Abstract
Mucin 1 (MUC1) is a type I transmembrane glycoprotein abundantly expressed on nearly all epithelial tissues and overexpressed by many cancer cells. Previous studies from our lab showed that progesterone receptor (PR)B is a strong stimulator of MUC1 gene expression. It is reported that liganded peroxisome proliferator-activated receptor γ (PPARγ) stimulates Muc1 expression in murine trophoblast. Here, we demonstrate that although the PPARγ ligand, rosiglitazone, stimulates the murine Muc1 promoter in HEC1A, a human uterine epithelial cell line, rosiglitazone alone, has no significant effect on basal human MUC1 promoter activity. In fact, rosiglitazone treatment antagonizes progesterone-stimulated human MUC1 promoter activity and protein expression in two human uterine epithelial cell lines and T47D human breast cancer cells. This response is antagonized by the PPARγ antagonist, GW9662, as well as a dominant-negative form of PPARγ, demonstrating the response is mediated by PPARγ. Additional studies indicate that PPARγ activation does not change PR binding to the MUC1 promoter but generally antagonizes progesterone activity by stimulating PRB degradation and inhibiting progesterone-induced PRB phosphorylation. Collectively, these studies indicate that PPARγ activation inhibits PRB activity through both acute (phosphorylation) and long-term (PRB degradation) pathways.
This report demonstrates a novel form of crosstalk between nuclear receptors and demonstrates an inhibition of progesterone-stimulated human MUC1 expression by PPARγ.
Mucins are large molecular weight glycoproteins expressed on the apical surface of most epithelia. A characteristic feature of mucins is the tandem repeat motifs in their ectodomains, typically consisting of 20–30 amino acids that are rich in serine (Ser), threonine, and proline residues providing many sites for O-glycosylation ( 1). Mucin 1 (MUC1) is a type I transmembrane glycoprotein abundantly expressed on nearly all epithelial tissues, including those of the stomach, pancreas, trachea, lung, kidney salivary, mammary glands, and female reproductive tract and overexpressed by many cancer cells ( 2,3,4). In the uterine lumen, the extended ectodomain of MUC1 forms a barrier that protects the mucosa from infection and prevents embryo implantation ( 5,6). In cancer cells, MUC1 contributes to cancer progression by immunosuppression ( 7,8), facilitation of tumor cell migration ( 9,10), and protection against hypoxia ( 11). Therefore, identifying means to decrease MUC1 expression would be beneficial for both infertility treatment and cancer therapy. Nonetheless, no pharmacologically useful agents have been shown to reduce MUC1 expression.
Human MUC1 gene expression is regulated by multiple hormones and cytokines ( 12,13). Several important regulatory elements have been found in the 1.4-kb 5′-sequence flanking the human MUC1 gene, a region that is sufficient to drive normal patterns of MUC1 expression in epithelia in the absence of introns and the 3′-flanking region in transgenic mice ( 14). Previous studies from our laboratory have shown that TNFα-stimulated MUC1 gene expression is mediated by nuclear factor-κB binding to the κB site at −589/−580 ( 12), interferon-γ activates MUC1 expression through signal transducers and activators of transcription (STAT)1α binding to the STAT-binding site at −503/−495 ( 12), and progesterone-stimulated MUC1 expression requires the region from −570 to −523 of the human MUC1 promoter ( 13). Consistent with this, human MUC1 expression in the uterus is maximal during the receptive phase of the cycle when the progesterone level is high ( 15). Progesterone receptor (PR)B stimulates MUC1 expression, whereas PRA antagonizes PRB action, in this regard ( 13). Differences in PRA:PRB ratios in uterine epithelia in mice and humans appear to account for differences in progesterone responsiveness (P-responsiveness) between these species. In addition, the mouse promoter has a deletion of 21 bp in the region corresponding to P-responsiveness in the human gene and which also may contribute to lack of P-responsiveness in the mouse. In vitro studies have shown that human MUC1 is lost locally at the site of implantation ( 16), suggesting that other signaling pathways might antagonize progesterone action and down-regulate MUC1 at the site of implantation and/or cause local loss of MUC1 at the protein level. In the latter case, cell surface proteases, i.e. TNFα converting enzyme/a disintegrin and metalloprotease-like 17 and membrane-type 1 matrix metalloprotease, have been identified that mediate MUC1 cell surface shedding in cell lines and are found at the apical aspect of uterine epithelial cells in situ ( 17,18).
Peroxisome proliferator-activated receptors (PPARα, PPARβ/δ, and PPARγ) belong to the nuclear hormone receptor superfamily and play important roles in multiple biological processes. Liganded PPARs enter the nucleus and heterodimerize with retinoid X receptors (RXRs), recruit cofactors, and bind to a PPAR-responsive element (AGGTCA N AGGTCA), in the regulatory regions of target genes ( 19,20,21). Differential tissue distribution and ligand-binding ability, in part, may contribute to different PPAR functions ( 22,23). The two PPARγ isoforms, γ1 and γ2, act in white and brown adipose tissues to promote adipocyte differentiation, macrophage differentiation, and lipid storage ( 24). Thiazolidinediones are PPARγ agonists that not only directly modulate adipocyte glucose uptake but also induce expression of the insulin-sensitizing factor, adiponectin, and simultaneously reduce several insulin resistance-promoting polypeptides in adipocytes ( 25). Therefore, two thiazolidinedione compounds, rosiglitazone and pioglitazone, are currently prescribed for the treatment of type 2 diabetes. It was reported that PPARγ modulates Muc1 expression in murine trophoblasts ( 26) and regulates implantation in mice (27). However, the function of PPARs in modulating MUC1 expression in other systems has not been examined. Although the mechanism is not clear, clinical trials have shown that a PPARγ agonist, rosiglitazone, improves fertility of patients with polycystic ovary syndrome, a defect in ovarian function ( 28). Although there is no clear evidence that PPARγ agonists are useful therapies for implantation failure, we examined the possibility that PPARγ agonists modulate MUC1 expression in human uterine cell lines and thereby could be useful tools to influence embryo implantation.
Results
PPARs and RXRs are expressed in uterine epithelial cell lines, HES and HEC-1A
PPAR family members and their requisite partners, the RXRs, have broad, but tissue-specific, distributions ( 22). To examine their expression in two human uterine epithelial cell lines, total RNA from HES and HEC-1A cell lines was extracted and analyzed by RT-PCR using specific primers for each subtype (Fig. 1). PPARα, PPARβ/δ, PPARγ, RXRα, and RXRβ mRNAs were readily detected in both cell lines. However, RXRγ was barely detectable (Fig. 1). Because PPARγ has been reported to control murine Muc1 gene expression in trophoblast ( 26), we next considered that PPARs also played a role in modulating human MUC1 gene expression in human uterine epithelial cell lines. Two independently derived cell lines were routinely used for these studies to avoid artifacts that may be peculiar to any one cell line.
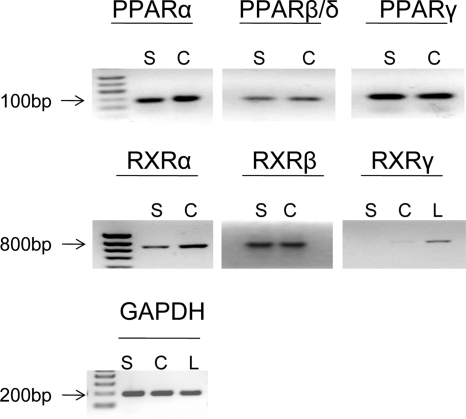
Figure 1.
