Abstract
Increased calcium transport has been observed in vitamin D-deficient pregnant and lactating rats, indicating that another factor besides 1,25-Dihydroxyvitamin D3 (1,25(OH)2D3) is involved in intestinal calcium transport. To investigate prolactin as a hormone involved in calcium homeostasis, vitamin D-deficient male mice were injected with 1,25(OH)2D3, prolactin, or prolactin + 1,25(OH)2D3. Prolactin alone (1 μg/g body weight 48, 24, and 4 h before termination) significantly induced duodenal transient receptor potential vanilloid type 6 (TRPV6) mRNA (4-fold) but caused no change in calbindin-D9k. Combined treatment with 1,25(OH)2D3 and prolactin resulted in an enhancement of the 1,25(OH)2D3 induction of duodenal TRPV6 mRNA, calbindin-D9k mRNA, and an induction of duodenal calcium transport [P < 0.05 compared with 1,25(OH)2D3 alone]. Because lactation is associated with an increase in circulating 1,25(OH)2D3, experiments were done to determine whether prolactin also has a direct effect on induction of 25-hydroxyvitamin D3 1α hydroxylase [1α(OH)ase]. Using AOK B-50 cells cotransfected with the prolactin receptor and the mouse 1α(OH)ase promoter −1651/+22 cooperative effects between prolactin and signal transducer and activator of transcription 5 were observed in the regulation of 1α(OH)ase. In addition, in prolactin receptor transfected AOK B-50 cells, prolactin treatment (400 ng/ml) and signal transducer and activator of transcription 5 significantly induced 1α(OH)ase protein as determined by Western blot analysis. Thus, prolactin, by multiple mechanisms, including regulation of vitamin D metabolism, induction of TRPV6 mRNA, and cooperation with 1,25(OH)2D3 in induction of intestinal calcium transport genes and intestinal calcium transport, can act as an important modulator of vitamin D-regulated calcium homeostasis.
Prolactin can act as a modulator of vitamin D-regulated calcium homeostasis.
Calcium, an essential ion in all organisms, is an indispensable constituent of diverse physiological processes (1). To serve these multiple functions it is imperative that the body maintain a tight regulation of calcium homeostasis. Maintenance of calcium homeostasis involves coordinated interplay between intestine, bone, and kidney (2). The intestine is the sole source of new calcium, supplying dietary calcium to the body. 1,25-Dihydroxyvitamin D3 [1,25(OH)2D3] is the principal hormone regulating intestinal calcium absorption (2,3,4). 1,25(OH)2D3, the biologically active form of vitamin D, is produced by two sequential hydroxylations of vitamin D: first, in the liver by vitamin D 25-hydroxylase; and then, in the kidney by 25-hydroxyvitamin D3 1α hydroxylase [1α(OH)ase]. The actions of 1,25(OH)2D3 are mediated by the vitamin D receptor (VDR), which acts as a heterodimer with the retinoid X receptor, binds to vitamin D-response elements within the promoter of target genes, and together with coactivators, affects gene transcription (2,3,4). Active intestinal calcium transport is a transcellular process involving three 1,25(OH)2D3-regulated steps: apical entry of calcium via the apical calcium channel, transient receptor potential vanilloid type 6 (TRPV6), cytoplasmic translocation of calcium through the interior of the enterocyte (it has been suggested that the calcium-binding protein, calbindin-D9k, acts to facilitate calcium diffusion), and basolateral extrusion of calcium by the intestinal plasma membrane calcium pump plasma membrane Ca2+- ATPase 1b (2,3,4,5,6). As the body’s demand for calcium increases from pregnancy, lactation, and/or from a diet low in calcium, the synthesis of 1,25(OH)2D3 is increased, stimulating active intestinal calcium absorption (2,6).
Although 1,25(OH)2D3 is the principal hormone regulating intestinal calcium absorption, increased intestinal calcium transport has been observed in vitamin D-deficient pregnant and lactating rats, suggesting that hormonal factors besides 1,25(OH)2D3 are involved in intestinal calcium absorption (7,8,9). Studies by Van Cromphaut et al. (10) showed that pregnancy and lactation enhance TRPV6 expression in the duodenum equally in wild-type and VDR null mutant mice, further suggesting vitamin D-independent effects during pregnancy and lactation on duodenal calcium absorption mechanisms. It has been suggested that prolactin, a lactogenic polypeptide hormone of the anterior pituitary that is elevated during pregnancy and lactation, acts as a calcium regulating hormone (11). Besides mammary gland, prolactin receptors or binding sites are widely distributed in vertebrates and include binding sites in duodenum, jejunum, ileum, osteoblasts, and kidney cortex (12). The prolactin receptor most commonly signals through the Janus kinase 2 (JAK2)/signal transducer and activator of transcription (STAT)5 pathway (13,14). Prolactin has been shown to stimulate active intestinal calcium transport in vitamin D-deficient rats (15). In addition, a direct effect of prolactin on duodenal transcellular calcium transport was reported in studies using the Ussing chamber technique and prolactin applied to the incubation solution (16). Prolactin has also been reported to increase paracellular calcium permeability and passive calcium transport in the intestine (17,18). In addition to direct effects on the intestine, an effect of prolactin on stimulating the production of 1,25(OH)2D3 has been suggested. Lactation is associated with an increase in circulating 1,25(OH)2D3 (7,19,20). Bromocriptine, which inhibits pituitary prolactin secretion, was reported to significantly reduce plasma 1,25(OH)2D3 levels in lactating animals and prolactin administered with bromocriptine resulted in a restoration of the 1,25(OH)2D3 plasma levels (21). Thus, it has been suggested that prolactin can stimulate 1,25(OH)2D3 production, which would lead to enhanced intestinal calcium absorption. Although it has been shown that prolactin can affect intestinal calcium transport, mechanisms whereby prolactin modulates vitamin D-mediated calcium homeostasis are largely unknown. In this study, we used vitamin D-deficient mice injected with prolactin alone or prolactin and 1,25(OH)2D3 to determine whether prolactin may up-regulate intestinal vitamin D target genes and whether this regulation is dependent or independent of 1,25(OH)2D3. In addition, because lactation is associated with an increase in circulating 1,25(OH)2D3, experiments were done to determine whether prolactin has a direct effect on induction of 1α(OH)ase. Our findings indicate that prolactin can regulate TRPV6 and the 1α(OH)ase gene and that prolactin has cooperative effects with 1,25(OH)2D3 in regulating intestinal calcium transport proteins, as well as intestinal calcium transport. Thus, prolactin, by different mechanisms, can act as a calcium regulating hormone and modulate the effect of 1,25(OH)2D3.
