Abstract
The pineal hormone melatonin (MEL) is the key initiator in regulating seasonal photoperiodic responses; however, the central sites that mediate short day (SD) winter-like responses, such as testicular regression and decreases in white adipose tissue (WAT) mass, by Siberian hamsters are not precisely known. WAT is innervated by the sympathetic nervous system, and several forebrain sites that are part of the sympathetic nervous system outflow to WAT coexpress MEL1a receptor mRNA [e.g. suprachiasmatic nucleus, subzona incerta (SubZi), dorsomedial nucleus of the hypothalamus, nucleus reunions and paraventricular nuclei of the thalamus]. We tested the involvement of these sites in MEL-triggered SD responses. A long duration, SD-like MEL signal was applied site specifically for 5 wk, with sc and third ventricle MEL application serving as positive controls. Whereas SD MEL signals delivered to each of these sites were able to induce testicular regression, all but the paraventricular nuclei of the thalamus also trigger SD-induced decreases in body mass. Third ventricle, sc, suprachiasmatic nucleus, or SubZi MEL application also decreased WAT mass, and only sc and SubZi MEL application decreased food intake. Collectively these data suggest a distributed system of MEL-sensitive brain sites sufficient to mediate these SD responses, the redundancy of which suggests its importance for appropriate seasonal responses critical for overwintering.
The present data argue for a distributed forebrain system that mediates short day (SD) responses in Siberian hamsters, rather than one site being responsible for all SD responses or relatively separate sites for each SD response.
Obesity is one of the fastest growing health threats in America, promoting secondary health consequences such as stroke, cardiovascular disease, certain types of cancer, and diabetes mellitus (e.g. Refs. 1,2,3). Whereas the development of obesity results from increased food intake and/or decreased energy expenditure, the opposite holds true for its reversal. The precise mechanisms underlying the latter are less well defined, especially concerning lipid mobilization, although increases in the sympathetic nervous system (SNS) drive to white adipose tissue (WAT) is the principal initiator of lipolysis (for review see Refs. 4,5,6).
Siberian hamsters provide an ideal model to study the natural reversal of obesity because it is a seasonal animal and exhibits a suite of responses governed by the photoperiod. In long summer-like days (LDs), Siberian hamsters accrue large amounts of lipid reserves [∼50% body fat (7,8)], are reproductively active, and have unremarkable thermogenic responses (for review see Refs. 9 and 10). By contrast, in short winter-like days (SDs), the LD responses are reversed, exhibiting profound decreases in body mass and fat (∼−30%), testicular regression, marked increases in thermogenic responses (nonshivering thermogenesis), expression of shallow daily torpor, change to a white-winter pelage color (for review see Refs. 11,12,13,14), and decreases in humoral immunity (15,16). These and other SD responses are triggered by decreases in the daylength naturally (17,18) that can be mimicked in the laboratory by decreases in the vivarium photoperiod (19) or by manipulating the neuroendocrine transducer of the seasonal photoperiod information, the duration of the nocturnal secretion of melatonin [MEL (19,20,21)]. Specifically, timed systemic injections of MEL occurring about 3–4 h before lights off in LD-housed, pineal-intact hamsters generate a MEL signal that summates with the naturally occurring, short nocturnal duration of MEL secretion associated with LDs, thereby lengthening it and triggering SD responses in Siberian hamsters (22). Even though such timed MEL injections produce nonphysiological circulating concentrations of MEL, the SD-like responses are quite similar to that achieved with timed daily MEL sc infusions in pinealectomized Siberian hamsters that mimic SD nocturnal durations of the hormone and that create circulating MEL concentrations that are physiological (for review see Ref. 21). Collectively it is the duration of the MEL signal that is important for inducing SD responses once a threshold value has been reached or exceeded (23,24). Moreover, these more physiologically relevant MEL signals reduce body fat (WAT mass) equivalent to that seen with transfer from LDs to SDs (7,25); however, reductions in WAT mass have not, to our knowledge, been assessed using the timed MEL injection model.
