Abstract
Estrogens have potent suppressive effects on food intake and body weight in many species, including humans. Compelling evidence suggests estrogen’s anorectic action is through an indirect mechanism by enhancing the strength of other physiological signals that reduce meal size such as apolipoprotein A-IV (apo A-IV), a satiation factor from the gut and brain. We determined whether estradiol, the primary form of estrogen, modulates the anorectic effect of apo A-IV. Intrafourth ventricular administration of low doses of apo A-IV reduced food intake to a greater extent in ovariectomized (OVX) rats cyclically treated with estradiol than in vehicle-treated OVX controls, implying that cyclic estradiol replacement increases the satiating potency of apo A-IV. OVX significantly increased food intake and body weight but decreased apo A-IV gene expression in the nucleus tractus solitarius (NTS). All of these alterations were reversed by cyclic regimen of estradiol treatment. The finding of colocalization of apo A-IV with estrogen receptor-α in the NTS suggests that estradiol might act locally in the NTS to up-regulate apo A-IV gene expression. Finally, OVX apo A-IV knockout mice had a smaller feeding response to estradiol because they ate significantly more food and gained more body weight than OVX wild-type controls during the period of cyclic estradiol replacement. These data indicate that an increased signaling of endogenous apo A-IV may partially mediate estradiol-induced inhibitory effect on feeding.
Estradiol-induced increase of apolipoprotein A-IV signaling in the nucleus tractus solitarius may partially mediate the inhibitory effect of estradiol on feeding.
The ovarian hormone estradiol suppresses food intake in animals and humans (1). Adult female mice and rats eat different amounts of food across the 4-d estrous cycle, consuming the least during the estrus phase, which occurs just after estradiol peaks, and the most during diestrus, when estradiol levels are lower (1,2). Disruption of rat ovarian cycling by ovariectomy (OVX) dramatically increases daily food intake and promotes weight gain (3,4), and a cyclic regimen of estradiol replacement at a physiological dose, designed to mimic the normal changes in plasma estradiol levels across the estrous cycle, normalizes meal size and body weight to the levels observed in gonadally intact rats (5). Food intake in women varies across the menstrual cycle, on the average being lowest during the periovulatory period when estrogen levels are highest (6,7). Many women increase their visceral fat after menopause because estrogen levels naturally decrease (8). A role of estrogen in maintenance of body weight is further supported by the observation that the increased body weight gain that occurs at menopause is prevented in women given hormone replacement therapy (8,9).
Estradiol has been identified as an indirect controller of food intake (9), i.e. it inhibits feeding, at least in part, by increasing the potency of satiation signals involved in meal termination (10). The most extensively studied such interaction involves cholecystokinin, a peptide released from the small intestine during meals (11). However, blocking cholecystokinin signaling only partially attenuated estradiol’s action on feeding (11), implying that estradiol may also enhance the potency of other satiation factor(s). One putative candidate is the gut-brain peptide, apolipoprotein A-IV (apo A-IV). Compelling evidence implicates central apo A-IV in the control of meal termination, or satiation. In rodents, apo A-IV dose-dependently reduces food intake after central administration, and blocking its endogenous action with its specific antibody increases meal size, implying that endogenous apo A-IV plays a role in the tonic inhibition of food intake (12,13). Thus, the goal of this series of experiments was to determine a possible interaction of estradiol and brain apo A-IV in the control of food intake in female rats and mice. We focused on the nucleus tractus solitarius (NTS) in the brain stem as a target area because recent evidence demonstrates that estradiol acts there to influence food intake and body weight (14,15). The results collectively indicate that the increased endogenous apo A-IV signaling in the NTS is one of the mechanisms mediating estradiol’s inhibitory effect on feeding.
Materials and Methods
Animals
Adult female Long-Evans rats (Harlan, Indianapolis, IN) and adult female apo A-IV knockout (KO) and wild-type (WT) C57BL/6J mice bred in our laboratory were individually housed in a temperature-controlled vivarium on a 12-h light, 12-h dark cycle (lights on at 0400 h). Laboratory chow (Teklad 7912, Madison, WI) and water were provided ad libitum except where noted. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Materials
17β-Estradiol-3-benzoate (estradiol) and sesame oil were purchased from Sigma (St. Louis, MO). Antiserum against apo A-IV was raised from goat and tested previously (16,17). Rabbit antiestrogen receptor (ER)-α antiserum was purchased from Upstate (Temecula, CA).