Materials and Methods
Materials
45Ca (39.49 mCi/ml), γ[32P] deoxy-ATP [3000 Ci (111 TBq)/mmol] was purchased from PerkinElmer Life Sciences (Waltham, MA). Random Primers DNA Labeling kit was purchased from Life Technologies, Inc. (Gaithersburg, MD). Prestained protein molecular weight markers and electrochemiluminescent detection system were obtained from Invitrogen (Carlsbad, CA) and Denville Scientific (Metuchen, NJ), respectively. T4 polynucleotide kinase for labeling double-stranded oligonucleotides for EMSA was purchased from Invitrogen. Ovine prolactin was obtained from Sigma (St. Louis, MO). Human prolactin was obtained from A. F. Parlow (The National Hormone and Peptide Program, Harbor-University of California, Los Angeles, Medical Center, Torrance, CA). Antiserum against rat calbindin-D9k was obtained from Swant Swiss Antibodies (Bellinzona, Switzerland). STAT5 antibody (9363) was purchased from Cell Signaling Technologies (Danvers, MA). β-Actin antisera and secondary antibodies against mouse and rabbit antisera were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The 1α(OH)ase antiserum was provided by Harvey J. Armbrecht (St. Louis Veterans Affairs Hospital, St. Louis, MO). Chemically synthesized 1,25(OH)2D3 was a gift from M. Uskokovic of Hoffmann-La Roche (Nutley, NJ). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Animals
C57BL/6J mice (Taconic Farms, Germantown, NY) were exposed to a 12-h light, 12-h dark cycle. Food and water were given ad libitum. All of the animal experiments were approved by the University of Medicine and Dentistry of New Jersey Animal Care and Use Committee. For prolactin and vitamin D administration studies, 60-d-old male mice were fed a 0.8% strontium, 0.02% calcium, vitamin D-deficient diet (Teklad diet TD00562) for 7 d to inhibit endogenous renal synthesis of 1,25(OH)2D3 (22). Male mice were used to avoid alterations due to fluctuations of female hormones. The serum calcium levels of the strontium fed mice before injection with vehicle or homone/s were less than 7.5 mg/dl. In one experiment the mice fed the strontium vitamin D-deficient diet were randomly separated into three groups and injected ip with either 0.1 ml vehicle (9:1 mix of propylene glycol and ethanol; −D mice), 1,25(OH)2D3 [1 ng/g body weight (bw) in a 9:1 mix of propylene glycol and ethanol] or prolactin (1 μg/g bw in saline) three times over the next 48 h (48, 24, and 4 h before being terminated). The three dose protocol was used to study both short-term and long-term effects of 1,25(OH)2D3 or prolactin. In another study, vitamin D-deficient mice were randomly separated into four groups and were injected with either vehicle, 1,25(OH)2D3 (ip 2 ng/g bw), or prolactin (1 μg/g bw at 12 or 4 h before termination, respectively) or 1,25(OH)2D3 + prolactin (at 12 and 4 h before termination, respectively). For 1,25(OH)2D3, 12 h was chosen because suboptimal induction of intestinal TRPV6 and calbindin-D9k mRNA results after one injection and termination after 12 h (23). For prolactin, 4 h was chosen because previous studies noted changes in gene expression within 4 h of in vivo prolactin treatment (24). Tissues were harvested, and RNA and protein were isolated as previously described (23,25). Duodenum of mice under the same protocol but with 6-h treatment with prolactin before termination were also used for the determination of intestinal calcium transport using the everted gut sac assay. A longer time for prolactin treatment was used in these studies because previous studies related to the effect of prolactin on intestinal calcium absorption indicated that between 4 and 8 h after prolactin injection intestinal calcium transport is maximal (15). An additional group of mice injected with 1,25(OH)2D3 (1 ng/g bw at 48, 24, and 6 h before being terminated) was included in the intestinal calcium transport studies as a positive control.
