecipitated with mouse anti-V5 antibody. Note that radioactive Runx2 (lanes 2 and 6–8), but not OPN (lanes 1 and 3–5), is coprecipitated by pull-down of V5 HIPK3. B, CMV-MINT was transfected into C3H10T1/2 cells and localization compared with that of endogenous HIPK3 (upper row) or coexpressed EYFP-HIPK3 (lower row) by confocal microscopy. The two proteins extensively colocalize in the nucleus and exclude the nucleoli. C, In vitro synthesized and unlabeled V5-tagged-HIPK3 was incubated with radioactively labeled OPN or T7-tagged MID MINT and immunoprecipitated with mouse anti-V5 antibody. Note that radioactive MID MINT (lanes 2, 5, and 6), but not OPN (lanes 1, 3, and 4), is coprecipitated by pull-down of V5 HIPK3. IP, Immunoprecipitated.
Recombinant HIPK3 recognizes Runx2 AD3 as a kinase substrate in vitro
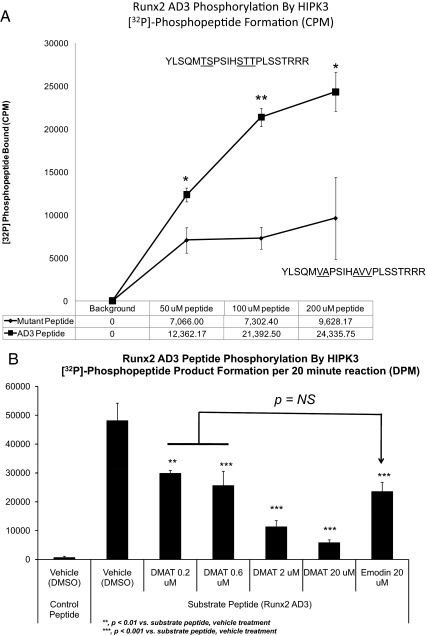
Because 1) DMAT treatment and HIPK3 RNAi inhibited MINT+FGF2 activation of Runx2 AD3; 2) intact Ser/Thr-Pro PDPK cognates in this region were required for AD3 activation by MINT+FGF2; and 3) phosphomimetic Asp substitutions at these Runx2 AD3 Ser/Thr-Pro motifs enhanced activation by MINT, we hypothesized that the PDPK activity of HIPK3 would recognize Runx2 AD3 as a substrate. To directly test this notion, we examined whether recombinant purified HIPK3 could catalyze radiolabeled phosphate transfer from γ-[32P]-ATP to a Runx2 AD3 synthetic peptide corresponding to residues Tyr313 to Arg332, thus encompassing the proline-directed kinase cognates specific to Runx2 AD3 and not shared with Runx1(Fig. 8A). Two additional Arg residues were added to the C terminus to facilitate interaction of the peptide to phosphocellulose in this filter binding assay. As shown in Fig. 8A, HIPK3 increases [32P]-Runx2(313-332) formation in a time- and concentration-dependent fashion. Moreover, replacement of all hydroxyl amino acids immediately upstream of the Pro residues in this AD3 segment significantly reduced radiolabeled phosphate incorporation (Fig. 8A). Heating HIPK3 for 5 min at 95 C before addition of peptide substrate inactivated the enzyme and reduced radiolabeled phosphate incorporation to background (data not shown). Moreover, DMAT dose dependently inhibited HIPK3 phosphorylation of the Runx2 AD3 domain, with an IC50 approximately 100-fold lower than that of the potent CK2 inhibitor emodin (Fig. 8B). Thus, the PDPK activity of HIPK3 phosphorylates Runx2 AD3 residues (313-332) in vitro, and phosphorylation is potently inhibited by DMAT.
Figure 8.
Recombinant HIPK3 recognizes Runx2 AD3 peptide as a kinase substrate. A, A synthetic peptide encompassing the unique Runx2 AD3 residues contributing to Runx1:Runx2(316-421) chimera response to MINT+FGF2 was tested as a substrate for phosphorylation by HIPK3. A control peptide lacking proline-directed kinase cognates served as a control for background. WT Runx2 AD3 is phosphorylated to a significant extent above background by HIPK3. *, P < 0.05 vs. mutant; **, P < 0.01 vs. mutant. B, DMAT inhibits HIPK3-dependent phosphorylation of Runx2 AD3, with an IC50 that is about 100-fold lower than that of the potent CK2 inhibitor, emodin. NS, Not significant.