PPAR and RXR expression by HEC-1A and HES cells. Total RNA was isolated from human liver (L), HES (S), and HEC-1A (C) cells, and amplicons for PPARα, PPARβ, PPARγ, RXRα, RXRβ, and RXRγ were generated by RT-PCR as described in Materials and Methods. A molecular weight ladder is indicated to the left of the panels. Note that all three PPAR transcripts, as well as those for RXRα and RXRβ, are expressed in both cell lines. However, RXRγ transcript is barely detectable in the cell lines. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
A PPARα agonist, fenofibrate, and a PPARγ agonist, rosiglitazone, antagonize progesterone-stimulated MUC1 promoter activity and protein expression
To study PPAR agonists’ effect on the human MUC1 promoter, HEC1A (Fig. 2, A and B) or HES (Fig. 2, C and D) cells were transiently cotransfected with human PRB (hPRB), pRL-TK, and a reporter plasmid containing the proximal 1.4 kb of the human MUC1 promoter upstream of the firefly luciferase gene and then treated with a PPARα agonist, fenofibrate, or a PPARγ agonist, rosiglitazone, for 24 h in the presence or absence of progesterone. Luciferase assays showed that neither agonist alone had a significant effect on MUC1 promoter activity. However, both rosiglitazone (Fig. 2, A and B) and fenofibrate (Fig. 2, C and D) antagonized progesterone-stimulated MUC1 promoter activity in a dose-dependent fashion. Compared with rosiglitazone, fenofibrate required a higher dose to completely antagonize progesterone-stimulated MUC1 promoter activity. Similar experiments with the PPARβ/δ agonist, prostacyclin, showed no significant effect on basal or progesterone-stimulated MUC1 promoter activity (data not shown).
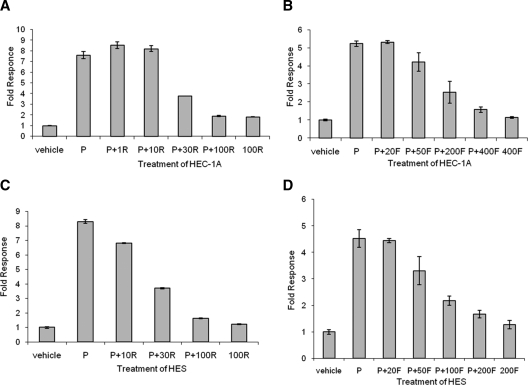
Figure 2.
PPAR agonists antagonize the progesterone-stimulated MUC1 promoter activity in HEC-1A and HES cells. Triplicate cultures of HEC-1A (A and B) or HES (C and D) cells were transiently cotransfected with hPRB, pRL-TK, and a reporter plasmid containing the proximal 1.4 kb of the human MUC-1 promoter upstream of the firefly luciferase gene (pFL-MUC1.4) and then treated with rosiglitazone (R) (A and C) or fenofibrate (F) (B and D), in the presence or absence of progesterone (P) for 24 h as indicated. Reporter activity was expressed as the ratio of firefly luciferase activity to Renilla luciferase activity and normalized to that of the vehicle control. Vehicle, 0.001% (vol/vol) ethanol + 0.1% (vol/vol) DMSO; P, 400 nm progesterone diluted in ethanol; R, rosiglitazone (1, 10, 30, and 100 μm) diluted in DMSO; F, fenofibrate (20, 50, 100, 200, and 400 μm) diluted in DMSO.
To determine PPAR agonists’ effect on MUC1 protein expression, HEC1A cells, stably transfected with PRB ( 13), were treated with fenofibrate (Fig. 3A), rosiglitazone (Fig. 3B), or prostacyclin (data not shown) in the presence or absence of progesterone. Cell lysates were probed with MUC1 and β-actin antibodies by Western blot analysis. By themselves, PPAR agonists had no significant effect on basal MUC1 protein expression. However, rosiglitazone or fenofibrate, but not prostacyclin, antagonized progesterone-stimulated MUC1 protein expression. Similarly, rosiglitazone also antagonized progesterone-stimulated MUC1 protein expression in HES cells stably transfected with PRB (Fig. 3C) and R5020-stimulated MUC1 protein expression in T47D cells, which express endogenous PRA and PRB at roughly equal levels (Fig. 3D).
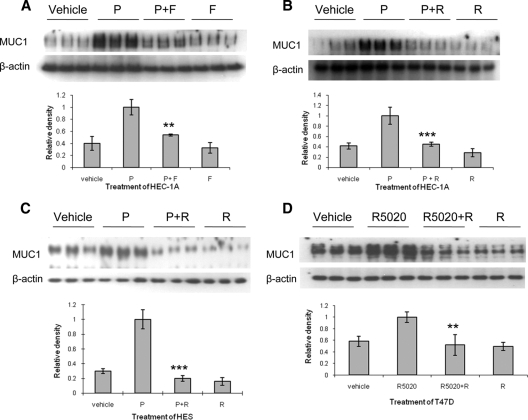
Figure 3.
PPAR agonists antagonize progesterone-stimulated MUC1 protein expression in HEC-1A, HES, and T47D cells. HEC-1A (A and B) and HES (C) cells stably transfected with hPRB, were treated with fenofibrate (F) or rosiglitazone (R), in the presence or absence of progesterone (P) for 48 h. T47D cells, expressing endogenous PRB, were treated with rosiglitazone, with or without 200 nm R5020 for 48 h (D). Cell lysates were probed with MUC1 and β-actin antibodies by Western blot analysis. The results of densitometric scans to determine the ratio of MUC1 to β-actin reactivity are presented below each set of blots. Vehicle, 0.001% (vol/vol) ethanol + 0.1% (vol/vol) DMSO; P, 400 nm progesterone; F, 400 μm fenofibrate; R, 100 μm rosiglitazone. **, P < 0.01, P vs. P+F; R5020 vs. R5020+R; ***, P < 0.001, P vs. P+R.
Rosiglitazone antagonism of progesterone-stimulated MUC1 expression is PPARγ dependent
To study the mechanism of PPARγ agonist’s effect on MUC1 promoter, HEC1A cells were transiently cotransfected with hPRB, pRL-TK, and a reporter plasmid containing the proximal 1.4 kb of the human MUC1 promoter upstream of the firefly luciferase gene and then treated with progesterone, rosiglitazone, and/or GW9662, a PPARγ antagonist ( 29), for 24 h as indicated (Fig. 4A). Rosiglitazone’s action on P-stimulated MUC1 promoter activity was antagonized by GW9662. In addition, transient transfection with a dominant-negative form of PPARγ ( 30) partially reversed rosiglitazone’s actions on the MUC1 promoter (Fig. 4B). Addition of twice the amount of dominant-negative PPARγ did not further restore the progesterone response (data not shown). Western blot analysis revealed that the levels of transfected PPARγ were roughly equivalent to that of endogenous PPARγ (Fig. 4C). This did not appear to be sufficient to fully reverse the effect of the endogenous PPARγ on MUC1 promoter activity. Therefore, we concluded that rosiglitazone’s actions were mediated by PPARγ and not by other side effects associated with this agent.
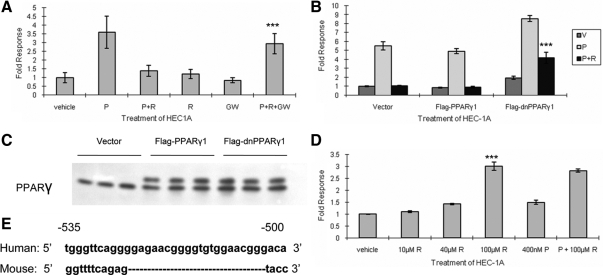
Figure 4.
