Abstract
The prostate gland develops from the urogenital sinus in response to circulating androgens. Androgens initiate and stimulate branching morphogenesis in the urogenital sinus via unknown mediators. Heparan sulfate proteoglycans are important extracellular molecules that sequester many growth factors in the extracellular matrix and facilitate signaling by some growth factors as part of ternary complexes that include growth factors, receptors, and heparan sulfate chains. Several enzymes modify the chemical structure of heparan sulfate to further regulate its activity. An examination of these enzymes for sexually dimorphic expression in the urogenital sinus identified Sulfatase 1 (Sulf1) as an enzyme that was down-regulated in the male urogenital sinus coincident with the initiation of prostatic morphogenesis. Down-regulation of Sulf1 was accompanied by an increase in the most highly sulfated forms of heparan sulfate, and a similar increase was observed in female urogenital sinuses treated with testosterone. Inhibiting de novo sulfation of heparan sulfate blocked prostatic morphogenesis, supporting the importance of heparan sulfate modification for prostate development. To functionally test the specific role of Sulf1 during prostate development, Sulf1 was ectopically expressed in the urogenital sinus. It partially inhibited testosterone-stimulated ductal morphogenesis, and it reduced the activation of fibroblast growth factor receptors as well as the ERK1 and ERK2 MAPKs. These data identify sulfatase 1 as an inhibitor of prostatic branching morphogenesis and growth factor signaling that is down-regulated as part of the normal response to androgen action in the male urogenital sinus.
Sulfatase 1 is an inhibitor of prostatic bud development and FGF signaling with sexually dimorphic repression in the male urogenital sinus.
In mammals, early embryogenesis includes the formation of several sexually ambiguous tissues that develop into male-specific or female-specific organs during the later stages of embryogenesis. The urogenital sinus (UGS) is the ambisexual precursor of the male-specific prostate gland. In the mouse at embryonic d 16 (e16), the male UGS is indistinguishable from the female UGS. By e18, androgens circulating in the male embryo trigger outgrowth of epithelial buds into the UGS mesenchyme (UGM) as the first morphological manifestation of prostate development in the male UGS (1). In the UGS, androgens stimulate mesenchymal androgen receptors (ARs) to trigger epithelial budding and subsequent branching morphogenesis. The critical role of mesenchymal ARs was demonstrated by classic tissue recombination experiments (2). Recombination of UGS epithelial (UGE) cells with UGM cells containing the wild-type AR initiated and sustained ductal branching morphogenesis in tissue graft experiments irrespective of the AR status in the UGE. However, UGE cells combined with UGM cells containing a nonfunctional AR did not initiate prostatic morphogenesis. The fact that epithelial morphogenesis requires a functional mesenchymal AR has led to the hypothesis that one or more androgen-regulated mesenchymal factors mediate the local action of androgens via paracrine signals that have not yet been conclusively identified.
Although the mechanisms that mediate local androgen responses in the developing prostate remain unclear, several paracrine growth factors have been implicated in the regulation of branching morphogenesis in the developing prostate. One of the critical paracrine signals is fibroblast growth factor (FGF)-10 that is expressed in the UGM and binds to receptors in the UGE (3). Although it remains controversial whether Fgf10 is regulated by androgens, FGF10 is a critical regulator of UGS development because male mice lacking Fgf10 expression (FGF10−/−) have rudimentary and nonfunctional prostates (4). However, FGF10 treatment of UGS tissues in organ culture is insufficient to promote branching morphogenesis in the absence of androgens, suggesting that multiple mesenchymal-to-epithelial paracrine signals are necessary for prostate development.
FGF10 binds the epithelial-specific FGF receptor (FGFR) 2 isoform iiib (FGFR2b) in a ternary complex with heparan sulfate (HS). HS is the glycosylaminoglycan component of HS proteoglycans (HSPGs) that contains specific patterns of sulfated disaccharide units. The HS disaccharide repeats are predominantly composed of N-acetyl-glucosamine and hexauronic acid units with multiple moieties that can be modified by sulfation. The composition of HS disaccharide units and the specific HS sulfation patterns determine the ability of HSPGs to modulate the interactions of WNT and bone morphogenetic protein ligands with their receptors by sequestering these growth factors and interfering with receptor binding (5,6). Similarly, the composition of HS disaccharide units and the specific HS sulfation patterns determine the ability of HSPGs to facilitate ligand-receptor interactions as a coreceptor, which occurs in the ternary complex of FGF10-HS-FGFR2b. Crystal structures of FGF-FGFR interactions suggest that HS interacts with the Ig2 loop of the receptor extracellular domain within the ligand-receptor complex to promote signaling (7). Inhibition of HS sulfation using sodium chlorate in organ culture of embryonic lung rudiments inhibited epithelial bud development and branching by inhibiting FGF10-FGFR2b signaling (8).
HS is conjugated to HSPGs during its synthesis in the Golgi complex as part of a series of processing reactions (9). Synthesis of HS includes steps to epimerize glucuronic acid to iduronic acid by a d-glucuronsyl C-5 epimerase, N-deacetylase/N-Sulfotransferase by NDST enzymes, and sulfation at the 2-O position of uronic acid and 6-O and 3-O positions of glucosamine by the 2-O, 6-O, and 3-O HS sulfotransferases (ST) (HS2ST, HS6ST, and HS3ST, respectively) (10). More recently, the 6-O endosulfatase enzymes [sulfatase 1 (SULF1) and SULF2] that selectively remove 6-O sulfation postsynthetically were discovered and have been investigated for their ability to interfere with growth factor signaling in vivo and in vitro (11,12,13). The functional significance of HS and the enzymes involved in its synthesis is evident in knockout mouse models, which have significant developmental abnormalities. Embryonic or neonatal lethality and developmental patterning defects are observed in mouse knockout models including: NDST1−/−, C5-epimerase−/−, and HS2ST−/− (14,15,16,17). Increased embryonic or neonatal lethality and severe developmental abnormalities are also observed in HS6ST1−/− and SULF1−/−;SULF2−/− knockout mice (18,19,20).
To investigate the role of HS in the developing prostate and its potential interaction with FGFR2 signaling, we analyzed mouse UGS tissues for the expression of enzymes that specifically modify HS 6-O sulfation including Hs6st1, Hs6st2, Hs6st3, Sulf1, and Sulf2. Here we show a sexually dimorphic expression pattern of Sulf1 in the UGS beginning at e18, and decreased Sulf1 expression correlated with increased 6-O sulfation patterns. Inhibition of de novo HS sulfation inhibited UGS bud formation in vitro, and overexpression of Sulf1 decreased UGS epithelial bud formation. To the best of our knowledge, we present the first evidence of androgen-dependent changes in HS sulfation patterns that facilitate epithelial bud outgrowth in the developing prostate gland.
Materials and Methods
Experimental animals
Female CD1 mice (Charles River Laboratories, Wilmington, MA) were obtained as timed pregnant animals and killed in the morning 16, 17, or 18 d after being bred. UGSs were dissected out of female and male embryos as previously described (21). All animal experimentation in this study was conducted in accord with accepted standards of humane animal care as outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the experimentation was approved by the Animal Care and Use Committee of the University of Wisconsin-Madison.