Intestinal calcium transport
Intestinal calcium transport was determined by the everted gut sac assay using a 5-cm segment of duodenum removed from the mice proximally to the pyloric junction as previously described (25). The active accumulation of 45Ca in the inside (serosal) fluid was expressed as a ratio of the final concentration of 45Ca inside/outside (external incubation medium). The fold stimulation of intestinal calcium transport observed in these studies in 1,25(OH)2D3-treated mice is consistent with previous studies (25). The everted gut sac assay selectively measures active intestinal calcium transport [the calcium transport is against a concentration gradient, is saturable, is sensitive to intestinal segment, dietary calcium, and 1,25(OH)2D3 treatment (25,26,27,28)].
Northern blot analysis
Total RNA was prepared with RNAzol RNA extraction solution according to the manufacturer’s instructions (Tel-Test, Friendswood, TX), and 20 μg of total RNA were analyzed by Northern blot analysis for calbindin-D9k gene expression as previously described (23). All membranes were stripped and rehybridized to [32P] β-actin cDNA. The relative OD obtained using the test probe was divided by the relative OD obtained after probing with β-actin to normalize for sample variation.
RT-PCR analysis
Two micrograms of total RNA, prepared as described above, were reverse transcribed using Superscript II Reverse Transciptase (Invitrogen) according to the manufacturer’s protocol. TRPV6 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were assessed by semiquantitative RT-PCR as previously described (25). For each primer set, PCR cycle numbers were chosen so that the amplification was in the linear range of amplification efficiency. Primers and annealing temperatures are the following: TRPV6, forward, 5′-ATCGATGGCCCTGCGAACT-3′ and reverse, 5′- CAGAGTAGAGGCCATCTTGTTGCTG-3′, Ta = 60 C; GAPDH, forward, 5′-TCACCATCTTCCAGGAGCG-3′ and reverse, 5′-CTGCTTCACCACCTTCTTGA-3′, Ta = 60 C.
Cell culture
Fetal bovine serum (FBS) and charcoal-stripped FBS were from Gemini Biological Products (Calabasas, CA). Cell culture media, 0.25% trypsin-EDTA and penicillin, streptomycin, and neomycin mixture were purchased from Invitrogen. Green monkey kidney cell lines (COS-1 and COS-7) and the human colon adenocarcinoma cell line (Caco-2) were obtained from the American Type Culture Collection (Manassas, VA). AOK-B50 cells, porcine renal proximal tubular cells [LLCPK1 cells that express PTH/PTHrP type I receptors] were obtained from F. R. Bringhurst (29). MPCT cells (mouse proximal tubule cells) were provided by Peter Friedman (30). Cells were cultured in DMEM supplemented with 10% heat-inactivated FBS and 1% penicillin, streptomycin, and neomycin antibiotic mixture. Caco-2 cells were further supplemented with 1% nonessential amino acids (Invitrogen). All cells were grown in a humidified incubator with an atmosphere of 95% air-5% CO2 at 37 C. For treatments, cells were grown to desired confluency, and their medium was changed to DMEM supplemented with 2% charcoal-dextran-treated FBS. Treatments with vehicle or test compounds were done for the durations and with concentrations mentioned in Results and figure legends.
Plasmids, transfections, and assay of luciferase activity
For transfection studies, the human TRPV6 promoter luciferase reporter constructs were from the J. W. Pike lab (31). The mouse 1α(OH)ase promoter −1651/+22 placed upstream of a luciferase reporter gene in the pGL2b vector and the deletion constructs −477/+22, −233/+22, −144/+22, −85/+22, as well as constructs containing various lengths of the distal 1α(OH)ase promoter region [−1631/−1064 (SR2) and −1631/−1386 (BDC5′)] were kindly provided by H. F. DeLuca (University of Wisconsin at Madison) (32). −2300/+490 β-casein promoter subcloned into pGL2 luciferase vector used as a positive control in some studies was from J. Rosen (Baylor College of Medicine, Houston, TX) (33). The glucocorticoid receptor expression vector, used in studies related to the casein promoter, was provided by Keith Yamamoto (University of California, San Francisco, San Francisco, CA). Prolactin receptor expression vector (pECE-PRL-R-L, which binds ovine and human lactogenic hormones) was from the lab of Paul Kelly (Institut National Santé Recherche Médicale, Paris, France). Expression vectors for STAT5a and STAT5b (pRcCMVSTAT5a and pcDNA3STAT5b) were from J. Rosen (Baylor College of Medicine). pMEX-CCAAT/enhancer-binding protein-β (C/EBP β) was a gift of Simon Williams (Texas Tech University, Lubbock, TX). STAT1 expression vector was provided by James Darnell (Rockefeller University, New York, NY). Dominant negative (DN) STAT5a (STAT5a truncated at amino acid 713 STAT5a DN) was from Jim Ihle. Cells were seeded in a 24-well culture dish 24 h before transfection at 70–80% confluence. Cells were transfected using Lipofectamine 2000 (Invitrogen) and treated as described in Results. Time-course studies with prolactin and STAT5 for activity of 1α(OH)ase transcription indicated a 5-fold induction in transcriptional activity using the −1651/+22 promoter construct at 4 h after prolactin treatment (400 ng/ml) and a peak at 12 h. Studies were done at 24 h (suboptimal conditions) consistent with previous studies examining transcriptional effects of prolactin (33,34). After treatment, cells were harvested, and dual luciferase assay was performed as previously described (35).