Rosiglitazone antagonism of MUC1 promoter activity is PPARγ dependent and different between the human and murine promoters. A, The PPARγ antagonist, GW9662, antagonizes rosiglitazone action on MUC1 promoter activity. Triplicate cultures of HEC-1A cells were transiently cotransfected with hPRB, pRL-TK, and pFL-MUC1.4 in the presence of vehicle, progesterone (P), rosiglitazone (R), GW9662 (GW), or combinations of these treatments for 24 h. Note that GW9662 treatment largely reverses rosiglitazone inhibition. B, Dominant-negative PPARγ (dnPPARγ) partially blocks rosiglitazone action on MUC1 promoter activity. Triplicates cultures of HEC-1A cells were transiently cotransfected with hPRB, pRL-TK, pFL-MUC1.4, and pcDNA3 (vector), wild-type PPARγ1 (flag-PPARγ1), or dominant-negative PPARγ1 (Flag-dnPPARγ1), then treated with progesterone in the presence or absence of rosiglitazone as indicated. Reporter activity was expressed as the ratio of firefly luciferase activity to Renilla luciferase activity and normalized to that of vehicle-treated vector. Note that dominant-negative PPARγ1 partially reverses the inhibition of promoter activity observed in vector or PPARγ1 wild type. C, Level of transiently expressed PPARγ1 is similar to that of endogenous PPARγ1. Cell lysates used in B were precipitated with 10% (wt/vol) trichloroacetic acid and then probed with PPARγ antibodies by Western blot analysis as described in Materials and Methods. Note that the transfected forms are larger than the endogenous forms due to addition of the Flag tag and that the levels of the transfected protein are roughly equivalent to that of endogenous PPARγ. D, Rosiglitazone stimulates murine Muc1 promoter activity in HEC-1A cells. Triplicate cultures of HEC-1A cells were transiently cotransfected with hPRB, pRL-TK, and a reporter plasmid containing the 1.85-kb murine Muc1 promoter. Note that in contrast to the human promoter, the murine Muc1 promoter is stimulated in a dose-dependent fashion. E, Comparison of the PPARγ-responsive region of the murine Muc1 promoter with the corresponding region of the human MUC1 promoter. Note that there is a 21-nucleotide insertion in this region of the human promoter ( 26). Vehicle, 0.001% (vol/vol) ethanol + 0.1% (vol/vol) DMSO; P, 400 nm progesterone; R, 100 μm (or as indicated in the panel) rosiglitazone; GW, 100 μm GW9662. ***, P < 0.001, P+R+GW vs. P+R (A); Flag-dnPPARγ1 vs. vector or Flag-PPARγ1 (B); and 100 R vs. vehicle (D).
Rosiglitazone affects the human MUC1 and murine Muc1 promoters in opposite fashion
Rosiglitazone has been shown to stimulate Muc1 expression and promoter activity in murine trophoblast ( 26). However, as shown above, rosiglitazone alone has very little effect on human MUC1 expression. To determine whether this differential effect is due to cell context or structural differences between the mouse and human promoter, HEC-1A cells were transiently cotransfected with hPRB, pRL-TK, and a reporter plasmid containing 1.85 kb of the murine Muc1 promoter and treated with rosiglitazone or progesterone as indicated (Fig. 4D). In agreement with previous studies, rosiglitazone stimulated murine Muc1 promoter activity in HEC-1A cells. However, progesterone had no significant effect on murine Muc1 promoter activity either in the presence or absence of rosiglitazone. Comparison of the PPARγ-responsive region of the murine Muc1 promoter with the corresponding region of the human MUC1 promoter revealed a 21-nucleotide insertion in this region of the human promoter (Fig. 4E) ( 26). Chromatin immunoprecipitation (ChIP) assays were performed with samples obtained from HEC-1A or NMuMG cells, a normal murine mammary gland cell line, treated with rosiglitazone to compare PPARγ binding with the corresponding PPARγ-responsive regions of the human and mouse promoters (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals. org). We detected no PPARγ in association with this region of either the human or mouse promoters. Thus, it remains possible that the differential responsiveness of the human and mouse promoter regions are due to differential recruitment of PPARγ-modulated coregulators due to the structural differences in this region. Induction of murine Muc1 by PPARγ requires both the proximal DR1 (direct repeats of core motif spaced by base pair 1), 65 bp upstream of the transcriptional start site motif and the distal PPARγ-responsive region ( 26).
Rosiglitazone generally antagonizes progesterone activity without changing PR binding to the MUC1 promoter
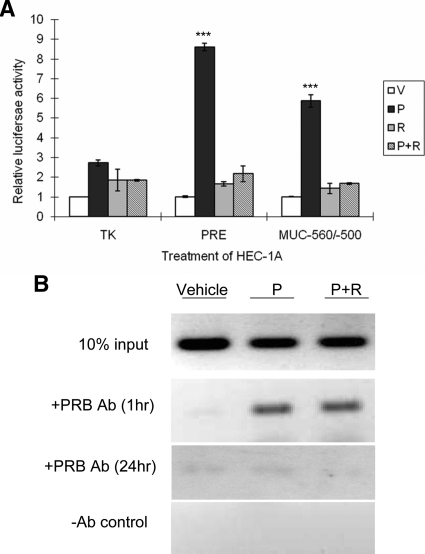
HEC-1A cells were transiently cotransfected with hPRB, pRL-TK, and a reporter plasmid containing thymidine kinase promoter driven by a consensus progesterone response element (PRE) or the P-responsive region of the MUC1 promoter (−560/−500) ( 13) upstream of the firefly luciferase gene and then treated with rosiglitazone in the presence or absence of progesterone. Interestingly, P-responsiveness was almost completely inhibited by rosiglitazone for both the consensus PRE and the P-responsive region of the MUC1 promoter (Fig. 5A). Thus, the action of rosiglitazone on transcription was not specific to the context of the MUC1 promoter. HEC-1A cells stably expressing hPRB were treated with rosiglitazone in the presence or absence of progesterone for 1 and 24 h. ChIP assays were performed to compare PRB binding with the P-responsive region of the MUC1 promoter. PRB was still found on MUC1 promoter after progesterone treatment in the presence of rosiglitazone (Fig. 5B). By 24 h, very little PRB was found associated with the MUC1 promoter in the presence of rosiglitazone by ChIP (Fig. 5B), presumably due to the drastically reduced levels of PRB found at this time (see below). Therefore, we concluded that PPARγ activation did not acutely inhibit P-responsiveness by reducing PRB’s ability to bind DNA target sequences.
Figure 5.
Rosiglitazone generally antagonizes PR function without inhibiting PR binding to the MUC1 promoter region. A, Triplicate cultures of HEC-1A cells were transiently cotransfected with hPRB, pRL-TK, and a reporter plasmid containing the TK promoter driven by a consensus PRE or −560/−500 region of MUC1 promoter upstream of the firefly luciferase gene and then treated with 100 μm rosiglitazone (R), in the presence or absence of progesterone (P) for 24 h as indicated. Reporter activity was expressed as the ratio of firefly luciferase activity to Renilla luciferase activity and normalized to that of the vehicle. B, HEC-1A cells expressing hPRB were treated with progesterone, in the presence or absence of rosiglitazone for 1 or 24 h as indicated. Subsequently, cells were processed for ChIP assays using either the PR antibody or no antibody (−Ab) control as described in Materials and Methods. Vehicle, 0.001% (vol/vol) ethanol + 0.1% (vol/vol) DMSO; P, 400 nm progesterone; R, 100 μm rosiglitazone. ***, P < 0.001, P vs. other treatments in the indicated group.