Sample processing and HPLC analysis of HS disaccharides
Pools of 16–20 UGSs were processed exactly as described in Chen et al. (22) and loaded onto the HPLC using conditions previously described (23). Briefly, tissues were rinsed in PBS, lyophilized, and resuspended in 50 mm Tris-HCl (pH 8), 1 mm CaCl2, and 1% Triton X-100. The samples were sonicated, digested with protease, heat inactivated, and further digested with benzonase. Insoluble material was removed by centrifugation, and the supernatant was applied to a Vivapure Mini D membrane (Sartorius AG, Goettingen, Germany). The fractions eluted with 1.0 m NaCl were collected, desalted, lyophilized, and resuspended in 0.03 m sodium acetate buffer (pH 7.0) with 3.33 mm calcium acetate, 0.33 mIU heparinase, 0.33 mIU heparitinase II, and 0.33 mIU heparitinase I (Seikagaku America, East Falmouth, MA). After lyophilization, the mixtures were resuspended in water. Samples were analyzed using reversed-phase ion-pair chromatography with postcolumn fluorescence detection. The area of each peak was determined and then divided by total HS present for normalization.
RNA isolation, RT-PCR, and quantitative real-time PCR
Pools of 6–10 UGSs were dissected out of male and female embryos, rinsed in PBS, and then crushed in Trizol (Invitrogen, Carlsbad, CA) using a pestle. RNA was isolated using manufacturer’s instructions. For RT and PCR, 2 μg total RNA was deoxyribonuclease treated with deoxyribonuclease I using the manufacturer’s instructions (New England Biolabs, Ipswich, MA). After treatment, RT was completed using random primers (Roche Applied Science, Indianapolis, IN), SSRT II (Invitrogen), MgCl2, RNAsin (Promega, Madison, WI), and dNTPs (Roche Applied Science, Indianapolis, IN). Real-time PCR was completed using the LightCycler (Roche Applied Science). Briefly, LightCycler FastStart DNA MasterPLUS SYBR Green I kit (Roche Applied Science) was used with 5 μl cDNA from the reverse transcriptase reaction. Relative mRNA expression levels were calculated as described previously (24).
For epithelial and mesenchymal expression, approximately 20 e16 UGS tissues were pooled, trypsinized, and manually separated as previously described in Joesting et al. (25).
Primer sequences were as follows: mSulf1 forward, TGC TCA CTG GGA AGT ACG TG, and reverse, AGG GAT GTA GCT GCC ATT GT; mShh forward, AAT GCC TTG GCC ATC TCT GT, and reverse, GCT CGA CCC TCA TAG TGT AGA GAC T; and mGli1 forward, GGA AGT CCT ATT CAC GCC TTG A, and reverse, CAA CCT TCT TGC TCA CAC ATG TAA G. Transcript levels were normalized to m18S; primer sequences were forward GCC GCT AGA GGT GAA ATT CTT G and reverse CAT TCT TGG CAA ATG CTT TCG.
In vitro organ cultures and UGS transfections
UGSs were dissected out of mice in basal medium and cultured at 5% CO2 and 37 C on Millicell-CM culture plate inserts (30 mm, 0.4-μm pore size; Millipore Corp., Billerica, MA) in four-well plates (Nunc A/S, Roskilde, Denmark) at the air/medium interface. Plate inserts were floated over 0.5 ml basal medium consisting of DMEM/F12 50/50 mix supplemented with 0.37 g/liter l-glutamine, 10 U/ml penicillin, 10 μg/ml streptomycin, 1× insulin, transferrin, and selenium, and 0.5% dimethylsulfoxide. Additional supplements sometimes included 10−8 m testosterone, 50 mm sodium chlorate (403016; Sigma-Aldrich, St. Louis, MO), and 0.001 mg/ml heparin (H9399; Sigma-Aldrich) as noted in experiments. A concentration curve with increasing amounts of heparin was completed on e17 male UGSs cultured with testosterone to determine the optimal dose (0.001 mg/ml heparin) at which epithelial bud development of male e17 UGSs was not affected positively or negatively (data not shown). Culture medium was changed every 48 h during the culture period. To monitor growth, pictures were taken of organs every 48 h.
UGS (e16) tissues were transiently transfected by injecting concentrated plasmid DNA into the luminal urethra of the UGS during fine dissection, electroporated, and cultured. Plasmid constructs were obtained as follows: pCMV-Sport6 containing the full-length Sulf1 cDNA (clone 4500594; Open Biosystems, Huntsville, AL) or the full-length Shh cDNA (clone 6516263; Open Biosystems), and the pCMVβ plasmid (BD Biosciences, San Jose, CA). Concentrated plasmid (5–15 μg/μl) was mixed with 2 mg/ml BSA and 0.1% (wt/vol) Fast Green FCF dye (Sigma-Aldrich) and injected into the urethra of the isolated UGS tissues using a 36-gauge needle and 10-μl syringe (World Precision Instruments, Sarasota, FL). Approximately 0.1 μl plasmid solution was injected per UGS as indicated by the presence of the dye. Tissues were immediately electroporated (Gene Pulser Xcell; Bio-Rad Instruments, Hercules, CA) in 10 mm Tris-HCl (pH 7.5), with 1 mm EDTA buffer using a square-wave profile (100 V, 20-msec pulse/1-sec interval, seven pulses). After electroporation, tissues were placed directly into culture as above.
Immunohistochemistry
Female e17 UGSs were cultured for 4 d under stated conditions, rinsed in PBS, fixed in 10% buffered formalin for 1 h at room temperature, and then dehydrated before embedding into paraffin blocks. Sections were cut at 8 μm thickness, deparaffinized, and rehydrated through a series of graded ethanol solutions. Slides underwent antigen retrieval by boiling for 30 min in antigen unmasking solution (Vector Laboratories, Burlingame, CA). Endogenous peroxidases were quenched with 3% (vol/vol) hydrogen peroxide solution for 10 min. Slides were blocked with 2.5% (vol/vol) sheep serum at room temperature for 3 h before incubating overnight with primary antibody anti-cytokeratin (wide spectrum screening) at a 1:250 dilution (DakoCytomation, Carpinteria, CA). After a series of washes, slides were incubated with secondary biotinylated antirabbit antibody diluted to 1:500 in 2.5% (vol/vol) sheep serum for 2 h at room temperature. Slides were washed and then incubated with the Vectastain ABC kit (Vector) for 30 min at room temperature, washed again, and stained using a diaminobenzidine kit (Vector). Finally, slides were counterstained with hematoxylin, dehydrated, and mounted.
Whole-mount X-gal staining for β-gal expression and immunofluorescent staining
Tissues were cultured for 48 h after electroporation, rinsed in PBS, and fixed with fresh 4% (wt/vol) paraformaldehyde solution. Tissues were stained with 1 mg/ml X-gal, 4 mm potassium ferrocyanide, 4 mm potassium ferricyanide, 0.1 mg/ml sodium deoxycholate, and 0.02% (vol/vol) Nonidet P-40 in 20 mm Tris buffer. After staining, tissues were excessively rinsed and fixed in 4% (wt/vol) paraformaldehyde in PBS. Tissues were imaged in 30% (vol/vol) glycerol.
UGS and bladder tissues were rinsed with PBS, dehydrated in a graded isopropanol series, and embedded into paraffin. Tissues were cut in 8-μm sections and processed for anti-cytokeratin staining as above. For immunofluorescent imaging, a 1:5000 dilution of the tetramethylrhodamine isothiocyanate-labeled antirabbit IgG secondary antibody (T6778; Sigma Aldrich) was applied to sections, followed by extensive washes in PBS, and sections were mounted with Vectashield mounting media (Vector).
