Abstract
The inability of the uterine epithelium to enter a state of receptivity for the embryo to implant is a significant underlying cause of early pregnancy loss. We previously showed that mice null for the progesterone receptor (PGR)-interacting protein Krüppel-like factor (KLF) 9 are subfertile and exhibit reduced uterine progesterone sensitivity. KLF9 expression is high in predecidual stroma, undetectable in decidua, and enhanced in uteri of mice with conditional ablation of bone morphogenetic protein 2 (BMP2). Given the individual importance of KLF9 and BMP2 for implantation success, we hypothesized that the establishment of uterine receptivity involves KLF9 and BMP2 functional cross-regulation. To address this, we used early pregnant wild-type and Klf9 null mice and KLF9 small interfering RNA-transfected human endometrial stromal cells (HESCs) induced to differentiate under standard conditions. Loss of KLF9 in mice and HESCs enhanced BMP2 expression, whereas recombinant BMP2 treatment of HESCs attenuated KLF9 mRNA levels. IGFBP1 and KLF9-related KLF13 expression were positively associated with BMP2 and inversely associated with KLF9. Prolonged, but not short-term, knockdown of KLF9 in HESCs reduced IGFBP1 expression. Mouse uterine Igfbp1 expression was similarly reduced with Klf9 ablation. PGR-A and PGR-B expression were positively associated with KLF9 in predecidual HESCs but not decidualizing HESCs. KLF13 knockdown attenuated BMP2 and PGR-B and abrogated BMP2-mediated inhibition of KLF9 expression. Results support cross-regulation among BMP2, KLF9, and KLF13 to maintain progesterone sensitivity in stromal cells undergoing differentiation and suggest that loss of this regulatory network compromises establishment of uterine receptivity and implantation success.
Uterine stromal BMP2 signaling can be dynamically influenced by the two highly related transcription factors KLF9 and KLF13 to maintain progesterone sensitivity and support endometrial receptivity for embryo implantation.
Infertility and early pregnancy loss affect 10–15% of couples of fertility age (1). A significant underlying cause of these reproductive dysfunctions is developmental asynchrony between the uterus and the implantation-ready embryo, due largely to the inability of the uterine epithelium to enter a state of receptivity for embryo attachment and subsequent implantation (2,3). In the transition from the nonreceptive to receptive state, uterine endometrial cells undergo dynamic changes in cellular organization and differentiation, events largely controlled by the pregnancy hormone progesterone (P) in concert with estradiol (E2), each acting through cognate receptors and coregulators (3,4,5,6). Although the initial stage of implantation takes place in the luminal epithelium, one absolute requirement for successful implantation is the sequential proliferation and subsequent differentiation of endometrial stromal cells into multinucleated, enlarged, and phenotypically distinct decidual cells, a process termed endometrial decidualization (7). Perturbations in the multicomponent signaling pathways that temporally direct and integrate early molecular changes in stromal as well as neighboring epithelial cells are anticipated to compromise events requisite for embryo implantation.
P, working through P receptor (PGR) isoforms A and B, mediates the production of paracrine factors in stromal cells that enhance epithelial growth and differentiation (8). Given that loss of stromal P sensitivity renders the uterine endometrium refractory to implantation (9), mechanisms that predispose the dysfunctional response of endometrial stromal cells to P, resulting in faulty communication between the embryo and the mother, require further study. Whereas loss or altered ratio of PGR isoform expression are established to underlie aberrant ligand-activated PGR signaling (10,11,12,13,14), the network of PGR-regulated genes and attendant regulatory pathways in the uterus remain poorly understood.
Our group has identified the specificity protein 1 (Sp1)-related transcription factor Krüppel-like factor (KLF)-9 (previously designated basic transcription element binding protein-1) (15) as a PGR coactivator in the uterus (16,17). KLF9, although predominantly expressed in uterine stromal cells (18), directs the responsiveness of neighboring luminal epithelial cells to P (19), consistent with its regulation of P-responsive stromal-derived factors mediating epithelial-mesenchymal interactions. Klf9 female null mice are subfertile with reduced numbers of postimplantation embryos, due to defects at the level of the uterus, and exhibit partial P resistance (18,19). These findings raise the question of whether and, if so, how KLF9 regulates the sequential program of events involving PGR in stromal cells during the transition from prereceptive to receptive uterus.
One key molecule that is critical for implantation success in mice is bone morphogenetic protein (BMP)-2, a member of the TGFβ superfamily of growth regulators that are implicated in many aspects of embryonic, postnatal, and adult tissue development (20,21,22,23,24). Targeted deletion of Bmp2 in mice was embryo lethal (25), whereas female mice with conditional ablation of Bmp2 in the uterus were infertile due to failure to decidualize (26). Microarray analyses of Bmp2 null uteri indicated major changes in gene expression, specifically genes involved in Wnt, PGR, and prostaglandin signaling pathways (26). Interestingly, Klf9 expression was up-regulated in Bmp2 null uteri (26).
Given the positive association between KLF9 and PGR on the one hand and BMP2 and PGR on the other hand, coupled with the lack of BMP2 expression in the preattachment uterus when KLF9 expression is highest (18,19,27,28), we hypothesized the existence of a negative cross-regulation between KLF9 and BMP2 to maintain PGR function in stromal cells for successful implantation. Here we report that KLF9 and BMP2 negatively regulate each other to prevent premature BMP2 expression in predecidual stroma and to promote loss of KLF9 expression in decidualizing stroma. We demonstrate BMP2 inhibition of KLF9 expression that is mediated in part by KLF13, the most highly related KLF9 family member (29). Furthermore, we show that stromal PGR-B expression is maintained by the sequential expression of KLF9 and KLF13. Together, our studies define a new model for the integration of PGR signaling pathways and BMP2 action involving KLFs, the deregulation of which may compromise the attainment of uterine receptivity, leading to infertility.
Materials and Methods
Animals
Animal studies were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences. Wild-type (WT) and Klf9 knockout (KO) mice (C57/BL6J) were propagated, genotyped, and mated for timed pregnancies as described previously (18,19). WT and Klf9 KO females (8–12 wk old) were mated with Klf9 KO and WT males, respectively, to preclude the contribution of embryo genotype (all embryos were heterozygous) to any observed differences in uterine phenotype. The morning of the vaginal plug was designated as 0.5 d postcoitum (dpc). Uterine tissues were isolated at the indicated day postcoitum (2.5, 3.5, 4.5; n = 4–5 mice/genotype per days postcoitum). For each mouse, the left uterine horn was homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA) for RNA isolation, whereas the right uterine horn was processed for immunohistochemistry.
