Abstract
TNFα is an inflammatory-related cytokine that has inhibitory effects on gonadotropin- and cAMP-stimulated steroidogenesis and folliculogenesis. Because ovulation is an inflammatory reaction and TNF specifically induces serum amyloid A3 (SAA3) in mouse granulosa cells, the effect of cAMP on TNF-induced SAA3 promoter activity, mRNA and protein was investigated. Granulosa cells from immature mice were cultured with TNF and/or cAMP. TNF increased SAA3 promoter activity, mRNA, and protein, which were further increased by cAMP. cAMP alone increased SAA3 promoter activity, but SAA3 mRNA and protein remained undetectable. Thus, there appeared to be different mechanisms by which TNF and cAMP regulated SAA3 expression. SAA3 promoters lacking a nuclear factor (NF)-κB-like site or containing its mutant were not responsive to TNF but were responsive to cAMP. Among four CCAAT-enhancing binding protein (C/EBP) sites in the SAA3 promoter, the C/EBP site nearest the NF-κB-like site was required for TNF-induced SAA3. The C/EBP site at −75/−67 was necessary for responsiveness to cAMP. Dominant-negative C/EBP and cAMP response element-binding protein or short interfering RNA of C/EBPβ blocked TNF- or cAMP-induced SAA3 promoter activity. The combination of TNF and cAMP increased C/EBPβ protein above that induced by TNF or cAMP alone. Thus, cAMP in combination with TNF specifically induced C/EBPβ protein, leading to enhanced SAA3 expression but requiring NF-κB in mouse granulose cells. In addition, like TNF, SAA inhibited cAMP-induced estradiol accumulation and CYP19 levels. These data indicate SAA may play a role in events occurring during the ovulation process.
C/EBPβ and NF-κB cooperate at the level of the promoter in inducing granulosal serum amyloid A3 expression.
TNFα, a multifunctional hormone-like polypeptide, modulates many genes involved in inflammation, infection, and malignancy. TNF protein and mRNA have been localized to several ovarian compartments including the granulosa, theca, oocyte, follicular fluid, and corpus luteum and in multiple species including human, mouse, rat, pig, cow, rabbit, chicken, and sheep (1,2,3,4,5,6,7,8,9,10,11,12,13).
Several studies indicate ovarian and systemic levels of TNF fluctuate during the cycle. During the menstrual cycle in women, serum levels of TNF were increased during the late follicular and midluteal phases (14). Another study demonstrated higher TNF levels in the luteal phase compared with the early follicular phase. This study also found serum levels of TNF were higher in women compared with men (15). TNF levels in follicular fluid were higher after controlled ovarian hyperstimulation compared with during a natural cycle indicating potential gonadotropin regulation of ovarian TNF secretion (16). In the rat, ovarian levels of TNF fluctuated at the first ovulation during puberty during which levels were high before and then decreased after the first ovulation (17). In addition, in the immature equine chorionic gonadotropin (eCG)/human chorionic gonadotropin (hCG)-treated rat, ovarian levels of TNF decreased after eCG administration, and both ovarian and serum levels of TNF increased after hCG administration (17). TNF levels within corpora lutea also changed, dependent on stage of the cycle. TNF mRNA was significantly higher in corpora lutea collected during the late luteal phase compared with early luteal phase in macaques (18).
In regard to biology, TNF can affect many ovarian processes. TNF inhibits gonadotropin/cAMP-stimulated steroidogenesis (19,20,21,22,23) and supports folliculogenesis by modulating granulosal proliferation (24,25). Increased levels of TNF in the follicular fluid of preovulatory follicles in sheep after GnRH synchronization were shown to regulate collagenolytic bioactivity necessary for ovulation (26). A role for TNF in ovulation was further demonstrated using the perfused rat ovary in which TNF alone induced ovulation and TNF enhanced ovulation in response to LH, whereas injection of antibodies to TNF inhibited ovulation (27,28,29). The role of TNF in luteal function is also complex. TNF can be luteotropic or luteolytic dependent at least in part on the balance of prostaglandin synthesis in response to TNF (18,30,31). The association of altered TNF and infertility further highlights the importance of TNF in normal ovarian function. For example, serum concentrations of TNF were lower in women with premature ovarian failure compared with normal cycling women (32). Follicular fluid levels of TNF were elevated in women with infertility due to immunological reasons (presence of antisperm or antinuclear antibodies) compared with women with tubal factors. In addition, higher TNF levels correlated with lower follicular fluid estradiol and significantly lower fertilization rates (33). Another study reported poor oocyte quality in patients with elevated TNF in the follicular fluid (34). Additional studies in cattle demonstrated TNF negatively affects oocyte maturation (35). Elevated serum levels of TNF are also found in women with polycystic ovarian syndrome, including both normal-weight and obese women compared with women without polycystic ovarian syndrome (36,37). Thus, abnormalities in ovarian function are associated with altered levels of TNF.
A previous study exploring the mechanisms of TNF action in mouse granulosa cells found that TNF specifically increased serum amyloid A3 (SAA3) (38). Before that study SAA mRNA had been observed in the whole ovaries of hamster, rabbit, and mink (39,40,41,42,43). Subsequently, array analyses of mouse cumulus oocyte complexes demonstrated increased SAA3 mRNA after eCG/hCG administration (44). SAA3 is an acute-phase protein that has classically been associated with inflammatory states (45). The SAA family of genes and proteins has been described in mammals, marsupials, and fish; however, human and mouse have been most thoroughly studied (45). The SAA family consists of four genes (SAA 1, -2, -3, -4) and three proteins (SAA1, -2, -4) in the human and five genes (Saa1, -2, -3, -4, ps1) and four proteins (Saa1, -2, -3, -4) in the mouse; each has one pseudogene. These apolipoproteins serve diverse functions; SAA has been shown to promote the reverse transport of cholesterol from damaged tissue; activate cholesterol ester hydrolase; serve as a chemoattractant to monocytes, leukocytes, mast cells, and T lymphocytes; induce cytokine production; and induce expression of enzymes including collagenase, stromelysin, and matrix metalloproteinases. Although the significance is not yet understood, it is interesting to note serum levels of SAA, like TNF, are higher in women compared with men (46).
Because ovulation is an inflammatory process (47,48,49) and TNF levels increase around ovulation, SAA production within the ovary in response to gonadotropin and TNF may play a role in the changes occurring during the ovulatory process. Therefore, the interaction of cAMP and TNF in the regulation of SAA3 was addressed in the present studies. Results from these studies indicate granulosal SAA3 expression is regulated by both cAMP and TNF via CCAAT-enhancing binding protein (C/EBP)-β and nuclear factor-κB (NF-κB) p65. Furthermore, like TNF, SAA inhibited cAMP-induced estradiol accumulation and CYP19 mRNA expression by granulosa cells.
Materials and Methods
Reagents
Recombinant murine TNF was obtained from R&D Systems (Minneapolis, MN). The following reagents were purchased from Sigma (St. Louis, MO): penicillin G/streptomycin, fibronectin, 17β-estradiol, progesterone, eCG, hCG, lipopolysaccharide (LPS), and 8-bromo cAMP. Lipofectamine Plus, TRIzol, and all liquid culture media were acquired from Invitrogen (Grand Island, NY). Antisense and sense oligonucleotides were obtained from Integrated DNA Technologies (Coralville, IA). Human SAA1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used at a concentration of 10 μg/ml, a dose correlating to plasma levels measured in women (46) and mice (50,51). The SAA3 antibody was kindly provided by Dr. Philipp Scherer (Albert Einstein College of Medicine, Bronx, NY). The expression plasmid for pMSV-C/EBPβ was kindly obtained from Dr. Steven L. McKnight (The Ohio State University, Columbus, OH). The dominant negative-cAMP response element-binding protein (CREB) and dominant negative-C/EBP were provided by Dr. Charles Vinson (National Cancer Institute/National Institutes of Health, Bethesda, MD). Both were prepared by fusing an acidic amphipathic protein sequence onto the N terminus of the CREB or C/EBP leucine zipper domain. The acidic extension of the dominant negative-protein interacts with the basic region of the endogenous protein forming a coiled-coil extension of the leucine zipper and preventing the basic region of the endogenous protein from binding to DNA (52,53). C/EBP, p65, and β-actin antibodies and short interfering RNA (siRNA) for C/EBPβ were purchased from Santa Cruz Biotechnology. pCRE-luc vector was purchased from BD Biosciences (San Jose, CA). The phospho-inhibitory-κB (IκB; Ser 32/36) and inhibitory-κB antibodies were purchased from Cell Signaling Technology (Beverly, MA). The Luciferase reporter assay system and siRNA transfection reagent were obtained from Promega (Madison, WI).