Whole-mount immunohistochemistry and UGS bud counting
Imaging of epithelial bud development in transfected tissues was obtained using anti-E-cadherin immunostaining with confocal imaging and three-dimensional (3D) image reconstruction. The whole-mount immunohistochemistry protocol was previously described (26). Transfected UGS tissues were culture for 4 d, rinsed in PBS, and fixed with 4% (wt/vol) paraformaldehyde. The fixed UGSs were dehydrated with graded methanol solutions, frozen overnight in methanol, quenched with 5% (vol/vol) H2O2/methanol, and rehydrated. UGS tissues were incubated with anti-E-cadherin (clone ECCD-2; Invitrogen). After extensive washing steps, UGSs were incubated with a biotin-conjugated antirat IgG (mouse adsorbed; Vector) and subsequently treated with fluorescein tyramide reagent (PerkinElmer, Waltham, MA). The immunostained UGSs were imaged with z-stack acquisition on the Olympus BX61 microscope equipped with the Fluoview1000 confocal imaging system and the Olympus Fluoview version 1.7b software package (Olympus Corp., Tokyo, Japan).
Individual UGS tissues were reconstructed as 3D images with z-stack image frames using maximum intensity projection 3D rendering on the Olympus Fluoview version 1.7b software. Two people blinded to the treatment conditions counted the individual bud tips of each imaged UGS using the rendered 3D images. For each UGS, the number of bud tips was averaged for the two analysts. Statistical analysis was performed using a nonparametric t test.
Western blotting
Protein was obtained from pools of approximately five UGS tissues after 48 h culture. Tissues were lysed by sonication in modified RIPAS buffer (50 mm Tris-HCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, and 10 mm NaF) with protease inhibitor cocktail (Roche). Eighty micrograms of protein were run on a polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (GE Healthcare, Piscataway, NJ). Protein was visualized using the Supersignal West Femto Chemiluminescent substrate (Pierce, Rockford, IL) on film. Antibodies (dilution; catalog number) for phospho-MAPK42/44 (1:1000; 4376), phospho-FGFR (1:1000; 3476), and total-ERK(1:1000; 9102) were obtained from Cell Signaling Technology (Beverly, MA). The anti-SULF1 (1:500; sc98325) antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Statistical analyses
Statistics were evaluated by an ANOVA test using StatView 4.1 software program (Abacus Concepts, Inc., Berkeley, CA) or a t test using the Prism v5.0b software program (GraphPad Software, Inc., San Diego, CA).
Results
Sulf1 expression is down-regulated coincident with the onset of prostatic morphogenesis
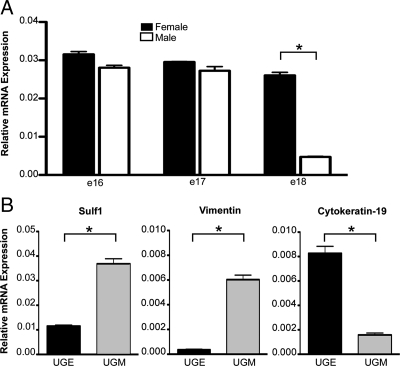
To determine whether HS modifications might play a role in the sexually divergent development of the male and female UGS, mRNA expression levels of the HS-modifying enzymes HS 6-O sulfotransferase 1, 2, and 3 (Hs6st1, Hs6st2, and Hs6st3) and the 6-O sulfatases, Sulf1 and Sulf2, were compared in male and female UGSs at e18 when epithelial bud outgrowth was commencing in the male UGS. This experiment was repeated four times using independent pools of e18 male and female UGSs for each experiment. In all four experiments, Sulf1 expression was significantly lower in the male UGS relative to the female UGS. Sulf1 mRNA levels were not different in the male and female UGS at e16 or e17, but expression of Sulf1 was dramatically decreased at e18 in the male UGS correlating with the morphological changes in prostatic epithelial bud outgrowth in the developing male UGS (Fig. 1A). The expression of Hs6st1 and Sulf2 were not significantly different in the male and female e18 UGSs in the four experiments. Statistically significant decreases in expression of Hs6st2 and Hs6st3 in the male e18 UGS relative to the female e18 UGS were observed in two of the four experiments (data not shown). Before the sexually dimorphic repression of Sulf1 in the male UGS, e16 Sulf1 expression was significantly higher in the UGS mesenchyme compared with the UGS epithelium (Fig. 1B) which is consistent with expression of Sulf1 in mesenchyme of other tissues (27,28).
Figure 1.
Sulf1 expression is down-regulated in the developing male UGS. Expression of Sulf1 in the UGS was quantified by real-time RT-PCR with expression values normalized to the 18S mRNA. A, Sulf1 expression was not significantly different in the male and female UGS at e16 or e17, but expression was dramatically down-regulated in the male UGS by e18. To further clarify the expression of Sulf1 in the developing UGS, e16 UGSs were dissected to separate the UGE and UGM, and Sulf1 expression was measured in the separated tissues by real-time RT PCR. The UGM-specific gene vimentin and UGE-specific gene cytokeratin-19 were also assayed to determine the relative purity of the separated tissues. B, Sulf1 expression was primarily in the UGM. Expression of the control genes indicated that the UGM was approximately 94% pure and the UGE approximately 84% pure. Error bars indicate sem. Statistical significance was determined by ANOVA with Bonferroni correction post hoc analysis. *, Comparisons where statistically significant (P < 0.001) differences in expression were observed.
Sexually dimorphic patterns of HS sulfation correlate with Sulf1 expression
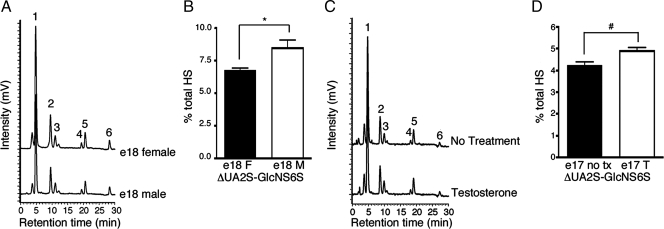
SULF1 specifically cleaves 6-O sulfates from trisulfated HS disaccharides in vivo. To determine whether down-regulation of Sulf1 in the male UGS led to an increase in 6-O sulfated HS disaccharides, the relative abundance of different HS disaccharides was measured in the developing UGS using a previously validated HPLC method (23,29,30,31,32). Female and male e18 UGS tissues were isolated, and HS-containing material was partially purified, digested into disaccharide units, and separated by reverse-phase ion-pair HPLC. Samples were then labeled with postcolumn fluorescent derivatization. Six peaks were collected corresponding to six disaccharide species identified based on the elution time compared with known HS disaccharide standards (Fig. 2A). Peak areas were determined, and each disaccharide species was normalized to the total HS present in the sample (Table 1). The trisulfated disaccharide unit (ΔUA2S-GlcNS6S: 2-O sulfated uronic acid, N- and 6-O sulfated N-acetylglucosamine) represented by peak six was increased in the male UGS compared with the female UGS (Fig. 2B).
Figure 2.
Highly sulfated HS disaccharides are increased in the e18 male UGS. The pattern of HS sulfation was determined using previously established methods (22,23) to isolate and quantify HS disaccharides by HPLC. A, Representative HPLC traces of e18 female (top trace) or male UGSs (bottom trace) are shown. The identity of each peak was determined by comparisons with known standards. The six peaks represent disaccharide species denoted by numbers 1–6: peak 1, ΔUA-GlcNAc; peak 2, ΔUA-GlcNS; peak 3, ΔUA-GlcNAc6S; peak 4, ΔUA-GlcNS6S; peak 5, ΔUA2S-GlcNS; and peak 6, ΔUA2S-GlcNS6S. B, The normalized area under the curve for peak 6 corresponding to the ΔUA2S-GlcNS6S disaccharide is shown in the graph. The increased level of ΔUA2S-GlcNS6S in the e18 male (M) UGS compared with the e18 female (F) UGS was statistically significant: *, P = 0.046 by ANOVA with least significant difference post hoc analysis. C, Representative HPLC traces for in vitro cultured e17 female UGSs with testosterone (bottom trace) or without testosterone (top trace) are shown. D, The normalized area under the curve for peak 6 corresponding to the ΔUA2S-GlcNS6S disaccharide is shown in the graph. The increased level of ΔUA2S-GlcNS6S in the e17 female UGS treated with testosterone (T) compared with the untreated (no tx) e17 female UGS was statistically significant: #, P = 0.0116 by ANOVA with least significant difference post hoc analysis. Normalized peak area values for all HS disaccharides are reported in Table 1. Error bars in the graphs represent sem.