Cell culture and treatments
The human endometrial stromal cell line (HESC) was a generous gift from Drs. Graciela Krikun and Charles Lockwood (Yale University, New Haven, CT). HESCs were maintained and propagated in phenol red-free DMEM and Ham’s F12 [1:1 (vol/vol)] supplemented with 10% charcoal-stripped bovine calf serum (CS-BCS) and 1% antibiotic/antimycotic solution in a 37 C incubator with humidified atmosphere of 5% CO2 (30). For experiments involving hormonal treatments, cells were grown to near confluence, and the medium was substituted with phenol red-free DMEM/2% CS-BCS with added 8-bromoadenosine-cAMP (8-Br-cAMP) [0.05 or 0.5 mm, as indicated for each study (Sigma-Aldrich, St. Louis, MO)], progestin [medroxyprogesterone acetate (MPA), 1 μm; (Sigma)] and E2 [10 nm (Sigma) (henceforth designated cAME)]. Recombinant human BMP2 (R&D Systems, Minneapolis, MN) was added at a final concentration of 100 ng/ml. For experiments lasting more than 2 d, the medium with the added treatments was replenished every other day.
RNA interference
Transfections of HESCs with small interfering RNAs (siRNAs) targeting human KLF9 or human KLF13 (siGeNOME SMART pool) and nontargeting (siCONTROL) siRNAs (Dharmacon, Waltham, MA) were performed with Lipofectamine 2000 reagent (Invitrogen) in OPTI-MEM 1 reduced serum-containing medium (Invitrogen) when cells were approximately 60% confluent, as previously described (31). siRNAs were added at a final concentration of 50 nm. After 6 h, cells were washed, and the medium was replaced with phenol red-free DMEM containing 2% CS-BCS. After 24 h, medium was replaced with phenol red-free DMEM/2% CS-BCS supplemented with cAME (cAMP added at 0.05 or 0.5 mm), and cells were further incubated for 48 h. Cells were collected and processed for RNA or protein isolation.
RNA isolation and real-time quantitative PCR (QPCR)
Total RNA was isolated from tissues or cells using TRIzol (Invitrogen) according to the manufacturer’s instructions. RNA (1 μg) was reverse transcribed to cDNA using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). cDNA was diluted 1:5 (vol/vol), and 5 μl were used in a total reaction volume of 20 μl containing SYBR-Green mixture (Bio-Rad) and 0.3 μm of each primer. All primers were designed to span introns using PrimerExpress software (Applied Biosystems, Foster City, CA) and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The sequences of primers for real-time QPCR amplification and amplicon sizes for each primer set are listed in Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Amplification was performed under previously described thermal conditions (32) using an ABI Prism 7000 sequence detection system (Applied Biosystems). Transcript levels were normalized to corresponding levels of cyclophilin A, TATA box binding protein (TBP), or 18S rRNA as indicated for each study and were calibrated to a standard curve generated using pooled cDNA stocks.
Western blot analyses
Total cell lysate and nuclear fractions were prepared following the manufacturer’s instructions (NE-PER; Pierce Biotechnology, Rockford, IL), and protein concentrations were quantified by bicinchoninic acid assay (Bio-Rad) using BSA as standard. Proteins (50–80 μg) were separated on SDS-10% PAGE and transferred to nitrocellulose membranes (Millipore, Billerica, MA). The membranes were incubated with specific antibodies against rabbit antirat KLF9 (generated in-house) (16); goat antihuman KLF13 [RFLAT (A-19), sc-9605; Santa Cruz Biotechnology, Santa Cruz, CA], and mouse antihuman PGR (Pgr-1294; Dako, Carpinteria, CA) at 4 C overnight and further incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature. Immunoreactive proteins were visualized using the enhanced chemiluminescence detection system Amersham ECL Plus (GE Healthcare Life Sciences, Piscataway, NJ). Membranes were stripped and reprobed for α-actinin (Sigma) or lamin A (Sigma) as loading controls for whole-cell and nuclear extracts, respectively. Densitometric values of immunoreactive bands were quantified using the Bio-Rad molecular analyst detection system and Quantity One software (Bio-Rad). All experiments were performed at least twice.
Alkaline phosphatase activity
Treated HESCs were harvested in 0.2% Triton X-100 (Sigma) at room temperature with shaking. Cell lysates (100 μl) were diluted with the same volume of SigmaFAST p-nitrophenyl phosphate (Sigma) reagent, and absorbance (410 nm) was read after incubation at room temperature for 20 min, using the Bio-Rad molecular analyst detection system.
Immunohistochemistry
Paraffin-embedded uteri from pregnant time-mated mice were serially sectioned, dewaxed with xylene and rehydrated through a graded alcohol series as previously described (19). Sections were then treated with 3% hydrogen peroxide to quench endogenous peroxidase activity; microwaved sequentially (105 sec at power 10 and then 10 min at power 1) in Citra Plus (Biogenex, San Ramon, CA) to unmask antigen; and incubated in blocking solution with goat IgG (Vectastain ABC kit, Vector Laboratories Inc., Burlingame, CA) for 1 h. Sections were then incubated overnight at 4 C with goat antihuman KLF13 antibody [RFLAT (A-19)] (1:200) or goat antihuman BMP2 antibody (1:100, sc-6895; Santa Cruz Biotechnology). After incubation with antigoat secondary antibodies (Vectastain ABC kit) for 30 min, sections were stained with 3,3′-diaminobenzidine tetrahydrochloride (Dako) and counterstained with hematoxylin. Control sections were processed similarly with omission of primary antibody. Approximately 1000 stromal, 300 luminal epithelial, and 200 glandular epithelial cells were counted on average from at least three randomly selected fields (×400 magnification) per slide; one to two slides/mouse (n = 3–5 mice/genotype) were evaluated. Results are expressed as percent nuclear-immunopositive cells [(number of nuclear positively staining cells/number of total cells counted) × 100].
Statistical analysis
Data are presented as means ± sem from at least two independent experiments, each performed in triplicate or quadruplicate per treatment group. Statistical analysis by one- or two-way ANOVA and a two-tailed Student’s t test was performed using SigmaStat3.5 software (SPSS, Chicago, IL). P ≤ 0.05 was considered to be significant.
