Abstract
Small molecule inverse agonists for the TSH receptor (TSHR) may be used as probes of the role of basal (or agonist-independent or constitutive) signaling and may have therapeutic potential as orally active drugs to inhibit basal signaling in patients with thyroid cancer and in some patients with hyperthyroidism. We describe the first small-molecule ligand [1;2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one] that exhibits inverse agonist properties at TSHR. 1 inhibits basal and TSH-stimulated signaling, measured as cAMP production, by TSHRs in HEK-EM 293 cells stably expressing wild-type TSHRs; the antagonism of TSH-mediated signaling is competitive. 1 also inhibits basal signaling by wild-type TSHRs, and four constitutively active mutants of TSHR expressed transiently in HEK-EM 293 cells. 1 was active under more physiologically relevant conditions in primary cultures of human thyrocytes expressing endogenous TSHRs where it inhibited basal levels of mRNA transcripts for thyroglobulin, thyroperoxidase, sodium iodide symporter, and TSHR. These data serve as proof of principle that small, drug-like molecules can inhibit basal signaling by TSHR. We suggest that this small molecule is a lead compound for the development of higher-potency inverse agonists that can be used as probes of TSHR biology with therapeutic potential.
A small molecule inverse agonist for TSH receptor was identified, and the effect of this ligand on clinically important TSH receptor-sensitive biologic endpoints was demonstrated.
Discovery of novel ligands for the human TSH receptor (TSHR), which is a seven transmembrane-spanning receptor (7TMR or G protein-coupled receptor) (1), has been an area of active research for several decades (for review, see Ref. 2). Different types of ligands, recombinant TSH, TSH analogs, antibodies, and small-molecule, drug-like compounds, have been reported that are agonists that activate TSHR or antagonists that inhibit stimulation of TSHR by TSH and thyroid-stimulating antibodies. Native TSHR, distinct from the receptors for LH/chorionic gonadotropin and FSH (3), the two other members of the glycoprotein hormone receptor subfamily of class A, rhodopsin-like 7TMRs, exhibits basal (or agonist-independent or constitutive) signaling (4). It has been found that some 7TMR antagonists exhibit the additional property of inhibiting basal signaling and are referred to as inverse agonists (5); antagonists that inhibit agonist-stimulated signaling but do not inhibit agonist-independent signaling are termed neutral antagonists (6). In fact, several thyroid-blocking antibodies (TBAbs) have been shown to exhibit inverse agonist properties at TSHR. Chen et al. (7,8,9) generated a mouse monoclonal TBAb, and Sanders et al. (10) and Moriyama et al. (11) generated human monoclonal TBAbs with inverse agonist properties. Rossi et al. (12) showed that the insecticide 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT), which had been described as a thyroid disruptor (13), inhibited cAMP accumulation in Chinese hamster ovary (CHO) cells expressing TSHRs. However, because they also found that DDT inhibited cAMP accumulation stimulated by forskolin, it is not correct to conclude that DDT is a TSHR inverse agonist. It has been suggested that monoclonal antibodies that are inverse agonists may be used therapeutically to inhibit TSHR signaling in patients with recurrent or metastatic thyroid cancer who are receiving thyroid hormone to suppress TSH and in patients with germline constitutively activating mutations (CAMs) of TSHR causing nonautoimmune hyperthyroidism.
Over the last several years, we have been involved in studies to discover/develop small-molecule ligands (SMLs) for TSHR (for review, see Ref. 2). SMLs are attractive agents because they are more easily employed as probes and drugs compared with TSH, its analogs, or anti-TSHR antibodies; can be synthesized chemically in large amounts at moderate cost; and can be given orally because they are not degraded within and can be absorbed from the gastrointestinal tract. We previously reported a SML partial agonist (14), a full agonist that we could use in mice (15), and a SML neutral antagonist that does not exhibit inverse agonist properties (16). Here we describe the first SML inverse agonist [1; 2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one; National Institutes of Health Chemical Genomics Center number NCGC00161856] that inhibits basal signaling by wild-type TSHR and several CAMs that may be a lead drug for probes of TSHR biology and for treatment of patients with thyroid cancer and of some patients with hyperthyroidism.
Materials and Methods
Synthesis of 1 [2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one (NCGC00161856)] (Fig. 1)
Figure 1.