Table 1.
UGS HS disaccharide profiles (percent total HS)
| Disaccharide |
In vivoa
|
In vitro culture (4 d)b
|
||
|---|---|---|---|---|
| e18 Female | e18 Male | No treatment | Testosterone | |
| ΔUA-GlcNAc | 45.420 ± 0.86 | 46.325 ± 0.56 | 45.919 ± 0.66 | 45.875 ± 1.00 |
| ΔUA-GlcN | 22.639 ± 0.24 | 23.248 ± 1.22 | 22.344 ± 0.34 | 22.886 ± 0.50 |
| ΔUA-GlcNAc6S | 4.710 ± 0.33 | 3.602 ± 1.32 | 4.498 ± 0.21 | 4.710 ± 0.30 |
| ΔUA-GlcN6S | 2.961 ± 0.36 | 3.271 ± 0.29 | 2.854 ± 0.33 | 3.539 ± 0.36 |
| ΔUA2S-GlcNS | 17.521 ± 0.46 | 15.078 ± 1.24 | 20.151 ± 0.98 | 18.096 ± 1.28 |
| ΔUA2S-GlcNS6S | 6.748 ± 0.16 | 8.476 ± 0.58c | 4.234 ± 0.15 | 4.896 ± 0.14d |
Each sample contained about 20 UGSs.
Values are the averages from three independent experiments ± sem.
Values are the averages from five independent experiments ± sem.
ΔUA2S-GlcNS6S content in e18 male UGSs is significant relative to e18 female UGSs (P = 0.046).
ΔUA2S-GlcNS6S content in e17 female UGSs cultured with testosterone is significant relative to e17 female UGSs cultured without testosterone (P = 0.0116).
To further investigate the potential role of androgens in HS regulation, e17 female UGS tissues were cultured with or without testosterone for 4 d. Female e17 UGS tissues cultured in the presence of testosterone exhibited a distribution of HS species similar to the male in vivo pattern with increased ΔUA2S-GlcNS6S disaccharide content compared with the no-hormone controls (Fig. 2, C and D, and Table 1). This difference was statistically significant (ANOVA with least significant difference post hoc analysis). The analysis of sulfation states indicated that only 6-O sulfation was significantly increased in the e17 female UGS treated with testosterone compared with the untreated control UGS tissues, whereas 2-O and N-sulfation content did not exhibit statistically significant differences in the cultured UGS tissues.
Inhibition of de novo HS sulfation inhibits prostatic bud formation
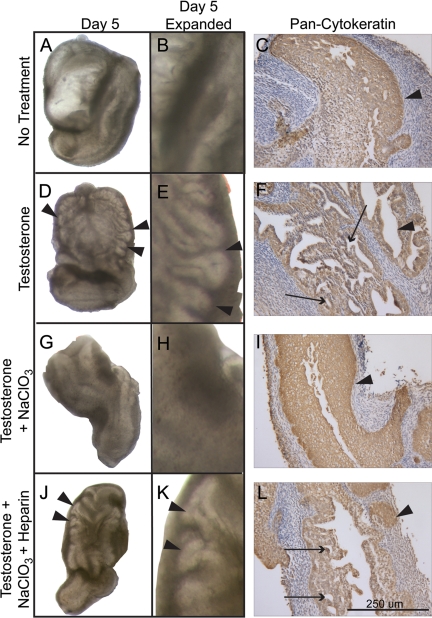
The increase in HS 6-O sulfation in the developing male UGS may facilitate epithelial bud development and branching morphogenesis by affecting growth factor signaling in the UGS. Previous reports have shown that 50 mm sodium chlorate blocks de novo HS O-sulfation in tissue culture (33). To test the requirement for de novo HS O-sulfation during early prostate development, female e17 UGS tissues were cultured for 5 d without treatment, with testosterone, with testosterone and sodium chlorate, or with testosterone, sodium chlorate, and exogenous heparin. The UGS tissues left untreated did not develop buds (Fig. 3, A and B), whereas the UGS tissues cultured with testosterone underwent prostatic epithelial bud development (Fig. 3, D and E). Immunohistochemical analysis further showed the development of epithelial duct lumens in the testosterone-treated UGS tissues that did not develop in the untreated tissues (Fig. 3, C and F). UGS tissues cultured with testosterone and sodium chlorate failed to develop epithelial buds (Fig. 3, G and H) or undergo lumen formation (Fig. 3I). Heparin is a highly sulfated glycosylaminoglycan that can substitute for HS to act as a coreceptor for HS-dependent growth factors. Heparin treatment recovered the formation of epithelial buds and changes in the UGS epithelial architecture (Fig. 3, J–L). An observer blinded to the treatment groups scored the presence or absence of bud development in sodium chlorate-treated and sodium chlorate- plus heparin-treated cultures. Sodium chlorate inhibited prostatic epithelial bud formation, and exogenous heparin treatment led to a statistically significant recovery of bud formation in the presence of sodium chlorate demonstrating that the block in epithelial bud formation caused by sodium chlorate was due to its inhibition of HS sulfation (Table 2).
Figure 3.
De novo sulfation of HS is required for prostatic epithelial bud formation. Female e17 UGS tissues cultured for 5 d without treatment did not develop epithelial buds (A, enlarged view in B), whereas tissues cultured with testosterone underwent bud development as indicated by arrowheads (D, enlarged view in E). Immunohistochemical staining for epithelial cytokeratin (wide-spectrum staining) indicated areas of lumen formation in the growing prostatic epithelial ducts in tissues cultured with testosterone (F), whereas the epithelium of untreated tissues did not undergo epithelial duct formation (C). UGS tissues cultured with sodium chlorate (NaClO3) and testosterone did not develop epithelial buds (G, enlarged view in H). Epithelial immunostaining revealed a smooth edge on the central epithelium (I, arrowhead) and the absence of prostatic duct lumens, indicating that branching morphogenesis was not initiated when de novo HS sulfation was blocked by sodium chlorate treatment. Exogenous heparin that can substitute for highly sulfated HS to facilitate growth factor signaling rescued bud formation in e17 female UGS tissues cultured with testosterone, sodium chlorate, and exogenous heparin (J). An enlarged view of the UGS shows the presence of prostatic epithelial buds (arrowheads in K). Epithelial immunostaining revealed areas where the UGS epithelium had invaded the surrounding mesenchyme (L, arrowhead) and prostatic ductal lumens formed in the UGS epithelium (L, arrows). Arrowheads in pan-cytokeratin images (C, F, I, and L) indicate epithelial-mesenchymal interface. Bar, 250 μm in each cytokeratin image.
Table 2.
Branching morphogenesis in cultured e17 female UGSs
| Treatment group | n | No. of branched UGSs | % Branched UGSs | P value |
|---|---|---|---|---|
| Testosterone | 27a | 27 | 100 | |
| Testosterone + sodium chlorate | 12b | 3 | 25 | <0.0001c |
| Testosterone + sodium chlorate + heparin | 13b | 8 | 61.5 | 0.0002d |
Combined data from six independent experiments.