Because MEL is unable to stimulate white adipocyte lipolysis directly (26), we reasoned that MEL may be having its effects on lipid mobilization via a secondary hormone. Therefore, we previously tested hormones that change in Siberian hamsters moved from LDs to SDs and, moreover, that either stimulate lipolysis or decrease lipolytic inhibition. No hormone (e.g. glucocorticoids, insulin, prolactin, gonadal steroids, thyroid hormones) was able to account for the SD-induced increases in lipolysis (for review see Ref. 12). Photoperiod/MEL stimulation of adrenal medullary epinephrine, a well-known lipolytic initiator (27), is not necessary for SD-induced increases in lipid mobilization because adrenal demedullation, and thus the removal of the sole source of circulating epinephrine, does not block SD-induced decreases in body fat (27,28). We reported previously, however, that the central nervous system is connected to WAT through neural circuits, revealed through the use of a transneuronal viral tract tracer, pseudorabies virus (28). Moreover, gene expression for the functional MEL receptor subtype (MEL1a-R) that underlies all MEL-induced changes in photoperiodic responses in Siberian hamsters is colocalized on some of the SNS outflow neurons from brain to WAT (29). Functionally, the sympathetic drive to WAT increases after SD exposure, as measured neurochemically by norepinephrine turnover (30). Norepinephrine is a potent lipolytic stimulus (31) that is released from sympathetic nerve terminals. For rodent WAT, it stimulates β3-adrenoceptors located on WAT cell membranes to initiate a cascade of internal signaling, resulting ultimately in the hydrolysis of triacylglycerol into free fatty acids and glycerol (for review see Ref. 32). WAT SNS denervation blocks SD-induced increases in lipid mobilization, demonstrating its importance in the loss of body fat functionally (33). Thus, MEL driving the SNS innervation of WAT appears to underlie the SD-induced increases in lipid mobilization. What is not precisely known is which population of central MEL1a-R-bearing neurons is important for this response.
Brain sites showing high amounts of colocalization of WAT SNS outflow neurons expressing MEL1a-R mRNA include the suprachiasmatic nucleus (SCN), dorsomedial nucleus of the hypothalamus (DMH), subzona incerta (SubZi), paraventricular nucleus of the thalamus (PVT) and nucleus reunions [ReN (29)] and thus are potential sites underlying the SD-triggered increases in WAT lipolysis. Some of these target sites (SCN, ReN, PVT) previously have been suggested to be involved in photoperiodic seasonal reproductive responses in this species (34,35,36,37,38,39) or seasonal changes in immunity (40), but their potential contribution to SD-induced decreases in body mass has not been tested except for the SCN (35,38,41). In terms of the latter, pinealectomized Siberian hamsters given sc daily MEL infusions of a long duration (10 h; SD like) exhibit decreases in WAT mass indicative of lipid mobilization (lipolysis), but in animals bearing SCN lesions, this and all photoperiodic responses are blocked despite the animals receiving an appropriate SD-type MEL signal (35,38,41). Thus, an intact SCN is necessary to receive SD. MEL signals and thereby trigger SD responses.
Therefore, the purpose of the present study was to test whether SD MEL signals delivered to brain sites that contain WAT SNS outflow neurons possessing MEL1a-R mRNA will trigger SD-like decreases in WAT mass. This was accomplished by site-specific delivery of SD-like MEL signals to brain sites containing high populations of SNS outflow neurons possessing MEL1a-R mRNA (SCN, DMH, SubZi, PVT, or ReN). This was done by inserting MEL-filled cannulae separately into each site in different sets of pineal-intact Siberian hamsters 3 h before lights off and removing them 4 h later when the naturally occurring LD short nocturnal duration of MEL secretion by the pineal would be well underway. Thus, only the MEL cannulation sites would receive lengthened, SD-like, long-duration MEL signals, with the remainder of the brain receiving the naturally occurring, LD short-duration MEL signals. After 5 wk, we measured changes in body and fat pad mass, food intake, serum testosterone, and testicular mass, the latter being a MEL-sensitive, integrative indicator of reproductive status.
Materials and Methods
Animal housing
All experiments were approved by the Georgia State University Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health and Department of Agriculture guidelines. Siberian hamsters (Phodopus sungorus) were housed in a vivarium in which the temperature, relative humidity, and photocycle were held constant at 20 ± 1.5 C, 50 ± 5%, and 16 h light, 8 h dark (lights off at 1030 h), respectively, throughout all experiments. Animals were given Purina Lab Chow 5001 (TestDiets, Brentwood, MO) and tap water ad libitum. Hamsters were weaned at 21 d postpartum and singly housed in polypropylene cages (27.8 × 17.5 × 1 3.0 cm) containing corn cob bedding (The Andersons, Maumee, OH) and cotton nestlets (Ancare, Belmore, NY) until used in the experiments.