Intrafourth ventricular (i4vt) cannulation
Rats were anesthetized with ip ketamine (87 mg/kg) and xylazine (13 mg/kg) and placed in a stereotaxic instrument with lambda and bregma at the same vertical coordinate. A 24-gauge stainless steel guide cannula (Plastics One, Roanoke, VA) was implanted on the midline of the skull, 2.5 mm anterior to the occipital suture, and with its tip 4.5 mm below the dura (18,19). Cannulas were attached to the skull with dental acrylic and jeweler’s screws and sealed with an obturator. At least 7 d after surgery, cannula placement was verified through measurement of the hyperglycemic response to 210 μg of 5-thio-d-glucose in 2 μl of saline. 5-Thio-d-glucose is a nonmetabolizable glucose isomer that causes NTS neurons to initiate counterregulatory responses to increase plasma glucose levels (20). Only animals responding with an increase of plasma glucose concentration of at least 100% after 30 min were used for the next procedure (18,19).
Ovariectomy and cyclic estradiol replacement
Rats with viable i4vt cannulas were food deprived overnight, anesthetized with ketamine and xylazine, and given bilateral OVX through a midline abdominal incision using sterile surgical technique as described previously (21). The OVX rats were divided into two groups beginning 7 d after surgery. Group 1 rats were injected sc every fourth day with estradiol (2 μg) in 100 μl sesame oil for eight cycles (total 32 d) (5), and group 2 rats were injected sc with vehicle (100 μl sesame oil) for the same period. Injections were done between 0900 and 1000 h. The dose of 2.0 μg estradiol was selected because it produced plasma estradiol levels similar to peak levels occurring during the ovarian cycle in intact rats and, when administered over an extended period of time, normalized daily food intake and body weight of OVX rats (5).
Effects of cyclic estradiol replacement on apo A-IV’s anorectic effect in OVX rats
Both groups of OVX rats were fasted for 4 h and then received i4vt apo A-IV (0.5 or 1 μg) or vehicle (saline) at the onset of dark, which was 30 h after the final sc injection of estradiol or oil. We selected these doses of apo A-IV because 0.5 and 1 μg apo A-IV were subthreshold or minimally effective doses in male rats, respectively, in a pilot study. Food intake was measured after apo A-IV administration.
Effects of cyclic estradiol replacement on apo A-IV gene expression in the NTS of OVX rats
Another cohort of female rats received OVX or sham-operation surgery. The sham-operation procedure consisted of exposure of the ovaries on both sides but leaving them intact. Half of the OVX rats then received cyclic estradiol (2 μg) replacement and another half received vehicle (oil) for eight cycles. One group of OVX-vehicle rats was pair fed by being provided with the same amount of food consumed by estradiol-treated OVX rats each day. The rats in the sham-operated group were allowed to freely eat and treated with oil. Body weight and daily food intake were monitored daily.
Quantitative real-time PCR for apo A-IV mRNA measurement
The OVX rats were killed at the onset of dark on the second day after the last estradiol or vehicle injection. The sham-operated rats were killed at the onset of dark when they were in the estrous phase. The phase of the ovarian cycle was determined by examination of vaginal smears taken daily 4 h before dark onset, and estrus was characterized by large clumps of nonnucleated squamous cornified cells (22). Rat NTS was micropunched (19,23,24), total RNA was extracted with RNAquous-micro kit (Ambion, Austin, TX), and apo A-IV mRNA levels were measured by quantitative real-time PCR as described previously (25).
Immunohistochemistry
Potential colocalization of apo A-IV with ERα in the NTS was determined using dual-labeled immunohistochemistry. Female rats were anesthetized and perfused with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4) at the onset of dark when they were in estrus. Free-floating sections (30 μm) were cut through the NTS of brains. After washing, the sections were blocked with 5% normal donkey serum in PBS containing 0.3% Triton X-100 for 2 h. Then the sections were incubated with a goat antirat apo A-IV antiserum (1:400 dilutions) and rabbit anti-ERα antibody (1:2000) overnight at 4 C. Secondary antibodies were used as appropriate, including 1:200 diluted donkey antigoat (for apo A-IV) and donkey antirabbit (for ERα) Ig conjugated to fluorescein, Alexa 488, or Alexa 594 (Invitrogen Co., Carlsbad, CA), respectively. Confocal imaging was performed on a Zeiss 510 microscope system (Zeiss, New York, NY). Omission of the primary antibodies as well as substituting the primary antibody with apo A-IV preabsorbed antiserum or normal rabbit serum for ERα antibody was used to determine the specificity of these antibodies.