Animals
C57BL6 mice (Harlan, Inc., Indianapolis, IN) and p55 TNF receptor type 1 null mice on a C57BL6 background (Immunex, Seattle, WA) were established as breeding colonies in our laboratory. All mice were given commercial feed and drinking water ad libitum and housed in a controlled light-dark cycle (12 h each) under pathogen-free conditions. For assessment of ovarian SAA3 mRNA after in vivo gonadotropin, eCG (2.5 IU) was administered on d 25 of age followed by hCG (2.5 IU) 52 h later. Ovaries were isolated 48 and 72 h after eCG. In a separate group of mice, LPS (2.5 mg/kg body weight, ip) was administered on d 25, and ovaries were collected 48 h later and were used as a positive control for SAA3 mRNA. Total ovarian RNA was extracted and subjected to RT-PCR as described. All handling of animals and procedures conformed to the guidelines set forth by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
Cell cultures and treatments
Ovaries were collected under a laminar flow hood from mice 28 d of age and placed in cold DMEM/F12 supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml). The ovaries were cleaned of connective tissues, and fat and follicles were punctured using a 27-gauge needle attached to a 1-ml syringe to extrude granulosa cells. Before culture, plates were coated overnight with 4 μg fibronectin/ml of Hanks’ solution. Granulosa cells (2 × 105 cells/ml; viability was determined to be ∼90% by the exclusion of trypan blue) were cultured at 37 C in a water-saturated atmosphere of 95% air and 5% CO2 in fibronectin-coated 24- or six-well culture plates with serum-free DMEM/F12 medium containing penicillin/streptomycin. All experiments were initiated after overnight culture (18–20 h) to allow for cellular attachment; when the medium was removed, fresh medium was added, and treatments were initiated as outlined in Results. Androstenedione (1 μm) was added to cultures as substrate when steroid end points were measured.
RT-PCR and Northern blot
Total RNA was isolated using TRIzol reagent (Invitrogen). RT-PCR for SAA3 was as previously described (38). RT-PCR for CYP19 was carried out using the same conditions and specific primers for mouse CYP19, 5′-TCA ATA CCA GGT CCT GGC TA-3′ (forward) and 5′-TGC TTG ATG GAC TCC ACA CA-3′ (reversed). L19 was used as a loading control as previously described (54). Amplified PCR products were purified using a spin-column (QIAGEN, Valencia, CA), and the PCR products were inserted into pGEM-T vector. Antisense and sense linear template DNAs were made by restricting with SpeI and NcoI, respectively, and were labeled with digoxigenin-uridine 5-triphosphate (Roche, Indianapolis, IN) using SP6 and T7 RNA polymerase. Total RNA (5 μg) was separated on a 1.0% agarose-formaldehyde gel in 1× 3[N-morholino]propanesulfonic acid buffer, and the gel was photographed under UV light to visualize 28S and 18S rRNA as size markers, transferred onto a nylon membrane, and UV cross-linked. The membrane was subsequently prehybridized and hybridized with SAA3 RNA probe and washed with 2× saline sodium citrate plus 0.1% sodium dodecyl sulfate followed by 0.1× saline sodium citrate plus 0.1% sodium dodecyl sulfate. The membrane was rinsed briefly in maleic acid buffer [0.1 m maleic acid and 0.15 m NaCl (pH 7.5)] with 0.3% Tween 20 and incubated in 1× blocking solution (Roche) followed by incubating for 30 min with antidigoxigenin-alkaline phosphatase (1:10,000) in blocking solution. The membrane was washed in maleic acid buffer with Tween 20 and equilibrated in detection buffer [0.1 m Tris-HCl and 0.1 m NaCl (pH 9.5)]. After placing the membrane in disodium 3-(4-methoxyspiro[1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13, 7]decan]-4-yl) phenyl phosphate (1:100) in detection buffer, the membrane was incubated for 10 min at 37 C to enhance luminescence and then exposed to x-ray film. Bands then were quantitated by image analyses using Gel Pro software (Media Cybernetics, Bethesda, MD). 28S and 18S rRNAs were used as loading controls.
Construction of the SAA3 promoter-luciferase gene
The mSAA3-198LUC, mSAA3-172LUC, mSAA3-143LUC, and mSAA3-109LUC promoters were constructed as previously described (38). Additional deletion mutants were generated from the mSAA3–198LUC using the following primers: 5′-GCA CTC GAG GAA GAC TTC AGA AAG TC-3′ for −92/+73 deleted construct (mSAA3–92LUC), 5′-GCG CTC GAG TAT CTT CTG AAA GAG AA-3′ for −78/+73 deleted construct (mSAA3-78LUC), and 5′-GCG CTC GAG TAT CTT CTG AAA GAG AA-3′ for −36/+73 deleted construct (mSAA3–36LUC). Site-directed mutants were generated from the mSAA3-172LUC with the following sites (lowercase letters): GctAATGCCT for atypical mutant NF-κB site (−164/−154), TGGCGCttT for C/EBP-4 site (−150/−142), TGGGGAttG for C/EBP-3 site (−140/−132), TTCTGAttG for C/EBP-2 site (−105/−97), and TTATG CttG for C/EBP-1 site (−75/−67). In addition, C/EBP mutants of mSAA3–78LUC of the same size (−78/+73) were generated with TTATG CttG for C/EBP-1 site (−75/−67).
Transient transfection and the luciferase assay
Granulosa cells at approximately 50% confluency in 24-well plates were washed once with fresh DMEM/F12 (without additives) and were transiently transfected with vectors for 3.5 h at 37 C using Lipofectamine Plus solution (Invitrogen). Transfected cells were treated as outlined in Results and incubated for 16 h. After rinsing cells with ice-cold PBS and adding lysis buffer, cell lysates were centrifuged at 12,000 × g for 1 min at room temperature. The supernatant fluid was then used for determination of luciferase activity using a microplate luminometer. Luciferase activity expressed as relative light units was normalized to the protein level. Proteins were measured using the method of Bradford (55).
Western blot
Western blot was carried out as previously described (38) using primary antibodies at 1:1000 dilution. β-Actin was immunologically detected to serve as an internal loading control.
DNA microarray
After isolating total RNA, an Mouse DNA microarray (Murine Genome U74A version 2; Affymetrix, Santa Clara, CA) was performed as previously described (38).
In situ hybridization
Nonradioactive methods for in situ hybridization in cultured cells were performed as previously described (56) using digoxigenin-labeled SAA3 RNA probes (antisense and sense) as described for Northern blot.
Immunohistochemistry
Granulosa cells (2 × 104) were seeded into eight-chamber slides and after overnight incubation to allow for attachment, treatments were initiated. Immunostaining was carried out as previously described (57,58) using C/EBPβ antibody at a dilution of 1:1000.
Steroid analysis
Concentrations of estradiol and progesterone in culture media were determined by RIA as previously described (59). Data presented (mean ± sem from three to five replicates) are results from a single experiment and are representative of the results from at least three independent experiments.
Statistics
Data were analyzed by Student’s t test or one-way ANOVA. If statistical significance (P ≤ 0.05) was detected by ANOVA, the data were further analyzed by Tukey’s test to detect specific differences between groups. All experiments were repeated at least three independent times unless indicated differently.
Results
SAA inhibits cAMP-induced estradiol production and CYP19 expression in mouse granulosa cells
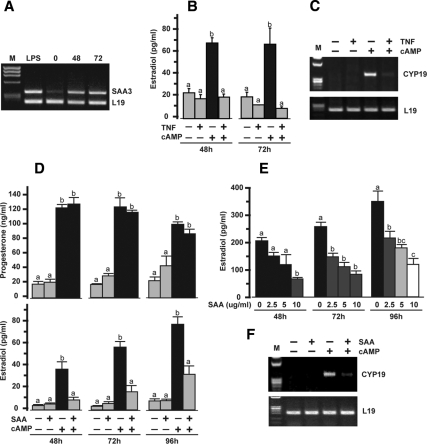
Ovarian expression of SAA3 mRNA was increased 48 h after the administration of eCG to immature mice. Expression remained elevated 20 h after an ovulatory dose of hCG (Fig. 1A). The ability of TNF to inhibit cAMP-stimulated estradiol production and granulosal CYP19 mRNA in cultured mouse granulosa cells is illustrated in Fig. 1, B and C. These results in the mouse granulosa culture system are similar to previous findings in other systems (19,20,21,22,23). Our previous studies further demonstrated TNF induced SAA3 production by mouse granulosa cells (38). Thus, the possibility that TNF’s action on granulosal estradiol production was mediated by TNF-induced SAA was first tested by determining the effects of SAA on granulosal steroid production. Effects of SAA were examined using 10 μg/ml SAA, similar to the systemic mean level of 10.7 mg/liter reported in women (46) and similar to the range of 9.2 and 78 μg/ml reported in mice (50,51). Treatment of mouse granulosa cells with cAMP resulted in increased progesterone and estradiol production (Fig. 1D). cAMP-stimulated estradiol production was significantly inhibited by the addition of SAA to the cultures. On the other hand, SAA had no effect on cAMP-stimulated progesterone (Fig. 1D). In addition, SAA treatment had no effect on basal production of either progesterone or estradiol (Fig. 1D). Effects of SAA on cAMP-stimulated estradiol production were dose dependent (Fig. 1E). Expression of CYP19 mRNA was increased after treatment of granulosa with cAMP, and the cAMP-induced increase was inhibited by the addition of SAA to the culture (Fig. 1F). SAA treatment alone had no detectable effect on levels of CYP19 mRNA.