Cannulation and MEL treatment
At about 2.5 months of age, 122 male Siberian hamsters were used across these experiments. Animals were anesthetized under isoflurane anesthesia, hair removed from the top of the head, an incision made to reveal the skull, and guide cannulae were stereotaxically implanted bilaterally into one of the following structures: SCN (n = 15; anterior-posterior: +0.1 mm, medial-lateral: ±0.28 mm, dorsal-ventral: −6.7 below dura at a 21.5° angle), SubZi (n = 15; anterior-posterior: −0.3 mm, medial-lateral: ±0.75 mm, dorsal-ventral: −5.4 mm below dura), DMH (n = 10; anterior-posterior: −1.1 mm, medial-lateral: ±0.6 mm, dorsal-ventral: −5.8 mm below dura). Cannulae were stereotaxically implanted unilaterally into one of the following midline structures: ReN (n = 19; anterior-posterior: −0.1 mm, medial-lateral: 0 mm, dorsal-ventral: −5 mm below dura), PVT (n = 16; anterior-posterior: −0.1 mm, medial lateral: 0 mm, dorsal-ventral: −4.2 mm below dura) or third ventricle [third ventricle, n = 28 (to control for possible MEL diffusion into the third ventricle); anterior-posterior: −0.8 mm, medial-lateral: 0 mm, dorsal-ventral: −5.5 mm below dura]. Animals were allowed to recover for 1 wk with an obturator inserted into the outer cannulae. Internal cannulae (0.5 mm extension beyond tip of external cannulae) were filled with beeswax or MEL in beeswax [1:4 parts (42,43)] and inserted daily 3 h before lights off for 5 wk. This was done to extend the nocturnal peak of MEL that begins shortly after lights out to mimic SD-like MEL signals. The cannulae were then removed daily under dim red light 4 h into the dark period, a time when the peak of the nocturnal MEL secretion profile occurs (21). Thus, only the targeted area received daily SD-like MEL signals but not the whole brain. Upon removal, the cannulae were cleaned and maintained in a 37 C water bath until reinserted the next day. New MEL-filled cannulae replaced the old cannulae every 2 wk. As a negative control, beeswax-filled cannulae were used and inserted and removed as above. As a positive control, another set of 19 male Siberian hamsters were injected sc in the interscapular area daily 3 h before lights off for 5 wk with either ethanolic saline (1:9 parts) or MEL (5 μg in 0.1 ml ethanolic saline, 1:9 parts), prepared fresh daily from a stock solution of 500 μg/ml MEL in 100% ethanol (44), a treatment that triggers SD-like body and lipid mass as well as reproductive responses (22,45,46). Body mass and food intake were monitored weekly, the latter corrected for spillage and pouching.
Blood serum analysis
After 5 wk, animals were anesthetized between 2100 and 0200 h with isoflurane, and orbital sinus blood was taken for assay of testosterone concentrations. Blood (∼500 μl) was collected with heparinized glass capillary tubes, stored in culture tubes overnight at 4 C, and centrifuged the next day at 4 C for 20 min at 2000 rpm. Serum was removed and stored at −80 C until analyzed. A commercially available testosterone RIA kit (Diagnostic Systems Laboratories, Webster, TX) was used according to the manufacturer’s instructions. For all testosterone assays, the overall correlation coefficient was 99.16%, intraassay reliability 9.2%, and interassay reliability was 7.5%.
Perfusions and cannula verification
After orbital sinus blood collection, animals were anesthetized with an overdose of pentobarbital sodium (300 mg/kg), and the right inguinal WAT (IWAT), bilateral retroperitoneal WAT (RWAT), and epididymal WAT (EWAT) depots and testes were rapidly removed and weighed. The animals were then perfused transcardially first with about 125 ml isotonic saline, followed by about 125 ml of 4% paraformaldehyde in 0.1 m PBS solution (pH 7.4) and the brains removed. All brains were stored in 4% paraformaldehyde and transferred to sucrose (30 and 0.1% sodium azide) after 24 h until sectioned. The brains were sectioned in the coronal plane using a freezing microtome at a thickness of 80 μm. Finally, sections were mounted onto microscope slides (Superfrost; Fisher Scientific, Suwanee, GA) and dried overnight. The slides were then dehydrated and delipidated through a series of alcohol concentrations and xylene, stained with cresyl echt violet, and finally coverslipped using Permount (Fisher Scientific). The stained sections were then viewed by light microscopy to locate the cannulae tips, and this was recorded on copies of a mouse atlas (47) because a Siberian hamster atlas does not exist and the shapes of many brain structures for Syrian hamsters do not match well with this species.
Statistical analysis
Multivariate analyses for percentage changes from baseline (within animal control for food intake and body mass) and control (saline treatment or beeswax) were used independently for each brain site and combined for the following variables: EWAT, IWAT, and RWAT fat pads and testes and testosterone, with Bonferroni post hoc tests (GraphPad Prism version 4.00; GraphPad Software, San Diego, CA) when appropriate. Misplaced cannulae (misses) were defined as 400 μm or greater of the cannula tip away from the target site and were excluded from the analysis. Differences between means were considered significant if P < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of presentation.
Results
Cannulae site verification
Cannulae were implanted to deliver SD signals site-specifically to only one of six different target sites (Fig. 1) for 5 wk. One Siberian hamster with a PVT cannula had to be excluded due to unusually enlarged ventricles. Misplaced cannulae were used as a control for the spread of MEL from the target site (third ventricle, n = 1; SCN, n = 2; DMH, n = 1; ReN, n = 5; and PVT, n = 2) and were excluded from further analysis; note, however, that these misplaced cannulae yielded responses no different from control animals for all of the responses measured (data not shown). Cannulae misplacements for the SubZi (n = 3) were located in the ReN, third ventricle, and DMH; however, all were able to induce SD responses. Thus, the spread of MEL from the cannulae for the SubZi could not be accounted for, but there is no reason to expect it to be different from the other five sites.