Effects of cyclic estradiol replacement on food intake and body weight in OVX apo A-IV KO and WT mice
Adult female apo A-IV KO and WT (C57BL/6J) littermates received OVX surgery as described above for rats. Seven days after recovery, the mice were divided into two groups per genotype. One group received vehicle sc (sesame oil, 50 μl per 20 g body weight), and the other received estradiol (2 μg per 50 μl per 20 g) every fourth day for seven cycles. Food intake and body weight were monitored daily.
Statistics
Data were analyzed using parametric statistics (SigmaStat, version 3.5; SyStat, San Jose, CA). Two-way ANOVA and two-way repeated-measures ANOVA followed by Student-Newman-Keuls test method was used for analysis of food intake and body weight. For other comparisons such as apo A-IV mRNA levels, a one-way ANOVA followed by Tukey’s test was used. P < 0.05 was considered statistically significant.
Results
Cyclic estradiol replacement increased the satiating potency of apo A-IV in OVX rats
There was no significant difference in food intake between the saline groups treated with estradiol and with oil (1.05 ± 0.07 vs. 0.99 ± 0.05 g, respectively). The dose of 0.5 μg apo A-IV, a subthreshold dose in male rats, significantly decreased food intake in estradiol-treated OVX rats (0.80 ± 0.04 g) but not oil-treated OVX rats (0.96 ± 0.07 g, Fig. 1). Although a larger dose of apo A-IV (1 μg) also decreased food intake in oil-treated rats (0.79 ± 0.06 g), the inhibitory effect in these rats was significantly less than that in estradiol-treated OVX rats (0.60 ± 0.06 g). Post hoc tests revealed main effects of hormone treatment [F(1,42) = 7.89, P < 0.01] and drug [F(2,42) = 14.96, P < 0.001]; the interaction effect was not significant.
Figure 1.
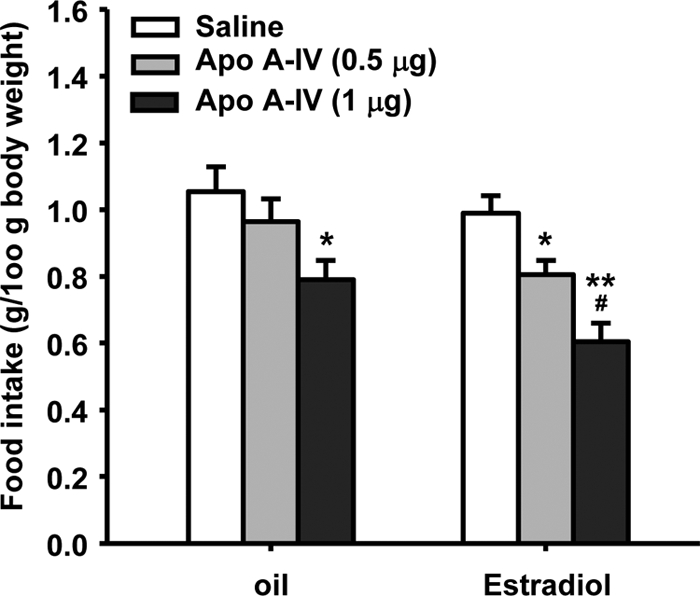
Cyclic estradiol replacement increases the anorectic effect of apo A-IV in OVX rats. Food intake was measured at 45 min after i4vt administration of apo A-IV (0.5 or 1 μg). Data are the mean ± sem (n = 8 rats/group). *, P < 0.05, **, P < 0.01, compared with saline controls; #, P < 0.05, compared with the oil-treated OVX rats receiving i4vt 0.5 μg of apo A-IV.