Figure 1.
Effects of TNF and SAA on mouse granulosa cell progesterone and estradiol production and CYP19 mRNA expression. A, Effects of eCG and hCG administration on mouse ovarian SAA3 mRNA. Ovaries were collected 0, 48, and 72 h after eCG (2.5 IU); hCG was administered 52 h after eCG. Ovaries were collected from LPS-treated mice and serve as a positive control. L19 was used as a loading control for RT-PCR. M, Molecular marker. B, Effects of TNF (10 ng/ml) in the presence and absence of cAMP (500 μm) on estradiol production after 48 and 72 h. C, Effects of TNF (10 ng/ml) in the presence and absence of cAMP (500 μm) on granulosal CYP19 expression after 72 h. L19 was used as a loading control for RT-PCR. M, Molecular marker. D, Effects of SAA (10 μg/ml) in the presence and absence of cAMP (500 μm) on progesterone and estradiol production after 48, 72, and 96 h. E, Dose-dependent effects of SAA (0–10 μg/ml) on cAMP (500 μm)-stimulated estradiol production after 48, 72, and 96 h. F, Effects of SAA (10 μg/ml) in the presence and absence of cAMP (500 μm) on CYP19 expression after 96 h. L19 was used as a loading control for RT-PCR. M, Molecular marker. Bars with different colors and letters are significantly different (P ≤ 0.05) by ANOVA and Tukey’s test.
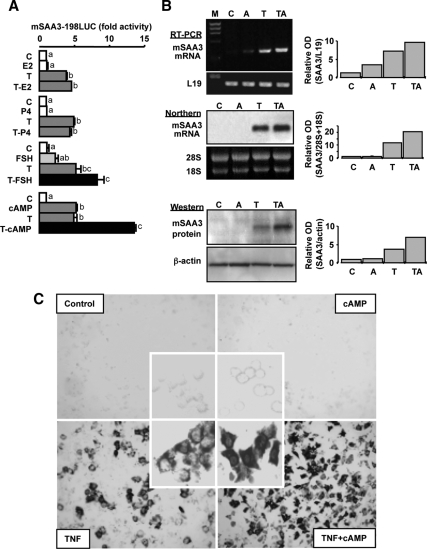
cAMP enhances TNF-induced SAA3 expression in granulosa cells
To begin to address the mechanisms whereby TNF and SAA interact in granulosal steroid production, effects of estradiol, progesterone, FSH, and cAMP on basal and TNF-induced SAA3 promoter activity were determined. The ovarian steroids estradiol and progesterone, in the presence or absence of TNF, had no effect on SAA3 promoter (mSAA3-198LUC) activity (Fig. 2A). Interestingly, FSH and cAMP each alone increased SAA3 promoter activity and further enhanced TNF-induced promoter activity (Fig. 2A). Although cAMP alone slightly increased SAA3 mRNA by RT-PCR (Fig. 2B), the increase was not sufficient to be detected by Northern and Western blots (Fig. 2B) and in situ hybridization (Fig. 2C). cAMP in combination with TNF further increased SAA3 mRNA and protein in mouse granulosa cells (Fig. 2B), and the effect on SAA3 mRNA was confirmed by in situ hybridization, showing intense signals in the cytoplasm (Fig. 2C).
Figure 2.
Effects of hormones and cAMP on TNF-induced SAA3. Panel A, Effects of estradiol, progesterone, FSH, and cAMP on TNF-induced SAA3 promoter (mSAA3-198LUC) activity in mouse granulosa cells. Cells were transfected for 3.5 h with SAA3 promoter luciferase reporter and then incubated with vehicle or TNF (10 ng/ml) in the absence or presence of steroids [estradiol (E2), 1 μm; progesterone (P4), 1 μm], FSH (10 ng/ml), or cAMP (500 μm) for 16 h. Luciferase activity was normalized using total protein concentrations and expressed as a fold increase compared with the control. Bars with different colors and letters are significantly different (P ≤ 0.05) by ANOVA and Tukey’s test. C, Control; T, TNF; LUC, luciferase. Panel B, Effect of cAMP on TNF-induced SAA3 mRNA and protein in mouse granulosa cells. After isolating total RNAs and proteins, RT-PCR, Northern and Western blots were performed using specific primers, cDNA probe, and antibody for SAA3, respectively. Loading controls, L19, 28S, and 18S, and β-actin were used for RT-PCR and Northern and Western blot, respectively. These figures represent data from duplicate experiments. M, Molecular marker; C, control; A, cAMP; T, TNF; TA, TNF+cAMP. Panel C, Effect of cAMP on TNF-induced SAA3 mRNA in cultured mouse granulosa cells determined by in situ hybridization.
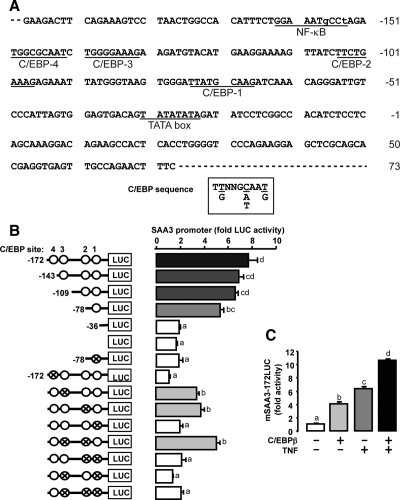
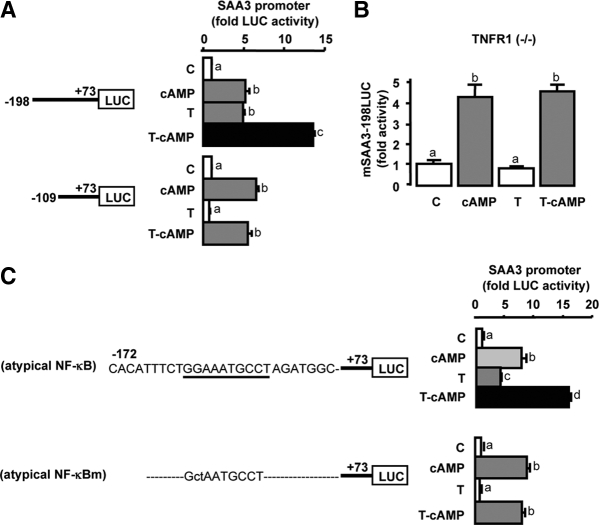
TNF and cAMP interact in regulating SAA3 promoter activity through cooperation of C/EBP and NF-κB sites
The nucleotide sequence of the mouse SAA3 promoter (−198/+73) contains one atypical NF-κB site and four C/EBP sites (see Fig. 4A). TNF-induced promoter activity when the atypical NF-κB site was present (mSAA3-198LUC) but had no effect on the promoter that lacked the atypical NF-κB binding site (mSAA3-109LUC) (Fig. 3A). However, cAMP treatment increased promoter activity in both SAA3 promoters independent of the presence of the atypical NF-κB site (mSAA3-198LUC and mSAA3-109LUC). In addition, cAMP further enhanced promoter activity induced by TNF on the mSAA3-198LUC promoter (including the atypical NF-κB site) but not the mSAA3-109LUC promoter lacking the atypical NF-κB binding site (Fig. 3A). In granulosa cells cultured from TNF receptor-1 knockout mice (−/−), cAMP increased SAA3 promoter (mSAA3-198LUC) activity, whereas TNF treatment had no effect (Fig. 3B). Furthermore, cAMP increased the activity of SAA3 promoters containing either the endogenous atypical NF-κB site or a mutant NF-κB binding site and further increased the activity of the TNF-induced mSAA3-172LUC promoter containing the atypical NF-κB binding site (Fig. 3C). On the other hand, TNF did not increase the activity of the SAA3 promoter containing a mutated (m) atypical NF-κB binding site (Fig. 3C). These differential results indicate TNF-mediated activity requires the atypical NF-κB site, whereas the atypical NF-κB site is not required for cAMP-mediated SAA3 promoter activity.
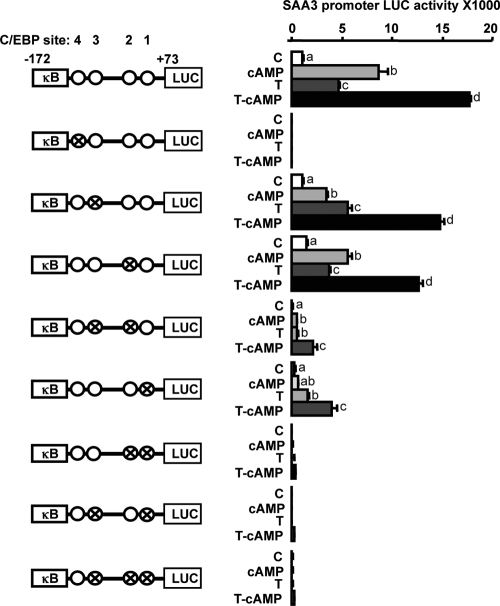
Figure 4.