Rosiglitazone stimulates PR degradation
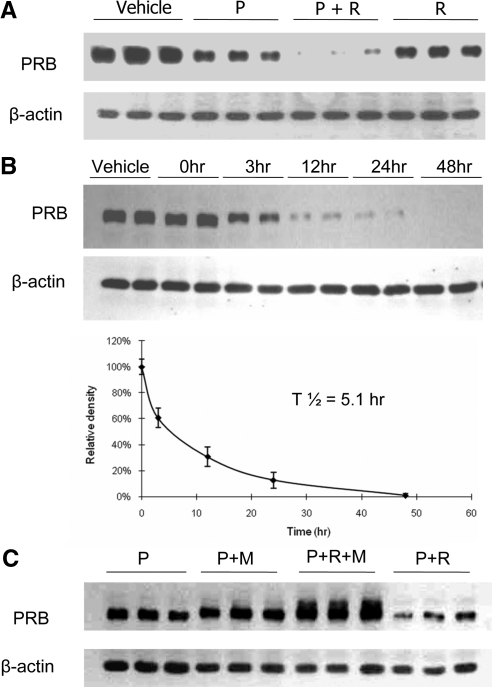
It has been reported that PPARγ activation can stimulate degradation of cyclin D1 ( 31), estrogen receptor α ( 31), and Aβ precursor protein (32). Therefore, we considered that PPARγ might reduce P-responsiveness by enhancing PRB degradation. As expected, 48 h of treatment with progesterone alone reduced PRB levels in HEC-1A cells stably expressing PRB. Treatment with rosiglitazone alone also reduced PRB levels modestly. However, the largest reductions in PRB levels were observed when rosiglitazone was used in the presence of progesterone (Fig. 6A). The synergy of progesterone and rosiglitazone in this regard was further demonstrated by performing time-course studies which showed a 50% reduction of PRB levels by 5.1 h of combined treatment (Fig. 6B). Progesterone addition stimulates ubiquitinylation of PR, a modification that not only leads to PR degradation but also is necessary for PR recycling and function ( 33,34). To study the mechanism of rosiglitazone-induced PR degradation, HEC-1A cells were treated with the proteasomal inhibitor, MG132, progesterone, and rosiglitazone for 24 h (Fig. 6C). PRB degradation triggered by combined progesterone, and rosiglitazone treatment was largely prevented by MG132. In addition, slower migrating forms of PR also were detected in the presence of MG132, consistent with the accumulation of ubiquitinylated forms. Collectively, these observations were consistent with an enhancement of ubiquitin-mediated PRB degradation in response to PPARγ activation.
Figure 6.
Rosiglitazone treatment enhances ubiquitin-mediated PR degradation. A, Triplicate cultures of HEC-1A cells stably transfected with hPRB were treated with rosiglitazone, in the presence or absence of progesterone for 48 h. Cell lysates were probed with PR or β-actin antibodies by Western blot analysis. Note the profound reduction in PRB in presence of both agents. B, Duplicate cultures of HEC-1A cells stably transfected with hPRB were treated with progesterone and rosiglitazone for 48 h. Cell lysates were probed with PR and β-actin antibodies by Western blot analysis. The bottom graph shows the densitometric analysis of the ratio of PRB to β-actin at the indicated times. A half time for degradation was calculated to be 5.1 h. C, Triplicate cultures of HEC-1A cells stably transfected with hPRB were treated with rosiglitazone, progesterone, or MG 132 for 24 h as indicated. Cell lysates were probed with PR and β-actin antibodies by Western blot analysis. Note that inclusion of MG132 not only completely reverses the loss of PRB otherwise seen in the presence of rosiglitazone and progesterone but also results in accumulation of some larger PR forms presumably ubiquitinylated. Vehicle, 0.001% (vol/vol) ethanol + 0.1% (vol/vol) DMSO; P, 400 nm progesterone; R, 100 μm rosiglitazone; M, 100 μm MG132.
Rosiglitazone inhibits progesterone-induced PRB phosphorylation
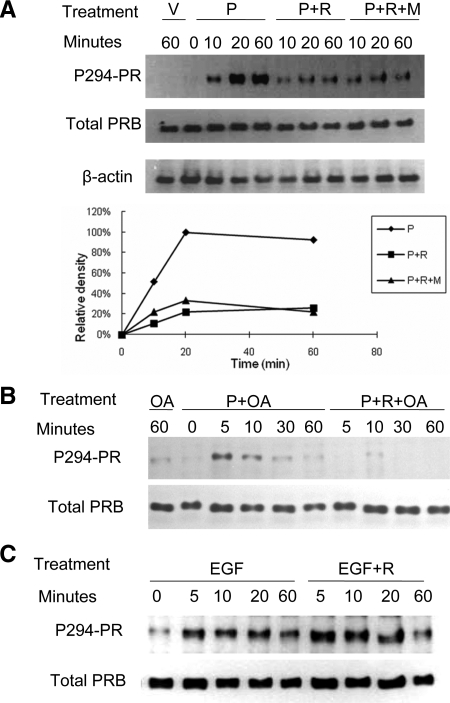
PR phosphorylation at Ser294 has been shown to be crucial for its function ( 35). Therefore, we also considered that PPARγ activation altered PRB phosphorylation. Again, HEC-1A cells stably expressing PRB were treated with progesterone and/or rosiglitazone as indicated and probed with an antibody specific to PRB phosphorylated at Ser294 (Fig. 7A). Progesterone induced PRB phosphorylation within 10 min, which became maximal by 20 min. In contrast, PRB phosphorylation in cells treated with both rosiglitazone and progesterone was barely detectable even after 60 min of combined treatment. This antagonism was not due to enhanced PRB degradation, because total PRB levels were similar among these samples. Furthermore, inclusion of the proteasomal inhibitor, MG132, did not preserve PRB phosphorylation. To test whether the decrease in PRB phosphorylation caused by PPARγ activation is due to an increase of phosphatase activity, the cells were treated with progesterone, rosiglitazone, and the general phosphatase inhibitor, okadaic acid ( 36). As shown in Fig. 7B, okadaic acid treatment led to a faster accumulation of phosphorylated PRB in the presence of progesterone but did not affect the reduction in phosphorylation caused by rosiglitazone treatment. Therefore, it seems likely that PPARγ activation inhibits PRB phosphorylation. Alternatively, rosiglitazone treatment may activate an okadaic acid-insensitive phosphatase. In either case, the net effect would be to reduce PRB phosphorylation and transcriptional activity. Epidermal growth factor (EGF) also has been shown to stimulate PRB phosphorylation at Ser residue 294 ( 37,38). However, rosiglitazone treatment had no effect on this response (Fig. 7C). Therefore, PPARγ inhibition displayed selectivity with regard to the particular kinase pathway involved in PRB phosphorylation. Although EGF stimulation of rapid PRB phosphorylation was not sensitive to rosiglitazone, EGF did not reverse rosiglitazone inhibition of MUC1 accumulation at 48 h, presumably due to the longer term actions of rosiglitazone on PRB stability (data not shown).
Figure 7.
Rosiglitazone treatment inhibits ligand-induced PRB phosphorylation in HEC1A-PRB cells. A, HEC-1A cells stably transfected with hPRB were treated with vehicle, rosiglitazone, progesterone, and MG132 for 0–60 min as indicated. Cell lysates were probed with antibodies specific for Ser294-phosphorylated PR (P294-PR) or total PR by Western blot analysis. The graph shows the densitometric analysis of the ratio of P294-PR to total PR at the indicated times. B, HEC-1A cells stably transfected with hPRB were treated with rosiglitazone, progesterone, and okadaic acid for 0–60 min as indicated. Cell lysates were probed with antibodies specific for Ser294-phosphorylated PR (P294-PR) or total PR by Western blot analysis. Note that rosiglitazone still can antagonize ligand-induced phosphorylation of PRB in the presence of okadaic acid. C, HEC-1A cells stably transfected with hPRB were treated with 30 ng EGF, in the presence or absence of rosiglitazone, for 0–60 min as indicated. Cell lysates were probed with antibodies specific for Ser294-phosphorylated PR (P294-PR) or total PR by Western blot analysis. Note that rosiglitazone did not inihibit EGF-induced phosphorylation of PRB. V, Vehicle, 0.001% (vol/vol) ethanol + 0.1% (vol/vol) DMSO; P, 400 nm progesterone; R, 100 μm rosiglitazone; M, 100 μm MG132; OA, 10 μm okadaic acid.