Cyclic estradiol replacement increased apo A-IV gene expression in the NTS of OVX rats
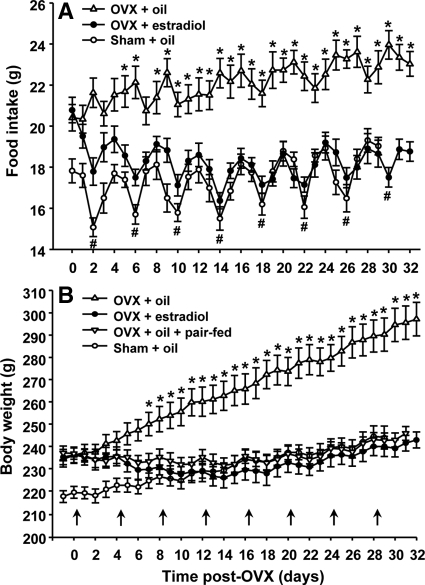
Consistent with previous reports (5), OVX induced a large, lasting increase in food intake and eliminated the cyclic decrease in food intake associated with estrus in intact rats, resulting in a rapid increase in body weight relative to sham-operated intact rats (Fig. 2). Oil-treated rats gained 62.8 ± 5.1 g between d 7 and 32 after ovariectomy, significantly more than the 26.3 ± 2.7 g gained in sham-operated rats during the same period. The estradiol-treated OVX rats slightly gained body weight at a rate, which was close to that of sham-operated rats after three cycles of estradiol treatment (Fig. 2B). There was a significant interaction effect between treatment and testing period [F(90,840) = 25.88, P < 0.001].
Figure 2.
Cyclic estradiol replacement normalizes postovariectomy food intake (A) and body weight (B). Data indicate values from sham-operated rats and OVX rats during seven consecutive cycles of treatment with estradiol (2 μg once each fourth day, indicated by arrows) or oil. Mean ± sem (n = 7–8). *, P < 0.05, compared with estradiol-treated OVX rats and sham-operated rats; #, P < 0.05, compared with diestrous 2 in sham-operated rats or d 2 of the same cycle in estradiol-treated OVX rats.
Although ovariectomy induced a marked increase in food intake and eliminated the cyclic decrease in food intake associated with estrus in intact rats, food intake in estradiol-treated OVX rats varied cyclically with frequencies and amplitudes similar to those of intact rats (Fig. 2A). These effects were statistically significant from cycle 2 through cycle 8 of estradiol treatment [interaction effect of treatment × testing period: F(87,809) = 4.534, P < 0.01].
Oil-treated OVX rats had significantly reduced apo A-IV mRNA in the NTS, compared with sham-operated females (63.1 ± 9.7 vs. 100 ± 10.2). This alteration induced by OVX was almost completely restored by cyclic replacement with estradiol (97.1 ± 8.1) but not by pair feeding (61.7 ± 5.1) [F(3,27) = 6.13, P < 0.01, Fig. 3], indicating that estradiol directly, but not secondary to the changes in energy intake, regulates apo A-IV gene expression in the NTS.
Figure 3.
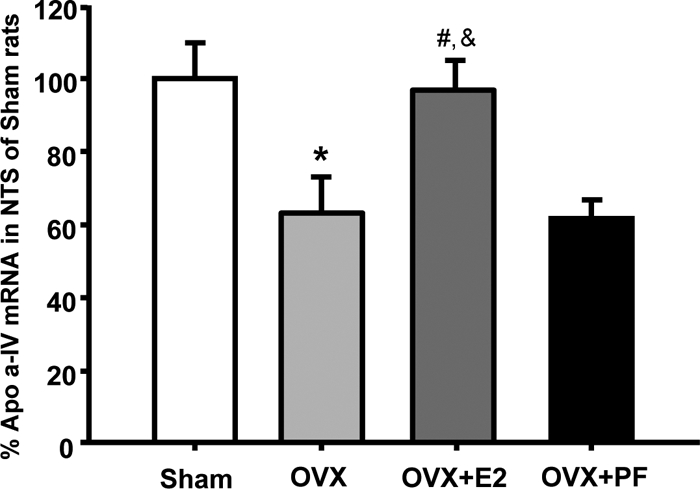
Comparison of apo A-IV mRNA levels in the NTS between sham-operated females and OVX rats treated with estradiol or oil. The pair-fed (PF) rats were provided with the same amount of food consumed by estradiol-treated OVX (OVX+E2) rats each day. E2, Estradiol. Mean ± sem (n = 8). *, P < 0.05, compared with sham-operated rats; #, P < 0.05, compared with OVX rats; &, P < 0.05, compared with OVX + pair-fed rats.