Involvement of C/EBP sites in cAMP-regulated SAA3 promoter activity. A, Nucleotide sequence of SAA3 promoter (−198/+73). The SAA3 promoter contained one atypical NF-κB and four C/EBP sites. Lowercase letters indicate base pairs different from consensus sites. B, Effects of cAMP on the activity of SAA3 promoters containing serially deleted and mutated C/EBP sites in mouse granulosa cells. Data for each promoter is fold change with cAMP treatment compared with control; statistical significance (P ≤ 0.05) by Student’s t test is indicated by a colored bar. Comparison of camp-stimulated activity between promoters was by ANOVA and Tukey’s test, and bars with different colors and numbers are statistically different (P ≤ 0.05). C, Effects of C/EBPβ on TNF-induced SAA3 promoter (mSAA3-172LUC) activity in mouse granulosa cells. Cells were transfected for 3.5 h with mSAA3 promoter with or without a C/EBPβ expression vector (100 ng/ml) and then incubated with or without cAMP (500 μm) for 16 h. Luciferase activity was normalized to total protein concentration and expressed as a fold change compared with the control. Bars with different colors and letters are significantly different (P ≤ 0.05) by ANOVA and Tukey’s test. Cross and open circles indicate mutated and normal C/EBP sites, respectively. LUC, Luciferase.
Figure 3.
Differential mechanisms between cAMP and TNF in regulating SAA3 promoter activity. A, Effects of cAMP on the luciferase activity of TNF-induced SAA3 promoters (mSAA3-198LUC and mSAA3-109LUC) in mouse granulosa cells. B, Effects of cAMP and TNF on SAA3 promoter (mSAA3-198LUC) activity in granulosa cells cultured from p55 TNF receptor type 1 (TNFR1) knockout mice (−/−). C, Effects of cAMP and TNF on the activity of SAA3 promoters containing an atypical NF-κB binding site and its mutant in mouse granulosa cells. Cells were transfected for 3.5 h with various mSAA3 promoters and then were incubated with or without TNF (10 ng/ml) in the absence or presence of cAMP (500 μm) for 16 h. Luciferase activity was normalized using total protein concentrations and expressed as a fold change in comparison with the control. Bars with different colors and letters are significantly different (P ≤ 0.05) by ANOVA and Tukey’s test. Atypical NF-κB sequence is underlined and its mutant (atypical NF-κBm) is indicated by lowe-case letters. C, Control; T, TNF; LUC, luciferase.
The significance of each C/EBP site in cAMP-induced promoter activity was examined by deletion and mutation analysis. Compared with pGL3 basic vector alone (LUC), cAMP increased the luciferase activity of mSAA3–172LUC (four C/EBP sites), mSAA3-143LUC (three C/EBP sites), mSAA3-109LUC (two C/EBP sites), and mSAA3-78LUC (one C/EBP site), whereas it did not increase that of mSAA3-36LUC (no C/EBP site) (Fig. 4B). The mSAA3-78LUC containing a mutant C/EBP-1 site was not responsive to cAMP. Furthermore, mSAA3-172LUC containing an intact C/EBP-1 site combined with mutation of either or both C/EBP sites 2 and 3 maintained responsiveness to cAMP indicating the C/EBP-1 site (−75/−67) was necessary for responsiveness to cAMP (Fig. 4B). The promoter containing a mutant C/EBP-4 site (−150/−142), which is located near the atypical NF-κB site, was not responsive to cAMP, whereas the deleted constructs containing at least the C/EBP-1 site but without the C/EBP-4 site were still responsive to cAMP (Fig. 4B). These data imply that the C/EBP-1 site is required for cAMP-stimulated promoter activity; however, there also appears to be an interaction with the C/EBP-4 site. Because granulosa cells expressed mainly the C/EBPβ isoform (Table 1), a C/EBPβ expression vector was selected to test its effects on SAA3 promoter activity. Overexpression of C/EBPβ in the presence and absence of TNF increased SAA3 promoter activity and the C/EBPβ further enhanced TNF-induced promoter activity (Fig. 4C).
Table 1.
Effects of TNF on C/EBP isoforms in mouse granulosa cells as determined by Affymetrix microarray
| Gene | Account | Control | TNF | Ratio |
|---|---|---|---|---|
| C/EBPα | M62362 | 724 (P) | 624 (P) | 0.86 |
| C/EBPβ | M61007 | 2571 (P) | 3615 (P) | 1.41 |
| C/EBPδ | X61800 | 179 (A) | 283 (A) | 1.58 |
Granulosa cells were cultured in the presence of vehicle (control) or TNF (10 ng/ml) for 24 h. Expression signals were conducted with a 3-μm pixel value and 570-nm wavelength. Target signal lower limit of detection is 500. A, Absent; P, present.
Because the cAMP-induced increase in SAA3 promoter activity apparently involved C/EBP sites (Fig. 4), the interaction of the C/EBP sites and the atypical NF-κB site were further investigated using cAMP and TNF in the mSAA3-172LUC promoter with specifically mutated C/EBP sites. Mutation of the C/EBP-4 site (−150/−142) located near the atypical NF-κB site resulted in complete loss of cAMP- and TNF-induced SAA3 promoter activity (Fig. 5). Mutation of C/EBP-3 (−140/−132) or C/EBP-2 (−105/−97) had no effect on SAA3 promoter activity; however, mutation of both sites resulted in reduced cAMP- and TNF-induced activity. The mutation of the C/EBP-1 site (−75/−67) resulted in reduced promoter activity and the combination of any other C/EBP mutation together with mutation of the C/EBP-1 site resulted in further loss of activity (Fig. 5). These data indicate the C/EBP-4 site is likely functioning as a bidirecting site involving the upstream atypical NF-κB site and the other downstream C/EBP sites and thus is a critical contributor in regulating SAA3 promoter activity.
Figure 5.
Identification of C/EBP sites regulating SAA3 promoter activity. Interaction of cAMP and TNF on the activity of mSAA3-172LUC containing mutated C/EBP sites in mouse granulosa cells is shown. Cross and open circles indicate mutated and intact C/EBP sites, respectively. Cells were transfected for 3.5 h with mSAA3 promoters and then incubated with vehicle or TNF (10 ng/ml) in the absence or presence of cAMP (500 μm) for 16 h. Luciferase activity is reported and was normalized to total protein concentrations. Bars with different colors and letters are significantly different (P ≤ 0.05) by ANOVA and Tukey’s Test. κB, NF-κB site; C, control; T, TNF; LUC, luciferase.
C/EBP and CREB mediate cAMP- and TNF-induced SAA3 promoter activity
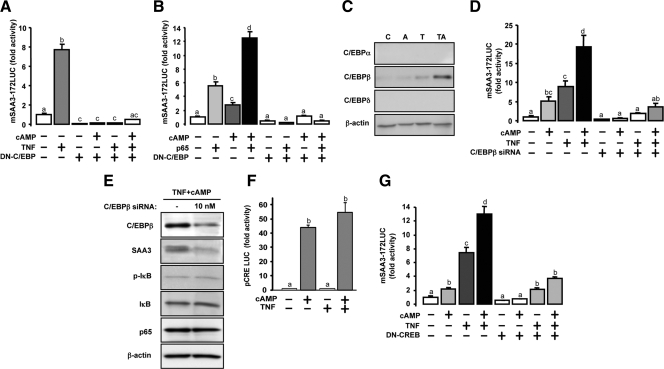
Transient transfection of mouse granulosa cells with a dominant-negative C/EBP resulted in reduced basal and cAMP- and TNF-induced SAA3 promoter activity (Fig. 6A). Because the p65 (not p50) component of NF-κB increased SAA3 promoter activity in mouse granulosa cells (38), the interaction between cAMP and p65 on SAA3 promoter activity was further investigated. Similar to TNF, NF-κB p65 increased SAA3 promoter activity, which was further increased by cAMP (Fig. 6B). Transfection with a dominant-negative C/EBP blocked the stimulatory effect of cAMP and p65, each alone and in combination, on SAA3 promoter activity (Fig. 6B). These results indicate C/EBP is a critical contributor in regulating cAMP- and TNF-induced SAA3 promoter activity.
Figure 6.