Results
Loss of Klf9 disrupts the temporal pattern of Bmp2 expression in early pregnancy mouse uteri
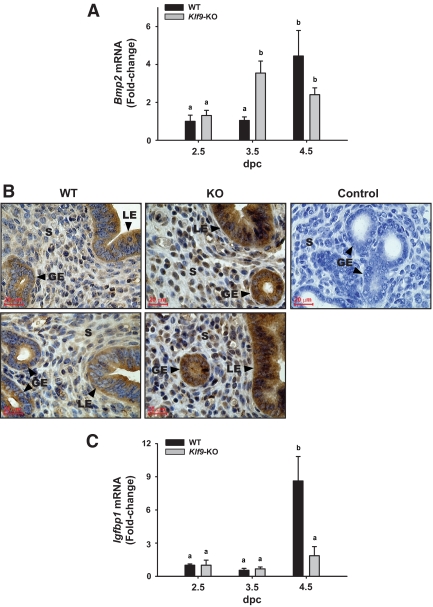
Previous work has shown, using null mutant mice, that BMP2 (26) and KLF9 (18) are functionally important to early pregnancy events in the uterus. Loss of Bmp2 expression leads to total infertility due to failure of mutant uteri to undergo the decidual reaction (26), whereas Klf9 null mutation results in subfertility due to diminished uterine P sensitivity (18). To investigate the functional association of BMP2 and KLF9 in the progression of stromal cell differentiation important for embryo implantation, we evaluated Bmp2 transcript levels in uteri of WT and Klf9 null (KO) mice at dpc 2.5, 3.5, and 4.5 by QPCR. Bmp2 transcript levels were low at dpc 2.5 and 3.5 and significantly increased by 4-fold at dpc 4.5 in WT mice (Fig. 1A), consistent with a previous report (28). Interestingly, loss of Klf9 resulted in a significant increase of Bmp2 transcript levels at dpc 3.5 to the levels found for dpc 4.5 WT uteri (Fig. 1A). Klf9 null mutation had no effect on Bmp2 transcript levels at dpc 2.5, whereas at dpc 4.5, Bmp2 expression was comparable for WT and KO uteri. The premature Bmp2 increase in Klf9 null uteri at dpc 3.5 was confirmed at the level of the BMP2 protein by immunohistochemistry (Fig. 1B). Whereas BMP2 immunopositive cells were barely detectable in all endometrial compartments of WT uteri, loss of Klf9 expression resulted in a substantial increase in stromal localization of BMP2 immunoreactivity, with some localization detected in luminal epithelial and in glandular epithelial cells (Fig. 1B). In stroma as in both epithelial cell types, immunoreactivity was localized to both cytoplasmic and nuclear compartments. To evaluate the functional consequence of the altered pattern of Bmp2 expression with Klf9 null mutation, we compared the temporal expression of the decidualization marker Igfbp1 in WT and KO uteri. Whereas uteri of WT mice showed a dramatic induction in Igfbp1 levels from dpc 2.5 and 3.5 to dpc 4.5, the corresponding rise for KO mice at dpc 4.5 was not observed (Fig. 1C). These findings show a transient negative association between Klf9 and Bmp2 during the initiation of embryo attachment at dpc 3.5 and suggest that disruption of the temporal sequence of Bmp2 expression impairs the normal progression of stromal differentiation.
Figure 1.
Uterine expression of Bmp2 and Igfbp1 in WT and Klf9 null mice during early pregnancy. Transcript levels (means ± sem) of Bmp2 (A) and Igfbp1 (C) were quantified by QPCR, normalized to that of 18S RNA, and renormalized to values of dpc 2.5 endometrium of WT mice. Each bar represents the mean from four to five mice per day postcoitum per genotype; fold change is relative to dpc 2.5 values. Means with different superscripts indicate significant difference at P < 0.05 by two-way ANOVA. B, Representative BMP2 immunostaining of glandular epithelium (GE), luminal epithelium (LE), and stromal (S) compartments of WT and Klf9 KO mice at dpc 3.5 is shown at ×400 magnification. Each panel for WT and KO represents uterine sections from an individual mouse. Control shows lack of immunostaining (WT uteri) in the absence of primary antibody.
KLF9 expression precedes BMP2 expression in human endometrial stromal cells in vitro
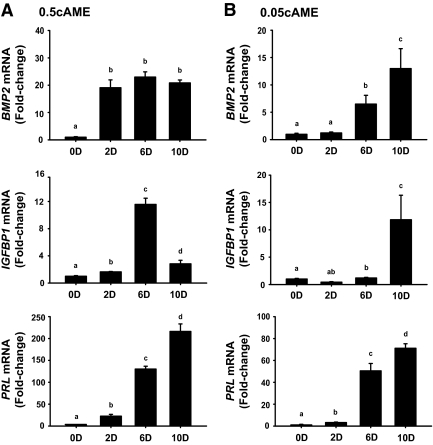
Because human and mouse endometrial stromal cells similarly undergo predecidualization in response to P (3) and KLF9 is expressed predominantly in endometrial stromal cells in mice (18), a human endometrial stromal cell line (HESC) was used to further explore the functional importance of the negative association between KLF9 and BMP2 expression during progression of stromal differentiation. In previous studies, treatment of endometrial stromal cells for 48–72 h with a cocktail of 8-Br-cAMP (0.5 mm), MPA (1 μm), and E2 (10 nm) in vitro resulted in their differentiation and a pattern of gene expression recapitulating that noted in vivo for human and mouse decidualizing cells (33,34). Control (untreated, d 0) HESCs and HESCs treated with 0.5 mm 8-Br-cAMP, MPA, and E2 (henceforth designated 0.5cAME) from d 2 to 10 were evaluated for expression of various genes known to be important in decidualization, using QPCR. Within 2 d of 0.5cAME treatment, the levels of BMP2 transcript increased by 18-fold and those of IGFBP1 and PRL transcripts by 2- and 22-fold, respectively (Fig. 2A). Although IGFBP1 transcript levels dramatically decreased at d 10 from peak levels at d 6, these levels were nevertheless higher by at least 3-fold from those at d 0 (Fig. 2A). Given the rapid and dramatic increase in BMP2 transcript levels with 0.5 mm cAMP, cells were treated with a 10-fold lower dose (0.05 mm) in the presence of E2 and MPA (0.05cAME) to more carefully dissect the inverse association of KLF9 and BMP2. Under these treatment conditions, BMP2 transcript levels did not increase from basal (d 0) levels until treatment d 6, coincident with (PRL) or just before (IGFBP1) markers of progression to decidualization (Fig. 2B); this is consistent with an early role of BMP2 in stromal differentiation (33).