HIPK3 supports Runx2 protein accumulation and OC gene expression
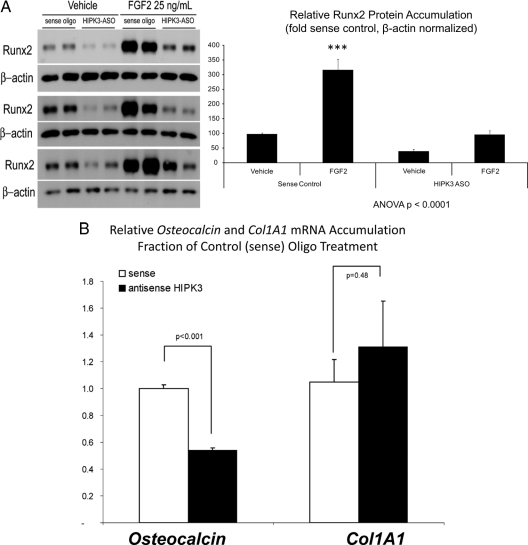
The steady-state accumulation of Runx2 protein is an important aspect of Runx2-dependent gene regulation ( 31,32,33). FGF2 treatment increases Runx2 protein levels in C3H10T1/2 cells, and this increase is inhibited by DMAT treatment, suggesting a HIPK3-dependent mechanism (Supplemental Fig. 8). To test this notion, we examined the effect of depleting HIPK3 in this same assay. Transfection of cells with HIPK3 antisense oligodeoxynucleotide phosphorothioate (ASO) recapitulated the effects of DMAT treatment, inhibiting basal and FGF2-stimulated Runx2 protein accumulation (Fig. 9A). Moreover, HIPK3 ASO resulted in a 46% decrease in endogenous OC mRNA expression (P < 0.001) compared with cells transfected with the control sense-strand oligonucleotide (Fig. 9A). Col1A1 mRNA accumulation was unaffected by HIPK3 ASO (Fig. 9B). Thus, depletion of HIPK3 selectively inhibits OC gene expression and FGF2-regulated Runx2 protein accumulation in C3H10T1/2 mesenchymal cells.
Figure 9.
HIPK3 maintains Runx2 protein accumulation and OC gene expression. A, HIPK3 knock-down by an ASO directed toward HIPK3 recapitulates the effects of DMAT on Runx2 protein accumulation. B, As compared with the sense strand control oligo, HIPK3 ASO also significantly reduces endogenous OC gene expression in cells transfected with CMV-Runx2. The Col1A1 gene was not affected by HIPK3 ASO. ***, P < 0.001 vs. vehicle.
Discussion
The large size of MINT has made detailed structure-function analyses of this nuclear matrix transcription factor very difficult ( 13). However, based upon protein-protein interactions and protein-nucleic acid interactions, five domains [the C-terminal SPOC domain (3410-3576; murine sequence), the N-terminal RRM-containing domain (269-522), the central RBP-J-interacting domain RAM7 (2638-2777), the adjacent MID (2070-2394), and the overlapping nuclear receptor-interacting domain (RID) (2148-2637)] have been identified as playing critical roles in MINT-regulated gene transcription (Fig. 1C). Honjo and co-workers ( 22) first identified the important role of MINT as an inhibitor of Notch1 signaling; via its RAM7 domain, MINT sequesters and prevents RBP-J DNA binding, thus precluding trans-activation directed by the RBP-J coadapter, Notch1 intracellular domain ( 22). As such, MINT fulfills a function of Drosophilan Hairless, a negative regulator of RBP-J that is not present in the vertebrate genome. The C-terminal SPOC domain participates in transcriptional suppression via type I histone deacetylase recruitment ( 23,34), and we have confirmed the results of Honjo and co-workers ( 22) in our own mammalian one-hybrid assays (our unpublished observations). The MINT SPOC domain may also be involved in Notch inhibition ( 34). However, our data demonstrate that the MINT SPOC domain is not required for Runx2 regulation of the OCFRE. The N-terminal RRM domain of human MINT was shown by Evans and co-workers ( 23) to recognize steroid receptor RNA activator, an RNA-based nuclear receptor coactivator. Their studies also identified a nuclear receptor binding motif called RID located in MINT residues 2148-2637 (numbered per murine sequence for coregistration) ( 23). The RID segment of MINT partially overlaps the MID that we identified upon the interaction cloning of MINT ( 14). We now identify that the MINT MID segment also encompasses the core activity for MINT+FGF2 activation of the Runx2 AD3. Although contributions are certainly made by the N-terminal RRM domain, the MID domain encompasses the core function of MINT-mediated Runx2 activation. Consistent with this, MINT+FGF2 activation of OCFRE7-RSVLUC is exquisitely sensitive to even very low levels of Msx2 ( 14), inhibited by concentrations of Msx2 expression plasmid 2 log units lower than those required for Runx2 or MINT responses (our unpublished observations).
Our structure-function studies also newly indentify HIPK3 in the signaling pathway by which FGF2 and MINT stimulate Runx2 trans-activation. HIPK3 is required for OCFRE activation, binds Runx2 and MINT, phosphorylates Runx2 AD3, controls AD3 activation by MINT+FGF2, and is necessary for FGF2-mediated enhancement of Runx2 protein accumulation. Thus, we conclude that this functionally and physically interacting Runx2 kinase is a key mediator of MINT+FGF2-regulated OC gene expression (Supplemental Fig. 9). Whether the MINT MID domain is also phosphorylated by HIPK3 is being investigated. Based upon the data of Franceschi and co-workers ( 36) and Franceschi et al. (4,37), we anticipate that osteoblast HIPK3 and ERK1/2 signaling will sequentially participate in the stage-specific control of OC gene expression. Because DMAT is much more effective than PD98059 in inhibiting Runx2 actions in C3H10T1/2 osteoprogenitors, HIPK3 is like to play a critical role upstream of ERK1/2 during the sequential elaboration of the osteoblast phenotype. However, this notion remains to be tested in vivo.