Figure 1.
Representative schematic drawings of cannulation placement in the SCN (n = 15), SubZi (n = 15), third ventricle (3rd V; n = 28), DMH (n = 10), PVT (n = 16), and the ReN (n = 18). Black dot, Correct placement (hit, depicted unilaterally or as midline structure); summing junction, incorrect placement (miss; SCN, n = 2; SubZi, n = 3; third ventricle (3rd V), n = 1; DMH, n = 1; PVT, n = 2; ReN, n = 5). Not indicated is one ReN cannulated individual with cannulation misplacement too far anterior into the anterior commissure, and the misplacements for the SubZi were located in the ReN, 3rd V, and DMH.
Site-specific SD-like signals and testicular regression
Extended MEL application to deliver SD signals site- specifically to SCN, SubZi, DMH, ReN, and PVT, and more generally via the third ventricle all were able to induce testicular regression at 5 wk (P values < 0.05; Fig. 2A). The testicular regression associated with MEL application to each of these sites was comparable with the SD-like responses triggered by sc MEL injections for 5 wk (Fig. 2A). After 5 wk of site-specific MEL application, testicular mass was significantly decreased compared with controls for all brain sites (P < 0.05; Fig. 2A). This level of regression (∼<300 mg paired testes mass) was well below normal functioning paired testes masses because paired testes masses less than 300 mg normally have undetectable serum testosterone concentrations (Bartness, T. J., unpublished observations). The effects of MEL in the PVT on testicular regression were more variable, and although significantly different from control animals (P < 0.05), testicular regression was not complete and paired testes mass (∼>300 mg) indicated they still were functional. We found a significant correlation between testicular mass and serum testosterone concentrations across all animals (r2 = 0.6; P < 0.05; Fig. 2B).
Figure 2.
A, Mean ± sem percent change of testes mass (grams) from MEL-treated Siberian hamsters vs. their controls (saline or beeswax treated) in the SCN [n = 13 (six MEL)], SubZi [n = 12 (five MEL)], third ventricle [3rd V; n = 27 (15 MEL)], DMH [n = 9 (four MEL)], PVT [n = 13 (six MEL)], and the ReN [n = 14 (six MEL)]. *, P < 0.05 vs. controls. B, Correlation of percent change of testes mass and serum testosterone concentrations (nanograms per milliliter) for all animals [absolute values of control animals for testes and testosterone, respectively: sc: 0.84 ± 0.06 g, 5.32 ± 1.32 ng/ml; 3rd V: 0.69 ± 0.05 g, 6.36 ± 1.14 ng/ml; SCN: 0.79 ± 0.09 g, 2.93 ± 1.29 ng/ml; SubZi: 0.77 ± 0.06 g, 1.15 ± 0.29 ng/ml; DMH: 0.66 ± 0.08 g, 2.19 ± 0.87 ng/ml; PVT: 0.82 ± 0.05 g, 4.09 ± 1.52 ng/ml; ReN: 0.94 ± 0.08 g, 3.32 ± 0.89 ng/ml].
Site-specific SD-like signals and body mass and food intake
Five weeks of site-specific SD-like MEL application resulted in significantly decreased body mass when MEL was delivered sc to the third ventricle or site specifically to the SCN, SubZi, DMH, and ReN compared with preexperimental values (P < 0.5; Table 1). Body mass loss occurred largely without decreases in food intake, with food intake only significantly decreased when MEL was delivered sc or site specifically to the SubZi [P < 0.05; almost reaching significance for ReN MEL application (P = 0.07), Table 1)]. Changes in body mass, however, were not dependent on serum testosterone concentrations (r2 = 0.1; P > 0.05, data not shown).
Table 1.
Mean ± sem percent change of body mass and food intake for MEL-treated Siberian hamsters and their controls (saline or beeswax treated) for wk 5 vs. the start of the experiment (wk 0)
| Site | Body mass
|
Food intake
|
||
|---|---|---|---|---|
| Control | MEL | Control | MEL | |
| Subcutaneous | 0.4 ± 2.0 | −13.2 ± 1.4a | 31.5 ± 5.8 | 14.0 ± 7.4a |
| Third ventricle | 3.8 ± 2.0 | −23.2 ± 1.9a | −1.9 ± 5.8 | −0.7 ± 8.2 |
| SCN | −3.0 ± 4.1 | −22.3 ± 5.0a | 5.6 ± 10.2 | 23.9 ± 33.8 |
| SubZi | −3.8 ± 2.4 | −19.8 ± 2.0a | 0.7 ± 8.3 | −28.7 ± 10.6a |
| DMH | −2.5 ± 4.6 | −23.5 ± 5.5a | −2.2 ± 4.3 | −7.1 ± 7.1 |
| ReN | −0.8 ± 2.7 | −13.9 ± 4.3a | −13.2 ± 2.7 | −25.5 ± 6.8 |
| PVT | −5.2 ± 1.7 | −4.0 ± 4.5 | −9.2 ± 7.8 | −11.3 ± 6.5 |
Hamsters received implants in the SCN [n = 13 (six MEL)], SubZi [n = 12 (five MEL)], third ventricle [n = 27 (15 MEL)], DMH [n = 9 (four MEL)], PVT [n = 13 (six MEL)], and the ReN [n = 14 (six MEL)].