Synthesis of 1 [2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one (NCGC00161856)]. ACN, Acetonitrile; EtOH, ethanol; Me, methyl; MW, microwave.
To a solution of 3-(chloromethyl)-4-methoxybenzaldehyde (85 mg, 0.46 mmol, 1.0 equivalent) and 2,6-dimethylphenol (62 mg, 0.51 mmol, 1.1 equivalent) in 3.0 ml anhydrous acetonitrile, K2CO3 (320 mg, 2.3 mmol, 5.0 equivalents) was added, and the mixture was heated in a microwave reactor for 30 min at 150 C. After filtering off the solid and removing the solvent, 2-amino-N-(furan-2-ylmethyl)benzamide (110 mg, 0.51 μmol, 1.1 equivalent) in 5 ml ethanol was added followed by addition of ytterbium trifluoromethanesulfonate (57 mg, 0.02 μmol, 0.2 equivalent). The mixture was heated at 80 C for 2 h. The product was isolated via preparative HPLC purification, and solvent was removed via reduced pressure lyophilization to give 2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one (64.5 mg, 30%) as a white solid after triturating with diethyl ether. 1H nuclear magnetic resonance (400 MHz, dimethylsulfoxide-d6) δ ppm 2.16 (s, 6 H), 3.77 (s, 3 H), 3.86 (d, J = 15.6 Hz, 1 H), 4.69 (d, J = 1.9 Hz, 2 H), 5.19 (d, J = 15.4 Hz, 1 H), 5.75 (d, J = 2.3 Hz, 1 H), 6.30 (d, J = 3.2 Hz, 1 H), 6.39 (dd, J = 3.2, 1.9 Hz, 1 H), 6.62–6.70 (m, 2 H), 6.86–6.97 (m, 1 H), 6.99–7.04 (m, 3 H), 7.19–7.29 (m, 2 H), 7.32 (d, J = 2.3 Hz, 1 H), 7.49 (d, J = 2.4 Hz, 1 H), 7.57 (dd, J = 1.8, 0.7 Hz, 1 H), 7.68 (dd, J = 7.9, 1.5 Hz, 1 H); HPLC: tR = 6.88 min, UV254 = 97%. High resolution mass spectroscopy for this compound revealed a mass to charge ratio (M+H+) of 496.2138 (the calculated value for C29H29N2O4+ is 496.2132).
Culture of HEK-EM 293 cell lines and transient transfection
HEK-EM 293 cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 10 μg/ml streptomycin (Life Technologies Inc., Rockville, MD) at 37 C in a humidified 5% CO2 incubator. The generation of a stable HEK-EM 293 cell line expressing TSHRs was described previously (16). Cells were transiently transfected with wild-type TSHR or mutant receptors in 24-well plates (7.5 × 104 cells per well) with 0.2 μg DNA per well using FuGENE 6 reagent (Roche, Indianapolis, IN).
Site-directed mutagenesis of TSHR
S281N, M453T, I568T, and F631I mutations were introduced into wild-type TSHR-pcDNA3.1 via the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The constructs were verified by sequencing (MWG Biotech, High Point, NC).
Culture of primary human thyrocytes
Thyroid tissue samples were collected from normal thyroid tissue from patients undergoing total thyroidectomy for thyroid cancer at the National Institutes of Health Clinical Center as described previously (15). Surgical specimens were maintained in Hanks’ balanced salt solution (HBSS) on ice, minced into small pieces, and digested with 3 mg/ml collagenase type IV (Life Technologies). Monodispersed cells were plated in 10 ml DMEM with 10% FBS in 10-cm tissue culture dishes and incubated at 37 C in a humidified 5% CO2 incubator, and after 24 h, the primary cultures of adherent thyroid cells were obtained. For determination of thyroglobulin (TG), thyroid peroxidase (TPO), TSHR, sodium iodide symporter (NIS), or deiodinase type 2 (DIO2) mRNA expression, thyrocytes were seeded into 12-well plates at a density of 1 × 105 cells per well. The cells were incubated in DMEM with 2% FBS and 1 mm 3-isobutyl-1-methylxanthine (IBMX) for 48 h in the presence or absence of 1.