Site-directed mutagenesis and EMSA
Putative STAT5-binding sites were mutated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The mutated constructs were confirmed by DNA sequencing. For EMSAs, complementary oligonucleotides were synthesized based on the region of the mouse 1α(OH)ase promoter containing the putative wild-type or the mutated STAT5 sites (prepared by the University of Medicine and Dentistry of New Jersey Molecular Resource Facility). The complementary oligonucleotides were annealed, end-radiolabeled, and purified as described before and were used for the EMSA (35,36).
Western blot analysis
Total protein were prepared and analyzed for protein concentration by the Bradford method (37), and 50 μg of protein from total cell or tissue extracts was loaded onto a 15% sodium dodecyl sulfate-polyacrylamide gel, separated by electrophoresis, and transferred onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA). Western blot analysis of calbindin-D9k in intestine and 1α(OH)ase in AOK-B50 cells was performed as previously described (23,38). All membranes were stripped and reprobed with β-actin antibody.
Serum calcium
Serum concentrations of calcium were determined using a standard calcium chloride solution (Fisher Scientific, Fair Lawn, NJ) by atomic absorption spectrophotometry (39).
Statistical analysis
Results are expressed as means ± se, and significance was determined by analysis by Student’s t test for two-group comparison or by ANOVA for multiple-group comparison.
Results
Prolactin induces duodenal TRPV6 mRNA
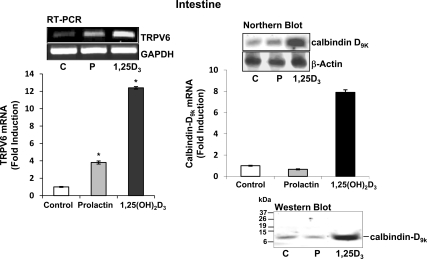
To investigate possible mechanisms involved in prolactin as a hormone involved in calcium homeostasis, vitamin D-deficient male mice were injected with vehicle, 1,25(OH)2D3 (as a positive control), or prolactin at 48, 24, and 4 h before termination to study both short-term and long-term effects of 1,25(OH)2D3 or prolactin administration. Three injections of 1,25(OH)2D3 significantly induced duodenal TRPV6 mRNA and calbindin-D9k mRNA and protein as previously reported (23,25). Three injections of prolactin significantly induced duodenal TRPV6 mRNA (4-fold; P < 0.05 compared with control) (Fig. 1, left panel). Using the same protocol, duodenal calbindin-D9k mRNA and protein levels were unaltered by prolactin (Fig. 1, right panel).
Figure 1.
The effect of repeated administration of prolactin or 1,25(OH)2D3 on duodenal TRPV6 and calbindin-D9k mRNA levels. Sixty-day-old mice were made 1,25(OH)2D3 deplete by feeding 0.8% strontium diet for 7 d. Mice were injected with prolactin [1 μg/g bw, vehicle (control), or 1,25(OH)2D3 (1 ng/g bw as a positive control)] three times over 48 h (48, 24, and 4 h before termination). Serum calcium levels: control −D mice, 6.3 ± 0.04; prolactin-treated mice, 7.1 ± 0.1; and 1,25(OH)2D3-treated mice, 8.2 ± 1.5 mg/dl [P < 0.05 prolactin and 1,25(OH)2D3-treated mice vs. control −D]. Left panel, RT-PCR analysis of TRPV6 mRNA in duodenum. Right panel, Summary of densitometric scans of Northern blot analyses of calbindin-D9k mRNA in duodenum. Data were corrected for GAPDH or β-actin mRNA expression and are presented relative to control (vitamin D-depleted mice). Bottom right, Western blot analysis of calbindin-D9k protein C, Control; P, prolactin; 1,25D3, 1,25(OH)2D3. Bars represent the mean ± sem, n = 6–8/group. *, P < 0.05 compared with control.