Of the HIPK family members described to date, HIPK1 and HIPK2 have been the most extensively studied. Ito recently demonstrated that Runx1 is phosphorylated by HIPK2, a crucial step in sequential Runx1-p300 modifications that direct subnuclear localization of transcriptional complexes ( 38). HIPK1 and HIPK2 knockout mice are grossly normal, but HIPK1−/−;HIPK2−/− mouse embryos die in utero due to severe defects in vasculogenesis and angiogenesis ( 39). This embryonic-lethal phenotype likely reflects impaired regulation of Runx1-dependent transcriptional programs ( 39). By comparison, HIPK3 has been only superficially studied. Known functions of HIPK3 include augmentation of androgen receptor ( 40) and c-Jun (41) transcriptional responses. Via an independent line of biochemical investigation, we have identified that HIPK3 also participates in Runx2 transcriptional regulation by MINT+FGF2. This adds to emerging data indicating an important role for the HIPK family in Runx-dependent gene transcription ( 38). To date, the phenotype of HIPK3-null mice has not been reported. Given the inability of the intact HIPK3 gene to support early development in HIPK1−/−;HIPK2−/− mice ( 39), and the unique contributions of HIPK3 to Runx2-dependent trans-activation, we anticipate that HIPK3 will serve unique physiological roles during later vertebrate development, including skeletogenesis.
Of note, FGF2 ( 6,42), Runx2 (43), and Msx2 ( 44,45,46,47) signals significantly contribute to diseases of heterotopic calcification ( 48,49), as well as to orthotopic bone formation ( 50,51). All of these signals, along with osteoregulatory Notch signaling ( 48), functionally converge upon MINT. Thus, targeting the HIPK3-MINT interaction with novel antagonists, or DMAT-like kinase inhibitors, may offer new therapeutic strategies for limiting aberrant osteogenic mineralization as occurs in calcific aortic stenosis ( 52,53). Our future studies will examine the potential impact of HIPK3 inhibition on valvular and vascular calcification, using in vitro and in vivo models of disease.
Materials and Methods
Chemicals, reagents, and antibodies
Protein kinase inhibitors emodin, apigenin, 1,3-dihydro-3-[(2,4,6-trimethoxyphenyl) methylene]-2H-indol-2-one, DMAT, and PD98059 were obtained from Sigma Chemical Co. (St. Louis, MO). The following antibodies were used: mouse anti-Runx2 8G5 was from MBL International, Inc. (Naka-ku Nagoya, Japan). Mouse anti-V5 epitope tag Clone V5–10, mouse M-2 anti-Flag, and rabbit anti-Runx1 antibody (N terminal; R-0406) were obtained from Sigma-Aldrich (St. Louis, MO). Rabbit anti-His tag (sc-803), goat polyclonal anti-HIPK3 (sc-46360), and goat polyclonal anti-CK2 (sc-6480) were obtained from Santa Cruz Biotechnology, Inc. (San Diego, CA). All other biochemical reagents were purchased from Fisher Scientific (Pittsburgh, PA), Invitrogen Corp. (Carlsbad, CA), Millipore Corp. (Billerica, MA), QIAGEN (Valencia, CA), or Sigma Chemical Co. as indicated.
Reporter and expression plasmids
As previously detailed ( 11,15), the reporter for OCFRE activity is a seven-copy concatemer of rat OC promoter nucleotides −154 to −113 that places cognates for Runx2 and Ku antigen upstream of the minimal RSV promoter and LUC cDNA. The cytomegalovirus (CMV)-LacZ control vector ( 14,15) and the OC-LUC ( 54) and RANKL-LUC (55) promoter-reporter constructs been previously described. The latter kindly provided by Xu Feng. NFAT-LUC and NF-κB-LUC reporters were purchased from Stratagene (La Jolla, CA). Full-length MINT (amino acids 1-3576; also known as murine SHARP or SPEN) corresponds to RefSeq NM_019763 as initially reported and cloned by us ( 13,14). The cDNAs for wild-type Runx2 (Osf2/Cbfa1; amino acids 1-528; Expasy Q08775-5 or National Center for Biotechnology Information NP_001139510) ( 16,56) and Runx1 (PEBP2αB1, murine AML1b; amino acids 1-451; Expasy isoform 1 Q03347-1, National Center for Biotechnology Information isoform 3 NM_001111023.1) ( 57,58) have been previously reported. The pFR-LUC reporter and G4-Runx2 and G4-USF1 expression plasmids used in one hybrid analyses have been previously described ( 14,59).
To prepare N- or C-terminal truncation variants of MINT, full-length MINT cDNA originally cloned from MC3T3-E1 calvarial osteoblasts ( 14) was used as a template in high-fidelity PCRs, and fragments were ligated into pCMV-HA (cytomegalovirus promoter-HA tag) vector as previously detailed (Fig. 1D) ( 14). Likewise, to study the contribution of short stretches of Runx2 within the context of Runx1 and their interaction with MINT, the distinct regions of Runx2 indicated in Fig. 2 were PCR amplified and inserted in-frame into unique endonuclease restriction sites found along Runx1 isoform 1 cDNA (BamH1, SacI, SalI, and SphI). Murine HIPK3 ( 17) was PCR amplified using IMAGE clone no. 40110751 as a template, then cloned into pcDNA3.1-V5-His vector. HIPK3 was also cloned in open reading frame onto the C terminus of the EGFP plasmid (CLONTECH, Mountain View, CA) and used in transient transfection to image the intracellular distribution of EGFP-HIPK3. Vectors and methods for one-hybrid analysis of Runx2 have been previously described ( 14). All plasmid inserts were sequenced to verify fidelity, and protein expression validated by coupled transcription/translation (Promega, Inc., Madison, WI), Western blot analysis, and/or fluorescence imaging.