P < 0.05 vs. controls [absolute values of body mass (wk 0) and food intake (wk 0), respectively: sc: 40.4 ± 0.9, 26.6 ± 0.9 g; third ventricle: 38.5 ± 0.7, 36.4 ± 2.0 g; SCN: 38.4 ± 1.8, 29.8 ± 3.7 g; SubZi: 41.0 ± 1.6, 35.0 ± 1.0 g; DMH: 40.0 ± 2.8, 33.5 ± 1.4 g; PVT: 44.4 ± 1.7, 38.5 ± 2.6 g; ReN: 43.3 ± 1.9, 39.1 ± 1.6 g].
Site-specific SD-like signals and WAT responses
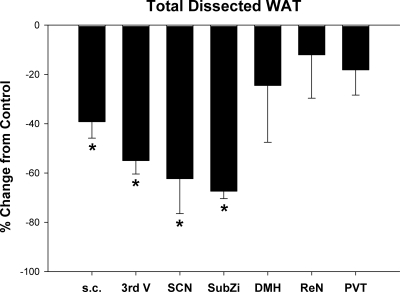
Total dissected WAT mass (IWAT, EWAT, RWAT) was significantly decreased in hamsters receiving sc MEL as well as site specifically when applied to the SCN or SubZi as well as more generally centrally with third ventricle administration compared with group controls (Fig. 3, P < 0.05).
Figure 3.
Mean ± sem percent change of fat pad mass (grams) in MEL-treated Siberian hamsters compared with controls (saline or beeswax treated) in the SCN [n = 13 (six MEL)], SubZi [n = 12 (five MEL)], third ventricle [3rd V; n = 19(8 MEL)], DMH [n = 9 (four MEL)], PVT [n = 13 (six MEL)], and the ReN [n = 12 (four MEL)]. *, P < 0.05 vs. controls [absolute values of controls for EWAT, IWAT, and RWAT mass, respectively: sc: 0.95 ± 0.08, 0.74 ± 0.05, 0.2 ± 0.03 g; 3rd V: 0.88 ± 0.06, 0.65 ± 0.04, 0.18 ± 0.01 g; SCN: 0.92 ± 0.08, 0.56 ± 0.06, 0.15 ± 0.02 g; SubZi: 0.76 ± 0.02, 0.52 ± 0.06, 0.15 ± 0.02 g; DMH: 0.78 ± 0.09, 0.57 ± 0.09, 0.19 ± 0.03; PVT: 0.89 ± 0.05, 0.73 ± 0.05, 0.19 ± 0.02; ReN: 0.78 ± 0.06, 0.54 ± 0.04, 0.13 ± 0.01 g].
Discussion
Five weeks of daily timed insertion/removal of MEL-filled cannulae into the SCN, DMH, SubZi, ReN, but not the PVT, decreased body mass, as did sc MEL injections or third ventricle implants (the latter two positive controls). MEL administered to the SCN and SubZi also decreased total WAT mass and especially EWAT mass; these effects were mimicked by the positive controls of timed sc MEL injections or timed insertion/removal of third ventricle MEL-filled cannulae. Just as timed daily sc MEL injections in LDs experimentally creates SD-like MEL signals that result in testicular regression (7), we found for the first time that timed daily site-specific application of SD MEL signals to the DMH and SubZi and third ventricle also triggered testicular regression associated with significant decreases in serum testosterone concentrations. In addition, we also confirmed the importance of some midline thalamic structures (i.e. ReN and PVT) and the SCN for the control of gonadal status by the current methodology. That is, previously it was found that SD-like MEL signals, created by administering MEL via reverse microdialysis, applied to the ReN, PVT, or SCN inhibit gonadal maturation of juvenile Siberian hamsters just as SDs do naturally (36). In the present experiment, gonadal regression was induced by applying SD MEL signals to these same structures in adult Siberian hamsters. Collectively these (36), other data to be discussed below (34), and the present data argue for a distributed forebrain system that mediates SD responses in Siberian hamsters, rather than one site being responsible for all SD responses or relatively separate sites for each SD response.