Combined data from four independent experiments.
Testosterone vs. testosterone + sodium chlorate.
Testosterone + sodium chlorate vs. testosterone + sodium chlorate + heparin.
A new method for ectopic gene expression in the UGS
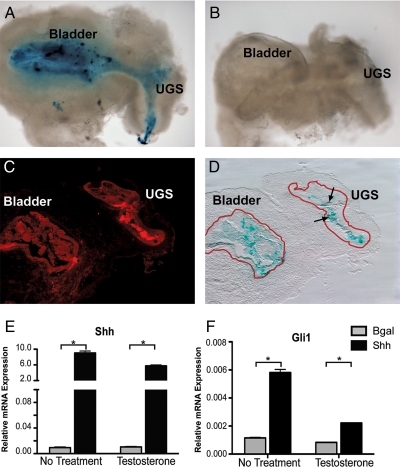
Although several developmental systems routinely achieve ectopic gene expression in organ culture, ectopic gene expression methods for investigating UGS development have not been previously reported. For this study, an in vitro transfection method for the transient expression of genes carried on plasmid DNA was developed for UGSs grown in serum-free organ culture. Concentrated plasmid DNA was injected into the urethral opening of UGS tissues during fine dissection using a 36-gauge needle. Tissues were immediately electroporated using a square-wave protocol and placed into tissue culture with or without testosterone. To validate the UGS transfection method, plasmids carrying cytomegalovirus (CMV) promoter regulated expression of the full-length cDNAs for β-galactosidase (βgal) and Shh were electroporated into UGSs. UGSs transfected with the βgal plasmid and mock-transfected UGSs were cultured for 48 h and then processed for X-gal staining. βgal-transfected UGS tissues and bladders expressed β-galactosidase, but electroporated control UGSs did not stain positive for β-galactosidase activity (Fig. 4, A and B). Immunofluorescent analysis of tissue sections from β-galactosidase-transfected UGSs revealed that the extent of β-galactosidase activity varied in both intensity and localization among transfected UGSs, but expression of β-galactosidase was in the epithelial layer of the UGS (Fig. 4, C and D).
Figure 4.
Square-wave electroporation achieves functional gene expression in the UGS. The common lumen of the UGS and bladder dissected from e16 CD1 mice was injected with pCMVβ plasmid DNA, and the tissues were transfected using a square-wave electroporation protocol. Tissues were cultured for 48 h, and β-galactosidase activity was determined by X-gal oxidation (blue coloration). A, X-gal staining revealed extensive β-galactosidase expression in the tissues surrounding the lumen of both the UGS and bladder. B, Control tissues that were electroporated without pCMVβ plasmid DNA did not stain positive for β-galactosidase activity. The X-gal-stained tissues were sectioned and immunostained for anti-cytokeratin (wide spectrum) to delineate epithelial localization and preserve the intensity of the X-gal stain. C, The immunofluorescent stained tissues (tetramethylrhodamine isothiocyanate-labeled, red staining) were imaged with both bright-field and fluorescent microscopy. The fluorescently labeled epithelium was traced and overlaid in red on the correlating bright-field image to indicate the epithelial border of the bladder and UGS. D, The X-gal stain localized to the epithelial region of the bladder and UGS. Arrows indicate X-gal staining in the UGS epithelium. In a separate experiment, UGSs (without the bladder) were electroporated with a plasmid expressing Shh or a plasmid expressing βgal and cultured for 24 h. E, Transfection increased Shh mRNA expression 5- to 10-fold in UGS tissues relative to βgal-transfected UGSs as determined by real-time RT-PCR. F, mRNA transcripts for Gli1, a SHH-responsive gene, also increased in tissues overexpressing Shh relative to βgal-expressing UGS tissues. All expression values are normalized to 18s mRNA values. Error bars indicate sem. Statistically significant differences in gene expression were determined by ANOVA with least significant difference post hoc analysis. *, P < 0.001.
To further determine the capacity of electroporation-mediated gene transfer to alter the overall activity of paracrine signaling pathways in the intact UGS, UGSs transfected with the Shh plasmid were also compared with βgal-transfected UGSs after 24 h in serum-free organ culture. Transfection with Shh increased Shh mRNA levels by 5- to 10-fold in the cultured UGS relative to βgal plasmid-transfected UGS tissues (Fig. 4E). Shh transfection also led to an increase in SHH signaling activity as measured by a significant increase in Gli1 expression, a gene known to be a transcriptional target for SHH signaling (Fig. 4F). These data confirmed that UGS electroporation achieved sufficient gene expression to alter the overall activity of a paracrine signaling pathway in the UGS.
Sulf1 overexpression in the developing UGS inhibits UGS bud formation
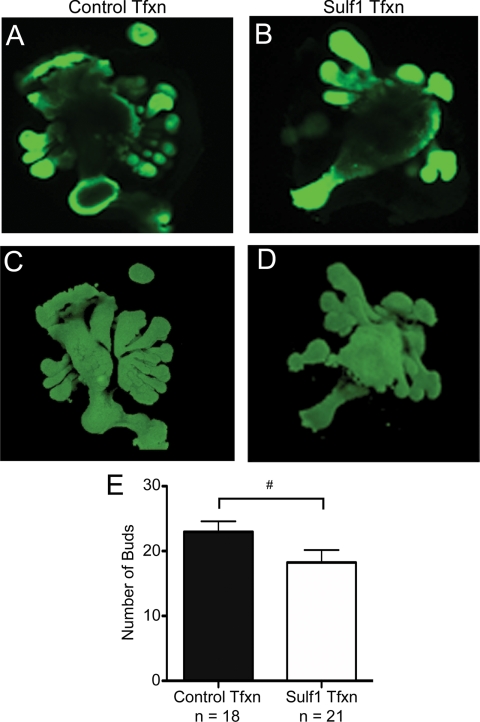
To test the importance of Sulf1 down-regulation in prostatic epithelial bud development in the male UGS, male e16 UGSs were transfected with plasmids containing the cDNA constructs for Sulf1 or βgal (control) to partially restore Sulf1 expression in the male UGS during epithelial bud outgrowth. Transfected UGSs were cultured with testosterone for 4 d to stimulate ductal morphogenesis. At the conclusion of the culture period, UGSs were processed for whole-mount immunofluorescent staining using an anti-E-cadherin antibody that stained the epithelium of the UGS. Whole-mount stained organs were then imaged using confocal microscopy to obtain optical sections of each UGS (Fig. 5, A and B). Olympus Fluoview version 1.7b software was then used to assemble the optical sections into 3D images of each UGS, which allowed for optimal visualization of UGS bud development of the cultured tissues (Fig. 5, C and D). Observers blinded to the treatment conditions counted the number of epithelial buds emerging from each UGS based on the reconstructed 3D images (Fig. 5E). The decrease in the number buds in Sulf1-transfected UGSs relative to controls was statistically significant (t test with Mann-Whitney analysis), indicating that expression of Sulf1 in the male UGS impaired testosterone-dependent bud development.
Figure 5.