Discussion
During embryo implantation and through gestation, progesterone levels increase in both mice and humans. However, uterine MUC1 gene expression is regulated in opposite fashion in response to progesterone in these species. Progesterone down-regulates Muc1 in the murine uterus but stimulates MUC1 in human uterine epithelial cells ( 13,39). This differential response appears to be due to the dominance of PRA in mice vs. PRB in humans ( 13). These observations provide a cautionary tale in predicting how human gene expression will respond to ligands based on mouse models without confirmation of the roles of mediators of these responses between the species. Moreover, structural differences between promoters, as well as differences in the transcriptional context of human and mouse uterine epithelial cells, may contribute to differential responsiveness.
In the current studies, we report that human MUC1 expression in response to progesterone is strongly inhibited by rosiglitazone in a PPARγ-dependent fashion. This observation is in apparent contradiction to previous studies of the mouse Muc1 expression in response to PPARγ activation ( 26). The proximal promoters of murine and human Muc1 genes exhibit 74% homology, and various hormone response elements are not conserved ( 40). Microarray screening revealed that Muc1 expression is markedly reduced in PPARγ-null murine placenta ( 26). These authors determined that liganded PPARγ stimulates murine Muc1 expression through a small PPAR-binding site in the proximal promoter and an essential cooperation with a composite upstream enhancer element at −535/−480 ( 26). In agreement with these observations, we found that the PPARγ ligand, rosiglitazone, stimulates the murine Muc1 promoter in a human uterine epithelial cell line. Thus, the differential responsiveness does not appear to be due to differences in the cellular complement of transcription factors. Rather, the differences must be due to differences between the structures of human and mouse promoters. We mapped the PPARγ-inhibited region of the human promoter to the same minimal region identified as the P-responsive region and corresponding to the essential upstream enhancer element described in the mouse promoter. Both the P-responsive motif of the human promoter and the PPARγ-responsive motif of the mouse promoter fall within the same complex region containing multiple, overlapping regulatory sites ( 41). The human MUC1 promoter contains a 21-nucleotide insertion in the motif corresponding to the PPARγ-responsive enhancer sequence in the mouse promoter. We suggest that this difference is critical for PPARγ activation and accounts for the lack of stimulation by liganded PPARγ in humans. ChIP assays reveal that PPARγ does not bind directly to this promoter region in either the human or mouse promoters. Regulation by PPARγ requires the proximal DR1, as well as this distal element in the mouse promoter ( 26). The PPARγ-responsive region corresponding to the human MUC1 promoter encompasses multiple response elements and transcription factor binding sites, including those for PR, nuclear factor-κB, and STATs among others ( 12,13,41). Thus, it is possible that PPARγ modulates transcription not by binding directly to this region but rather by modulating the activities of other factors that bind directly to the distal motif as is the case for PRB. The 21-bp insertion in humans may impact the association of such factors in this region. The identity of these factors remains to be determined.
PPARγ agonists induce ubiquitin-mediated degradation of cyclin D1 and estrogen receptor α ( 31). Furthermore, overexpression of PPARγ in HEKAPP+ cells induces ubiquitin-mediated degradation of Aβ precursor protein ( 32). Our results demonstrate that the PPARγ agonist, rosiglitazone, stimulates ubiquitin-mediated degradation of PRB in HEC-1A cells. PRB is a substrate for the ubiquitin/proteasome pathway. Although ubiquitinylation increases the degradation of PRB, it also is necessary to recycle PRB onto the promoter ( 42). When proteasomal degradation is inhibited, PRB fails to recruit RNA polymerase II and loses transactivation ability ( 34). PRB ligands also induce PRB phosphorylation, stimulate PRB translocation to the promoter, recruit cofactors to initiate transcription, and then stimulate ubiquitinylation to remove PRB from the promoter, so that new liganded PRB can bind to the promoter and initiate the next round of transcription. Because we used cells stably transfected with PRB driven by the SV40 promoter, these experiments avoided the potential complication introduced by the normal down-regulation of PR expression caused by progesterone-dependent repression of the PR gene expression ( 43). As shown by our data, rosiglitazone activates PPARγ, antagonizes PRB phosphorylation, and accelerates the PRB removal through proteasomal degradation (Fig. 8). Based on insensitivity to the general phosphatase inhibitor, okadaic acid, rosiglitazone does not appear to reduce PRB phosphorylation by activating a phosphotase but is more likely to act via inhibition of a kinase that phosphorylates PRB at Ser294 in response to progesterone binding to PRB ( 44). The identity of this kinase is presently unknown, and so we were not able to explore this process further. Phosphorylation may promote cofactor recruitment to PRB. However, when we compared samples treated with progesterone alone to the samples treated with both progesterone and rosiglitazone by ChIP assay, we found no significant difference in the recruitment of steroid receptor coactivator-1, steroid receptor coactivator-3, nuclear receptor corepressor, or mediator complex subunit 1 at 1 h of treatment looking at the same −618/−500 region used in our other ChIP assays (data not shown). Further examination of other cofactors might elucidate the crucial cofactors recruited to the MUC1 promoter by phosphorylated PRB.
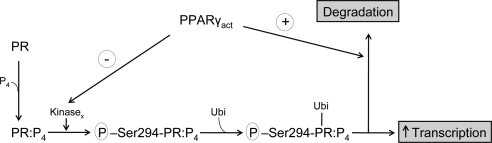
Figure 8.
Model of PPARγ action on PR. Progesterone (P4) binding to PR results in a PR form capable of being phosphorylated on Ser294 and activated by an, as yet, unidentified kinase (Kinasex). The phosphorylated PR:P4 complex is not only transcriptionally active but also a substrate for ubiquitination (Ubi), which, in turn, both makes PR more transcriptionally active and enhances its degradation. Activated PPARγ (PPARγact) inhibits this pathway at two levels: 1) inhibition of PR phosphorylation, and 2) enhancement of PR ubiquitination and degradation.
Mutation of PRB at Ser294 to Ala destroys ligand-induced transcriptional activity without changing ligand-induced PRE binding. Conversely, mutation of PRB at Ser294 to Asp, which mimics phosphorylation at this site, heightens ligand-induced transcription ( 35). Therefore, PRB phosphorylation at Ser294 is critical for its transactivation ability, but not necessary for promoter binding. Consistent with this, we found that PPARγ activation inhibited acute PRB phosphorylation but did not reduce PRB binding to the MUC1 promoter. Moreover, EGF also induces PRB phosphorylation through the MAPK pathway ( 37,38), which connects this steroid hormone pathway with kinase signaling pathways ( 45). MAPK kinase 1/MAPK kinase 2 inhibitors inhibit EGF-induced PRB phosphorylation and nuclear translocation but have no effect on ligand-induced PRB phosphorylation and nuclear translocation ( 38). Rosiglitazone failed to antagonize EGF-induced phosphorylation of PRB. Therefore, PPARγ activation selectively antagonizes PRB phosphorylation through the PRB ligand-activated pathway.
Recently, PPARγ has been found to mediate cross talk of signaling pathways and regulate whole-body homeostasis ( 22). It is an important therapeutic target for many diseases, including diabetes, cancer, and atherosclerosis. PPARγ is essential for placenta differentiation ( 46). Progesterone induces 12/15-lipoxygenases that produce lipid mediators, including 12-hydroxyeicosatetraenoic acid, 15-hydroxyeicosatetraenoic acid, and 13-hydroxyoctadecadienoic acids, that not only activate PPARγ but also control reproductive tract function during embryo implantation ( 27). Our study provides novel insight as to how progesterone action can be attenuated by PPARγ in humans. Furthermore, the specific ability of PPARγ to attenuate MUC1 expression may have useful applications in reproductive and cancer therapies.