Effects of dominant-negative C/EBP, C/EBPβ siRNA, and dominant-negative CREB on cAMP-, TNF-, and p65-induced SAA3 promoter activity. Panel A, Effect of dominant-negative C/EBP on TNF-induced SAA3 promoter activity in mouse granulosa cells. DN, Dominant-negative. Panel B, Effect of dominant-negative C/EBP on interaction between cAMP and p65 in increasing SAA3 promoter activity. Cells were transfected for 3.5 h with mSAA3-172LUC alone or with dominant-negative C/EBP vector (100 ng/ml) and p65 expression vector (10 ng/ml) and then incubated with TNF (10 ng/ml) alone and/or with cAMP (500 μm) for 16 h. The luciferase activity was normalized using total protein concentrations and expressed as a fold change in comparison with the control. DN, Dominant negative; LUC, luciferase. Panel C, Effects of TNF and cAMP on C/EBP isoforms in mouse granulosa cells. Cells were cultured with TNF (10 ng/ml) and cAMP (500 μm) alone and in combination for 24 h. Each lane was loaded with 20 μg of total protein using whole-cell lysates. β-Actin was used as a loading control. C, Control; A, cAMP; T, TNF; TA, TNF+cAMP. Panel D, Inhibitory effect of C/EBPβ siRNA on cAMP and TNF in enhancing SAA3 promoter activity. Cells were transfected for 24 h with C/EBPβ siRNA (10 nm) and then further transfected for 3.5 h with mSAA3-172LUC. Transfected cells were incubated with TNF (10 ng/ml) alone, cAMP (500 μm) alone, and in combination for 16 h. Panel E, Effect of C/EBPβ siRNA on C/EBPβ, SAA3, phospho-IκB, IκB, and p65 protein expression after cAMP and TNF treatments in mouse granulosa cells. Cells were transfected for 24 h with C/EBPβ siRNA (10 nm) and then incubated with TNF (10 ng/ml) and cAMP (500 μm) in combination for 24 h. Panel F, Effect of cAMP and TNF on pCRE-luc promoter activity in granulosa cells. Cells were transfected for 3.5 h with pCRE-luc and then incubated with TNF (10 ng/ml) alone, cAMP (500 μm) alone, and in combination for 16 h. Luciferase activity was normalized using total protein concentrations and expressed as a fold change in comparison with the control. Panel G, Effect of dominant-negative CREB on interaction between cAMP and TNF in increasing SAA3 promoter activity. Cells were transfected for 3.5 h with mSAA3–172LUC alone or with dominant-negative CREB vector (100 ng/ml) and then incubated with TNF (10 ng/ml) alone and/or with cAMP (500 μm) for 16 h. Luciferase activity was normalized using total protein concentrations and expressed as a fold change in comparison with the control. Different color bars indicate significant differences (P ≤ 0.05) among groups. DN, Dominant-negative; LUC, luciferase.
The combination of cAMP and TNF increased mouse granulosal C/EBPβ protein greater than either cAMP or TNF alone (Fig. 6C). Although C/EBPα mRNA was expressed at a marginal level when compared with the target signal in array analysis, its protein was not detectable by Western blot. In addition, C/EBPδ isoform protein expression was not detectable in granulosa cells (Table 1 and Fig. 6C). C/EBPβ siRNA inhibited SAA3 promoter activity induced by cAMP and TNF (Fig. 6D). Granulosa cells treated with C/EBPβ siRNA and stimulated with cAMP and TNF expressed reduced C/EBPβ and SAA3 proteins (Fig. 6E). In contrast, phospho-IκB, IκB, and p65 were not affected by C/EBPβ siRNA (Fig. 6E). Because C/EBPβ is regulated by the protein kinase A (PKA)/CREB pathway (60), effects of cAMP on cAMP response element (CRE) reporter activity and effects of a dominant-negative CREB was used to clarify whether CREB plays a role in the cAMP- and TNF-induced SAA3 promoter activity. CRE reporter activity was significantly increased when mouse granulosa cells were treated with cAMP. Treatment with TNF had no effect on basal or cAMP-stimulated activity (Fig. 6F). Overexpression of dominant-negative CREB blocked SAA3 promoter activity induced by TNF or cAMP when compared with its empty vector (Fig. 6G). These results indicate the C/EBPβ isoform regulates cAMP- and TNF-induced SAA3 promoter activity via the CREB pathway in mouse granulosa cells.
C/EBPβ protein expression is enhanced by cAMP and TNF in combination
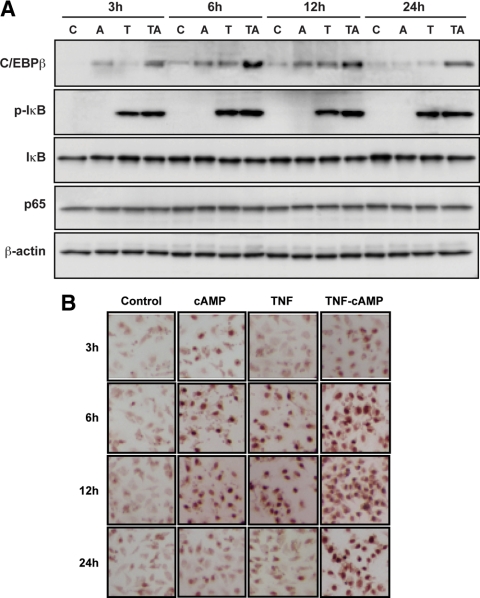
Mouse granulosa cells treated with TNF expressed increased phospho-IκB. Addition of cAMP had no significant effect on TNF-induced phospho-IκB expression (Fig. 7A). IκB and p65 proteins were not altered throughout 24 h (Fig. 7A). The pattern of C/EBPβ protein expression was different after treatment with cAMP and TNF. cAMP induced an increase in C/EBPβ protein at 3 h, whereas a TNF-induced increase was not evident until 6 h after treatment. C/EBPβ protein remained elevated at 12 h after treatment with either cAMP or TNF and was then reduced to control levels at 24 h (Fig. 7A). Interestingly, when cAMP and TNF were added simultaneously, C/EBPβ protein was highly induced, greater than by cAMP or TNF alone. The induction of C/EBPβ protein by cAMP and TNF observed at 3 h was maintained throughout 24 h (Fig. 7A). The effects of cAMP and TNF were confirmed with increased nuclear localization of immunoreactive C/EBPβ protein in mouse granulosa cells (Fig. 7B). These results indicate the combination of cAMP and TNF increases C/EBPβ protein, and this increase likely further enhanced SAA3 promoter activity in mouse granulosa cells.
Figure 7.
Time course of C/EBPβ, phospho-IκB (p-IκB), IκB, and p65 proteins in response to cAMP and TNF. Panel A, Effects of cAMP and TNF on the time course of C/EBPβ, p-IκB, IκB, and p65 proteins in mouse granulosa cells. Cells were incubated with TNF (10 ng/ml) alone, cAMP (500 μm) alone, and in combination for 3, 6, 12, and 24 h. Each gel lane was loaded with 20 μg of total protein using whole-cell lysates. β-Actin was used as a loading control. Panel B, Nuclear localization of C/EBPβ protein by immunocytochemistry after cAMP and/or TNF in mouse granulosa cells. The experiments were repeated twice and a representative figure is shown. C, Control; A, cAMP; T, TNF; TA, TNF+cAMP.
Discussion
The most significant finding of the present study is that in mouse granulosa cells, cAMP and TNF synergized in inducing SAA3 expression by increasing C/EBPβ protein (Figs. 6C and 7, A and B) and that cAMP used the PKA-CREB pathway in enhancing the expression of TNF-induced SAA3. This conclusion is based on several observations. First, dominant-negative CREB blocked the ability of cAMP and TNF, each alone and in combination, to increase SAA3 promoter activity. Second, C/EBPβ siRNA reduced SAA3 promoter activity (Fig. 6D) and protein (Fig. 6E) induced by cAMP and TNF.
Previous studies demonstrated administration of an ovulatory dose of hCG results in up-regulation of granulosal C/EBPβ (61,62). The essential role of C/EBPβ in ovarian function has been demonstrated by the loss of fertility with targeted deletion (63,64). The ability of an ovulatory surge of LH/hCG to induce the transition of granulosa cells to luteal cells was lost with deletion of C/EBPβ; for example, LH/hCG-induced inhibition of aromatase and induction of steroidogenic acute regulatory protein were lost. In this case, C/EBPβ activation was primarily mediated via LH-induced ERK1/2 activity. However, in the absence of ERK1/2, C/EBPβ expression was not completely lost, indicating additional ERK1/2-independent regulation of C/EBPβ (63). In the present study, cAMP treatment of mouse granulosa cells in vitro increased C/EBPβ, and this occurred via the PKA/CREB pathway because dominant-negative CREB inhibited cAMP-induced SAA promoter activity (Fig. 6). Previous studies demonstrated in vitro treatment with TNF does not activate ERK1/2 in mouse granulosa in vitro (57); however, contribution of cAMP-induced ERK1/2 in the induction of C/EBPβ and a subsequent interaction with TNF in inducing SAA3 cannot be ruled out.
Treatment of granulosa cells with TNF alone also increased C/EBPβ protein (Fig. 7). Although not previously described in granulosa cells, studies in other systems demonstrated TNF’s effects on gene expression can be mediated by C/EBPβ. In hepatocytes, TNF stimulates 11β-hydroxysteroid dehydrogenase-1 via MAPK-mediated induction of CREB activity and subsequent induction of C/EBPβ (65). Another study in hepatic stellate cells demonstrated TNF’s inhibition of the α1-collagen gene occurred by TNF-mediated induction of C/EBPβ protein and increased binding of C/EBPβ to the α1-collagen promoter (66). Interestingly, in the present study, cAMP and TNF in combination resulted in a greater increase in C/EBPβ protein levels and an apparent increase in protein stability (Figs. 6 and 7). Although the mechanism for this is currently unknown, a recent study has shown that phosphorylation by c-Abl tyrosine kinase resulted in increased stability and transcriptional activity of C/EBPβ (67). Thus, the cooperative interaction of TNF and cAMP at the level of the SAA3 promoter may be enhanced by an increased stability of C/EBPβ.