Previous studies in our laboratory (48) showed that 5 wk of SD exposure are sufficient to induce reduction in body fat mass, change circulating factors, or any other seasonal responses (e.g. Refs. 8,30,48 and 49,50). Therefore, we used this time point to assess responsiveness to SD-like MEL signals, and although not all SD responses are fully manifested at this time, the majority of changes are occurring by 5 wk. Thus, a lack of a significant change represents a nonresponse according to our previous work, not a failure to treat the animals for a sufficient period of time. It is highly unlikely that the ability of our timed site-specific MEL implants to trigger SD-like responses at these sites is due to diffusion of the MEL from the implant throughout the brain or to neighboring MEL-sensitive sites because of the failure to trigger such responses by nearby misplaced cannulae. Thus, hamsters with MEL-filled cannulae outside any of these target areas showed no evidence of testicular regression or decreases in body mass, supporting the restricted local release of MEL. Indeed, we used MEL-filled cannulae made identically with those used by others that were shown to release MEL only locally (42). Therefore, the use of these MEL-filled implants in a timed daily manner appears to be a specific method to probe MEL-sensitive sites in the brain. Note that timed insertion and withdrawal of intracerebral MEL implants identical with those used here triggered SD-like regression in pinealectomized white-footed mice when placed in the basomedial hypothalamus if the duration of the MEL signals were long (10 h) and therefore SD-like, but not if they were short (5 h) and therefore LD-like (43). The present model has the benefit of not having to additionally pinealectomize the hamsters. In our experienced hands, pinealectomy combined with daily insertion and withdrawal of the cannulae produces nonspecific stress/trauma-induced decreases in body and lipid mass with the control beeswax-filled cannulae (Leitner, C, and T. J. Bartness, unpublished observations). This is not the case for the pineal intact hamsters undergoing the daily insertion and withdrawal of beeswax-filled cannulae in the present experiment.
The SD-induced decreases in body and fat mass in Siberian hamsters typically is not accompanied by decreases in food intake during the first 5–7 wk of SD exposure (e.g. Refs. 7 and 51). When we mimicked SD-like MEL signals in pinealectomized Siberian hamsters receiving long-duration timed daily sc MEL infusions, food intake was not significantly decreased (52), nor was it decreased in the present study except for the hamsters that received MEL implants into the SubZi or sc MEL injections. This was not, however, the only response elicited from this site because SD-like MEL signals given to the SubZi significantly decreased paired testes mass as well. Thus, for the remaining groups in which body/lipid mass was decreased but food intake was not, an increase in energy expenditure must have occurred, although this was not measured in these animals.
The notion that SD (MEL induced) winter-like responses are controlled by a distributed system in the brain is reminiscent of the distributed neural system controlling SD-induced photorefractoriness (i.e. insensitivity to SD long duration MEL signals34). That is, prolonged SD exposure (∼16–20 wk) triggers gonadal recrudescence after the initial gonadal regression (e.g. Ref. 53) that can be mimicked by chronically implanted MEL-filled cannulae (rather than timed insertion-withdrawal of such cannulae as in the present study) into the SCN, ReN, or PVT (34). This causes each site separately to become refractory to MEL without imparting refractoriness to the rest of the brain (34). Thus, the chronically implanted MEL implants into each site causes an initial gonadal regression and subsequent gonadal recrudescence. Most importantly, a second gonadal regression can be triggered in the same animals by giving them timed daily long duration (SD-like) MEL infusions sc (34). This demonstrates that MEL-sensitive brain sites involved in the control of this gonadal response also are distributed because they did not become refractory due to the chronic application of SD MEL signals at the implant site (34). Finally, a distributed system of MEL-sensitive sites action exists for the inhibition of gonadal maturation, as noted above (36).
Collectively we tested brain sites that contain neurons that are part of the SNS outflow to WAT and that also express MEL1a-R mRNA (SCN, SubZi, DMH, ReN, and PVT) by applying timed daily MEL implants site specifically to generate SD-like MEL signals in attempts to trigger SD-like decreases in body and lipid mass. SD-like MEL signals applied to all these sites triggered SD-like decreases in body mass except for the PVT, whereas SD-like MEL signals given to each site-induced testicular regression. SD-like decreases in WAT mass occurred when SD-like MEL signals were applied to the SCN, SubZi, or third ventricle. Food intake only was decreased by SD-like MEL signals applied to the SubZi or sc. Together, these data argue in favor of distributed neuronal sites governing the suite of these SDs, MEL-induced overwintering responses.
Acknowledgments
The authors thank Mary Karom for help measuring testosterone in the Dr. H. Elliott Albers laboratory and Drs. Brett Teubner and David Freeman for helpful guidance and comments undertaking this project.
Footnotes
This work was supported in part by National Institutes of Health Grant R37 DK35254 (to T.J.B.) and a Brains and Behavior Fellowship (to C.L.).
Disclosure Summary: C.L. has nothing to declare. T.J.B. is recipient of a National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant, which supported this work.