Determination of cAMP production
Transiently transfected HEK-EM 293 cells or cells stably expressing TSHRs were seeded into 96-well plates at a density of 7 × 104 cells per well in DMEM containing 10% FBS. Thyrocytes were plated in 24-well plates at a density of 8 × 104 cells per well in DMEM containing 10% FBS. Cells were cultured for 24 h before incubation for 20 min in HBSS/10 mm HEPES (pH 7.4). To determine basal cAMP production, cells were incubated for 1 h at 37 C in a humidified incubator in HBSS/HEPES containing 1 mm IBMX (Sigma Chemical Co., St. Louis, MO) in the presence or absence of 1. To determine the effect of 1 on TSH-induced TSHR activity, cells were incubated for 20 min with 1 in HBSS/HEPES without IBMX and subsequently for 40 min with bovine TSH (Sigma) and 1 in HBSS/HEPES supplemented with 1 mm IBMX at 37 C. The levels of cAMP in cells incubated in HBSS/HEPES without IBMX were subtracted in all experiments. After aspiration of the incubation medium, cells were lysed using lysis buffer of the cAMP-Screen Direct system (Applied Biosystems, Foster City, CA). The cAMP content of the cell lysate was determined using the method described in the manufacturer’s protocol. The chemiluminescence signal was measured in a VICTOR3 V 1420 multilabel counter (PerkinElmer, Norwalk, CT). The potencies (EC50 and IC50) of the ligands were obtained from dose-response curves by data analysis with GraphPad Prism 4 for Windows.
Effect of 1 on [125I]TSH binding
HEK-EM 293 cells stably expressing TSHRs were seeded into 24-well plates at a density of 2.2 × 105 cells per well. Cell surface binding was measured by incubation in 0.25 ml binding buffer (HBSS containing 2.5% milk powder and 0.2% BSA) containing 60,000 cpm bovine [125I]TSH (Brahms Aktiengesellschaft, Hennigsdorf, Germany) without or with 30 μm 1 for 2 h at room temperature; nonspecific binding was measured in the presence of 1.8 μm unlabeled bovine TSH (17). Cells were washed three times with 0.5 ml ice-cold HBSS and lysed with 0.5 ml 0.4 n NaOH, and the cell-associated radioactivity was counted in a 1470 Wallac Wizard γ-counter (PerkinElmer).
Quantitative RT-PCR
Total RNA was purified using RNeasy Micro kits (QIAGEN, Valencia, CA). cDNA was prepared using a high-capacity cDNA archive kit (Applied Biosystems). RT-PCR was performed in 25-μl reactions using cDNA prepared from 100 ng or less of total RNA and Universal PCR Master Mix (Applied Biosystems). mRNA levels were measured using primers and probes from Applied Biosystems. Quantitative RT-PCR results were normalized to GAPDH to correct for differences in RNA input.
Statistical analysis
Data are expressed as mean ± se. The data were analyzed by Student’s t test or one-way ANOVA; P < 0.05 was considered significant.
Results and Discussion
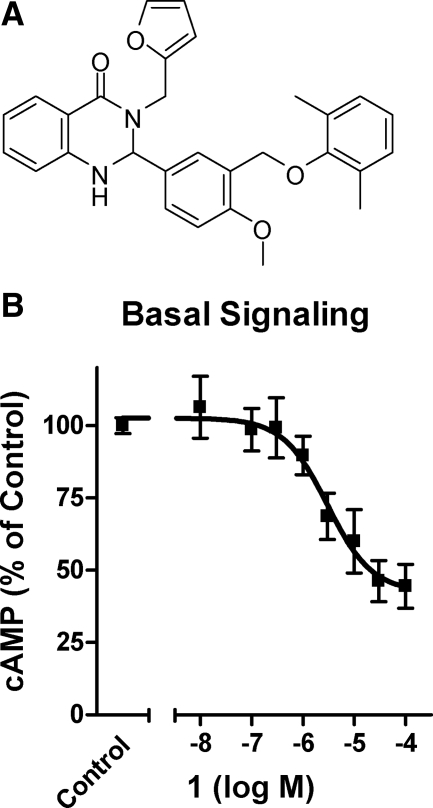
In a previous search for SMLs for TSHR, we synthesized over 100 analogs of an agonist that we had identified by quantitative high-throughput screening (15). We found 66 compounds that did not activate TSHR. Of these, 11 compounds inhibited TSH-stimulated signaling, measured as cAMP production over 60 min in HEK-EM 293 cells stably expressing TSHRs, but only 1 [2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one (NCGC00161856)] was found to be an inverse agonist (Fig. 2). 1 inhibited basal cAMP production by TSHRs by 58% with an IC50 = 3.0 μm. Figure 3A illustrates, as expected, that 1 also inhibits TSH-stimulated cAMP production by 86%; IC50 = 0.78 μm. Moreover, a Schild analysis of TSH-stimulated cAMP production shows that 1 acts as a competitive antagonist of TSH signaling (Fig. 3B). The Schild plot of these data were linear with a slope not different from 1.0 (data not shown), which is typical for a competitive ligand. Competitive antagonism is often caused by binding competition between the antagonist and agonist. However, 1 had no effect on [125I]TSH binding to TSHRs on the surface of HEK-EM 293 cells (data not shown). A lack of effect of 1 on TSH binding was expected because we have previously shown that the parent compound of 1 does not affect TSH binding and provided evidence that it binds in the transmembrane domain of TSHR (15). We assume that 1 binds in the same domain of TSHR as we predicted for all the SMLs we have discovered (2). 