The data in the present study also indicate an interaction between NF-κB and C/EBPβ at the level of SAA3 promoter activity (Figs. 3–5). Mutation of the atypical NF-κB site, although all C/EBP sites were intact, resulted in loss of TNF-inducible promoter activity. Similarly, mutation of either of two C/EBP sites individually (either site 1 or 4) with the NF-κB site intact resulted in loss of cAMP-induced activity. Thus, it appears a cooperative interaction exists between NF-κB and C/EBPβ to induce promoter activity. cAMP treatment alone induced SAA3 promoter activity and some detectable product by PCR was measured; however, both Northern and Western blot analyses were unable to detect SAA3 RNA/protein. This may be due to the increased sensitivities of promoter-reporter and RT-PCR analyses compared with Northern and Western blot or may indicate rapid turnover of transcript before translation occurs. Alternatively, promoter studies such as the present carried out with a small piece of upstream DNA could negate any repressive factors that may be further upstream and that are active in the endogenous gene. Currently the mechanisms whereby cAMP induces SAA3 promoter activity in granulosa cells without inducing protein expression are unknown.
Additional interaction between p65 and C/EBPβ is evidenced by the mutation of C/EBPβ site 4 and the resulting total loss of promoter activity with TNF or cAMP (Fig. 5) and the loss of TNF-inducible promoter activity in the presence of C/EBPβ dominant-negative form and siRNA (Fig. 6). Interestingly, in liver development and regeneration, TNF-induced hepatocyte growth factor receptor (Met) up-regulation is mediated by both p65 and C/EBPβ at the level of the Met promoter in which NF-κB and C/EBPβ binding sites are adjacent to each other (68). In that system C/EBPβ binding to the promoter is dependent on NF-κB (p65) binding to the adjacent NF-κB site. The p65 was the primary factor driving Met gene transcription and C/EBPβ partnered with p65 to boost transcription (68). Other studies demonstrating an interaction between p65 and C/EBPβ indicated TNF-mediated expression of manganous superoxide dismutase is primarily dependent on C/EBPβ and secondarily on p65. In that case C/EBPβ is required for NF-κB p65 binding to the manganous superoxide dismutase promoter (69). Another study demonstrated TNF-mediated human Mediterranean fever promoter requires both p65 and C/EBPβ in which C/EBPβ is the key regulatory factor, although p65 is required for full activity (70). The cooperative interaction between p65 and C/EBPβ is likely to be critical for full SAA3 promoter activity in response to TNF and cAMP.
SAA, an acute-phase protein, is rapidly produced by the liver in response to infection, injury, inflammation, and neoplasia (45,71). However, SAA is also produced by nonhepatic sites, and it is generally believed that locally expressed SAAs are produced under conditions that do not evoke the systemic acute-phase response and are likely to play a role related to site of expression. Transcriptional regulation of SAA has been studied largely in the context of the acute phase of inflammation and chronic settings such as rheumatoid arthritis. A study assessing cardiovascular risk factors in Finnish young adults found mean levels of SAA in women (mean 32 yr of age) to be 10.70 mg/liter, significantly higher than those measured in age-matched men (46); similar sex differences have also been reported in obese individuals (72). The reasons for the differences in serum SAA levels are not yet understood; however, it is interesting that similar gender differences have been described in SAA production by adipose cells in vitro (72,73). Furthermore, as previously indicated, TNF levels are higher in women compared with men (46). Cytokines including TNF are the principal inducers of the SAA family (71). Several studies investigated the cis-acting and trans-activating factors regulating transcription of the SAAs after an acute stimulation (45). NF-κB and C/EBP transcription factor recognition sequences that confer cytokine responsiveness and cell specificity of expression have been identified in the SAA promoters (74,75). In the mouse SAA3 promoter a 350-bp region upstream from the transcription start site allows cytokine-induced and cell-specific expression of the gene in hepatocytes (76). IL-1α responsiveness of the SAA3 promoter is regulated by a region between −128 and −169 containing two C/EBP sites and a site that binds a novel constitutive transcription factor, SAA enhancer factor 1, and between −155 and −164 containing an atypical NF-κB binding site (77). Our previous study demonstrated the atypical NF-κB site was important in mediating SAA3 expression in granulosa cells (38). In the context of SAA regulation in the granulosa cell, it is important to indicate consensus CRE sites are not present in the SAA3 promoter.
The present study indicates in granulosa cells SAA3 gene transcription and protein expression are regulated by both TNF and cAMP, together playing a role in optimal expression. Although our understanding of the physiological role of SAA in the ovary is currently limited, SAA may be important in the ovulatory process and the transition of a granulosa cell to a luteal cell. TNF levels, both systemic and ovarian, increase around the time of ovulation (14,17). C/EBPβ is induced in granulosa cells in response to the ovulatory surge of LH (78). The ovulatory surge of LH inhibits granulosal estradiol and aromatase expression. TNF inhibits granulosal estradiol production and aromatase expression (19,20,21,22,23). The present data indicate TNF-induced NF-κB p65 and C/EBPβ are important for induction of SAA3. In addition, SAA inhibits granulosal estradiol production and aromatase expression (Fig. 1). It is interesting to note that as shown in other systems, SAA can increase extracellular matrix-degrading enzymes such as collagenase, stromelysin, and matrix metalloproteinase-2 and -3 (79,80,81) and play a role in modulating platelet adhesion at vascular injury sites (82), both processes important for the normal ovulatory event. Taken together, these data imply SAA may play a role in the physiological events occurring during the ovulatory process as well as pathological events such as inflammation and infection.
In summary, the present study demonstrates that in mouse granulosa cells, cAMP and TNF synergized in inducing SAA3 by increasing C/EBPβ protein through both the PKA-CREB and NF-κB pathways. Furthermore, enhanced SAA3 protein expression is at least in part mediated by an interaction at the SAA3 promoter between p65 and C/EBPβ.
Acknowledgments
We thank Dr. Philipp Scherer (Albert Einstein College of Medicine, Bronx, NY) for SAA3 antibody, Dr. Steven L. McKnight (The Ohio State University, Columbus, OH) for pMSV-C/EBPβ expression vectors, and Dr. Charles Vinson (National Cancer Institute/National Institutes of Health, Bethesda, MD) for dominant-negative C/EBP and CREB vectors.
Footnotes
This work was supported by Grant CA50616 from the National Cancer Institute (to P.F.T.); National Institute of Child Health and Human Development (Specialized Cooperative Center Program in Reproduction Research, Grant U54HD33994 (to P.F.T.); and Kansas IDeA Network of Biomedical Research Excellence Grant RR 16475 from the National Center for Research Resources of the National Institutes of Health (to D.-S.S.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 5, 2010
Abbreviations: C/EBP, CCAAT-enhancing binding protein; CRE, cAMP response element; CREB, CRE-binding protein; eCG, equine chorionic gonadotropin; hCG, human chorionic gonadotropin; IκB, inhibitory-κB; LPS, lipopolysaccharide; m, mutated; Met, hepatocyte growth factor receptor; NF-κB, nuclear factor-κB; PKA, protein kinase A; SAA3, serum amyloid A3; siRNA, short interfering RNA.