Figure 2.
Temporal pattern of expression of differentiation-associated genes in HESCs treated with 0.5 mm 8-Br-cAMP (A) or 0.05 mm 8-Br-cAMP (B), in the presence of 10 nm E2 and 1 μm MPA from d 0–10. Treated cells were harvested at the indicated time points and analyzed for expression of BMP2, IGFBP1, and PRL transcript levels by QPCR, normalized to control gene TBP, and renormalized to values at d 0. Results (mean ± sem) are expressed as fold changes over OD values. Different superscripts are significantly different at P < 0.05 by one-way ANOVA.
Comparison of the in vivo (Fig. 1, A and C) and in vitro (Fig. 2A and B) patterns of BMP2 (Bmp2) and IGBP1 (Igfbp1) expression indicated that HESCs treated with 0.5cAME for 2 d or 0.05cAME for 6 d mimic stromal cells between dpc 3.5 and 4.5, whereas those treated with 0.05cAME for 2 d mimic stromal cells at dpc 2.5. Thus, we considered HESCs treated for 2 d with 0.05cAME as predecidual stroma, whereas those treated with 0.5cAME for 2 d or 0.05cAME for 6 d as undergoing differentiation.
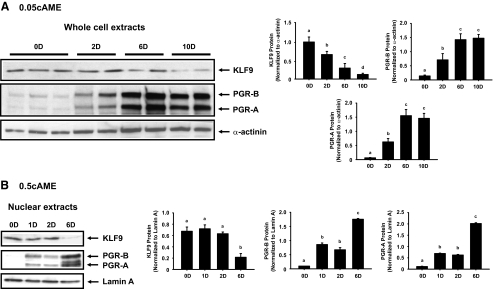
We evaluated KLF9 and PGR protein levels by Western blots in stromal cells treated with either 0.05cAME or 0.5cAME to determine their temporal association with BMP2 expression. At d 0 and d 2 of 0.05cAME treatment, KLF9 protein was readily detectable in whole-cell extracts coincident with increasing PGR-A and PGR-B protein levels (Fig. 3A). Interestingly, when the amounts of KLF9 protein began to decline at d 6 to almost undetectable levels by d 10, PGR isoform protein levels continued to increase (Fig. 3A). To determine whether treatment with a higher dose of cAMP results in similar expression patterns for KLF9 and PGR, nuclear extracts isolated from 0.5cAME-treated HESCs were analyzed. KLF9 and PGR are both transcription factors; hence, their nuclear localization indicates functionality. KLF9 protein in nuclear fractions were comparable at d 0 to d 2 and decreased dramatically to almost undetectable levels by d 6, whereas PGR-A and PGR-B expression progressively increased during this period, reaching highest levels at d 6 (Fig. 3B). These results demonstrate a decline in KLF9 protein levels with increasing BMP2 expression, with the higher dose of cAMP eliciting a more rapid loss in KLF9 coincident with accelerated induction of BMP2, IGFBP1, and PRL expression (Fig. 2, A and B).
Figure 3.
Loss of KLF9 protein expression accompanies progression of stromal cells to decidualization. Whole-cell extracts from HESCs treated with 0.05 mm 8-Br-cAMP + E2 + MPA (A) or nuclear extracts from HESCs treated with 0.5 mm 8-Br-cAMP + E2 + MPA (B) for the indicated time points were analyzed for KLF9, PGR-A, and PGR-B protein levels by Western blots. Loading controls were α-actinin (A) and lamin A (B), respectively. A representative Western blot is shown, with each lane representing an individual sample. Bar graphs represent densitometric scans of immunoreactive bands, normalized to respective loading controls from two independent experiments. Fold change is relative to OD values. Means (±sem) with different superscripts indicate significant difference at P < 0.05 by one-way ANOVA.
KLF9 and BMP2 negatively regulate each other’s expression in human endometrial stromal cells
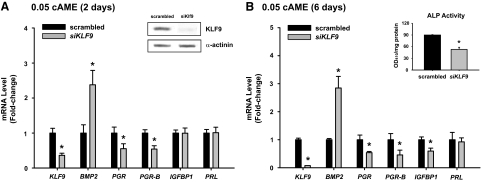
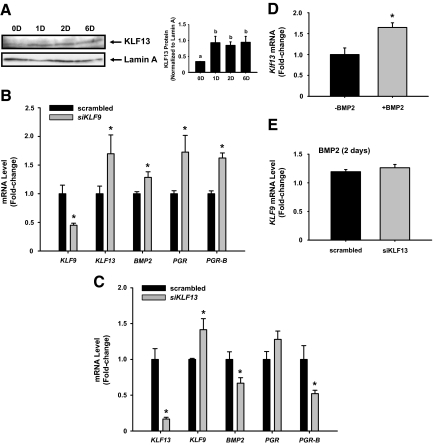
We next examined whether increased BMP2 transcript levels is a direct consequence of loss of KLF9 expression, as suggested for uteri of Klf9 null mice at dpc 3.5. In HESCs treated for 2 d with 0.05cAME (predecidual condition), transfection of siRNAs (50 nm) targeting KLF9 resulted in a greater than 90% reduction in KLF9 protein (Fig. 4A, inset) and a 2.5-fold up-regulation of BMP2 transcript levels (Fig. 4A). KLF9 knockdown decreased total PGR and PGR-B expression, consistent with their positive regulation by KLF9 (19); this was observed in the absence of effects on IGFBP1 and PRL expression (Fig. 4A). In cells similarly treated with KLF9 siRNAs and analyzed after exposure to 0.05cAME for 6 d (decidualizing condition), attenuated KLF9 expression likewise resulted in increased BMP2 (by 3-fold) and decreased total PGR and PGR-B transcript levels (Fig. 4B). IGFBP1 transcript levels also decreased (Fig. 4B), and this was accompanied by a significant reduction in the activity of alkaline phosphatase, a functional measure of decidualization (Fig. 4B, inset). These results recapitulate that found with Klf9 null mice in vivo (Fig. 1). Prolactin (PRL) levels, however, remained unaffected by KLF9 knockdown (Fig. 4B).
Figure 4.