Cell culture and transient transfection assays
Our methods of cell culture and transiently transfect ion have been previously described ( 14,15). Briefly, mouse C3H10T1/2 cells were passaged in 10% fetal bovine serum and basal medium eagle and then plated at 70% of confluence in 24-well plates (1.9-cm2 area/well) for transfection studies. Next day, cells were transiently transfected with 600 ng of total plasmid DNA/well with 2.5 μl of Superfect dendrimer reagent (QIAGEN) in 30 μl of OptiMEM (Invitrogen Corp.). After 4 h of transfection, cultures were allowed to recover overnight in complete growth medium, then treated with 25 ng/ml of human FGF2 (R&D Systems, Minneapolis, MN) for another 18 h. Cultures were harvested for luciferase reporter activity (routine β-galactosidase normalization) as previously detailed ( 14,15).
Small interfering RNA- and antisense-mediated knock-down methods
To systematically screen the DMAT-sensitive Dyrk family protein kinases that might participate in MINT+FGF2 activation of the OCFRE, siRNAs were transiently cotransfected along with the indicated Runx2 expression and LUC-reporter plasmids. Anti-LUC siRNA (Dharmacon, ThermoFisher Scientific, Inc., Lafayette, CO) served as positive control for RNA interference and was taken to define maximum silencing. The AllStars control siRNA (QIAGEN) was used as the negative control for initial screening, with subsequent revalidation using scrambled siRNA control sequence for HIPK3 (see below). Briefly, siRNAs were dissolved in serum-free medium containing Mirrus Transit-TKO reagent (Mirrus Co., Madison, WI), incubated, and pooled with plasmid-liposome mixture prepared separately, using diluted Superfect dendrimer transfection reagent and plasmid dissolved on OptiMEM. Complete growth medium was added to bring siRNA concentration to 100 nm, gently mixed, and then added to adherent C3H10T1/2 cells. After a 6-h incubation, cultures were turned to growth media and then replaced the next morning with fresh medium supplemented with either vehicle or FGF2 as previously detailed. Cell cultures were harvested for either LUC reporter activity or RT-qPCR using methods previously described (n = 3–6 per treatment condition, repeated at least twice) ( 14,15). Gene silencing was confirmed by RT-qPCR or by Western blot analysis. For assessing knock-down of the EGFP-HIPK3 fusion protein, cultures cotransfected with CMV-EGFP-HIPK3 and either control or anti-Hipk3 siRNA as above, then fixed with 2% paraformaldehyde and evaluated by fluorescence microscopy in lieu of extract preparation. The siRNA duplexes were directed against the following murine DNA sequence targets: CK-2α, CAGAAGATTTATATGACTATA; CDK2, GTTGTGGCGCTTAAGAAGATC; Hipk1, CTGATTCATGCTGACCTTAAA; Hipk2, CGGGAGTTCATTGACCTGTTA; and Hipk3, CAGGAGTAATTCATTGCAGAA. Control siRNAs were either the QIAGEN AllStars RNAi control (CAGGGTATCGACGATTACAAA) or a scrambled Hipk3 duplex sequence (ATACCAGTGGCATAAGTAGAT). All other siRNA duplex sequences were obtained commercially via QIAGEN GenomeWide Design. These sequences are available upon request. For quantifying Hipk family members, specific TaqMan reagents purchased from Applied Biosystems (Foster City, CA) were Mm00501689_m1 (Hipk1), Mm00439329_m1 (Hipk2), and Mm00468880_m1 (Hipk3). Other primer set sequences are available upon request.
Gene silencing using antisense oligonucleotides
Phosphorothioate antisense oligonucleotide (GGTAGACCAAGACTTGTGAG) directed against HIPK3 or the sense-strand control (CTCACAAGTCTTGGTCTACC) were transfected similarly to siRNA methods described above, but the concentration used was 40 nm. pCMV-Runx2 was cotransfected at 0.1 μg/cm2·well. Forty-eight hours after transfection, cells were treated with FGF2 (25 ng/ml) and then harvested 24 h later. Whole-cell lysates harvested in 2×Laemmli sodium dodecyl sulfate sample buffer were processed for immunodetection of Runx2 and the housekeeping gene eIF2α as indicated.