Colocalization of apo A-IV with ERα in neuronal cells of the NTS
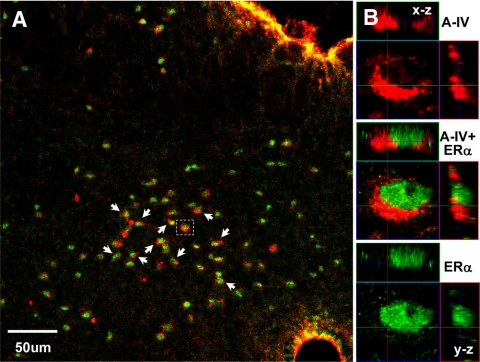
In previous studies, we demonstrated that apo A-IV is expressed in the NTS (26). To assess whether apo A-IV is present in ERα-positive cells, we performed double-labeling immunohistochemistry. As seen in Fig. 4, nuclear ERα staining (green) and the cytoplasmic Apo A-IV staining (red) are in the same cells (indicated by the red circles or crescents surrounding the green nuclei).
Figure 4.
Localization of apo A-IV and ERα in the same cells of the NTS. A, Low-magnification view. The colocalization of apo A-IV with ERα proteins is indicated by arrows. B, High-magnification confocal three-dimensional reconstruction of the area boxed in A, depicting that apo A-IV staining (red) and ERα staining (green) are in the same cells. Top, x-z plane; right, y-z plane.
Estradiol is less anorectic in apo A-IV KO mice
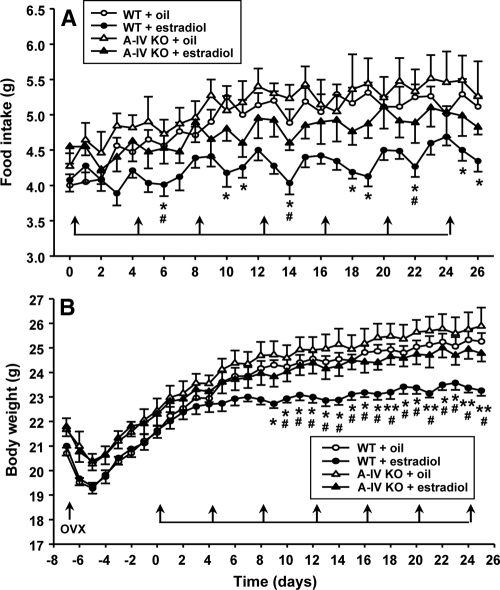
Consistent with what we found in OVX rats, estradiol-treated OVX WT mice ate significantly less food [F(1,389) = 44.58, P < 0.001] and gained significantly less body weight than oil-treated OVX WT mice [F(1,389) = 13.21, P < 0.01, Fig. 5, A and B]. However, these effects were greatly attenuated in OVX apo A-IV KO mice. During the period of cyclic estradiol replacement (total 28 d), the apo A-IV KO mice had significantly increased food intake and gained more body weight, compared with estradiol-treated OVX WT mice. Post hoc tests revealed a main effect of hormone treatment [F(3,726) = 8.761, P < 0.001]; and the interaction effect of treatment × testing period was not significant. No significant difference in food intake [F(1,338) = 2.36, P > 0.05] or body weight [F(1,325) = 1.12, P > 0.05] was found between the estradiol- and oil-treated OVX apo A-IV KO mice (Fig. 5).
Figure 5.
Comparison of food intake (A) and body weight (B) among OVX apo A-IV KO and OVX WT mice receiving cyclic treatment of estradiol or oil. As indicated by the arrows, estradiol or oil was administered sc every fourth day for seven cycles (total 28 d). The data are mean ± se (n = 7–9). *, P < 0.05, **, P < 0.01, compared with WT mice treated with oil; #, P < 0.05, compared with apo A-IV KO mice treated with estradiol.
Discussion
One of the many biological actions of estradiol is its suppressive effect on eating, and this action appears to occur centrally rather than peripherally (1). However, the specific brain area(s) mediating estradiol’s action on food intake remains unclear. Compelling evidence supports the possibility that one important brain site for estrogen’s anorectic action is the NTS (1,2,14,15). For example, estradiol treatment increases the satiating potency of intraduodenal infusions of lipid in OVX rats, and this increased satiation is associated with increased c-Fos expression in a circumscribed population of neurons in caudal NTS that express ERα (14). Administration of a low dose of estradiol (0.2 μg) onto the surface of the hindbrain over the caudal NTS, but not sc administration of the same estradiol dose, significantly decreased food intake and activated ERα-containing neurons in the NTS without a change in systemic plasma estradiol (15). These observations support our decision to focus on the NTS to identify the site of estradiol’s interaction with apo A-IV.