Expression levels of KLF9 and BMP2 are inversely associated in predecidual and decidualizing stromal cells. HESCs transfected with scrambled (negative control) siRNAs or siRNAs for KLF9 (50 nm each) were treated with 0.05 mm 8-Br-cAMP with MPA + E2 for 2 (A) and 6 (B) d and evaluated for transcript levels of the indicated genes. Insets: Western blot of nuclear extracts from treated cells for KLF9 and α-actinin (loading control) proteins, showing specificity of KLF9 knockdown with KLF9 siRNAs (A), and alkaline phosphatase (ALP) activity of cell lysates prepared from HESCs treated with 0.05cAME for 6 d (B). Gene expression was analyzed by QPCR, normalized to control gene TBP, and renormalized to scrambled group. Transcript levels (mean ± sem) are expressed as fold change. Results are representative of two to three independent experiments, with each experiment performed in quadruplicate. *, Significant difference between control siRNA- and KLF9 siRNA-treated groups at P < 0.05, using Student’s t test.
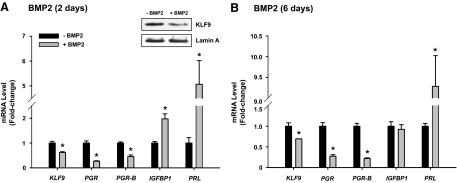
To determine whether BMP2 also negatively regulates KLF9 expression as suggested in vivo (26) and in vitro (compare Figs. 2 and 3), we quantified KLF9 mRNA levels in HESCs treated for 2 and 6 d with recombinant human BMP2 (100 ng/ml), in the absence of cAME. KLF9 transcript levels were down-regulated (by 30%) in cells treated with BMP2 for 2 d, together with those for total PGR and PGR-B (Fig. 5A). A similar reduction in KLF9, PGR, and PGR-B transcript levels was observed in cells treated with BMP2 for 6 d (Fig. 5B). Consistent with BMP2 as an early differentiation marker for stromal cells (33), BMP2-treated cells showed increased IGFBP1 and PRL mRNA expression at 2 d (Fig. 5A). With the longer treatment, however, the BMP2 effect on IGFBP1 expression, unlike that for PRL, was lost (Fig. 5B).
Figure 5.
BMP2 inhibits KLF9 expression in stromal cells. HESCs treated with BMP2 (100 ng/ml) for 2 (A) and 6 (B) d were quantified for expression of the indicated genes; QPCR values were normalized to those of control gene TBP. Inset: Western blot of nuclear extracts from HESC treated or not with BMP2. Transcript levels (mean ± sem) are expressed as fold change relative to control (untreated) group. Results are representative of two to three independent experiments, with each experiment conducted in quadruplicate. *, Significant difference at P < 0.05 using Student’s t test between untreated and BMP2-treated groups.
KLF9- and KLF13-negative cross-regulation in differentiating stromal cells involves BMP2
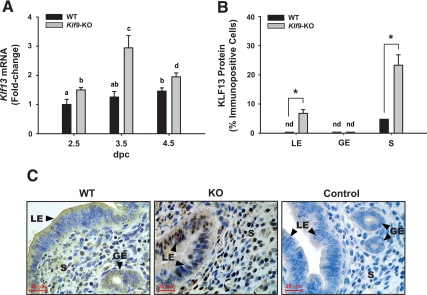
In a previous study, we showed that the KLF9-related protein, KLF13, had increased expression in uteri of nonpregnant Klf9 null mice (18). To evaluate the relationship between KLF9 and KLF13 during early pregnancy, we measured Klf13 transcript levels in uteri of WT and Klf9 null mice at dpc 2.5, 3.5, and 4.5. Klf13 transcript levels in WT uteri were low at dpc 2.5 and modestly but significantly increased by dpc 4.5 (Fig. 6A). Relative to WT uteri, Klf13 expression was significantly increased at all pregnancy days in Klf9 KO uteri, with the highest induction (by ∼3-fold) observed at dpc 3.5 (Fig. 6A). Immunohistochemical analyses of uterine sections from mice of both genotypes at dpc 3.5 confirmed the corresponding increase in levels of KLF13 protein, which was localized to the nuclear compartment of luminal epithelial and stromal cells, with KLF9 null mutation (Fig. 6, B and C). Glandular epithelial cells had undetectable KLF13 expression for both genotypes at dpc 3.5.
Figure 6.
Uterine KLF13 expression is altered by Klf9 null mutation in mice during early pregnancy. A, Klf13 transcript levels in uteri of WT and Klf9 null mice at dpc 2.5, 3.5, and 4.5 were quantified by QPCR and normalized to that of the control gene cyclophilin A. Transcript levels (mean ± sem) are expressed as fold change relative to WT (dpc 2.5) group (n = 4–5 mice per day postcoitum per genotype). Means with different superscripts indicate significant difference at P < 0.05 by two-way ANOVA. B, Percent of anti-KLF13 nuclear-staining cells in endometrial compartments of WT and Klf9 null mice at dpc 3.5 is presented as means ± sem (n = 3–4 mice/genotype). *, Significant difference between WT and Klf9 null mice for each endometrial compartment at P < 0.05, using Student’s t test. nd, Not detectable. C, Representative KLF13 immunostaining of endometrial glandular epithelium (GE), luminal epithelium (LE), and stromal (S) compartments of WT and KO mice at dpc 3.5 is shown at ×400 magnification. Control is WT uteri in the absence of antibody.
To further examine the inverse association of KLF13 and KLF9 transcript levels in stromal cells undergoing differentiation, KLF13 protein was measured in nuclear extracts prepared from 0.5cAME-treated HESCs. KLF13 protein, although barely detectable at d 0, showed significant expression at d 1, which was maintained until d 6 of treatment (Fig. 7A). This pattern of KLF13 protein expression was opposite to that of KLF9 protein (compare Figs. 7A and 3B). Next, HESCs were transfected with either KLF9 or KLF13 siRNAs and subsequently treated with 0.5cAME for 2 d. Repression of KLF9 expression resulted in a robust increase in KLF13 transcript levels (∼60%), coincident with a modest but significant increase in BMP2, as well as in total PGR and PGR-B levels (Fig. 7B). Conversely, the repression of KLF13 expression by siRNA targeting resulted in a modest increase (∼40%) in KLF9 transcript levels (Fig. 7C). Under these conditions, there was a 30% reduction in BMP2 expression and a corresponding 50% reduction for PGR-B, whereas no effects were noted for total PGR.
Figure 7.