Confocal microscopy
C3H10T1/2 cells were plated and transfected with CMV-EGFP-HIPK3, pCMV-HIPK3-V5, pCMV-Runx2, or p-CMV-HA-MINT-FLAG plasmids and incubated in normal growth conditions for 24 h. Cells were washed with ice-cold PBS and fixed with 4% paraformaldehyde in PBS for 10 min. After permeabilization in 0.1% Triton X-100/PBS, cells were washed and incubated in blocking buffer (2% BSA in PBS + 0.1% Triton X-100), and incubated with primary antibodies with gentle shaking at 4 C overnight. For Runx2, mouse monoclonal anti-Runx2 clone 8G5 (MBL International Corp., Woburn, MA) was used at 1:1000. Mouse monoclonal M2 anti-Flag antibody (1:500) was used to detect MINT (epitope tag added to the C terminus). For endogenous HIPK3, goat polyclonal anti-HIPK3 (sc-46360 N-13; Santa Cruz Biotechnology, Inc.) used at 1:100–200. Donkey anti-IgG secondary antibodies conjugated with fluorochromes Alexa 488 or Alexa 647 were added at 1:400 for 1.0 h and washed. Slides were mounted with Vecta-Mount aqueous media, sealed with nail polish, and imaged in a Bio-Rad Radiance 2000-Nikon TE300 inverted microscope. Confocal microscopy images were acquired by λ strobe sequential scanning at four-six zoom in 1024 × 1024 pixel files, and one-two Airy discs of pinhole. Grayscale picture files were deconvoluted with a constrained iteration deblurring algorithm set at 1.5 of brightness and two iterations (Microtome Deconvolution, VayTek, Fairfield, IA).
Coimmunoprecipitation reactions
Coprecipitation/pull-down assays were carried out essentially as described ( 14). Runx2, V5-His-tagged HIPK3, T7-tagged-MID MINT, or OPN cloned in T7-promoter driven plasmids were labeled in vitro with 35S-methionine in T7 polymerase-driven transcription-translation assays (Rabbit reticulocyte lysate; Promega, Inc.). Unlabeled proteins were prepared similarly using nonradioactive amino acids. Protein-protein interaction reactions were set up by mixing unlabeled HIPK3-V5 proteins (5 μl of in vitro reaction) with 5 μl of either radiolabeled OPN (as a negative control) or radiolabeled Runx2 and MID-MINT as indicated and incubated for 1.0 h on ice with occasional mixing in coimmunoprecipitation buffer [50 mm Tris-HCl (pH 7.5), 1.5 mm EGTA, 100 mm NaCl, 0.1% Triton X-100, 10 mm β-glycerol phosphate, 1 mm orthovanadate, and freshly added protease/phosphatase inhibitors]. Reciprocally, unlabeled Runx2 was incubated with radiolabeled OPN or HIPK3 and incubated as above. Immunoprecipitation directed toward the unlabeled protein partner was carried out by adding 2 μg of either mouse monoclonal anti-Runx2 antibody to the unlabeled Runx2 reactions or mouse monoclonal anti-V5 epitope tag or rabbit anti-His tag to pull-down unlabeled V5-tagged HIPK3 reactions and incubated for 1.0 h on ice. Subsequently, 50 μl of protein A/G-sepharose (25% bed volume) was added to each 400 μl coimmunoprecipitation reaction and mixed end-to-end for 1.0 h at 4 C. Immune complexes were washed three times in 1.0 ml in radio-immunoprecipitation assay buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate in Tris-buffer normal saline) and centrifuged at 3000 rpm for 1 min. Laemmli sample buffer was added, each sample heated for 5 min at 95 C, proteins resolved by SDS-PAGE, and visualized by autoradiography after fixation and fluoro-enhancement as previously detailed ( 60).
In vitro HIPK3 kinase assay
To determine whether Runx2 AD3 served as a substrate for phosphorylation by HIPK3, a peptide encompassing Runx2 residues 310-331 (YLSQMTSPSIHSTTPLSSTRRR) or a mutated control peptide (YLSQMVAPSIHAVVPLSSTRRR) were evaluated as kinase substrates in an in vitro assay using purified human HIPK3 (amino acids 161-562; Millipore Corp.). General guidelines were followed as described by Hardie ( 61). Briefly, 25 μl reactions were prepared in 8 mm 3[N-morholino]propanesulfonic acid-0.2 mm EDTA buffer (pH 7.0) with 10.7 mU of HIPK3 (10 ng), and either Runx2 AD3 or control peptide at 200 μm; reaction was started by adding 5 mm Mg/0.01 mm ATP. Radiolabeled γ [32P]-ATP was premixed with Mg-ATP to achieve a final specific activity of 2200 dpms/pmol·reaction. Reactions were incubated for 30 min at 30 C and stopped with 5 μl of 3% ortho-phosphoric acid. Half volume (15 μl) was spotted on a prewetted P81 phosphocellulose Millipore Multiscreen 96-well plates, and unincorporated radioactivity was removed by three 5-min washes of 75 mm ortho-phosphoric acid and a final methanol wash using a vacuum manifold (Millipore Corp.). Radioactivity was determined by Cerenkov counting of the P81 filters after collection with a Millipore Multiscreen well punch. For studies incorporating the kinase inhibitors DMAT and emodin, inhibitors were added to the reaction at the final concentration before addition of the substrate Runx2 AD3 peptide and kinase activity then quantified as described above.