First Published Online May 5, 2010
Abbreviations: DMH, Dorsomedial nucleus of the hypothalamus; EWAT, epididymal WAT; IWAT, inguinal WAT; LD, long day; MEL, melatonin; MEL1a-R, MEL receptor subtype; PVT, paraventricular nucleus of the thalamus; ReN, nucleus reunions; RWAT, retroperitoneal WAT; SCN, suprachiasmatic nucleus; SD, short day; SNS, sympathetic nervous system; SubZi, subzona incerta; WAT, white adipose tissue.
References
- Satcher D 2001 Overweight and obesity threaten U.S. health gains. www.surgeongeneral.gov/topics/obesity [Google Scholar]
- Flegal KM 2005 Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav 86:599–602 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Bamshad M 1998 Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol 275:R1399–R1411 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Song CK 2007 Brain-adipose tissue neural crosstalk. Physiol Behav 91:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P, Langin D 2007 The role of neutral lipases in human adipose tissue lipolysis. Curr Opin Lipidol 18:246–250 [DOI] [PubMed] [Google Scholar]
- Wade GN, Bartness TJ 1984 Effects of photoperiod and gonadectomy on food intake, body weight and body composition in Siberian hamsters. Am J Physiol 246:R26–R30 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Hamilton JM, Wade GN, Goldman BD 1989 Regional differences in fat pad responses to short days in Siberian hamsters. Am J Physiol 257:R1533–R1540 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN 1985 Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci Biobehav Rev 9:599–612 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Song CK 2007 Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 48:1655–1672 [DOI] [PubMed] [Google Scholar]
- Schuhler S, Ebling FJ 2006 Role of melanocortin in the long-term regulation of energy balance: lessons from a seasonal model. Peptides 27:301–309 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Demas GE, Song CK 2002 Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones and the sympathetic nervous system. Exp Biol Med 227:363–376 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN 1985 Body weight, food intake and energy regulation in exercising and melatonin-treated Siberian hamsters. Physiol Behav 35:805–808 [DOI] [PubMed] [Google Scholar]
- Goldman BD, Darrow JM, Duncan MJ, Yogev L 1986 Photoperiod, reproductive hormones, and winter torpor in three hamster species. In: Heller CH, Musacchia XJ, Wang LCH, eds. Living in the cold: physiological and biochemical adaptations. New York: Elsevier; 341–350 [Google Scholar]
- Drazen DL, Demas GE, Nelson RJ 2001 Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus). Endocrinology 142:2768–2775 [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Nelson RJ 2004 Photoperiod controls the induction, retention, and retrieval of antigen-specific immunological memory. Am J Physiol Regul Integr Comp Physiol 286:R54–R60 [DOI] [PubMed] [Google Scholar]
- Heldmaier G, Steinlechner S 1981 Seasonal pattern and energetics of short daily torpor in the Djungarian hamster, Phodopus sungorus. Oecologia 48:265–270 [DOI] [PubMed] [Google Scholar]
- Butler MP, Trumbull JJ, Turner KW, Zucker I 2007 Timing of puberty and synchronization of seasonal rhythms by simulated natural photoperiods in female Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 293:R413–R420 [DOI] [PubMed] [Google Scholar]
- Hoffmann K 1973 The influence of photoperiod and melatonin on testis size, body weight, and pelage colour in the Djungarian hamster (Phodopus sungorus). J Comp Physiol 85:267–282 [Google Scholar]
- Hoffmann K 1981 The role of the pineal gland in the photoperiodic control of seasonal cycles in hamsters. In: Follett BK, Follett DE, eds. Biological clocks in seasonal reproductive cycles. Bristol, UK: Wright; 237–250 [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD 1993 The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res 15:161–190 [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ 2002 Melatonin regulates energy balance and attenuates fever in Siberian hamsters. Endocrinology 143:2527–2533 [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD 1983 Progonadal role of the pineal in the Djungarian hamster (Phodopus sungorus sungorus): mediation by melatonin. Endocrinology 113:1268–1273 [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD 1983 Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology 113:1261–1267 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN 1984 Effects of interscapular brown adipose tissue denervation on estrogen-induced changes in food intake, body weight and energy metabolism. Behav Neurosci 98:674–685 [DOI] [PubMed] [Google Scholar]
- Ng TB, Wong CM 1986 Effects of pineal indoles and arginine vasotocin on lipolysis and lipogenesis in isolated adipocytes. J Pineal Res 3:55–66 [DOI] [PubMed] [Google Scholar]