References
- Bagavandoss P, Kunkel SL, Wiggins RC, Keyes PL 1988 Tumor necrosis factor-α (TNF-α) production and localization of macrophages and T lymphocytes in the rabbit corpus luteum. Endocrinology 122:1185–1187 [DOI] [PubMed] [Google Scholar]
- Barak V, Mordel N, Holzer H, Zajicek G, Treves AJ, Laufer N 1992 The correlation of interleukin 1 and tumor necrosis factor to oestradiol, progesterone and testosterone levels in periovulatory follicular fluid of in vitro fertilization patients. Hum Reprod 7:462–464 [DOI] [PubMed] [Google Scholar]
- Chen HL, Marcinkiewicz JL, Sancho-Tello M, Hunt JS, Terranova PF 1993 Tumor necrosis factor α gene expression in mouse oocytes and follicular cells. Biol Reprod 48:707–714 [DOI] [PubMed] [Google Scholar]
- Hehnke-Vagnoni KE, Clark CL, Taylor MJ, Ford SP 1995 Presence and localization of tumor necrosis factor-α in the corpus luteum of nonpregnant and pregnant pigs. Biol Reprod 53:1339–1344 [DOI] [PubMed] [Google Scholar]
- Ji I, Slaughter RG, Ellis JA, Ji TH, Murdoch WJ 1991 Analyses of ovine corpora lutea for tumor necrosis factor mRNA and bioactivity during prostaglandin-induced luteolysis. Mol Cell Endocrinol 81:77–80 [DOI] [PubMed] [Google Scholar]
- Judd A, MacLeod R 1992 The regulation of interleukin 6 and tumor necrosis factor release from primary cultures of ovarian cells. Prog Neuroendocrinimmunol 5:245–255 [Google Scholar]
- Marcinkiewicz JL, Krishna A, Cheung CM, Terranova PF 1994 Oocytic tumor necrosis factor α: localization in the neonatal ovary and throughout follicular development in the adult rat. Biol Reprod 50:1251–1260 [DOI] [PubMed] [Google Scholar]
- Onagbesan O, Bruggeman V, Decuypere E 2009 Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim Reprod Sci 111:121–140 [DOI] [PubMed] [Google Scholar]
- Punnonen J, Heinonen PK, Teisala K, Kujansuu E, Jansen CT, Punnonen R 1992 Demonstration of tumor necrosis factor-α in preovulatory follicular fluid: its association with serum 17β-estradiol and progesterone. Gynecol Obstet Invest 33:80–84 [DOI] [PubMed] [Google Scholar]
- Roby KF, Terranova PF 1989 Localization of tumor necrosis factor (TNF) in rat and bovine ovary using immunocytochemistry and cell blot: evidence for granulosal production. In: Hirshfield AN, ed. Growth factors and the ovary. New York: Plenum Press; 273–278 [Google Scholar]
- Roby KF, Weed J, Lyles R, Terranova PF 1990 Immunological evidence for a human ovarian tumor necrosis factor-α. J Clin Endocrinol Metab 71:1096–1102 [DOI] [PubMed] [Google Scholar]
- Shaw DW, Britt JH 1995 Concentrations of tumor necrosis factor-α and progesterone within the bovine corpus luteum sampled by continuous-flow microdialysis during luteolysis in vivo. Biol Reprod 53:847–854 [DOI] [PubMed] [Google Scholar]
- Zolti M, Meirom R, Shemesh M, Wollach D, Mashiach S, Shore L, Rafael ZB 1990 Granulosa cells as a source and target organ for tumor necrosis factor α. FEBS Lett 261:253–255 [DOI] [PubMed] [Google Scholar]
- Brännström M, Fridén BE, Jasper M, Norman RJ 1999 Variations in peripheral blood levels of immunoreactive tumor necrosis factor α (TNFα) throughout the menstrual cycle and secretion of TNFα from the human corpus luteum. Eur J Obstet Gynecol Reprod Biol 83:213–217 [DOI] [PubMed] [Google Scholar]
- O'Brien SM, Fitzgerald P, Scully P, Landers A, Scott LV, Dinan TG 2007 Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation 14:84–90 [DOI] [PubMed] [Google Scholar]
- Loret de Mola JR, Goldfarb JM, Hecht BR, Baumgardner GP, Babbo CJ, Friedlander MA 1998 Gonadotropins induce the release of interleukin-1β, interleukin-6 and tumor necrosis factor-α from the human preovulatory follicle. AJRI 39:387–390 [DOI] [PubMed] [Google Scholar]
- Rice VM, Limback SD, Roby KF, Terranova PF 1998 Changes in circulating and ovarian concentrations of bioactive tumour necrosis factor α during the first ovulation at puberty in rats and in gonadotrophin-treated immature rats. J Reprod Fertil 113:337–341 [DOI] [PubMed] [Google Scholar]
- Peluffo MC, Young KA, Hennebold JD, Stouffer RL 2009 Expression and regulation of tumor necrosis factor (TNF) and TNF-receptor family members in the macaque corpus luteum during the menstrual cycle. Mol Reprod Dev 76:367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adashi EY, Resnick CE, Croft CS, Payne DW 1989 Tumor necrosis factor-α inhibits gonadotrophin hormonal action in nontransformed ovarian granulosa cells. J Biol Chem 264:11591–11597 [PubMed] [Google Scholar]
- Ghersevich S, Isomaa V, Vihko P 2001 Cytokine regulation of the expression of estrogenic biosynthetic enzymes in cultured rat granulosa cells. Mol Cell Endocrinol 172:21–30 [DOI] [PubMed] [Google Scholar]
- Montgomery Rice V, Limback SD, Roby KF, Terranova PF 1999 Tumor necrosis factor α inhibition of follicle-stimulating hormone-induced granulosa cell estradiol secretion in the human does not involve reduction of cAMP secretion but inhibition at post-cAMP site(s). Endocrine 10:19–23 [DOI] [PubMed] [Google Scholar]
- Roby KF, Son DS, Terranova PF 1999 Alterations of events related to ovarian function in tumor necrosis factor receptor type I knockout mice. Biol Reprod 61:1616–1621 [DOI] [PubMed] [Google Scholar]
- Spicer LJ 1998 Tumor necrosis factor-α (TNF-α) inhibits steroidogenesis of bovine ovarian granulosa and thecal cells in vitro. Involvement of TNF-α receptors. Endocrine 8:109–115 [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Saragüeta PE, Bussmann UA, Barañao JL 2002 Paradoxical effects of tumour necrosis factor α on rat granulosa cell DNA synthesis. Reprod Fertil Dev 14:133–139 [DOI] [PubMed] [Google Scholar]
- Nakayama M, Manabe N, Inoue N, Matsui T, Miyamoto H 2003 Changes in the expression of tumor necrosis factor (TNF) α, TNFa receptor (TNFR) 2, and TNFR-associated factor 2 in granulosa cells during atresia in pig ovaries. Biol Reprod 68:530–535 [DOI] [PubMed] [Google Scholar]
- Johnson ML, Murdoch J, Van Kirk EA, Kaltenbach JE, Murdoch WJ 1999 Tumor necrosis factor α regulates collagenolytic activity in preovulatory ovine follicles: relationship to cytokine secretion by the oocyte-cumulus cell complex. Biol Reprod 61:1581–1585 [DOI] [PubMed] [Google Scholar]
- Brännström M, Bonello N, Wang LJ, Norman RJ 1995 Effects of tumour necrosis factor α (TNFα) on ovulation in the rat ovary. Reprod Fertil Dev 7:67–73 [DOI] [PubMed] [Google Scholar]
- Brännström M, Norman RJ, Seamark RF, Robertson SA 1994 Rat ovary produces cytokines during ovulation. Biol Reprod 50:88–94 [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Colgin DC, Ellis JA 1997 Role of tumor necrosis factor-α in the ovulatory mechanism of ewes. J Anim Sci 75:1601–1605 [DOI] [PubMed] [Google Scholar]
- Darbon JM, Oury F, Laredo J, Bayard F 1989 Tumor necrosis factor-α inhibits follicle-stimulating hormone-induced differentiation in cultured rat granulosa cells. Biochem Biophys Res Commun 163:1038–1046 [DOI] [PubMed] [Google Scholar]
- Skarzynski DJ, Woclawek-Potocka I, Korzekwa A, Bah MM, Piotrowska K, Barszczewska B, Okuda K 2007 Infusion of exogenous tumor necrosis factor dose dependently alters the length of the luteal phase in cattle: differential responses to treatment with indomethacin and L-NAME, a nitric oxide synthase inhibitor. Biol Reprod 76:619–627 [DOI] [PubMed] [Google Scholar]
- Naz RK, Thurston D, Santoro N 1995 Circulating tumor necrosis factor (TNF)-α in normally cycling women and patients with premature ovarian failure and polycystic ovaries. Am J Reprod Immunol 34:170–175 [DOI] [PubMed] [Google Scholar]
- Cianci A, Calogero AE, Palumbo MA, Burrello N, Ciotta L, Palumbo G, Bernardini R 1996 Relationship between tumour necrosis factor a and sex steroid concentrations in the follicular fluid of women with immunological infertility. Hum Reprod 11:265–268 [DOI] [PubMed] [Google Scholar]
- Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW 2000 Relationships between concentrations of tumor necrosis factor-α and nitric oxide in follicular fluid and oocyte quality. J Assist Reprod Genet 17:222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto P, Natzke RP, Hansen PJ 2003 Actions of tumor necrosis factor-α on oocyte maturation and embryonic development in cattle. Am J Reprod Immunol 50:380–388 [DOI] [PubMed] [Google Scholar]
- Samy N, Hashim M, Sayed M, Said M 2009 Clinical significance of inflammatory markers in polycystic ovary syndrome: their relationship to insulin resistance and body mass index. Dis Markers 26:163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkun I, Cetinarslan B, Türemen E, Cantürk Z, Biyikli M 2006 Association between circulating tumor necrosis factor-α, interleukin-6, and insulin resistance in normal-weight women with polycystic ovary syndrome. Metab Syndr Relat Disord 4:122–128 [DOI] [PubMed] [Google Scholar]
- Son DS, Roby KF, Terranova PF 2004 Tumor necrosis factor α (TNF) induces serum amyloid A3 in mouse granulosa cells. Endocrinology 145:2245–2252 [DOI] [PubMed] [Google Scholar]