Prolactin has cooperative effects with 1,25(OH)2D3 in regulating duodenal calcium transport genes and duodenal calcium transport
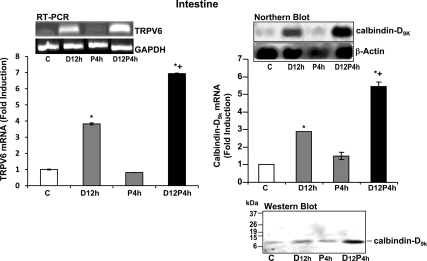
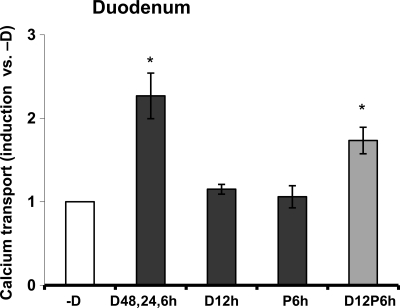
To determine whether there is a possible cooperative effect between 1,25(OH)2D3 and prolactin, vitamin D-deficient male mice were injected with 1,25(OH)2D3 or prolactin (12 or 4 h before termination, respectively) or 1,25(OH)2D3 + prolactin (at 12 and 4 h before termination). A single injection of prolactin alone was unable to induce TRPV6 mRNA or calbindin-D9k mRNA and protein (Fig. 2). 1,25(OH)2D3 given as a single injection significantly induced duodenal TRPV6 mRNA and duodenal calbindin-D9k mRNA and protein. Combined treatment with 1,25(OH)2D3 and prolactin resulted in a significant enhancement of the 1,25(OH)2D3 induction of duodenal TRPV6 mRNA and calbindin-D9k mRNA and protein [P < 0.05 compared with 1,25(OH)2D3 treatment alone] (Fig. 2). To evaluate the physiologic relevance of the enhanced gene and protein expression using combined treatment with 1,25(OH)2D3 and prolactin, everted gut sac assays were performed. As a positive control, three injections of 1,25(OH)2D3 administered to vitamin D-deficient mice resulted in a significant 2.3-fold induction in active duodenal calcium transport, as previously reported by our laboratory (25). Within the limits of sensitivity of our assay no change in active duodenal calcium transport was observed between vehicle injected vitamin D-deficient mice and vitamin D-deficient mice receiving a single injection of either prolactin or 1,25(OH)2D3 (Fig. 3). Combined treatment with 1,25(OH)2D3 and prolactin (at 12 and 6 h before termination, respectively) resulted in a significant induction in active duodenal calcium transport [P < 0.05 compared with vehicle or treatment with a single injection of 1,25(OH)2D3 alone or prolactin alone Fig. 3].
Figure 2.
Prolactin (P) has cooperative effects with 1,25(OH)2D3 (D) in regulating duodenal calcium transport genes. RT-PCR analysis of TRPV6 mRNA expression (left panel) or Northern blot analysis of calbindin-D9k mRNA (right panel) in the duodenum of vitamin D-deficient male mice injected with either vehicle (C, control), 1,25(OH)2D3 (2 ng/g bw D12 h), or prolactin (1 μg/g bw P4 h) at 12 or 4 h before termination, respectively, or 1,25(OH)2D3 + prolactin (D12, P4 h) at 12 and 4 h before termination, respectively. Bottom right, Western blot analysis of calbindin-D9k protein. Bars represent the mean ± sem, n = 4–6/group. *, P < 0.05 compared with control; +, P < 0.05 compared with D12 h. P4 h vs. C. P > 0.5; a single injection of prolactin and termination at 2, 6, or 12 h after prolactin injection also did not result in a significant effect on TRPV6 mRNA or calbindin-D9k mRNA (data not shown).
Figure 3.
Active intestinal calcium transport in the duodenum of vitamin D-deficient mice. Calcium transport was measured using everted intestinal sacs from the duodenum of vitamin D-deficient male mice injected with vehicle (-D) or 1,25(OH)2D3 (D; 1 ng/g bw) three times over 48 h (48, 24, and 6 h before termination) as a positive control. Mice were also injected with 1,25(OH)2D3 (2 ng/g bw D12 h) or prolactin (P; 1 μg/g bw P6 h) at 12 or 6 h before termination, respectively, or with a combination of both 1,25(OH)2D3 and prolactin (D12P6 h) at 12 and 6 h before termination, respectively. Data are expressed relative to duodenal calcium transport in the deficient mice injected with vehicle (-D). Values represent the mean ± sem, n = 12–16/ group. *, P < 0.05 compared with -D, vehicle-treated mice.
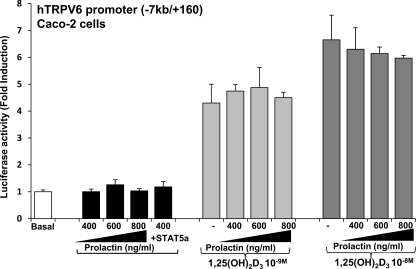
Lack of transcriptional activation of the −7 kb/+160 TRPV6 promoter construct by prolactin
To further investigate the nature of prolactin regulation of TRPV6, we examined the effects of prolactin on the activity of the human (h)TRPV6 −7000/+160 promoter construct. The prolactin receptor and the hTRPV6 −7 kb/+160 promoter construct were transiently cotransfected in Caco-2 cells. Caco-2 cells, human colon adenocarcinoma cells, were used because they are the only intestinal cell line that differentiates in culture and acquires the characteristics of intestinal enterocytes (40). They also have a functional VDR, and TRPV6 is regulated by 1,25(OH)2D3 in these cells (31,41). Treatment with increasing concentrations of prolactin or transfection of STAT5a followed by prolactin treatment had no significant effect on luciferase activity (Fig. 4). Similar results were observed using STAT5b (data not shown). As previously reported (31), 1,25(OH)2D3 treatment alone resulted in a 4- to 7-fold increase in TRPV6 transcription. A combination of prolactin and 1,25(OH)2D3 did not result in a change in promoter activity as compared with 1,25(OH)2D3 alone. Unlike prolactin and hydrocortisone regulation of the casein gene, which requires cooperation between STAT5, glucocorticoid receptor, and C/EBP β (33), a combination of prolactin and 1,25(OH)2D3 in the presence of STAT5a, as well as C/EBP β, also did not result in a change in promoter activity compared with 1,25(OH)2D3 alone (data not shown). Similar results were observed using a COS-1 or COS-7 cell reconstitution system and transfection of VDR and the prolactin receptor (data not shown). In studies in COS-1 cells the β-casein promoter was used as a positive control (for β-casein promoter activation, transcription factor expression and treatment were done as previously described) (33). These findings suggest that prolactin may be regulating TRPV6 at a posttranscriptional level or that TRPV6 is regulated by prolactin at other sites yet to be defined.