A novel finding in this study is that estradiol increased the anorectic effect of apo A-IV in OVX rats pretreated with a physiological regimen of estradiol. Apo A-IV, at a subthreshold dose (0.5 μg, i4vt), significantly reduced food intake in estradiol-treated rats but not oil-treated rats, suggesting that the minimally effective anorectic dose of apo A-IV is lower after cyclic estradiol replacement. Although a larger dose of apo A-IV (1 μg, i4vt) decreased food intake in oil-treated OVX rats, this anorectic effect was significantly less than that in estradiol-treated OVX rats (Fig. 1). These findings provide the first evidence that a physiological dose of estradiol is sufficient to increase apo A-IV-induced reduction of feeding. Importantly, rats received a regimen of estradiol treatment that mimics the changes in estradiol secretion in cycling female rats (5). This raises the possibility that the estrus-associated decrease in food intake, which is well characterized in intact female rats, may be mediated, at least in part, by an increase in apo A-IV signaling in the brain.
Additional studies are necessary to elucidate the mechanism by which estradiol interacts with apo A-IV to decrease food intake in female rats. One possibility is that estradiol, as a steroid, could modulate gene expression after coupling with one of its nuclear receptors, i.e. ERα, which has been proposed as the primary nuclear receptor mediating estrogen’s effect on energy homeostasis in female animals (27,28). ERα KO mice are obese, whereas ERβ-deficient mice remain lean (29,30). Inhibition of ERα by RNA interference in the neurons in the ventromedial hypothalamus results in severe obesity and symptoms of the metabolic syndrome (13). In fact, ERα is expressed in the NTS (14,31,32), supporting the possibility that estradiol interacts with apo A-IV neurons in the NTS. Consistent with this hypothesis, using dual-labeled immunohistochemistry, we found that apo A-IV is colocalized with ERα in the same cells of the NTS (Fig. 4). This observation provides structural support for investigating the mechanisms through which estrogen regulates apo A-IV expression and satiating activity in this brain area.
Using OVX and sham-operated rats, we further determined whether estradiol affects apo A-IV gene expression in the NTS. Consistent with previous reports (5), OVX induced a large increase in food intake and eliminated the cyclic decrease in food intake associated with estrus in intact rats, resulting in a rapid increase in body weight relative to sham-operated females (Fig. 2). More importantly, oil-treated OVX rats had significantly reduced apo A-IV mRNA levels in the NTS, compared with sham-operated female rats. These alterations induced by OVX, however, were almost completely restored after cyclic estradiol replacement but not by pair feeding (Fig. 3). Taken together, these results suggest that a physiological dose of estradiol may act on a population of cells within the NTS to up-regulate transcription of the gene that encodes apo A-IV and thereby to increase the anorectic effect of apo A-IV.
Given that apo A-IV signaling partially underlies estradiol-dependent changes in feeding and body weight, the lack of an apo A-IV gene in mice may cause them to be less responsive to estradiol, compared with WT controls. Consistent with this hypothesis, cyclically estradiol-treated OVX WT mice consumed food and gained body weight significantly less than oil-treated OVX WT mice over the course of treatment (Fig. 5). In contrast, the estradiol-treated OVX apo A-IV KO mice ate more food and gained more body weight than estradiol-treated OVX WT controls (Fig. 5). No significant difference in food intake or body weight was found between the estradiol-treated and oil-treated OVX apo A-IV KO mice, indicating that estradiol was significantly less efficacious in OVX apo A-IV KO mice. These results imply that endogenous apo A-IV, at least partially, mediates the inhibitory effects of estradiol on feeding. Further experiments will be needed to investigate the molecular mechanism(s) as to how central apo A-IV gene expression is regulated by estradiol.
In conclusion, the present studies demonstrate that cyclic estradiol replacement not only enhances the satiating potency of apo A-IV in OVX rats, but also increases the gene expression of apo A-IV in the NTS of OVX rats. The colocalization of apo A-IV with ERα in the NTS suggests that estradiol may act locally to activate neurons to express apo A-IV. Moreover, OVX apo A-IV KO mice had a smaller feeding response to estradiol than OVX WT littermates. These data collectively suggest that part of estradiol’s inhibitory action on food intake in normally cycling female rats may be mediated by modulation of apo A-IV signaling in the brain.