1 is an allosteric modulator of TSHR, which explains its effect on TSH-induced TSHR signaling without direct competition for the extracellular TSH binding site. The fact that allosteric ligands can affect affinity and/or efficacy of agonists for receptors is described for numerous GPCRs (18).
Figure 2.
Structure and effect of 1 on agonist-independent signaling by TSHR. A, Chemical structure of 1 [2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one (NCGC00161856)]. B, HEK-EM 293 cells stably expressing TSHRs were exposed to the noted concentrations of 1 for 60 min in HBSS with 1 mm IBMX (basal signaling) as described in Materials and Methods. Nontreated cells, incubated with IBMX only, were used as control. After 60 min, the cells were lysed, and cAMP levels were measured by ELISA. The data from two independent experiments with duplicate samples are shown as percentage of control.
Figure 3.
1 is a competitive antagonist of TSH-stimulated signaling. A, HEK-EM 293 cells stably expressing TSHRs were exposed to the noted concentrations of 1 20 min before the addition of a half-maximally effective concentration of TSH (1.8 nm, control) and 1 mm IBMX. After 40 min incubation with 1 and TSH, the cells were lysed and cAMP levels were measured by ELISA. B, HEK-EM 293 cells stably expressing TSHRs were incubated in the absence of 1 or in the presence of 3, 10, or 30 μm 1 that was added 20 min before the addition of increasing concentrations of TSH (0.18 nm to 1.8 μm) and 1 mm IBMX. After 40 min, the cells were lysed and cAMP levels were measured by ELISA. The data from two independent experiments with duplicate samples are shown as percentage of maximum. A Schild plot of these data was linear with a slope not different from 1.0 (not shown). bTSH, Bovine TSH.
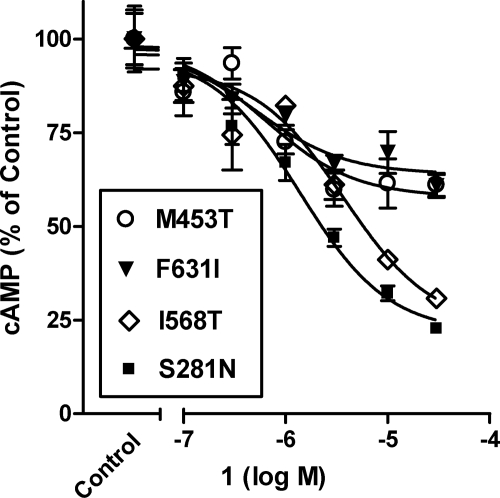
A small number of patients with hyperthyroidism exhibit increased thyroid function caused by basal signaling of CAMs (19). We measured basal activities and cAMP production over 60 min in HEK-EM 293 cells transiently expressing TSHRs of wild-type TSHR and four CAMs, S281N, M453T, I568T, and F631I, that were found in hyperthyroid patients and are located in different receptor domains. S281N is a mutant in the amino-terminal ectodomain of TSHR. The I568T mutant is located in extracellular loop 2, whereas the mutants M453T and F631I are located in transmembrane helices 2 and 6, respectively. These CAMs exhibit constitutive signaling between 15- and 27-fold higher than wild-type TSHR (see Fig. 4). Figure 4 illustrates that 1 inhibited basal signaling of all four CAMs tested with the following IC50 values and maximum levels of inhibition: 1.4 μm and 78% with S281N, 3.7 μm and 77% with I568T, 0.5 μm and 36% with F631I, and 0.6 μm and 42% with M453T. The levels of inhibition of basal signaling by 1 appear to segregate these CAMs into two groups with greater inhibition in one than in the other. It is noteworthy, therefore, that the CAMs that are inhibited to a greater extent have substitutions of residues in their amino-terminal ectodomains or extracellular loops (20), whereas those that are inhibited to a lesser degree have substitutions in their transmembrane domains (21). We suggest that the lesser degree of inhibition of M453T and F631I may be secondary to the altered conformation of the transmembrane helices in which 1 likely binds. Our findings also support the proposed model that in TSHR, the extracellular loops and a region of the ectodomain cooperate in the generation of a structural module functioning as an agonist of the serpentine domain (22).