KLF13 mediates BMP2 down-regulation of KLF9 expression in decidualizing stromal cells. A, Expression of KLF13 protein was analyzed in nuclear extracts isolated from HESCs treated with 0.5 mm 8-Br-cAMP + E2 + MPA for 0, 1, 2, and 6 d by Western blot. Bar graphs represent densitometric scans of immunoreactive band, normalized to loading control (lamin A) from two independent experiments. Means (±sem) with different superscripts indicate significant difference at P < 0.05 by one-way ANOVA. B, HESCs were transfected with scrambled (control) siRNAs and KLF9 siRNAs (50 nm each) and then treated with 0.5 mm 8-br-cAMP + E2 + MPA for 2 d. Harvested cells were analyzed for gene expression using QPCR and normalized to TBP. Transcript levels (mean ± sem) are expressed as fold change relative to control group. C, HESCs were transfected with scrambled (control) or KLF13 siRNAs (50 nm each) and then treated with 0.5 mm 8-br-cAMP + E2 + MPA for 2 d. Harvested cells were analyzed for gene expression using QPCR and normalized to TBP. Transcript levels (mean ± sem) are expressed as fold change relative to control group. D, HESCs were treated with recombinant BMP2 (100 ng/ml) in the presence of 0.5 mm 8-br-cAMP + E2 + MPA for 2 d. Harvested cells were analyzed for KLF13 transcript levels by QPCR and normalized to TBP. Transcript levels (mean ± sem) are expressed as fold change relative to non-BMP2-treated group. E, HESCs were transfected with scrambled (control) or KLF13 siRNAs (50 nm each) and treated with BMP2 and 0.5 mm 8-br-cAMP + E2 + MPA for 2 d. Harvested cells were analyzed for KLF9 transcript levels using QPCR and normalized to TBP. Transcript levels (mean ± sem) are expressed as fold change relative to control group. Results are representative of two independent experiments, with each experiment conducted in quadruplicate. *, Significant difference between control and treated cells at P < 0.05 using Student’s t test.
Because loss of KLF9 expression resulted in coincident increases in BMP2 and KLF13 expression, reduced KLF13 expression was accompanied by attenuated BMP2 and up-regulated KLF9 transcript levels, and BMP2 inhibited KLF9 expression, we tested whether KLF13 expression is up-regulated by BMP2 and, if so, whether KLF9 down-regulation by BMP2 is mediated by KLF13. HESCs treated with BMP2 (100 ng/ml) in the presence of 0.5cAME showed increased (by 50%) KLF13 transcript levels (Fig. 7D); by contrast, BMP2 in the absence of 0.5cAME had minimal effect on KLF13 expression (data not shown). Cells were transfected with scrambled (control) or KLF13 siRNAs and then incubated in media containing 0.5cAME and BMP2 (100 ng/ml). In the presence of KLF13 siRNAs, the previously observed BMP2 inhibition of KLF9 expression (Fig. 5) was lost, relative to BMP2-treated cells transfected with scrambled siRNAs (Fig. 7E). Thus, KLF13 acts downstream of BMP2 and mediates BMP2 inhibition of KLF9 expression in decidualizing stromal cells.
Discussion
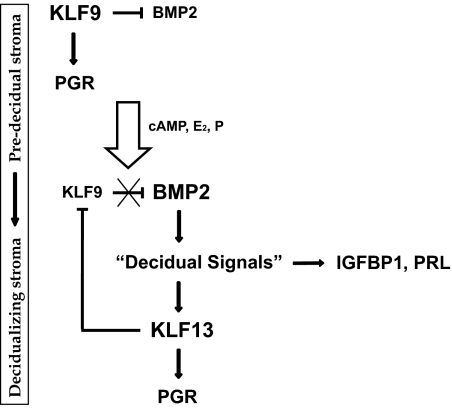
In the present study, we report novel roles for transcription factors KLF9 and KLF13 in the regulation of BMP2 signaling during progression of stromal cells to decidualization. Using early pregnant WT and Klf9 null mice and in vitro decidualizing human stromal cells transfected with siRNAs to KLF9 or KLF13, we showed an inverse association of KLF9 expression with those of BMP2 and KLF13 and a positive association between BMP2 and KLF13 expression. We provided mechanistic evidence to support the concept that disruption of these associations, initiated by the loss of KLF9 expression, resulted in the premature induction of BMP2 and KLF13 and compromised PGR and IGF binding protein (IGFBP)-1 expression, both measures of stromal progression to differentiation. Our findings suggest a novel pathway whereby stromal BMP2 signaling during periimplantation can be dynamically influenced by two highly related KLF proteins, in concert with other decidualizing signals (Fig. 8). To our knowledge, this study is the first report of a cross talk between BMP2 and KLF family members and reveals a network within the KLF family that may be important for early regulatory events requisite for uterine receptivity.
Figure 8.
Proposed model of cross-regulation among BMP2, KLF9, and KLF13 in stromal cells undergoing differentiation. KLF9 expression is highest in predecidual stroma and progressively decreases in decidualizing stroma, whereas the reverse is true for KLF13. In predecidual stroma, KLF9 inhibits BMP2 expression. The appearance of BMP2 induces the expression of decidual signals, which in turn promote KLF13 expression and eventually lead to loss of KLF9 expression. PGR expression and hence P sensitivity are maintained in part by the sequential temporal expression of KLF9 and KLF13. Arrows (→, ⊣) represent positive and negative linkages, respectively.
Our demonstration that BMP2 is an early target of KLF9, likely acting in concert with PGR (16,17), strongly supports the participation of KLF9 in the early events leading to the establishment of uterine receptivity and provides a molecular rationale for the subfertility phenotype of Klf9 null mice. In uterine stroma, BMP2 controls the activation and repression of numerous gene targets important for decidualization (26,33); indeed, female mice with targeted deletion of uterine Bmp2 are infertile due to failure of stromal cells to decidualize (26). However, during the transition from the prereceptive (dpc 2.5) to the receptive phase when the embryo initiates attachment to luminal epithelial cells (dpc 3.5), BMP2 expression is low to nondetectable (27,28), suggesting BMP2’s nonparticipation in this early event. Thus, the early inhibition of BMP2 expression by KLF9 and conversely, the silencing of KLF9 by BMP2 could be considered as a fail-safe mechanism to ensure that BMP2 is expressed in appropriate amounts and at the correct time to allow for the normal progression of stromal differentiation. In support of this, although recombinant BMP2 has been previously shown to inhibit cell proliferation and induce apoptosis in diverse cell types (35,36,37,38), response of target cells to BMP2 action is found to be biphasic, with low and high BMP2 levels eliciting distinct effects (23,24). Our data, however, do not preclude additional pathways independent of BMP2 by which KLF9 loss may lead to subfertility. Moreover, our investigation does not clarify whether KLF9 regulation of BMP2 expression is direct or indirect and by what mechanism. However, we suggest that the latter may occur at the level of BMP2 transcription given that the BMP2 promoter contains GC-rich sites that have been shown to bind the KLF9-related transcription factor Sp1 (39,40) and that Sp1 and KLF9 are reported to antagonize each other’s transactivity (41). Future studies at the level of the BMP2 promoter will address this predicted mechanism of molecular regulation.