Sulf1 overexpression decreases prostatic epithelial bud formation in the UGS. To test the potential role of Sulf1 in the developing UGS, e16 UGSs were electroporated with plasmids expressing Sulf1 or βgal as a control. After a 4-d culture period, UGSs were processed for whole-mount immunostaining with an anti-E-cadherin antibody, an epithelial-specific marker. Tissues were imaged with confocal microscopy, and a series of optical sections through each UGS was collected to allow all prostatic epithelial buds to be visualized. A, An optical section through a βgal-transfected UGS is shown. B, An optical section through a Sulf1-transfected UGS is shown. After collecting serial optical sections for the UGSs, Olympus Fluoview version 1.7b software was used to assemble the optical sections into 3D images of each UGS, and the number of UGS bud tips was counted by two observers blinded to the treatment group. C, A representative 3D reconstruction for a βgal-transfected UGS is shown. D, A representative 3D reconstruction for a Sulf1-transfected UGS is shown. E, The average number of prostatic epithelial buds is shown for each treatment group. The reduction in bud number for Sulf1-transfected UGSs relative to βgal (control) transfected UGSs was statistically significant: #, P = 0.042 by a t test with Mann-Whitney analysis. Error bars represent sem. Tfxn, Transfection.
Sulf1 overexpression inhibits Erk1/2 activation
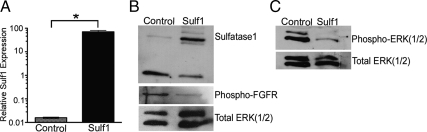
One potential mechanism for Sulf1 inhibition of epithelial ductal morphogenesis is the disruption of growth factor signaling. FGFR signaling through the MAPKs, ERK1/2, is known to be essential for prostatic epithelial bud formation in the UGS (34). The induced expression of Sulf1 in transfected UGSs after 48 h of culture with testosterone was confirmed by real-time RT-PCR and Western blotting (Fig. 6, A and B). The amount of phosphorylated ERK1/2 protein and phosphorylated FGFR were decreased in Sulf1-transfected UGSs relative to control (βgal) transfected UGSs (Fig. 6, B and C).
Figure 6.
Decreased ERK(1/2) phosphorylation in Sulf1-transfected UGS tissues. UGS tissues were cultured with testosterone for 48 h after transfection with the Sulf1- or βgal-expressing plasmids. A, Real-time RT-PCR analysis of mRNA extracts from transfected UGSs indicate that Sulf1 expression was significantly increased relative to control (βgal-transfected) UGS tissues. *, P = 0.0002 by unpaired t test. B, Western blot analysis of UGS protein extracts confirmed increased SULF1 protein content in the Sulf1-transfected UGSs relative to control (βgal) transfected tissues and phosphorylated FGFR (nonspecific for FGFR paralogs) is decreased in Sulf1-transfected UGS tissues. C, Phosphorylated ERK(1/2) protein is decreased in Sulf1-transfected tissues relative to control UGSs. Total ERK(1/2) protein content was used as a loading control in each blot.
Discussion
The development of the prostate from sexually ambiguous tissues is dependent on androgens that circulate in the male embryo. In addition to the critical role of androgens, several paracrine growth factor signaling cascades are known to coordinate prostatic branching morphogenesis via signaling events between the developing UGM and UGE. Despite decades of research, the molecular mechanisms that allow AR activation to influence growth factor signaling in the developing UGS remain unclear (35). Androgen-dependent changes in the composition of HS provide one potential mechanism for androgens to modulate the activity of growth factors like FGF10 in the developing UGS.
FGF10 has been identified as a critical early regulator of UGS ductal morphogenesis. In mice with engineered null mutations in the Fgf10 gene, the UGS does not develop into a prostate (4). Similarly, disrupting FGFR2b in the UGS using genetics or small molecules also inhibited testosterone-dependent UGS bud development and branching morphogenesis (34,36,37). The interaction of FGF10 with FGFR2b requires 6-O sulfated HS (7,38,39). Observations of branching morphogenesis in the lung and kidney also indicated that inhibition of HS sulfation disrupted FGF-dependent epithelial bud development (8,40). Inhibition of de novo HS sulfation with sodium chlorate inhibited testosterone-dependent UGS epithelial development and bud formation (Fig. 3). This disruption of UGS bud development could be recovered by treatment with exogenous heparin that can substitute for highly sulfated HS in ternary ligand and receptor complexes. These data established a critical role for de novo HS sulfation in testosterone-induced UGS ductal morphogenesis.
Regulation of HS sulfation involves multiple enzymes including intracellular sulfotransferases and extracellular endosulfatases that append and remove sulfates from HS, respectively. The 6-O endosulfatase, Sulf1, is repressed in the male UGS during e18 coinciding with epithelial bud outgrowth and the induction of branching morphogenesis (Fig. 1). Although decreased Sulf1 expression occurs later in embryonic development (e18) than the initial increases in fetal androgen production in male embryos (e13–e15), the repression of Sulf1 is sexually dimorphic in the UGS and is likely regulated indirectly by androgens through more direct gene expression changes and paracrine signaling events that are triggered when androgens initiate UGS ductal morphogenesis. Loss of Sulf1 in mice (SULF1−/−) resulted in increases in all 6-O sulfated HS disaccharide species (ΔUA-GlcNAc6S, ΔUA-GlcNS6S, and ΔUA2S-GlcNS6S) (19). The tissue-specific repression of Sulf1 in the e18 UGS correlated with increased trisulfated HS species (Fig. 2, A and B). Statistically significant differences in monosulfated and disulfated species were not observed in the UGS, further implicating that the most highly sulfated HS species have a necessary role in regulating the development of the prostate (Table 1). It is notable that the differences in HS modification between male and female e18 UGSs were more modest than the global increase in all sulfated disaccharide species observed in SULF1 knockout mice (19). This is likely explained by the spatially and temporally dynamic repression of Sulf1 that occurs in the male UGS to initiate a dynamic remodeling of HS sulfation that is distinct from the persistent and complete absence of SULF1 activity that occurs in the SULF1 knockout model. Furthermore, the increase in the 6-O sulfated HS species, ΔUA2S-GlcNS6S, in the UGS is testosterone dependent because female tissues cultured with testosterone had increased trisulfated 6-O HS species relative to UGSs cultured without hormone (Fig. 2, C and D). Although the increased level of trisulfated HS disaccharides in e18 male relative to female UGSs was statistically significant, it was a modest increase, raising the question of whether this increase was relevant for growth factor signaling. Data from other experimental systems has indicated that modest changes in HS sulfation can have dramatic and nonlinear affects on growth factor signaling pathways. For example, cultured fibroblasts isolated from SULF1−/− mice had a 45% increase in the trisulfated HS disaccharide species compared with wild-type fibroblasts (41). However, FGF2 stimulation of these SULF1−/− fibroblasts resulted in an approximately 5-fold increase in ERK1/2 activation relative to FGF2-stimulated wild-type cells, demonstrating a positive influence on FGF signaling that was disproportionate relative to the change in trisulfated HS disaccharides. Such studies indicate that even the modest changes in HS sulfation observed in the male UGS are likely to be highly relevant for paracrine growth factor signaling.