Figure 4.
1 inhibits the basal activities of constitutively active mutant TSHRs (CAMs). HEK-EM 293 cells transiently expressing wild-type TSHRs and mutant TSHRs S281N, M453T, I568T, or F631T, were incubated without 1 (control activity) or with the noted concentrations of 1 in HBSS with 1 mm IBMX for 60 min. Subsequently, cells were lysed and cAMP levels were measured by ELISA. The control activities of the CAMs were as follows: S281N, 15 ± 3.3-fold; M453T, 27 ± 7.3-fold; I568T, 24 ± 0.71-fold; and F631I, 20 ± 4.5-fold above wild-type TSHR basal activity. The data from two independent experiments with duplicate samples are shown as percentage of control activities.
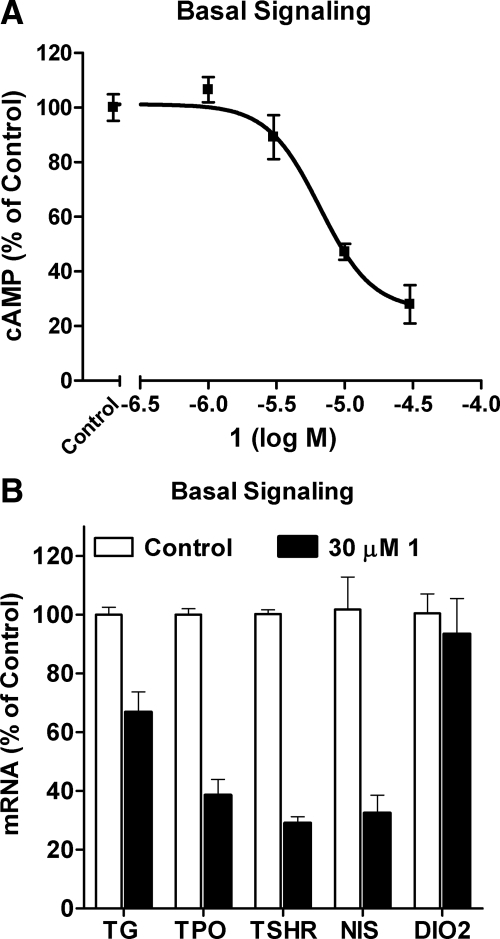
We sought to confirm the inverse agonist activities of 1 in human thyrocytes in primary culture, which is a more physiologically relevant cell system that allows for the determination of the effects of TSHR ligands on expression of genes important in differentiated thyroid function. This was important because it has been shown that TSHR mutants signal differently in different cell types (23). We first confirmed that 1 decreases cAMP accumulation in human thyrocytes by 72% with a potency of 6.6 μm (Fig. 5A). Because 1 did not decrease cAMP accumulation stimulated by isoproterenol, an agonist for the 7TMR β-adrenergic receptor, which couples to Gs; vasoactive intestinal peptide, which signals via a Gs-coupling 7TMR also; or forskolin, an adenylyl cyclase activator, in these cells (not shown), we conclude that 1 acts as a TSHR inverse agonist in human thyrocytes. We had shown that TSH and the SML agonist that we identified previously specifically increase expression of several genes in thyrocytes (15). We tested the effects of 1 on the expression of the mRNAs for the genes TPO, TSHR, TG, NIS, and DIO2 in the absence of any agonist (Fig. 5B). We treated thyrocytes without or with 1 alone in the presence of IBMX for 48 h. 1 decreased TPO, TSHR, TG, and NIS mRNA levels between 33 and 71% but did not decrease DIO2 mRNA levels. Thus, 1 is an inverse agonist in human thyrocytes that can decrease the levels of mRNAs for several genes expressed in differentiated thyrocytes. These observations support the idea that 1, or to-be-discovered analogs, could be used to suppress TSH-independent signaling in humans.