Figure 4.
Lack of transcriptional activation of the −7 kb/+160 TRPV6 promoter construct by prolactin. Caco-2 cells were transfected with 50 ng prolactin receptor and 0.3 μg hTRPV6 promoter (−7 kb/+160) in the presence or absence of STAT5. Empty vectors were used to keep the total DNA concentration the same. After 24 h, cells were treated with vehicle, prolactin, 1,25(OH)2D3 (10−9 or 10−8 m), or prolactin + 1,25(OH)2D3 for 24 h. TRPV6 promoter activity was normalized to values for Renilla luciferase activity as an internal control and is expressed as fold induction by comparison with basal levels; mean ± sem, n = 6–8 separate experiments. Prolactin alone (400–800 ng/ml) or in the presence of STAT5a (400 ng/ml prolactin + 50 ng STAT5a) did not affect TRPV6 transcriptional activation above basal levels (P > 0.5). In addition, prolactin did not enhance 1,25(OH)2D3-induced TRPV6 transcriptional activation [1,25(OH)2D3 (10−9 or 10−8 m) + prolactin (400, 600, or 800 ng/ml) vs. 1,25(OH)2D3 alone (10−9 or 10−8 m); P > 0.5]. Time-course studies (4–24 h) also did not indicate an effect of prolactin at earlier times in the presence or absence of 1,25(OH)2D3 on TRPV6 transcription (data not shown). When human prolactin, which is also an agonist for the long form of prolactin receptor, was substituted for ovine prolactin (used in these studies), similar results were observed.
Prolactin is a regulator of the 1α(OH)ase gene
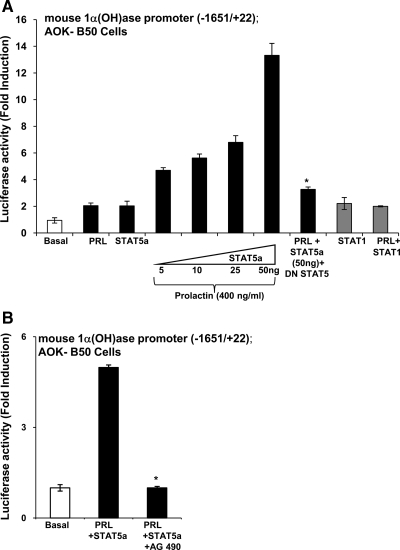
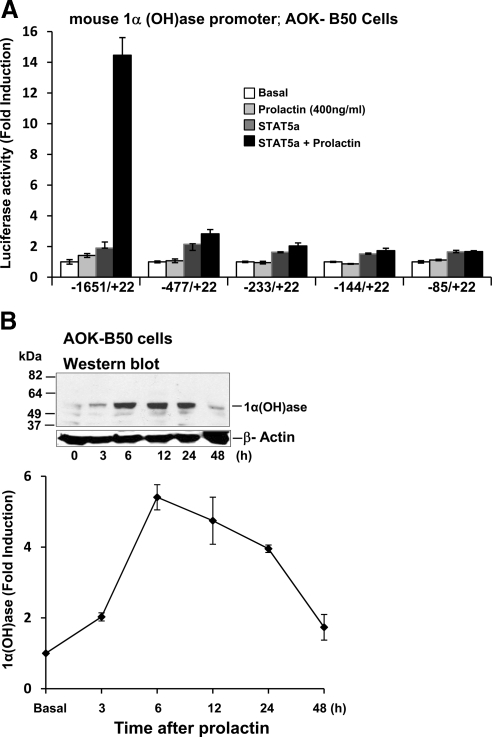
Because lactation is associated with an increase in plasma 1,25(OH)2D3 (7,19,20), in addition to effects on the intestine, the possibility that prolactin may have a direct effect on the transcription of the 1α(OH)ase gene was examined. AOK B-50 cells were cotransfected with the prolactin receptor and the mouse 1α(OH)ase promoter −1651/+22. Treatment with prolactin alone or transfection of STAT5a alone resulted in a 1.5- to 2-fold increase in luciferase activity. A combination of prolactin and STAT5a resulted in a maximum induction of 1α(OH)ase transcription of 13.2 ± 1.0 fold (P < 0.05 compared with prolactin alone or STAT5 alone), indicating a cooperative effects between STAT5 and prolactin in the regulation of 1α(OH)ase (Fig. 5A).Similar transactivation of 1α(OH)ase in the presence of STAT5b (50 ng) and prolactin was observed (data not shown). DN STAT5 inhibited prolactin/STAT5 induction of 1α(OH)ase transcription (Fig. 5A). Prolactin treatment in combination with STAT1 (PRL + STAT1) (Fig. 5A) did not affect 1α(OH)ase transcription above levels observed with STAT1 alone. The prolactin/STAT5 induction of 1α(OH)ase transcription was also inhibited by the AG490, an inhibitor of JAK2 (Fig. 5B). Cooperative effects between STAT5 and prolactin were observed using the −1651/+22 construct but not the deletion constructs (−477/+22, −233/+22, −144/+22, and −85/+22) (Fig. 6A), suggesting that the prolactin/STAT5 responsive region is in the distal region between −1651 and −477. To further determine which sequences in the distal region of the mouse 1α(OH)ase promoter are involved in the prolactin/STAT5 transactivation, constructs containing various lengths of the distal 1α(OH)ase promoter region fused to the minimal 1α(OH)ase promoter (−85/+22) upstream of the luciferase reporter gene were used (32). Cooperative effects between STAT5 and prolactin were observed using a 1α(OH)ase promoter construct containing −1631/−1064 (SR2) (32) but were not observed when a construct containing −1631/−1386 (BDC5′) (32) was used, suggesting that the prolactin/STAT5 responsive region is between −1386 and −1064. Consensus STAT5-binding sites (TTCNNNGAA) were not identified in the mouse 1α(OH)ase promoter (1621/+22) (TRANSFAC database). However, within the prolactin/STAT5 responsive region (−1386/−1064), we identified two regions that are similar to the STAT5 consensus sequence (−1297/−1287 TTCCCCAAGAA and −1236/−1229 TTCCAGAA). Although EMSAs indicated binding of STAT5a to these sites