- Demas GE, Bartness TJ 2001 Direct innervation of white fat and adrenal medullary catecholamines mediate photoperiodic changes in body fat. Am J Physiol 281:R1499–R1505 [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ 1998 Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol 275:R291–R299 [DOI] [PubMed] [Google Scholar]
- Song CK, Bartness TJ 2001 CNS sympathetic outflow neurons to white fat that express melatonin receptors may mediate seasonal adiposity. Am J Physiol 281:R666–R672 [DOI] [PubMed] [Google Scholar]
- Youngstrom TG, Bartness TJ 1995 Catecholaminergic innervation of white adipose tissue in the Siberian hamster. Am J Physiol 268:R744–R751 [DOI] [PubMed] [Google Scholar]
- White JE, Engel FL 1958 A lipolytic action of epinephrine and norepinephrine on rat adipose tissue in vitro. Proc Soc Exp Biol Med 99:375–378 [DOI] [PubMed] [Google Scholar]
- Holm C 2003 Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31:1120–1124 [DOI] [PubMed] [Google Scholar]
- Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ 2004 Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol 286:R1167–R1175 [DOI] [PubMed] [Google Scholar]
- Freeman DA, Zucker I 2001 Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. Proc Natl Acad Sci USA 98:6447–6452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CK, Bartness TJ 1998 Dorsocaudal SCN microknife-cuts do not block short day responses in Siberian hamsters given melatonin infusions. Brain Res Bull 45:239–246 [DOI] [PubMed] [Google Scholar]
- Badura LL, Goldman BD 1992 Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain Res 598:98–106 [DOI] [PubMed] [Google Scholar]
- Purvis CC, Duncan MJ 1997 Discrete thalamic lesions attenuate winter adaptations and increase body weight. Am J Physiol 273:R226–R235 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD, Bittman EL 1991 SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am J Physiol 260:R102–R112 [DOI] [PubMed] [Google Scholar]
- Teubner BJ, Smith CD, Freeman DA 2008 Multiple melatonin target tissues mediate termination of photorefractoriness by long day lengths in Siberian hamsters. J Biol Rhythms 23:502–510 [DOI] [PubMed] [Google Scholar]
- Freeman DA, Kampf-Lassin A, Galang J, Wen JC, Prendergast BJ 2007 Melatonin acts at the suprachiasmatic nucleus to attenuate behavioral symptoms of infection. Behav Neurosci 121:689–697 [DOI] [PubMed] [Google Scholar]
- Song CK, Bartness TJ 1996 The effects of anterior hypothalamic lesions on short-day responses in Siberian hamsters given timed melatonin infusions. J Biol Rhythms 11:14–26 [DOI] [PubMed] [Google Scholar]
- Glass JD, Lynch GR 1982 Evidence for a brain site of melatonin action in the white-footed mouse, Peromyscus leucopus. Neuroendocrinology 34:1–6 [DOI] [PubMed] [Google Scholar]
- Dowell SF, Lynch GR 1987 Duration of the melatonin pulse in the hypothalamus controls testicular function in pinealectomized mice (Peromyscus leucopus). Biol Reprod 36:1095–1101 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN 1984 Photoperiodic control of body weight and energy metabolism in Syrian hamsters (Mesocricetus auratus): role of pineal gland, melatonin, gonads, and diet. Endocrinology 114:492–498 [DOI] [PubMed] [Google Scholar]
- Anand S, Losee-Olson S, Turek FW, Horton TH 2002 Differential regulation of luteinizing hormone and follicle-stimulating hormone in male Siberian hamsters by exposure to females and photoperiod. Endocrinology 143:2178–2188 [DOI] [PubMed] [Google Scholar]
- Hira Y, Sakai Y, Matsushima S 2001 Effects of photoperiod and melatonin on the development of growth hormone cells and the pituitary-adrenal axis in the Djungarian hamster, Phodopus sungorus. Arch Histol Cytol 64:211–222 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ 2007 The mouse brain in stereotaxic coordinates. 2nd ed. New York: Academic Press [Google Scholar]
- Bowers RR, Gettys TW, Prpic V, Harris RBS, Bartness TJ 2005 Short photoperiod exposure increases adipocyte sensitivity to noradrenergic stimulation in Siberian hamsters. Am J Physiol 288:R1354–R1360 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, McGriff WR, Maharaj MP, Borer KT 1991 Photoperiodic control of serum insulin: effects on energy balance and reproductive status in Siberian hamsters. FASEB J 5:4409 (Abstract) [Google Scholar]
- Bartness TJ 1995 Short day-induced depletion of lipid stores is fat pad- and gender-specific in Siberian hamsters. Physiol Behav 58:539–550 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN, Goldman BD 1987 Are the short photoperiod decreases in serum prolactin responsible for the seasonal changes in energy balance in Syrian and Siberian hamsters? J Exp Zool 244:437–454 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD 1988 Peak duration of serum melatonin and short day responses in adult Siberian hamsters. Am J Physiol 255:R812–R822 [DOI] [PubMed] [Google Scholar]
- Weaver DR, Provencio I, Carlson LL, Reppert SM 1991 Melatonin receptors and signal transduction in photorefractory Siberian hamsters (Phodopus sungorus). Endocrinology 128:1086–1092 [DOI] [PubMed] [Google Scholar]