Materials and Methods
Antibodies and plasmids
MUC1 antibody, 214D4, was kindly provided by John Hilkens (The Netherlands Cancer Institute, Amsterdam, The Netherlands). β-Actin antibody was purchased from Abcam (Cambridge, MA; catalog no. ab8226-100). Total PR antibody for Western blot analysis was purchased from Neomarker (Fremont, CA; catalog no. MS298-p). Antibody specific for the PR phosphorylated at Ser294 was purchased from Abcam (catalog no. ab9896-25). PPARγ antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA; catalog no. SC-7196). PR antibody for ChIP assays was purchased from Santa Cruz Biotechnology, Inc. (catalog no. SC-539X). The pGL3-SV40 and pRL-TK plasmids were purchased from Promega (Madison, WI). The 1.4MUC promoter construct was the generous gift of Sandra Gendler (Mayo Clinic, Scottsdale, AZ). The mouse Muc1 promoter used in these studies contain 1.85 kb of the 5′ flanking sequence as described previously ( 47) inserted before a luciferase reporter. The human PRB expression plasmid was the generous gift of Pierre Chambon ( 48). The PRE reporter construct was made previously by cloning a single PRE fragment ( 49) into the pGL3-TK vector. Wild-type and dominant mutant (L468A/E471A) PPARγ1 ( 30) were generous gifts of V. K. K. Chatterjee (University of Cambridge, Cambridge, UK).
Cell culture and treatment
HEC-1A cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in phenol red-free DMEM/F12 medium (Invitrogen, Carlsbad, CA), supplemented with 10% (vol/vol) charcoal-stripped fetal bovine serum (FBS) (HyClone, Logan, UT). The human uterine epithelial cell line, HES, was kindly provided by Doug Kniss (Ohio State University, Columbus, OH). HES cells were derived from normal human endometrium and have many characteristics associated with normal uterine epithelia, including maintenance of high levels of MUC1 and support of blastocyst development in vitro ( 50). HES cells were maintained in DMEM (Invitrogen), supplemented with 10% (vol/vol) charcoal-stripped FBS (HyClone), and 100 μm sodium pyruvate (Sigma, St. Louis, MO). T47D cells were purchased from American Type Culture Collection and maintained in RPMI 1640 (Invitrogen), supplemented with 10% (vol/vol) heat-inactivated FBS. Before treatment, T47D cells were grown in RPMI 1640, supplemented with 10% (vol/vol) charcoal-stripped FBS and 1 nm estrogen for 2–3 d in 24-well plates. Normal murine mammary gland epithelial cells (NMuMG; American Type Tissue Collection, Manassas, VA) were maintained in DMEM/F12 (Invitrogen) supplemented with 10% (vol/vol) heat-inactivated FBS, 10 μg/ml bovine insulin (Sigma), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) in a humidified atmosphere with 5% (vol/vol) CO2 at 37 C.
For treatments, cells were plated in 24-well plates (for Western blot analysis) or six-well plates (for luciferase assay) and maintained as described until cells reach 80% confluence. Cells were serum-starved for 24 h before treatment and then treated with rosiglitazone (Cayman Chemical, Ann Arbor, MI), fenofibrate (Cayman Chemical), progesterone (Sigma), R5020 (PerkinElmer, Waltham, MA), GW9662 (Sigma), okadaic acid (Sigma), or MG132 (Sigma) as indicated.
RT-PCR
Total RNA from HEC-1A and HES cells was isolated using RNeasyR Mini kit purchased from QIAGEN (Valencia, CA). Contaminating genomic DNA was removed with a DNA-free kit (Ambion, Austin, TX). The synthesis of cDNA was performed with an Advantage RT-for-PCR kit purchased from BD Biosciences (San Jose, CA) according to the manufacturer’s instructions. Amplication of the partial cDNA encoding hPPARα, hPPARβ, hPPARγ, RXRα, RXRβ, and RXRγ was performed using HotStarTaq DNA polymerase kit purchased from QIAGEN according to the manufacturer’s instructions, with specific oligonucleotide primers as follows: hPPARα sense, 5′-ACTCTGCCCCCTCTCGCCACTC-3′ and antisense, 5′-GCCAAAGCTTCCAGAACTATCCTC-3′; hPPARβ sense, 5′-GAGCAGCCACAGGAGGAAGCC-3′ and antisense, 5′-GCTGTGGTCCCCCAT-3′; hPPARγ sense, 5′-AGAGATGCCATTCTGGCCCAC-3′ and antisense, 5′-GTGGAGTAGAAATGCTGGAGA-3′; hRXRα, sense, 5′-CTCCTCAAGCAAGCACTATG-3′, and antisense, 5′-AGAGCTTAGCGAACCTTCCC-3′; hRXRβ, sense, 5′-TCAGGCAAACACTACGGGGT-3′, and antisense, 5′-GCATACACTTTCTCCCGCAG-3′; and hRXRγ, sense, 5′-CTCAGGAAAGCACTACGGGG-3′ and antisense, 5′-CCGGATACTTCTGCTTGGTG-3′ ( 51,52).
Western blot analysis
After treatment, cells were lysed with sample extraction buffer [0.05 m Tris (pH 7), 8 m urea, 1% (wt/vol) sodium dodecyl sulfate (SDS), 0.01% (vol/vol) phenylmethylsulfonyl-fluoride, and 1% (vol/vol) β-mercaptoethanol], then diluted 1:1 in Laemmli sample buffer ( 53). Proteins were separated by SDS-PAGE using a 3.5% (wt/vol) Laemmli stacking gel ( 53) and a 10% (wt/vol) Porzio and Pearson resolving gel ( 54). Proteins then were transferred to a nitrocellulose membrane at 4 C. The membrane was washed with PBS with Tween 20 (PBST) [0.1% (vol/vol) Tween 20 in PBS] and probed with the MUC1 primary antibody 214D4 (1:1000 diluted), β-actin antibody (1:10,000), PPARγ antibody (1:10,000), total PR antibody (1:10,000), or antibody specific for the PR phosphorylated at Ser294 (1:1000) in incubation buffer [3% (wt/vol) BSA in PBST] overnight at 4 C. Subsequently, blots were washed with PBST and incubated for 1 h at room temperature with a secondary antibody, peroxidase-conjugated donkey antimouse IgG or peroxidase-conjugated goat antirabbit IgG (1:100,000 diluted in incubation buffer; Jackson ImmunoResearch, West Grove, PA). SuperSignal West Dura Extended Duration Substrate (Pierce, Rockford, IL) was used for detection per the manufacturer’s instructions. Blots were exposed to x-ray film, and signal intensities were quantitated using the Alpha Imager 1D-Multi Function (Alpha Innotech, San Leandro, CA).
Transient transfection and luciferase assays
HEC-1A or HES cells were plated in six-well plates and maintained as described until cells reached 80% confluence. Cells then were serum-starved for 24 h before transient transfection. Transient transfections were performed using Lipofectamine 2000 (Invitrogen, Gaithersburg, MD) according to the manufacturer’s instructions. Expression and reporter plasmid were added at 0.75 μg per well, and 0.025 μg of pRL-TK plasmid was used per well. After transfection, cells were allowed to recover in fresh medium containing 10% (vol/vol) charcoal stripped FBS for 12 h. Treatments were added as described in serum-free medium for 24 h. The Dual-Luciferase Assay kit (Promega) was used to lyse the cells and perform the luciferase assays according to the manufacturer’s instructions, using a Dynex MLX Microplate Luminometer (Dynex Technologies, Chantilly, VA). Reporter activity was expressed as the ratio of firefly luciferase activity to Renilla luciferase activity.
Stable transfection
HEC-1A cells were stably transfected with hPRB as described previously ( 13). HES cells were stably transfected with hPRB and pPUR plasmids with Lipofectamine 2000 as per the manufacturer’s instructions. Selection was done in DMEM containing 5% (vol/vol) FBS and 175 ng/ml of puromycin. Screening and characterization of clones was performed as described previously ( 13).