Statistical analysis
All data points are presented as the mean ± se of independent replicates (n ≥ 3), with each experiment carried out at least twice. All statistical analyses performed by one-way ANOVA or Chi-squared analysis as indicated using GraphPad InStat Software (version 3.06 for Windows; GraphPad, La Jolla, CA). When the ANOVA P was significant (P < 0.05), analysis for intergroup differences was then performed using either Student-Newman-Keuls (for all pair-wise comparisons) or Dunnett’s (for comparison vs. control) post hoc tests for multiple comparisons with GraphPad Instat. For Chi-squared analysis, two-sided Fisher’s exact test was implemented to evaluate statistical significance.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants AR43731 and HL81138 (to D.A.T.) and the Barnes-Jewish Hospital Foundation.
Disclosure Summary: D.A.T. serves as a paid consultant to the Department of Medicine, University of California, Los Angeles Geffen School of Medicine and to the Institute of Medicine/Committee to Review Dietary Reference Intakes for Calcium. O.L.S. has nothing to disclose.
First Published Online May 19, 2010
Abbreviations: AD, Activation domain; ASO, antisense oligodeoxynucleotide phosphorothioate; CMV, cytomegalovirus; CK2, casein kinase-2; DMAT, 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole; Dyrk, dual specificity tyrosine phosphorylation regulated kinase; EGFP, enhanced green fluorescent protein; EYFP, enhanced yellow fluorescence protein; FGF, fibroblast growth factor; HA, hemagglutinin; HIPK, homeodomain-interacting protein kinase; LUC, luciferase reporter gene; MID, Msx2-interacting domain; MINT, Msx2-interacting nuclear target; Msx2, muscle segment homeobox homolog 2; NF, nuclear factor; NFAT, NF of activated T cells; NMTS, nuclear matrix targeting signal; NLS, nuclear localization sequence; OC, osteocalcin; OCFRE, OC FGF response element; OPN, osteopontin; PDPK, proline-directed protein kinase; qPCR, quantitative PCR; RAM7, RBP-J binding domain; RANKL, receptor activator of NF-κB ligand; RBP-J, recombination signal-binding protein suppressor of hairless; RID, receptor-interacting domain; RRM, RNA recognition motif; RSV, Rous sarcoma virus; Runx2, Runt-related transcription factor 2; siRNA, small interfering RNA; SPOC, Spen paralog and ortholog C-terminal; TAF, trans-activation function; USF, upstream stimulatory factor.
References
- Jilka RL 2007 Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40:1434–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie PJ, Coffin JD, Hurley MM 2005 FGF and FGFR signaling in chondrodysplasias and craniosynostosis. J Cell Biochem 96:888–896 [DOI] [PubMed] [Google Scholar]
- Cancedda R, Bianchi G, Derubeis A, Quarto R 2003 Cell therapy for bone disease: a review of current status. Stem Cells 21:610–619 [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Xiao G 2003 Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem 88:446–454 [DOI] [PubMed] [Google Scholar]
- Khosla S, Westendorf JJ, Oursler MJ 2008 Building bone to reverse osteoporosis and repair fractures. J Clin Invest 118:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero A, Okada Y, Tomita M, Ito M, Tsurukami H, Nakamura T, Doetschman T, Coffin JD, Hurley MM 2000 Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J Clin Invest 105:1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MM, Okada Y, Xiao L, Tanaka Y, Ito M, Okimoto N, Nakamura T, Rosen CJ, Doetschman T, Coffin JD 2006 Impaired bone anabolic response to parathyroid hormone in Fgf2−/− and Fgf2+/− mice. Biochem Biophys Res Commun 341:989–994 [DOI] [PubMed] [Google Scholar]
- Naganawa T, Xiao L, Coffin JD, Doetschman T, Sabbieti MG, Agas D, Hurley MM 2008 Reduced expression and function of bone morphogenetic protein-2 in bones of Fgf2 null mice. J Cell Biochem 103:1975–1988 [DOI] [PubMed] [Google Scholar]
- Sabbieti MG, Agas D, Xiao L, Marchetti L, Coffin JD, Doetschman T, Hurley MM 2009 Endogenous FGF-2 is critically important in PTH anabolic effects on bone. J Cell Physiol 219:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue T, Naganawa T, Xiao L, Okada Y, Tanaka Y, Ito M, Okimoto N, Nakamura T, Coffin JD, Hurley MM 2005 Over-expression of fibroblast growth factor-2 causes defective bone mineralization and osteopenia in transgenic mice. J Cell Biochem 95:83–94 [DOI] [PubMed] [Google Scholar]
- Boudreaux JM, Towler DA 1996 Synergistic induction of osteocalcin gene expression: identification of a bipartite element conferring fibroblast growth factor 2 and cyclic AMP responsiveness in the rat osteocalcin promoter. J Biol Chem 271:7508–7515 [DOI] [PubMed] [Google Scholar]
- Newberry EP, Boudreaux JM, Towler DA 1996 The rat osteocalcin fibroblast growth factor (FGF)-responsive element: an okadaic acid-sensitive, FGF-selective transcriptional response motif. Mol Endocrinol 10:1029–1040 [DOI] [PubMed] [Google Scholar]
- Newberry EP, Latifi T, Towler DA 1999 The RRM domain of MINT, a novel Msx2 binding protein, recognizes and regulates the rat osteocalcin promoter. Biochemistry 38:10678–10690 [DOI] [PubMed] [Google Scholar]