Figure 5.
Inhibition of basal cAMP production and of the basal expression of mRNAs for TPO, TSHR, TG, NIS, and DIO2 by 1 in primary cultures of human thyrocytes. A, Thyrocytes were incubated in HBSS without or with 1 mm IBMX and without (control) or with increasing concentrations of 1 for 2 h at 37 C. Thereafter, the buffers were aspirated, the cells were lysed, and intracellular cAMP was measured by ELISA. The basal activity without IBMX was subtracted from all samples. B, Thyrocytes were incubated in DMEM containing 2% FBS and 1 mm IBMX without or with 30 μm 1 as described in Materials and Methods. After 48 h, the cells were lysed and the levels of the mRNAs were measured and normalized to GAPDH mRNA. The mRNA levels are presented as percentage of basal activity (control). The data from two independent experiments with duplicate samples are shown.
There are at least two patient groups in which inverse agonists could be used therapeutically as has been proposed previously for monoclonal antibodies with inverse agonist properties (7,8,9,10,11). Patients with nonautoimmune hyperthyroidism caused by CAMs (19), especially germline mutations, are an obvious group in whom inverse agonists could be effective because it has already been shown that 1 and TBAbs inhibit basal signaling by disease-associated CAMs expressed in cells in tissue culture. The use of SML inverse agonists would perhaps be most valuable in children with inherited forms of this disease in which radioiodine or surgical ablation is less attractive. Furthermore, one major advantage of SML in comparison with TBAbs is the possibility of oral administration. A larger patient group in which these drugs would be useful are patients with recurrent or metastatic thyroid cancer who are receiving thyroid hormones for TSH suppression but who may still have their cancer cells stimulated to proliferate and metastasize because of the agonist-independent signaling of TSHR. Although it has been shown that TSHR CAMs can stimulate proliferation of human thyrocytes in culture (24), to the best of our knowledge, there is no proof that constitutive signaling by wild-type TSHRs expressed on thyroid cancer cells stimulates proliferation or metastasis. This is an area of research that we think should be pursued vigorously.
Acknowledgments
We thank Francesco S. Celi and Anna Teresa Alberobello for providing human thyroid tissue, Noel T. Southall for informatics support, and William Leister for analytical support.
Footnotes
This research was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Human Genome Research Institute, National Institutes of Health and the Molecular Libraries Initiative of the National Institutes of Health Roadmap for Medical Research.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 28, 2010
Abbreviations: CAM, Constitutively activating mutation; DDT, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane; DIO2, deiodinase type 2; FBS, fetal bovine serum; HBSS, Hanks’ balanced salt solution; IBMX, 3-isobutyl-1-methylxanthine; NIS, sodium iodide symporter; SML, small-molecule ligand; TBAb, thyroid-blocking antibody; TG, thyroglobulin; 7TMR, seven transmembrane-spanning receptor; TPO, thyroid peroxidase; TSHR, TSH receptor.