ChIP assay
Formalin-cross-linked chromatin was extracted as described ( 55) and stored at −80 C. Chromatin was sonicated to an average length of 200–600 bp on ice. The samples were cleared by centrifugation at 14,000 rpm for 10 min at 4 C, diluted 1:1 with dilution buffer [0.01% (wt/vol) SDS, 1.1% (vol/vol) Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-Cl (pH 8.1), and 167 mm NaCl] plus PICS III (Calbiochem, EMD Bioscience, La Jolla, CA). The chromatin was precleared by addition of preblocked Protein A/G beads (Santa Cruz Biotechnology, Inc.). Chromatin was incubated with 2 μg anti-PR antibody C-20 (Santa Cruz Biotechnology, Inc.) at 4 C overnight. Blocked Protein A/G beads were added and the samples incubated 4 h at 4 C. The beads were washed twice with dialysis buffer [2 mm EDTA, 50 mm Tris (pH 8.0), and 0.2% (wt/vol) Sarkosyl] containing PICS III and six times with wash buffer [100 mm Tris-Cl (pH 9.0), 500 mm LiCl, 1% (vol/vol) Nonidet P-40, and 1% (wt/vol) deoxycholic acid] plus PICS III, and eluted in 50 mm NaHCO3 and 1% (wt/vol) SDS. RNase A (10 μg) and NaCl (final concentration of 0.3 m) were added, and the samples and inputs (1/10 total input chromatin) were incubated at 65 C overnight to reverse cross-linking. Samples were ethanol precipitated and resuspended in proteinase K buffer [10 mm Tris-Cl (pH 7.6), 5 mm EDTA, and 0.24% (wt/vol) SDS] plus 10 μg proteinase K (Promega) and incubated at 45 C overnight. DNA was purified with the QIAquick PCR purification kit (QIAGEN). PCR with primers specific to the −618/−500 (forward, 5′ CTTTCTCCAAGGAGGGAACC; reverse, 5′GGAATAGCCCCACCCTTCTA, 118-bp product) region of the MUC1 promoter was carried out under the following conditions: 94 C for 2 min; 35 cycles of: 94 C for 1 min, 59 C for 1 min, and 72 C for 1 min; final extension at 72 C for 10 min. NMuMG/HEC1-A cells were grown in 150-cm2 flasks until they attained 80% confluency. Cells then were treated with 0.1% (vol/vol) dimethylsulfoxide (DMSO) or 100 μm rosiglitazone (in DMSO) for 1 h. Formalin-cross-linked chromatin was extracted using ChIP-IT Express kit (Active Motif, Carlsbad, CA) as per the manufacturer’s instructions. ChIP reactions were carried out with affinity purified polyclonal antibody to PPARγ (Santa Cruz Biotechnology, Inc.) or appropriate control IgG overnight at 4 C PCR with primers specific to the −590/−443 (forward, 5′-AGAAAGCCAAAGAGAGACCCA; reverse, 5′-AGGTCGCTAGTTTTAGTCTCCCT, 147-bp product) region of the mouse Muc1 promoter was carried out under the following conditions at 94 C for 2 min; 35 cycles of: 94 C for 30 sec, 53 C for 30 sec, and 72 C for 30 sec; final extension at 72 C for 5 min. PCR products were resolved on a 2.0% (wt/vol) agarose gel containing ethidium bromide and visualized under UV light.
Data analysis
Data are shown as the means ± sd and are representative of at least two independent experiments. All data were analyzed by ANOVA followed by the Tukey-Kramer multiple comparisons test using the GraphPad InStat version 3.05 software (GraphPad Software, Inc., San Diego, CA).
Supplementary Material
Acknowledgments
We thank Dr. V. K. K. Chatterjee for providing wild-type and dominant-negative PPARγ1 plasmids and Dr. John Hilkens for 214D4 antibody; the secretarial and graphics assistance of Ms. Sharron Kingston; and JoAnne Julian and Dr. Mary C. Farach-Carson for many helpful discussions and suggestions.
Footnotes
Present address for P.W.: Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104.
This work was supported by National Institutes of Health Grant HD 29963 (to D.D.C).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 19, 2010
Abbreviations: ChIP, Chromatin immunoprecipitation; DMSO, dimethylsulfoxide; EGF, epidermal growth factor; FBS, fetal bovine serum; hPRB, human PRB; MUC1, mucin 1; PBST, PBS with Tween 20; PPAR, peroxisome proliferator-activated receptor; PR, progesterone receptor; PRE, progesterone response element; P-responsiveness, progesterone responsiveness; RXR, retinoid X receptor; SDS, sodium dodecyl sulfate; Ser, serine; STAT, signal transducer and activator of transcription.
References
- Lesuffleur T, Zweibaum A, Real FX 1994 Mucins in normal and neoplastic human gastrointestinal tissues. Cr Rev Oncol-Hem 17:153–180 [DOI] [PubMed] [Google Scholar]
- Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D 1990 Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem 265:15286–15293 [PubMed] [Google Scholar]
- Gendler SJ, Spicer AP 1995 Epithelial mucin genes. Ann Rev Physiol 57:607–634 [DOI] [PubMed] [Google Scholar]
- Gendler SJ 2001 MUC1, the renaissance molecule. J Mammary Gland Biol 6:339–353 [DOI] [PubMed] [Google Scholar]
- DeSouza MM, Surveyor GA, Price RE, Julian J, Kardon R, Zhou X, Gendler S, Hilkens J, Carson DD 1999 MUC1/episialin: a critical barrier in the female reproductive tract. J Reprod Immunol 45:127–158 [DOI] [PubMed] [Google Scholar]
- Brayman M, Thathiah A, Carson DD 2004 MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol 2:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal B, Krantz MJ, Reddish MA, Longenecker BM 1998 Cancer-associated MUC1 mucin inhibits human T-cell proliferation, which is reversible by IL-2. Nat Med 4:43–49 [DOI] [PubMed] [Google Scholar]
- Chang JF, Zhao HL, Phillips J, Greenburg G 2000 The epithelial mucin, MUC1, is expressed on resting T lymphocytes and can function as a negative regulator of T cell activation. Cell Immunol 201:83–88 [DOI] [PubMed] [Google Scholar]
- Ligtenberg MJ, Buijs F, Vos HL, Hilkens J 1992 Suppression of cellular aggregation by high levels of episialin. Cancer Res 52:2318–2324 [PubMed] [Google Scholar]
- Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J 1995 Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 129:255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Kharbanda S, Kufe D 2007 Mucin 1 oncoprotein blocks hypoxia-inducible factor 1α activation in a survival response to hypoxia. J Biol Chem 282:257–266 [DOI] [PubMed] [Google Scholar]
- Lagow EL, Carson DD 2002 Synergistic stimulation of MUC1 expression in normal breast epithelia and breast cancer cells by interferon-γ and tumor necrosis factor-α. J Cell Biochem 86:759–772 [DOI] [PubMed] [Google Scholar]
- Brayman MJ, Julian J, Mulac-Jericevic B, Conneely OM, Edwards DP, Carson DD 2006 Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol Endocrinol 20:2278–2291 [DOI] [PubMed] [Google Scholar]
- Kovarik A, Peat N, Wilson D, Gendler SJ, Taylor-Papadimitriou J 1993 Analysis of the tissue-specific promoter of the MUC1 gene. J Biol Chem 268:9917–9926 [PubMed] [Google Scholar]
- Hey NA, Graham RA, Seif MW, Aplin JD 1994 The polymorphic epithelial mucin MUC1 in human endometrium is regulated with maximal expression in the implantation phase. J Clin Endocrinol Metab 78:337–342 [DOI] [PubMed] [Google Scholar]
- Meseguer M, Aplin JD, Caballero-Campo P, O'Connor JE, Martín JC, Remohí J, Pellicer A, Simón C 2001 Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod 64:590–601 [DOI] [PubMed] [Google Scholar]
- Thathiah A, Blobel CP, Carson DD 2003 Tumor necrosis factor-α converting enzyme/ADAM 17 mediates MUC1 shedding. J Biol Chem 278:3386–3394 [DOI] [PubMed] [Google Scholar]
- Thathiah A, Carson DD 2004 MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem J 382:363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoete V, Grosdidier A, Michielin O 2007 Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta 1771:915–925 [DOI] [PubMed] [Google Scholar]