- Sierra OL, Cheng SL, Loewy AP, Charlton-Kachigian N, Towler DA 2004 MINT, the Msx2 interacting nuclear matrix target, enhances Runx2-dependent activation of the osteocalcin fibroblast growth factor response element. J Biol Chem 279:32913–32923 [DOI] [PubMed] [Google Scholar]
- Willis DM, Loewy AP, Charlton-Kachigian N, Shao JS, Ornitz DM, Towler DA 2002 Regulation of osteocalcin gene expression by a novel Ku antigen transcription factor complex. J Biol Chem 277:37280–37291 [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu K, Mahajan M, McLarren KW, Stifani S, Karsenty G 1998 Two domains unique to osteoblast-specific transcription factor Osf2/Cbfa1 contribute to its transactivation function and its inability to heterodimerize with Cbfβ. Mol Cell Biol 18:4197–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Choi CY, Lee SJ, Conti MA, Kim Y 1998 Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J Biol Chem 273:25875–25879 [DOI] [PubMed] [Google Scholar]
- Rinaldo C, Siepi F, Prodosmo A, Soddu S 2008 HIPKs: jack of all trades in basic nuclear activities. Biochim Biophys Acta 1783:2124–2129 [DOI] [PubMed] [Google Scholar]
- Miraoui H, Oudina K, Petite H, Tanimoto Y, Moriyama K, Marie PJ 2009 Fibroblast growth factor receptor 2 promotes osteogenic differentiation in mesenchymal cells via ERK1/2 and protein kinase C signaling. J Biol Chem 284:4897–4904 [DOI] [PubMed] [Google Scholar]
- Sánchez-Pulido L, Rojas AM, van Wely KH, Martinez-A C, Valencia A 2004 A SPOC: a widely distributed domain associated with cancer, apoptosis and transcription. BMC Bioinformatics 5:91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Shinkura R, Kuroda K, Yabe D, Honjo T 2007 Msx2-interacting nuclear target protein (Mint) deficiency reveals negative regulation of early thymocyte differentiation by Notch/RBP-J signaling. Proc Natl Acad Sci USA 104:1610–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Han H, Tani S, Tanigaki K, Tun T, Furukawa T, Taniguchi Y, Kurooka H, Hamada Y, Toyokuni S, Honjo T 2003 Regulation of marginal zone B cell development by MINT, a suppressor of Notch/RBP-J signaling pathway. Immunity 18:301–312 [DOI] [PubMed] [Google Scholar]
- Shi Y, Downes M, Xie W, Kao HY, Ordentlich P, Tsai CC, Hon M, Evans RM 2001 Sharp, an inducible cofactor that integrates nuclear receptor repression and activation. Genes Dev 15:1140–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SK, Javed A, Choi JY, van Wijnen AJ, Stein JL, Lian JB, Stein GS 2001 A specific targeting signal directs Runx2/Cbfa1 to subnuclear domains and contributes to transactivation of the osteocalcin gene. J Cell Sci 114:3093–3102 [DOI] [PubMed] [Google Scholar]
- Harrington KS, Javed A, Drissi H, McNeil S, Lian JB, Stein JL, Van Wijnen AJ, Wang YL, Stein GS 2002 Transcription factors RUNX1/AML1 and RUNX2/Cbfa1 dynamically associate with stationary subnuclear domains. J Cell Sci 115:4167–4176 [DOI] [PubMed] [Google Scholar]
- Lu KP, Liou YC, Zhou XZ 2002 Pinning down proline-directed phosphorylation signaling. Trends Cell Biol 12:164–172 [DOI] [PubMed] [Google Scholar]
- Sarno S, Pinna LA 2008 Protein kinase CK2 as a druggable target. Mol Biosyst 4:889–894 [DOI] [PubMed] [Google Scholar]
- Jensen ED, Gopalakrishnan R, Westendorf JJ 2009 Bone morphogenic protein 2 activates protein kinase D to regulate histone deacetylase 7 localization and repression of Runx2. J Biol Chem 284:2225–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann TG, Möller A, Sirma H, Zentgraf H, Taya Y, Dröge W, Will H, Schmitz ML 2002 Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat Cell Biol 4:1–10 [DOI] [PubMed] [Google Scholar]
- Himpel S, Tegge W, Frank R, Leder S, Joost HG, Becker W 2000 Specificity determinants of substrate recognition by the protein kinase DYRK1A. J Biol Chem 275:2431–2438 [DOI] [PubMed] [Google Scholar]
- Jones DC, Wein MN, Oukka M, Hofstaetter JG, Glimcher MJ, Glimcher LH 2006 Regulation of adult bone mass by the zinc finger adapter protein Schnurri-3. Science 312:1223–1227 [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL 2003 Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem 278:50259–50272 [DOI] [PubMed] [Google Scholar]
- Zhao M, Qiao M, Oyajobi BO, Mundy GR, Chen D 2003 E3 ubiquitin ligase Smurf1 mediates core-binding factor α1/Runx2 degradation and plays a specific role in osteoblast differentiation. J Biol Chem 278:27939–27944 [DOI] [PubMed] [Google Scholar]
- Ariyoshi M, Schwabe JW 2003 A conserved structural motif reveals the essential transcriptional repression function of Spen proteins and their role in developmental signaling. Genes Dev 17:1909–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KY, Kim HJ, Lee MH, Kwon TG, Nah HD, Furuichi T, Komori T, Nam SH, Kim YJ, Kim HJ, Ryoo HM 2005 Runx2 regulates FGF2-induced Bmp2 expression during cranial bone development. Dev Dyn 233:115–121 [DOI] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT 2002 Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem 277:36181–36187 [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E 2003 Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res 44(Suppl 1):109–116 [PMC free article] [PubMed] [Google Scholar]