Footnotes
This work was supported by National Institutes of Health Grants DK 63907 and DK 70992 (to M.L.); DK17844 (to S.C.W.); and DK54012 and DK73917 (to D.Q.-H.W.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 19, 2010
Abbreviations: Apo A-IV, Apolipoprotein A-IV; ER, estrogen receptor; i4vt, intrafourth ventricular; KO, knockout; NTS, nucleus tractus solitarius; OVX, ovariectomy; WT, wild type.
References
- Asarian L, Geary N 2006 Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361:1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Lovejoy L 2008 Sex differences in energy metabolism, obesity and eating behavior. In: Becker J, ed. Sex on the brain: from genes to behavior. New York: Oxford Publishers; 253–274 [Google Scholar]
- Mystkowski P, Schwartz MW 2000 Gonadal steroids and energy homeostasis in the leptin era. Nutrition 16:937–946 [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM 1979 Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav 22:583–593 [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N 2002 Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 42:461–471 [DOI] [PubMed] [Google Scholar]
- Lissner L, Stevens J, Levitsky DA, Rasmussen KM, Strupp BJ 1988 Variation in energy intake during the menstrual cycle: implications for food-intake research. Am J Clin Nutr 48:956–962 [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM 1995 Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav 58:1067–1077 [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, Genazzani AR 1997 Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab 82:414–417 [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Wurtman J, Rojansky N, Adler D, Stein P, Schenker JG, Brzezinski A 1995 Effects of hormone replacement therapy on weight, body composition, fat distribution, and food intake in early postmenopausal women: a prospective study. Fertil Steril 64:963–968 [DOI] [PubMed] [Google Scholar]
- Geary N 2000 Estradiol and appetite. Appetite 35:273–274 [DOI] [PubMed] [Google Scholar]
- Geary N 2001 Estradiol, CCK and satiation. Peptides 22:1251–1263 [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Fukagawa K, Sakata T, Tso P 1993 Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J Clin Invest 91:1830–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Doi T, Shen L, Woods SC, Seeley RJ, Zheng S, Jackman A, Tso P 2001 Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol 280:R1382–R1387 [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N 2007 Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 148:5656–5666 [DOI] [PubMed] [Google Scholar]
- Thammacharoen S, Lutz TA, Geary N, Asarian L 2008 Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 149:1609–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Liu M, Seeley RJ, Woods SC, Tso P 2001 Effect of leptin on intestinal apolipoprotein AIV in response to lipid feeding. Am J Physiol Regul Integr Comp Physiol 281:R753–R759 [DOI] [PubMed] [Google Scholar]
- Shen L, Pearson KJ, Xiong Y, Lo CM, Tso P, Woods SC, Davidson WS, Liu M 2008 Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol Behav 95:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Grill HJ 2008 Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149:4059–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Duos B, Patterson LM, Berthoud HR 2005 Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology 146:3739–3747 [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S 1981 Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452 [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N 2007 Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 56:1051–1058 [DOI] [PubMed] [Google Scholar]
- Eckel LA, Geary N 1999 Endogenous cholecystokinin’s satiating action increases during estrus in female rats. Peptides 20:451–456 [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ 1998 Maps and guide to microdissection of the rat brain. New York: Elsevier [Google Scholar]
- Li G, Zhang Y, Rodrigues E, Zheng D, Matheny M, Cheng KY, Scarpace PJ 2007 Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am J Physiol Endocrinol Metab 293:E252–E258 [DOI] [PubMed] [Google Scholar]
- Shen L, Tso P, Woods SC, Sakai RR, Davidson WS, Liu M 2007 Hypothalamic apolipoprotein A-IV is regulated by leptin. Endocrinology 148:2681–2689 [DOI] [PubMed] [Google Scholar]
- Liu M, Shen L, Doi T, Woods SC, Seeley RJ, Tso P 2003 Neuropeptide Y and lipid increase apolipoprotein AIV gene expression in rat hypothalamus. Brain Res 971:232–238 [DOI] [PubMed] [Google Scholar]
- Roesch DM 2006 Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav 87:39–44 [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S 2001 Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology 142:4751–4757 [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS 2000 Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly-YM, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA 2000 Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male mice. Biochem Biophys Res Commun 278:640–645 [DOI] [PubMed] [Google Scholar]
- Haywood SA, Simonian SX, van der Beek EM, Bicknell RJ, Herbison AE 1999 Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology 140:3255–3263 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]