but not to mutated sites, mutation of −1297/−1287 to TTtCCCAAaAA, mutation of −1236/−1229 to TTtCAaAA (similar to reported mutations of other STAT sites) (42), or mutation of both putative STAT5 sites within the −1651/+22 1α(OH)ase promoter construct did not affect the 13- to 15-fold induction in luciferase activity in response to prolactin/STAT5. Thus, these sites are not essential to activation by prolactin/STAT5 of 1α(OH)ase transcription. Although sites other than a STAT5 site or more divergent nonconsensus sites may be involved, these findings indicate for the first time that STAT5 and JAK2 mediated regulation by prolactin of 1α(OH)ase transcription. In addition, in AOK B-50 cells transfected with the prolactin receptor, prolactin treatment (400 ng/ml), and STAT5 transfection significantly induced 1α(OH)ase protein as determined by Western blot analysis (maximum induction 5.3-fold at 6 h after prolactin treatment) (Fig. 6B).
Figure 5.
Induction of 1α(OH)ase transcription by prolactin/STAT5. AOK B-50 cells were cotransfected with the prolactin receptor and the mouse 1α(OH)ase promoter construct (−1651/+22) in the presence or absence of STAT5a. After 24 h, cells were treated with vehicle or prolactin (400 ng/ml) for 24 h. For all transcription experiments, empty vectors were used to keep the total DNA concentration the same, Renilla luciferase was used as an internal control, and 1α(OH)ase promoter activity is represented as fold induction by comparison with basal levels. A, Prolactin alone (PRL) or STAT5 alone (50 ng) had a minimal effect on 1α(OH)ase transcription (1.5- to 2-fold increase in luciferase activity). However, a combination of prolactin and STAT5a resulted in a maximum induction of transcription of 13.2 ± 1.0 fold (P < 0.05 compared with prolactin alone or STAT5 alone at all concentrations of STAT5). Cotransfection with DN STAT5 (25 ng) resulted in a significant decrease in prolactin/STAT5 induction of 1α(OH)ase transcription (*, P < 0.05 PRL + STAT5a + DN STAT5 vs. PRL + STAT5a). There was no effect of DN STAT5 at the concentration used on basal levels of 1α(OH)ase transcription (data not shown). Prolactin treatment (400 ng/ml) in combination with STAT1 (PRL + STAT1) did not affect 1α(OH)ase transcription above levels observed with STAT1 alone [PRL + STAT1 (50 ng) vs. STAT1 (50 ng); P > 0.5)]. Results represent the mean ± sem, n = 4–10 observations/group. Similar results were observed using mouse proximal tubule (MPCT) cells (data not shown). B, The JAK2 inhibitor AG490 inhibits the prolactin/STAT5 induction of 1α(OH)ase transcription. AOK B-50 cells were cotransfected with the prolactin receptor and the mouse 1α(OH)ase promoter as described in A. Cells were also transfected with STAT5a expression vector (50 ng). AOK B-50 cells were pretreated for 1 h with JAK2 inhibitor AG490 (200 μm) followed by treatment with prolactin (PRL; 400 ng/ml) for 4 h. Results are expressed as mean ± sem of at least three observations/group. The JAK2 inhibitor AG490 significantly suppresses prolactin/STAT5 induction of 1α(OH)ase transcription (P < 0.05 PRL + STAT5a + AG490 vs. PRL + STAT5a).
Figure 6.
Regulatory region for prolactin/STAT5 stimulation of 1α(OH)ase transcription and induction of 1α(OH)ase protein by prolactin. A, AOK B-50 cells were transfected with 0.3 μg mouse 1α(OH)ase promoter −1651/+22 or deletion constructs −477/+22, −233/+22, −144/+22, and −85/+22. In some wells, STAT5a expression vector (50 ng) was also transfected. After 24 h, cells were treated with vehicle or prolactin (400 ng/ml) for 24 h. Results are the mean ± sem of six to eight observations/group. B, Western blot analysis was performed using total extracts from AOK B-50 cells. Cells were transfected with STAT5a and prolactin receptor and treated with vehicle (0) or prolactin (400 ng/ml) for 3–48 h. Prolactin treatment resulted in a significant increase in 1α(OH)ase protein levels at 3, 6, 12, and 24 h after prolactin treatment (P < 0.05 compared with vehicle).