- Palmer CN, Hsu MH, Griffin HJ, Johnson EF 1995 Novel sequence determinants in peroxisome proliferator signaling. J Biol Chem 270:16114–16121 [DOI] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Tudor C, Engelborghs Y, Wahli W, Desvergne B 2005 Fluorescence imaging reveals the nuclear behavior of peroxisome proliferator-activated receptor/retinoid X receptor heterodimers in the absence and presence of ligand. J Biol Chem 280:17880–17890 [DOI] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W 2006 From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45:120–159 [DOI] [PubMed] [Google Scholar]
- Tan NS, Michalik L, Desvergne B, Wahli W 2005 Multiple expression control mechanisms of peroxisome proliferator-activated receptors and their target genes. J Steroid Biochem 93:99–105 [DOI] [PubMed] [Google Scholar]
- Escher P, Wahli W 2000 Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res 448:121–138 [DOI] [PubMed] [Google Scholar]
- Rangwala SM, Lazar MA 2004 Peroxisome proliferator-activated receptor γ in diabetes and metabolism. Trends Pharmacol Sci 25:331–336 [DOI] [PubMed] [Google Scholar]
- Shalom-Barak T, Nicholas JM, Wang Y, Zhang X, Ong ES, Young TH, Gendler SJ, Evans RM, Barak Y 2004 Peroxisome proliferator-activated receptor γ controls Muc1 transcription in trophoblasts. Mol Cell Biol 24:10661–10669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Cheon YP, Kannan A, Shanker S, Bagchi IC, Bagchi MK 2004 A novel pathway involving progesterone receptor, 12/15-lipoxygenase-derived eicosanoids, and peroxisome proliferator-activated receptor γ regulates implantation in mice. J Biol Chem 279:11570–11581 [DOI] [PubMed] [Google Scholar]
- Sepilian V, Nagamani M 2005 Effects of rosiglitazone in obese women with polycystic ovary syndrome and severe insulin resistance. J Clin Endocrinol Metab 90:60–65 [DOI] [PubMed] [Google Scholar]
- Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK 1999 Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature 400:378–382 [DOI] [PubMed] [Google Scholar]
- Gurnell M, Wentworth JM, Agostini M, Adams M, Collingwood TN, Provenzano C, Browne PO, Rajanayagam O, Burris TP, Schwabe JW, Lazar MA, Chatterjee VK 2000 A dominant-negative peroxisome proliferator-activated receptor γ (PPARγ) mutant is a constitutive repressor and inhibits PPARγ-mediated adipogenesis. J Biol Chem 275:5754–5759 [DOI] [PubMed] [Google Scholar]
- Qin C, Burghardt R, Smith R, Wormke M, Stewart J, Safe S 2003 Peroxisome proliferator-activated receptor γ agonists induce proteasome-dependent degradation of cyclin D1 and estrogen receptor α in MCF-7 breast cancer cells. Cancer Res 63:958–964 [PubMed] [Google Scholar]
- d'Abramo C, Massone S, Zingg JM, Pizzuti A, Marambaud P, Dalla Piccola B, Azzi A, Marinari UM, Pronzato MA, Ricciarelli R 2005 Role of peroxisome proliferator-activated receptor γ in amyloid precursor protein processing and amyloid β-mediated cell death. Biochem J 391:693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick A, Katzenellenbogen BS 1986 Progesterone receptor synthesis and degradation in MCF-7 human breast cancer cells as studied by dense amino acid incorporation. Evidence for a non-hormone binding receptor precursor. J Biol Chem 261:13236–13246 [PubMed] [Google Scholar]
- Dennis AP, Lonard DM, Nawaz Z, O'Malley BW 2005 Inhibition of the 26S proteasome blocks progesterone receptor-dependent transcription through failed recruitment of RNA polymerase II. J Steroid Biochem 94:337–346 [DOI] [PubMed] [Google Scholar]
- Daniel AR, Qiu M, Faivre EJ, Ostrander JH, Skildum A, Lange CA 2007 Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids 72:188–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Holmes CF, Tsukitani Y 1990 Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci 15:98–102 [DOI] [PubMed] [Google Scholar]
- Lange CA, Richer JK, Horwitz KB 1999 Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol 13:829–836 [DOI] [PubMed] [Google Scholar]
- Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA 2003 Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol 17:628–642 [DOI] [PubMed] [Google Scholar]
- Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD 1995 Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 136:3639–3647 [DOI] [PubMed] [Google Scholar]
- Vos HL, de Vries Y, Hilkens J 1991 The mouse episialin (Muc1) gene and its promoter: rapid evolution of the repetitive domain in the protein. Biochem Biophys Res Commun 181:121–130 [DOI] [PubMed] [Google Scholar]
- Carson DD, Dharmaraj N, Wang P 2008 Transcriptional control of the expression of MUC1. Expert Rev Endocrinol Metab 3:463–471 [DOI] [PubMed] [Google Scholar]
- Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F 2003 Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Mol Cell 11:695–707 [DOI] [PubMed] [Google Scholar]
- Levy C, Robel P, Gautray JP, De Brux J, Verma U, Descomps B, Baulieu EE 1980 Estradiol and progesterone receptors in human endometrium: normal and abnormal menstrual cycles and early pregnancy. Am J Obstet Gynecol 136:646–651 [DOI] [PubMed] [Google Scholar]
- Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP 2000 Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol 14:52–65 [DOI] [PubMed] [Google Scholar]
- Shen T, Horwitz KB, Lange CA 2001 Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol 21:6122–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier T, Tsatsaris V, Handschuh K, Evain-Brion D 2007 PPARs and the placenta. Placenta 28:65–76 [DOI] [PubMed] [Google Scholar]
- Zhou X, DeSouza MM, Julian J, Gendler SJ, Carson DD 1998 Estrogen receptor does not directly regulate the murine Muc-1 promoter. Mol Cell Endocrinol 143:65–78 [DOI] [PubMed] [Google Scholar]
- Kastner P, Bocquel MT, Turcotte B, Garnier JM, Horwitz KB, Chambon P, Gronemeyer H 1990 Transient expression of human and chicken progesterone receptors does not support alternative translational initiation from a single mRNA as the mechanism generating two receptor isoforms. J Biol Chem 265:12163–12167 [PubMed] [Google Scholar]
- Klein-Hitpass L, Tsai SY, Greene GL, Clark JH, Tsai MJ, O'Malley BW 1989 Specific binding of estrogen receptor to the estrogen response element. Mol Cell Biol 9:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NN, Kennard EA, Kniss DA, Friedman CI 1994 Novel human endometrial cell line promotes blastocyst development. Fertil Steril 61:760–766 [PubMed] [Google Scholar]
- Fauconnet S, Lascombe I, Chabannes E, Adessi GL, Desvergne B, Wahli W, Bittard H 2002 Differential regulation of vascular endothelial growth factor expression by peroxisome proliferator-activated receptors in bladder cancer cells. J Biol Chem 277:23534–23543 [DOI] [PubMed] [Google Scholar]
- Brand C, Ségard P, Plouvier P, Formstecher P, Danzé PM, Lefebvre P 2002 Selective alteration of gene expression in response to natural and synthetic retinoids. BMC Pharmacol 2:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Porzio MA, Pearson AM 1977 Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta 490:27–34 [DOI] [PubMed] [Google Scholar]
- Boyd KE, Farnham PJ 1999 Coexamination of site-specific transcription factor binding and promoter activity in living cells. Mol Cell Biol 19:8393–8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.