In vitro culture of the UGS in serum-free media has been a valuable method to study the regulation of epithelial bud development and the role of growth factor signaling pathways in mediating mesenchymal-epithelial interactions (42,43). However, modulation of gene function in this system has previously been limited to treatment of UGS cultures with exogenous proteins or inhibitors in the media. Ectopic gene expression has been achieved in developing prostate using retroviral transduction of dissociated UGM and UGE followed by xenografting recombinants of the transduced tissues (44). Although useful, the low rate of transduced cells (1%) and the requirement for tissue separation and xenografting in this approach has prevented this method from being adopted for developmental studies. More recently, electroporation has been employed to modulate gene expression in developing tissues cultured in vitro. The isolated genital ridges of mouse embryos injected with plasmid DNA encoding green fluorescent protein sustained ectopic expression and fluorescence for 14 d in culture (45). Osumi and colleagues (46,47) recovered a Pax6 mutant phenotype by reintroducing Pax6 expression with electroporation of plasmid DNA in the hindbrain of developing rat embryos and subsequently culturing the whole embryo in vitro. For this study, we optimized methods for introducing plasmid DNA into the UGS by square-wave electroporation. Although we observed variability in the amount of gene expression in each transfected UGS, as indicated by varying intensity and localization of X-gal staining in βgal-transfected UGSs, ectopic gene expression was sufficient for a Shh expression plasmid to induce its transcriptional target, Gli1 (Fig. 4). Transfection of the Sulf1 expression plasmid also reduced UGS bud development and decreased phosphorylation of FGFR and ERK1/2 (Fig. 6). These data implicated the FGF signaling pathway as an important target for SULF1 action in the UGS. They also demonstrated the utility of square-wave electroporation for future functional studies of early prostate development.
In addition to our observations of the effects of Sulf1 on FGF signaling in UGS bud development, changes in 6-O HS sulfation in response to reduced SULF1 activity have the potential to modulate multiple growth factor pathways. The paracrine signaling pathways modulated by changes in 6-O sulfation that are also known to influence prostatic development include IGF-I, WNT, TGFβ, bone morphogenetic protein, and SHH signaling (5,12,48,49,50). Extracellular IGF-I binds a family of proteins, IGF-binding proteins, inhibiting the interaction of IGF-I with its receptor. The specific interaction of IGF-I with IGF-binding protein 5 is competitively inhibited by forms of highly sulfated heparin (51), making it plausible that changes in Sulf1 activity could alter IGF signaling. We compared activation of the IGF pathway in UGSs with and without ectopic Sulf1 expression using Western blots for the activated form of IGF type 1 receptor in a series of independent experiments. Although we observed modest activation of IGF type 1 receptor in some experiments, this result was not consistently reproduced in all experimental trials (data not shown). Similarly, WNT5a is a specific inhibitor of ventral prostate bud development (52), and WNT5a signaling is potentiated by HS (53). We also compared activation of the noncanonical WNT5a/c-Jun N-terminal kinase (JNK) pathway in UGSs with and without ectopic Sulf1 expression using Western blots for the activated form of JNK in a series of independent experiments. Although we observed modest activation of JNK in some experiments, this result was not consistently reproduced in all experimental trials (data not shown). The modulation of multiple growth factor pathways by ectopic Sulf1 expression in the UGS remains plausible, but our results remain inconclusive for pathways other than the FGF-ERK1/2 pathway. This may reflect a limited role for Sulf1 in modulating other pathways, or it may reflect limitations of the experimental model system used in the study.
The observations presented here indicate that testosterone-dependent UGS bud development is negatively regulated by the 6-O endosulfatase, SULF1. Sexually dimorphic down-regulation of Sulf1 occurs at e18 in the male UGS, coinciding with prostatic epithelial bud outgrowth in the UGS. Inhibition of de novo HS sulfation inhibited UGS bud development, and Sulf1 overexpression reduced the formation of UGS epithelial buds. SULF1 activity has the potential to modulate multiple growth factor signaling pathways, including several that regulate UGS ductal morphogenesis. Ectopic Sulf1 expression decreased FGFR and ERK1/2 activation, implicating modulation of FGF signaling through ERK1/2 as important targets for SULF1 inhibitory activity in the female UGS. Collectively, these observations identified a previously unrecognized role for sexually dimorphic expression of Sulf1 and the resulting changes in HS fine structure in regulating the sexually dimorphic development of the UGS.
Acknowledgments
We thank Rita Malinowski and Katsiaryna Frantskevich for assistance with data analysis.
Footnotes
This work was supported by Grant AG024278 from the National Institutes of Health (NIH) National Institute on Aging to P.C.M., Cancer Biology Training Grant CAO0138 from NIH to S.L.K., Award CA91290 from the National Cancer Institute to S.B.S., and NIH Grant GM054832 to S.B.S.
Current address for S.B.S.: Department of Biochemistry and Molecular Biology, The Pennsylvania State University, University Park, Pennsylvania 16802.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 21, 2010
Abbreviations: AR, Androgen receptor; 3D, three-dimensional; e16, embryonic d 16; FGF, fibroblast growth factor; FGFR, FGF receptor; HS, heparan sulfate; HSPG, HS proteoglycan; JNK, c-Jun N-terminal kinase; SULF1, sulfatase 1; UGE, UGS epithelial; UGM, UGS mesenchyme; UGS, urogenital sinus.
References
- Sugimura Y, Cunha GR, Donjacour AA 1986 Morphogenesis of ductal networks in the mouse prostate. Biol Reprod 34:961–971 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B 1978 The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool 205:181–193 [DOI] [PubMed] [Google Scholar]
- Thomson AA, Cunha GR 1999 Prostatic growth and development are regulated by FGF10. Development 126:3693–3701 [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Thomson AA, Cunha GR 2003 FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol 261:39–54 [DOI] [PubMed] [Google Scholar]
- Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson Jr CP 2003 QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol 162:341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S 2004 Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist noggin. J Biol Chem 279:5604–5611 [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA 2005 Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev 16:107–137 [DOI] [PubMed] [Google Scholar]
- Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV 2003 Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev Biol 258:185–200 [DOI] [PubMed] [Google Scholar]
- Sugahara K, Kitagawa H 2002 Heparin and heparan sulfate biosynthesis. IUBMB Life 54:163–175 [DOI] [PubMed] [Google Scholar]
- Selleck SB 2006 Shedding light on the distinct functions of proteoglycans. Sci STKE 2006:pe17 [DOI] [PubMed] [Google Scholar]
- Wang SW, Ai XB, Freeman SD, Pownall ME, Lu Q, Kessler DS, Emerson CP 2004 QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc Natl Acad Sci USA 101:4833–4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson Jr CP 2001 Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science 293:1663–1666 [DOI] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD 2002 Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem 277:49175–49185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock SL, Fletcher JM, Beddington RS, Wilson VA 1998 Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev 12:1894–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe K, Inatani M, Pallerla SR, Castagnola J, Yamaguchi Y, Esko JD 2005 Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development 132:3777–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringvall M, Ledin J, Holmborn K, van Kuppevelt T, Ellin F, Eriksson I, Olofsson AM, Kjellen L, Forsberg E 2000 Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem 275:25926–25930 [DOI] [PubMed] [Google Scholar]