- Wee HJ, Voon DC, Bae SC, Ito Y 2008 PEBP2-β/CBF-β-dependent phosphorylation of RUNX1 and p300 by HIPK2: implications for leukemogenesis. Blood 112:3777–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa Y, Nguyen LA, Isono K, Takakura N, Tagata Y, Schmitz ML, Koseki H, Kitabayashi I 2006 Roles of HIPK1 and HIPK2 in AML1- and p300-dependent transcription, hematopoiesis and blood vessel formation. EMBO J 25:3955–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen AM, Karvonen U, Poukka H, Jänne OA, Palvimo JJ 1998 Activation of androgen receptor function by a novel nuclear protein kinase. Mol Biol Cell 9:2527–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan HC, Li HJ, Lin G, Lai PY, Chung BC 2007 Cyclic AMP stimulates SF-1-dependent CYP11A1 expression through homeodomain-interacting protein kinase 3-mediated Jun N-terminal kinase and c-Jun phosphorylation. Mol Cell Biol 27:2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara T, Sato H, Shimizu T, Tanaka T, Matsui H, Kawai-Kowase K, Sato M, Iso T, Arai M, Kurabayashi M 2010 Fibroblast growth factor-2 induces osteogenic differentiation through a Runx2 activation in vascular smooth muscle cells. Biochem Biophys Res Commun 394:243–248 [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimuzu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T 1997 Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764 [DOI] [PubMed] [Google Scholar]
- Wilkie AO, Tang Z, Elanko N, Walsh S, Twigg SR, Hurst JA, Wall SA, Chrzanowska KH, Maxson Jr RE 2000 Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification. Nat Genet 24:387–390 [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R 2000 Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 24:391–395 [DOI] [PubMed] [Google Scholar]
- Liu YH, Tang Z, Kundu RK, Wu L, Luo W, Zhu D, Sangiorgi F, Snead ML, Maxson RE 1999 Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Dev Biol 205:260–274 [DOI] [PubMed] [Google Scholar]
- Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA 2008 Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem 283:20505–20522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao JS, Cheng SL, Sadhu J, Towler DA 2010 Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension 55:579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer LL, Tintut Y 2008 Vascular calcification: pathobiology of a multifaceted disease. Circulation 117:2938–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G 2008 Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet 9:183–196 [DOI] [PubMed] [Google Scholar]
- Cohen Jr MM 2006 The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A 140:2646–2706 [DOI] [PubMed] [Google Scholar]
- Rajamannan NM 2009 Calcific aortic stenosis: lessons learned from experimental and clinical studies. Arterioscler Thromb Vasc Biol 29:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler 3rd ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS 2001 Bone formation and inflammation in cardiac valves. Circulation 103:1522–1528 [DOI] [PubMed] [Google Scholar]
- Towler DA, Bennett CD, Rodan GA 1994 Activity of the rat osteocalcin basal promoter in osteoblastic cells is dependent upon homeodomain and CP1 binding motifs. Mol Endocrinol 8:614–624 [DOI] [PubMed] [Google Scholar]
- Liu J, Yang H, Liu W, Cao X, Feng X 2005 Sp1 and Sp3 regulate the basal transcription of receptor activator of nuclear factor κB ligand gene in osteoblasts and bone marrow stromal cells. J Cell Biochem 96:716–727 [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G 1997 Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754 [DOI] [PubMed] [Google Scholar]
- Bae SC, Ogawa E, Maruyama M, Oka H, Satake M, Shigesada K, Jenkins NA, Gilbert DJ, Copeland NG, Ito Y 1994 PEBP2 α B/mouse AML1 consists of multiple isoforms that possess differential transactivation potentials. Mol Cell Biol 14:3242–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Ohira M, Shimizu K, Mitani K, Hirai H, Imai T, Yokoyama K, Soeda E, Ohki M 1995 Alternative splicing and genomic structure of the AML1 gene involved in acute myeloid leukemia. Nucleic Acids Res 23:2762–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidder M, Shao JS, Charlton-Kachigian N, Loewy AP, Semenkovich CF, Towler DA 2002 Osteopontin transcription in aortic vascular smooth muscle cells is controlled by glucose-regulated upstream stimulatory factor and activator protein-1 activities. J Biol Chem 277:44485–44496 [DOI] [PubMed] [Google Scholar]
- Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA 2003 MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem 278:45969–45977 [DOI] [PubMed] [Google Scholar]
- Hardie DG 2000 Peptide assay of protein kinases and use of variant peptides to determine recognition motifs. In: Walker JM, Keyse SM, eds. Stress response: methods and protocols. Berlin: Springer-Verlag; 191–201 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.