Interestingly, BMP2 and KLF9 cross-regulation of their respective expression occurs without disrupting PGR expression levels, which progressively increase during the transition from predecidual to decidualizing stroma. Because the temporal decline of KLF9 accompanying this transition would be predicted to result in loss of its positive regulation of PGR expression, our findings that BMP2 induces KLF13 expression and that KLF13 takes over the role of KLF9 in promoting PGR expression, specifically of PGR-B, provide a novel mechanism by which the KLF regulatory network may avert pregnancy loss and by extension, the development of endometrial-associated dysfunction due to increased P resistance (9,10,42,43,44). In previous studies, we demonstrated that both KLF9 and KLF13 can functionally interact with PGR-B (16,17). Here we showed that KLF13, similar to KLF9, up-regulated PGR-B expression. Thus, our findings predict that conditions leading to coincident loss of KLF9 and KLF13 expression in stromal cells will be associated with poor pregnancy outcome and uterine disorders. Moreover, we expect that given the distinct stromal functions for PGR-B before and during differentiation, the shift in PGR-B partner from KLF9 to KLF13 in decidualizing stroma will lead to the induction of specific PGR-B targets with minimal overlap in regulation by KLF9 and KLF13. Studies on the uterine phenotypes of Klf9 null mice in the backgrounds of Klf13 haplo-insufficiency and total ablation will elucidate on these important questions.
Members of the Sp/KLF family are known to exhibit opposing functions in target cells (45,46,47,48), and evidence has emerged to indicate that these nuclear proteins can directly cross-regulate each other’s expression and activity (18,45). Here we show that the negative association of KLF9 and KLF13 expression is mediated in part by BMP2 in association with other decidual signals whose expression are likely induced during stromal differentiation (cAME addition). We suggest that BMP2 alone minimally influences KLF13 expression but may require additional effectors because we found no induction of KLF13 expression in cells treated with recombinant BMP2 in the absence of 0.5cAME (data not shown) in contrast to the up-regulation of levels of this transcript by BMP2 in the background of added cAME. This appears to be true as well for BMP2 inhibition of KLF9 expression because in the absence of added cAME, the observed BMP2 effect was relatively modest and was not further increased with prolonged (6 d) treatment. Whereas KLF9 does not appear to directly regulate KLF13 expression, our data suggest the possibility of KLF13 directly regulating the KLF9 gene. Further studies are needed to clarify whether this mechanism is true. By contrast, KLF9 and KLF13 regulation of PGR-B expression may occur within the same region of the PGR-B promoter because their respective expression do not overlap and are temporally defined by cellular context.
In conclusion, using an in vitro model for differentiating human stroma and a mouse model during early pregnancy, we report the existence of a BMP2/KLF regulatory axis during stromal transition from predecidual to decidualizing state. Albeit the important contribution of the embryo leading to a functionally differentiated stroma needs to be integrated when comparing in vivo and in vitro results (49), our studies provide experimental evidence to implicate dysregulated KLF expression in the etiology of uterine dysfunction leading to subfertility and infertility. Further investigations will address the relative position of the described KLF-mediated regulatory interactions within the broader network of BMP2 downstream pathways including that of Wnt-4 (33).
Supplementary Material
Acknowledgments
We are grateful to Amy Greenway and S. Renee Till for technical assistance with the immunohistochemistry analyses, Dr. Michael Velarde (Buck Institute for Age Research, Novato, CA) for insightful comments, and members of our laboratories for helpful discussions during the course of this study.
Footnotes
This work was supported by National Institutes of Health Grant RO1 HD-21961.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 21, 2010
Abbreviations: BMP, Bone morphogenetic protein; 8-Br-cAMP, 8-bromoadenosine-cAMP; cAME, 8-Br-cAMP, MPA, and E2; CS-BCS, charcoal-stripped bovine calf serum; dpc, day postcoitum; E2, estradiol; HESC, human endometrial stromal cell; IGFBP, IGF binding protein; KLF, Krüppel-like Factor; KO, knockout; MPA, medroxyprogesterone acetate; P, progesterone; PGR, progesterone receptor; PRL, prolactin; QPCR, quantitative RT-PCR; siRNA, small interfering RNA; Sp1, specificity protein 1; TBP, TATA box binding protein; WT, wild type.