- Li JP, Gong F, Hagner-McWhirter A, Forsberg E, Abrink M, Kisilevsky R, Zhang X, Lindahl U 2003 Targeted disruption of a murine glucuronyl C5-epimerase gene results in heparan sulfate lacking l-iduronic acid and in neonatal lethality. J Biol Chem 278:28363–28366 [DOI] [PubMed] [Google Scholar]
- Holst CR, Bou-Reslan H, Gore BB, Wong K, Grant D, Chalasani S, Carano RA, Frantz GD, Tessier-Lavigne M, Bolon B, French DM, Ashkenazi A 2007 Secreted sulfatases Sulf1 and Sulf2 have overlapping yet essential roles in mouse neonatal survival. PLoS ONE 2:e575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna WC, Baldwin RJ, Padva M, Kalus I, Ten Dam G, van Kuppevelt TH, Gallagher JT, von Figura K, Dierks T, Merry CL 2006 Heparan sulfate 6-O-endosulfatases: discrete in vivo activities and functional co-operativity. Biochem J 400:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi H, Nagai N, Sugaya N, Atsumi F, Stevens RL, Kimata K 2007 Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J Biol Chem 282:15578–15588 [DOI] [PubMed] [Google Scholar]
- Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P 2003 Mouse urogenital development: a practical approach. Differentiation 71:402–413 [DOI] [PubMed] [Google Scholar]
- Chen E, Stringer SE, Rusch MA, Selleck SB, Ekker SC 2005 A unique role for 6-O sulfation modification in zebrafish vascular development. Dev Biol 284:364–376 [DOI] [PubMed] [Google Scholar]
- Toyoda H, Kinoshita-Toyoda A, Selleck SB 2000 Structural analysis of glycosaminoglycans in Drosophila and Caenorhabditis elegans and demonstration that tout-velu, a Drosophila gene related to EXT tumor suppressors, affects heparan sulfate in vivo. J Biol Chem 275:2269–2275 [DOI] [PubMed] [Google Scholar]
- Wong ML, Medrano JF 2005 Real-time PCR for mRNA quantitation. Biotechniques 39:75–85 [DOI] [PubMed] [Google Scholar]
- Joesting MS, Cheever TR, Volzing KG, Yamaguchi TP, Wolf V, Naf D, Rubin JS, Marker PC 2008 Secreted frizzled related protein 1 is a paracrine modulator of epithelial branching morphogenesis, proliferation, and secretory gene expression in the prostate. Dev Biol 317:161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA 2008 The branching programme of mouse lung development. Nature 453:745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Sala-Newby GB, Dhoot GK 2006 Sulf1 expression pattern and its role in cartilage and joint development. Dev Dyn 235:3327–3335 [DOI] [PubMed] [Google Scholar]
- Lum DH, Tan J, Rosen SD, Werb Z 2007 Gene trap disruption of the mouse heparan sulfate 6-O-endosulfatase gene, Sulf2. Mol Cell Biol 27:678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledin J, Staatz W, Li JP, Götte M, Selleck S, Kjellén L, Spillmann D 2004 Heparan sulfate structure in mice with genetically modified heparan sulfate production. J Biol Chem 279:42732–42741 [DOI] [PubMed] [Google Scholar]
- Nairn AV, Kinoshita-Toyoda A, Toyoda H, Xie J, Harris K, Dalton S, Kulik M, Pierce JM, Toida T, Moremen KW, Linhardt RJ 2007 Glycomics of proteoglycan biosynthesis in murine embryonic stem cell differentiation. J Proteome Res 6:4374–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Okishio K, Ui-Tei K, Saigo K, Kinoshita-Toyoda A, Toyoda H, Nishimura T, Suda Y, Hayasaka M, Hanaoka K, Hitoshi S, Ikenaka K, Nishihara S 2008 Heparan sulfate regulates self-renewal and pluripotency of embryonic stem cells. J Biol Chem 283:3594–3606 [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Yoshida K, Shibata Y, Kimata K 2008 Tetrasulfated disaccharide unit in heparan sulfate enzymatic formation and tissue distribution. J Biol Chem 283:31237–31245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan F, Kolset SO, Prydz K, Gottfridsson E, Lindahl U, Salmivirta M 1999 Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. J Biol Chem 274:36267–36273 [DOI] [PubMed] [Google Scholar]
- Kuslak SL, Marker PC 2007 Fibroblast growth factor receptor signaling through MEK-ERK is required for prostate bud induction. Differentiation 75:638–651 [DOI] [PubMed] [Google Scholar]
- Thomson AA 2001 Role of androgens and fibroblast growth factors in prostatic development. Reproduction 121:187–195 [DOI] [PubMed] [Google Scholar]
- Kuslak SL, Thielen JL, Marker PC 2007 The mouse seminal vesicle shape mutation is allelic with Fgfr2. Development 134:557–565 [DOI] [PubMed] [Google Scholar]
- Lin Y, Liu G, Zhang Y, Hu YP, Yu K, Lin C, McKeehan K, Xuan JW, Ornitz DM, Shen MM, Greenberg N, McKeehan WL, Wang F 2007 Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development 134:723–734 [DOI] [PubMed] [Google Scholar]
- Ashikari-Hada S, Habuchi H, Kariya Y, Itoh N, Reddi AH, Kimata K 2004 Characterization of growth factor-binding structures in heparin/heparan sulfate using an octasaccharide library. J Biol Chem 279:12346–12354 [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Goetz R 2005 A protein canyon in the FGF-FGF receptor dimer selects from an a la carte menu of heparan sulfate motifs. Curr Opin Struct Biol 15:506–516 [DOI] [PubMed] [Google Scholar]
- Steer DL, Shah MM, Bush KT, Stuart RO, Sampogna RV, Meyer TN, Schwesinger C, Bai X, Esko JD, Nigam SK 2004 Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev Biol 272:310–327 [DOI] [PubMed] [Google Scholar]
- Lamanna WC, Frese MA, Balleininger M, Dierks T 2008 Sulf loss influences N-, 2-o-, and 6-o-sulfation of multiple heparan sulfate proteoglycans and modulates fibroblast growth factor signaling. J Biol Chem 283:27724–27735 [DOI] [PubMed] [Google Scholar]
- Doles JD, Vezina CM, Lipinski RJ, Peterson RE, Bushman W 2005 Growth, morphogenesis, and differentiation during mouse prostate development in situ, in renal grafts, and in vitro. Prostate 65:390–399 [DOI] [PubMed] [Google Scholar]
- Lipschutz JH, Foster BA, Cunha GR 1997 Differentiation of rat neonatal ventral prostates grown in a serum-free organ culture system. Prostate 32:35–42 [DOI] [PubMed] [Google Scholar]
- Thompson TC, Southgate J, Kitchener G, Land H 1989 Multistage carcinogenesis induced by Ras and Myc oncogenes in a reconstituted organ. Cell 56:917–930 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yamamoto M, Matsui Y 2002 Introduction and expression of foreign genes in cultured mouse embryonic gonads by electroporation. Reprod Fertil Dev 14:259–265 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Nomura T, Osumi N 2008 Transferring genes into cultured mammalian embryos by electroporation. Dev Growth Differ 50:485–497 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Osumi N 2002 Pax6 regulates specification of ventral neurone subtypes in the hindbrain by establishing progenitor domains. Development 129:1327–1338 [DOI] [PubMed] [Google Scholar]
- Carrasco H, Olivares GH, Faunes F, Oliva C, Larraín J 2005 Heparan sulfate proteoglycans exert positive and negative effects in Shh activity. J Cell Biochem 96:831–838 [DOI] [PubMed] [Google Scholar]
- Tang R, Rosen SD 2009 Functional consequences of the subdomain organization of the Sulfs. J Biol Chem 284:21505–21514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerde TJ, Bushman W 2009 IL-1 induces IGF-dependent epithelial proliferation in prostate development and reactive hyperplasia. Sci Signal 2:ra49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Parker A, Busby Jr W, Clemmons DR 1994 Heparin, heparan sulfate, and dermatan sulfate regulate formation of the insulin-like growth factor-I and insulin-like growth factor-binding protein complexes. J Biol Chem 269:20388–20393 [PubMed] [Google Scholar]
- Allgeier SH, Lin TM, Vezina CM, Moore RW, Fritz WA, Chiu SY, Zhang C, Peterson RE 2008 WNT5A selectively inhibits mouse ventral prostate development. Dev Biol 324:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MP, Fiori JL, Kershner EK, Frank BP, Indig FE, Taub DD, Hoek KS, Weeraratna AT 2009 Heparan sulfate proteoglycan modulation of Wnt5A signal transduction in metastatic melanoma cells. J Biol Chem 284:28704–28712 [DOI] [PMC free article] [PubMed] [Google Scholar]