- Hardardóttir I, Sipe J, Moser AH, Fielding CJ, Feingold KR, Grünfeld C 1997 LPS and cytokines regulate extra hepatic mRNA levels of apolipoproteins during the acute phase response in Syrian hamsters. Biochim Biophys Acta 1344:210–220 [DOI] [PubMed] [Google Scholar]
- Marhaug G, Hackett B, Dowton SB 1997 Serum amyloid gene expression in rabbit, mink and mouse. Clin Exp Immunol 107:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygg M, Husby G, Marhaug G 1993 Differential expression of rabbit serum amyloid A genes in response to various inflammatory agents. Scand J Immunol 38:417–422 [DOI] [PubMed] [Google Scholar]
- Rygg M, Nordstoga K, Husby G, Marhaug G 1993 Expression of serum amyloid A genes in mink during induction of inflammation and amyloidosis. Biochim Biophys Acta 1216:402–408 [DOI] [PubMed] [Google Scholar]
- Webb CF, Tucker PW, Dowton SB 1989 Expression and sequence analyses of serum amyloid A in the Syrian Hamster. Biochemistry 28:4785–4790 [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS 2006 Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 20:1300–1321 [DOI] [PubMed] [Google Scholar]
- Uhlar CM, Whitehead AS 1999 Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 265:501–523 [DOI] [PubMed] [Google Scholar]
- Jylhävä J, Haarala A, Eklund C, Pertovaara M, Kähönen M, Hutri-Kähönen N, Levula M, Lehtimäki T, Huupponen R, Jula A, Juonala M, Viikari J, Raitakari O, Hurme M 2009 Serum amyloid A is independently associated with metabolic risk factors but not with early atherosclerosis: the Cardiovascular Risk in Young Finns Study. J Intern Med 266:286–295 [DOI] [PubMed] [Google Scholar]
- Espey LL 1994 Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod 50:233–238 [DOI] [PubMed] [Google Scholar]
- Hillier SG, Tetsuka M 1998 An anti-inflammatory role for glucocorticoids in the ovaries? J Reprod Immunol 39:21–27 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL 2002 Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 64:69–92 [DOI] [PubMed] [Google Scholar]
- Ko KW, Corry DB, Brayton CF, Paul A, Chan L 2009 Extravascular inflammation does not increase atherosclerosis in apoE-deficient mice. Biochem Biophys Res Commun 384:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Whitin JC, Ling XB, Nayak NR, Cohen HJ, Jin J, Schilling J, Yu TT, Madan A 2009 Plasma biomarkers in a mouse model of preterm labor. Pediatr Res 66:11–16 [DOI] [PubMed] [Google Scholar]
- Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C 1998 A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol 18:967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylov D, Olive M, Vinson C 1995 Extending dimerization interfaces: the bZIP basic region can form a coiled coil. EMBO J 14:5329–5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Sarge OK, Mayo KE 1994 Regulation of the progesterone receptor gene by gonadotropins and cyclic adenosine 3′, 5′-monophosphate in rat granulosa cells. Endocrinology 134:709–718 [DOI] [PubMed] [Google Scholar]
- Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Son DS, Roby KF 2006 Interleukin-1α-induced chemokines in mouse granulosa cells: impact on keratinocyte chemoattractant (KC) chemokine, a CXC subfamily. Mol Endocrinol 20:2999–3013 [DOI] [PubMed] [Google Scholar]
- Son DS, Arai KY, Roby KF, Terranova PF 2004 Tumor necrosis factor α (TNF) increases granulosa cell proliferation: dependence on c-Jun and TNF receptor type-1. Endocrinology 145:1218–1226 [DOI] [PubMed] [Google Scholar]
- Son DS, Roby KF, Rozman KK, Terranova PF 2002 Estradiol enhances and estriol inhibits the expression of CYP1A1 induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in a mouse ovarian cancer cell line. Toxicology 176:229–243 [DOI] [PubMed] [Google Scholar]
- Terranova PF, Garza F 1983 Relationship between the preovulatory luteinizing hormone (LH) surge and androstenedione synthesis of preantral follicles in the cyclic hamster: detection by in vitro responses to LH. Biol Reprod 29:630–636 [DOI] [PubMed] [Google Scholar]
- Niehof M, Kubicka S, Zender L, Manns MP, Trautwein C 2001 Autoregulation enables different pathways to control CCAAT/enhancer binding protein beta (C/EBPβ) transcription. J Mol Biol 309:855–868 [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS 1998 Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip-1. Mol Endocrinol 12:924–940 [DOI] [PubMed] [Google Scholar]
- Sirois J, Richards JS 1993 Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for the role of a cis-acting C/EBPβ promoter element. J Biol Chem 268:21931–21938 [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS 2009 MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterneck E, Tessarollo L, Johnson PF 1997 An essential role for C/EBPβ in female reproduction. Genes Dev 11:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova ID, Kostadinova RM, Goldring CE, Nawrocki AR, Frey FJ, Frey BM 2009 Tumor necrosis factor-α upregulates 11β-hydroxysteroid dehydrogenase type 1 expression by CCAAT/enhancer binding protein-β in HepG2 cells. Am J Physiol Endocrinol Metab 296:E367–E377 [DOI] [PubMed] [Google Scholar]
- Iraburu MJ, Domínguez-Rosales JA, Fontana L, Auster A, García-Trevijano ER, Covarrubias-Pinedo A, Rivas-Estilla AM, Greenwel P, Rojkind M 2000 Tumor necrosis factor α down-regulates expression of the α1(I) collagen gene in rat hepatic stellate cells through a p20C/EBPβ- and C/EBPΔ-dependent mechanism. Hepatology 31:1086–1093 [DOI] [PubMed] [Google Scholar]
- Li X, Liu X, Wang G, Zhu X, Qu X, Li X, Yang Y, Peng L, Li C, Li P, Huang W, Ma Q, Cao C 2009 Non-receptor tyrosine kinases c-Abl and Arg regulate the activity of C/EBPβ. J Mol Biol 391:729–743 [DOI] [PubMed] [Google Scholar]
- Dai JY, DeFrances MC, Zou C, Johnson CJ, Zarnegar R 2009 The Met protooncogene is a transcriptional target of NFκB: implications for cell survival. J Cell Biochem 107:1222–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan P, Boss JM 2006 C/EBPβ regulates TNF induced MnSOD expression and protection against apoptosis. Apoptosis 11:1837–1849 [DOI] [PubMed] [Google Scholar]
- Papin S, Cazeneuve C, Duquesnoy P, Jeru I, Sahali D, Amselem S 2003 The tumor necrosis factor α-dependent activation of the human Mediterranean fever (MEFV) promoter is mediated by a synergistic interaction between C/EBPβ and NFκB p65. J Biol Chem 278:48839–48847 [DOI] [PubMed] [Google Scholar]
- Steel DM, Whitehead AS 1994 The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today 15:81–88 [DOI] [PubMed] [Google Scholar]
- Lappalainen T, Kolehmainen M, Schwab U, Pulkkinen L, Laaksonen DE, Rauramaa R, Uusitupa M, Gylling H 2008 Serum concentrations and expressions of serum amyloid A and leptin in adipose tissue are interrelated: the Genobin Study. Eur J Endocrinol 158:333–341 [DOI] [PubMed] [Google Scholar]
- Sjöholm K, Palming J, Olofsson LE, Gummesson A, Svensson PA, Lystig TC, Jennische E, Brandberg J, Torgerson JS, Carlsson B, Carlsson LM 2005 A microarray search for genes predominantly expressed in human omental adipocytes: adipose tissue as a major production site of serum amyloid A. J Clin Endocrinol Metab 90:2233–2239 [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB 1998 NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260 [DOI] [PubMed] [Google Scholar]
- Poli V 1998 The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem 273:29279–29282 [DOI] [PubMed] [Google Scholar]
- Huang JH, Rienhoff Jr HY, Liao WS 1990 Regulation of mouse serum amyloid A gene expression in transfected hepatoma cells. Mol Cell Biol 10:3619–3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Yamamoto K 1994 NF-κB and C/EBP transcription factor families synergistically function in mouse serum amyloid A gene expression induced by inflammatory cytokines. Gene 149:305–310 [DOI] [PubMed] [Google Scholar]
- Pall M, Hellberg P, Brännström M, Mikuni M, Peterson CM, Sundfeldt K, Nordén B, Hedin L, Enerbäck S 1997 The transcription factor C/EBP-β and its role in ovarian function; evidence for direct involvement in the ovulatory process. EMBO J 16:5273–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita K, Kawabe Y, Tominaga M, Origuchi T, Aoyagi T, Eguchi K 1998 Serum amyloid A protein induces production of matrix metalloproteinases by human synovial fibroblasts. Lab Invest 78:535–539 [PubMed] [Google Scholar]
- Mitchell TI, Coon CI, Brinckerhoff CE 1991 Serum amyloid A (SAA3) produced by rabbit synovial fibroblasts treated with phorbol esters or interleukin 1 induces synthesis of collagenase and is neutralized with specific antiserum. J Clin Invest 87:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strissel KJ, Girard MT, West-Mays JA, Rinehart WB, Cook JR, Brinckerhoff CE, Fini ME 1997 Role of serum amyloid A as an intermediate in the IL-1 and PMA-stimulated signalling pathways regulating expression of rabbit fibroblast collagenase. Exp Cell Res 237:275–287 [DOI] [PubMed] [Google Scholar]
- Uriel-Shoval S, Meek RL, Hanson RH, Eriksen N, Benditt EP 1994 Human serum amyloid A genes are expressed in monocyte/macrophage cell lines. Am J Pathol 145:650–660 [PMC free article] [PubMed] [Google Scholar]