References
- Matzuk MM, Lamb DJ 2002 Genetic dissection of mammalian fertility pathways. Nat Cell Biol 4(Suppl):S41–S49 [DOI] [PubMed] [Google Scholar]
- Psychoyos A 1973 Hormonal control of ovoimplantation. Vitam Horm 31:201–256 [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H 2004 Molecular cues to implantation. Endocr Rev 25:341–373 [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK 2006 Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7:185–199 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW 2002 Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 [DOI] [PubMed] [Google Scholar]
- Lonard DM, O'Malley BW 2006 The expanding cosmos of nuclear receptor coactivators. Cell 125:411–414 [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ 2007 Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM 2000 Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett-Mansfield RL, DeFazio A, Mote PA, Clarke CL 2004 Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J Clin Endocrinol Metab 89:1429–1442 [DOI] [PubMed] [Google Scholar]
- Balleine RL, Earls PJ, Webster LR, Mote PA, deFazio A, Harnett PR, Clarke CL 2004 Expression of progesterone receptors A and B isoforms in low-grade endometrial stromal sarcoma. Int J Gynecol Pathol 23:138–144 [DOI] [PubMed] [Google Scholar]
- Mote PA, Bartow S, Tran N, Clarke CL 2002 Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat 72:163–172 [DOI] [PubMed] [Google Scholar]
- Imataka H, Sogawa K, Yasumoto K, Kikuchi Y, Sasano K, Kobayashi A, Hayami M, Fujii-Kuriyama Y 1992 Two regulatory proteins that bind to the basic transcription element (BTE), a GC box sequence in the promoter region of the rat P-4501A1 gene. EMBO J 11:3663–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RCM 2002 Direct interaction of the Krüppel-like family (KLF) member, BTEB1, and PR mediates progesterone responsive gene expression in endometrial epithelial cells. Endocrinology 143:62–73 [DOI] [PubMed] [Google Scholar]
- Zhang XL, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RCM 2003 Selective interactions of KLF9/BTEB1 with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem 278:21474–21482 [DOI] [PubMed] [Google Scholar]
- Simmen RCM, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman Jr L, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP 2004 Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Krüppel-like factor 9/basic transcription element binding protein 1 (BTEB1) gene. J Biol Chem 279:29286–29294 [DOI] [PubMed] [Google Scholar]
- Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RCM 2005 Null mutation of Krüppel-like factor 9/basic transcription element binding protein 1 alters peri-implantation uterine development in mice. Biol Reprod 73:472–481 [DOI] [PubMed] [Google Scholar]
- Nifuji A, Kellermann O, Kuboki Y, Wozney JM, Noda Masaki 1997 Perturbation of BMP signaling in somatogenesis resulted in vertebral and rib malformations in the axial skeletal formation. J Bone Miner Res 12:332–342 [DOI] [PubMed] [Google Scholar]
- Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O'Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC 2000 Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res 15:663–673 [DOI] [PubMed] [Google Scholar]
- Canalis E, Economides AN, Gazzerro E 2003 Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 24:218–235 [DOI] [PubMed] [Google Scholar]
- James RG, Schultheiss TM 2005 BMP signaling promotes intermediate mesoderm gene expression in a dose-dependent, cell autonomous and translation-dependent manner. Dev Biol 288:113–125 [DOI] [PubMed] [Google Scholar]
- Schlueter J, Männer J, Brand T 2006 BMP is an important regulator of proepicardial identity in the chick embryo. Dev Biol 295:546–558 [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A 1996 Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122:2977–2986 [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ 2007 BMP2 is critical for the murine uterine decidual response. Mol Cell Biol 27:5468–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ 2000 Detection of multiple bone morphogenetic protein messenger ribonucleic acids and their signal transducer, smad1, during mouse decidualization. Biol Reprod 63:1781–1786 [DOI] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BLM 2001 Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci USA 98:1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suske G, Bruford E, Philipsen S 2005 Mammalian SP/KLF transcription factors: bring in the family. Genomics 85:551–556 [DOI] [PubMed] [Google Scholar]
- Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ 2004 A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology 145:2291–2296 [DOI] [PubMed] [Google Scholar]
- Pabona JMP, Velarde MC, Zeng Z, Simmen FA, Simmen RCM 2009 Nuclear receptor co-regulator Krüppel-like factor 9 and prohibitin 2 expression in estrogen-induced epithelial cell proliferation in the mouse uterus. J Endocrinol 200:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde MC, Iruthayanathan M, Eason RR, Zhang D, Simmen FA, Simmen RCM 2006 Progesterone receptor transactivation of the secretory leukocyte protease inhibitor gene in Ishikawa endometrial epithelial cells involves recruitment of Krüppel-like factor 9/basic transcription element binding protein 1 (BTEB1). Endocrinology 147:1969–1978 [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC 2007 Bone morphogenetic 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282:31725–31732 [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Stoikos C, Baca M, Fairlie WD, McCoubrie JE, Salamonsen LA 2005 Relaxin and prostaglandin E2 regulate interleukin 11 during human endometrial stromal cell decidualization. J Clin Endocrinol Metab 90:3458–3465 [DOI] [PubMed] [Google Scholar]
- Glozak MA, Rogers MB 1996 Specific induction of apoptosis in P19 embryonal carcinoma cells by retinoic acid and BMP2 or BMP4. Dev Biol 179:458–470 [DOI] [PubMed] [Google Scholar]
- Kawamura C, Kizaki M, Yamato K, Uchida H, Fukuchi Y, Hattori Y, Koseki T, Nishihara T, Ikeda Y 2000 Bone morphogenetic protein-2 induces apoptosis in human myeloma cells with modulation of STAT3. Blood 96:2005–2011 [PubMed] [Google Scholar]
- Hallahan AR, Pritchard JI, Chandraratna RA, Ellenbogen RG, Geyer JR, Overland RP, Strand AD, Tapscott SJ, Olson JM 2003 BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med 9:1033–1038 [DOI] [PubMed] [Google Scholar]
- Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, Kriett JM, Yung G, Rubin LJ, Yuan JX 2003 Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 285:L740–L754 [DOI] [PubMed] [Google Scholar]
- Abrams KL, Xu J, Nativelle-Serpentini C, Dabirshahsahebi S, Rogers MB 2004 An evolutionary and molecular analysis of Bmp2 expression. J Biol Chem 279:15916–15928 [DOI] [PubMed] [Google Scholar]
- Xu J, Rogers MB 2007 Modulation of bone morphogenetic (BMP) 2 gene expression by Sp1 transcription factors. Gene 392:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde MC, Zeng Z, McQuown JR, Simmen FA, Simmen RCM 2007 Krüppel-like factor 9 is a negative regulator of ligand-dependent estrogen receptor α signaling in Ishikawa endometrial adenocarcinoma cells. Mol Endocrinol 21:2988–3001 [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC 2007 Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE 2000 Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 85:2897–2902 [DOI] [PubMed] [Google Scholar]
- Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, Osteen KG 2005 Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-ρ-dioxin. Fertil Steril 84:67–74 [DOI] [PubMed] [Google Scholar]
- Eaton SA, Funnell AP, Sue N, Nicholas H, Pearson RC, Crossley M 2008 A network of Krüppel-like factors (Klfs). Klf8 is repressed by Klf3 and activated by Klf1 in vivo. J Biol Chem 283:26937–26947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, Suzuki R, Kitamura T, Akira S, Kadowaki T, Nagai R 2005 Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab 1:27–39 [DOI] [PubMed] [Google Scholar]
- Lomberk G, Urrutia R 2005 The family feud: turning off Sp1 by Sp1-like KLF proteins. Biochem J 392:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen RCM, Pabona JMP, Velarde MC, Simmons C, Rahal O, Simmen FA 2010 The emerging role of Krüppel-like factors in endocrine-responsive cancers of female reproductive tissues. J Endocrinol 204:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, Giudice LC 2007 Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod 76:102–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.