References
- Pierce KL, Premont RT, Lefkowitz RJ 2002 Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3:639–650 [DOI] [PubMed] [Google Scholar]
- Neumann S, Raaka BM, Gershengorn MC 2009 Development of human TSH receptor ligands as pharmacological probes and for potential clinical application. Expert Rev Endocrinol Metab 4:669–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman LH, Ijzerman AP 2008 G protein-coupled receptors of the hypothalamic-pituitary-gonadal axis: a case for Gnrh, LH, FSH, and GPR54 receptor ligands. Med Res Rev 28:975–1011 [DOI] [PubMed] [Google Scholar]
- Vassart G, Dumont JE 1992 The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 [DOI] [PubMed] [Google Scholar]
- Kenakin T 2001 Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J 15:598–611 [DOI] [PubMed] [Google Scholar]
- Bond RA, Ijzerman AP 2006 Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci 27:92–96 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2007 Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology 148:2375–2382 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2008 Identification of key amino acid residues in a thyrotropin receptor monoclonal antibody epitope provides insight into its inverse agonist and antagonist properties. Endocrinology 149:3427–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2009 A monoclonal antibody with TSH receptor inverse agonist and TSH antagonist activities binds to the receptor hinge region as well as to the leucine-rich domain. Endocrinology 150:3401–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J, Evans M, Betterle C, Sanders P, Bhardwaja A, Young S, Roberts E, Wilmot J, Richards T, Kiddie A, Small K, Platt H, Summerhayes S, Harris R, Reeve M, Coco G, Zanchetta R, Chen S, Furmaniak J, Smith BR 2008 A human monoclonal autoantibody to the thyrotropin receptor with thyroid-stimulating blocking activity. Thyroid 18:735–746 [DOI] [PubMed] [Google Scholar]
- Moriyama K, Okuda J, Saijo M, Hattori Y, Kanamoto N, Hataya Y, Matsuda F, Mori T, Nakao K, Akamizu T 2003 Recombinant monoclonal thyrotropin-stimulation blocking antibody (TSBAb) established from peripheral lymphocytes of a hypothyroid patient with primary myxedema. J Endocrinol Invest 26:1076–1080 [DOI] [PubMed] [Google Scholar]
- Rossi M, Dimida A, Dell'anno MT, Trincavelli ML, Agretti P, Giorgi F, Corsini GU, Pinchera A, Vitti P, Tonacchera M, Maggio R 2007 The thyroid disruptor 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane appears to be an uncompetitive inverse agonist for the thyrotropin receptor. J Pharmacol Exp Ther 320:465–474 [DOI] [PubMed] [Google Scholar]
- Santini F, Vitti P, Ceccarini G, Mammoli C, Rosellini V, Pelosini C, Marsili A, Tonacchera M, Agretti P, Santoni T, Chiovato L, Pinchera A 2003 In vitro assay of thyroid disruptors affecting TSH-stimulated adenylate cyclase activity. J Endocrinol Invest 26:950–955 [DOI] [PubMed] [Google Scholar]
- Moore S, Jaeschke H, Kleinau G, Neumann S, Costanzi S, Jiang JK, Childress J, Raaka BM, Colson A, Paschke R, Krause G, Thomas CJ, Gershengorn MC 2006 Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: structure-activity relationships and selective binding patterns. J Med Chem 49:3888–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM, Gershengorn MC 2009 Small molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci USA 106:12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Kleinau G, Costanzi S, Moore S, Jiang JK, Raaka BM, Thomas CJ, Krause G, Gershengorn MC 2008 A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology 149:5945–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Krause G, Chey S, Paschke R 2001 A free carboxylate oxygen in the side chain of position 674 in transmembrane domain 7 is necessary for TSH receptor activation. Mol Endocrinol 15:1294–1305 [DOI] [PubMed] [Google Scholar]
- Kenakin T 2004 Allosteric modulators: the new generation of receptor antagonist. Mol Interv 4:222–229 [DOI] [PubMed] [Google Scholar]
- Davies TF, Ando T, Lin RY, Tomer Y, Latif R 2005 Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest 115:1972–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinau G, Jaeschke H, Mueller S, Raaka BM, Neumann S, Paschke R, Krause G 2008 Evidence for cooperative signal triggering at the extracellular loops of the TSH receptor. FASEB J 22:2798–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinau G, Krause G 2009 Thyrotropin and homologous glycoprotein hormone receptors: structural and functional aspects of extracellular signaling mechanisms. Endocr Rev 30:133–151 [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S 2004 A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci 29:119–126 [DOI] [PubMed] [Google Scholar]
- Fuhrer D, Lewis MD, Alkhafaji F, Starkey K, Paschke R, Wynford-Thomas D, Eggo M, Ludgate M 2003 Biological activity of activating thyroid-stimulating hormone receptor mutants depends on the cellular context. Endocrinology 144:4018–4030 [DOI] [PubMed] [Google Scholar]
- Ludgate M, Gire V, Crisp M, Ajjan R, Weetman A, Ivan M, Wynford-Thomas D 1999 Contrasting effects of activating mutations of GαS and the thyrotropin receptor on proliferation and differentiation of thyroid follicular cells. Oncogene 18:4798–4807 [DOI] [PubMed] [Google Scholar]