Discussion
In this study, we demonstrate novel mechanisms whereby prolactin can act as a calcium regulating hormone. Prolactin can induce TRPV6 mRNA in the intestine and has cooperative effects with 1,25(OH)2D3 in regulating intestinal calcium transport proteins, as well as intestinal calcium transport. In addition, we report for the first time that prolactin has a direct effect on the transcription of the 1α(OH)ase gene and that prolactin and STAT5 cooperate in regulating 1α(OH)ase. Thus, prolactin, by different mechanisms, can modulate the effects of 1,25(OH)2D3.
Earlier reports have shown regulatory effects of prolactin on duodenal calcium transport (11,15,43). Studies in vitamin D-deficient male rats indicate that the effects of prolactin on duodenal active calcium transport can occur in a vitamin D-independent manner (15). This is supported by studies demonstrating increased calcium absorption in pregnant and lactating vitamin D-deficient animals (7,8,9). However, the mechanisms involved in the effects of prolactin on calcium transport are not well defined and whether the expression of calcium transporter genes is affected by prolactin was not known. Studies using female vitamin D replete rats have suggested a nongenomic action of prolactin on duodenal active and passive transport (prolactin enhanced calcium uptake within 8 min, and effects on active duodenal calcium transport were observed within 1 h of ip injection of prolactin) (16,44,45). In our studies, we did not examine rapid actions of prolactin on the intestine but rather the effects of prolactin over a time period consistent with genomic actions. Although TRPV6 mRNA expression in the duodenum is known to be induced by low dietary calcium and 1,25(OH)2D3 (23,25), in this study, we show that prolactin can also regulate duodenal TRPV6 mRNA. Three injections of prolactin over 48 h were required to observe induction of TRPV6 mRNA, suggesting that more long-term exposure, rather than acute administration, is needed for prolactin mediated regulation of TRPV6. Administration of prolactin alone either acutely or over 48 h was unable to affect duodenal calbindin-D9k expression however. This is consistent with previous findings in VDR null mutant mice, indicating that duodenal TRPV6 mRNA expression, but not calbindin-D9k expression, is markedly induced during lactation and late pregnancy (10,46). Prolactin and PTHrP, which remain high during lactation, were suggested as candidate hormones that result in the major up-regulation of TRPV6 (10). Although it is possible that PTHrP may also affect TRPV6 expression, our findings indicate that at least part of the marked vitamin D-independent up-regulation of TRPV6 mRNA observed in the intestine during lactation is due to an effect of prolactin.
In addition to vitamin D-independent effects of prolactin on TRPV6 expression, cooperative effects were noted between prolactin and 1,25(OH)2D3 in the regulation of both TRPV6 and calbindin-D9k. Also, at concentrations that did not result in a measureable effect for each hormone alone, combined treatment with 1,25(OH)2D3 and prolactin resulted in a significant induction in duodenal calcium transport as measured by the everted gut sac assay. Because both 1,25(OH)2D3 and prolactin serum levels are increased during lactation (7,19,20,47), prolactin may act together with 1,25(OH)2D3 for some of its effects related to calcium homeostasis. Cooperative effects between prolactin and 1,25(OH)2D3 have previously been reported for growth suppression of prostate cancer cells (48). Studies in prostate cancer cells showed an induction of VDR by prolactin (48). In our studies, however, we did not observe an increase in VDR in the intestine of vitamin D-deficient mice injected with prolactin alone, 1,25(OH)2D3 alone (as previously reported) (49,50), or 1,25(OH)2D3 + prolactin, suggesting that the mechanism of the enhancement was not due to an induction of VDR in the intestine. Because the binding of prolactin to its receptor regulates transcriptional activation of milk protein genes (51), we examined the possibility that prolactin may transcriptionally activate the −7000/+160 TRPV6 promoter construct. The expression of TRPV6 is regulated by 1,25(OH)2D3/VDR at the transcriptional level through two active vitamin D-response elements at −2.1 and −4.3 kb (31). Although a cooperative effect was observed between prolactin and 1,25(OH)2D3 in the regulation of TRPV6 mRNA, a combination of prolactin and 1,25(OH)2D3 in the presence or absence of C/EBP β and STAT5 did not affect 1,25(OH)2D3-induced TRPV6 promoter activity. It is possible that prolactin regulates TRPV6 at other sites in the TRPV6 promoter yet to be identified. It is also possible that prolactin may affect TRPV6 expression by posttranscriptional mechanisms. Prolactin induces casein gene transcription by both transcriptional and posttranscriptional mechanisms. Prolactin has been reported to induce a 2- to 3-fold increase in the rate of casein mRNA transcripton and a 17- to 25-fold increase in the half life of casein mRNA (52). Thus, both mechanisms are involved in the marked accumulation of casein mRNA after prolactin treatment of mammary organ culture. More recent studies have shown that prolactin plus insulin can induce poly(A) elongation of β-casein (53). A previous study, which examined PTH regulation of 25-hydroxyvitamin D3 24-hydroxylase, also noted posttranscriptional regulation of a 1,25(OH)2D3 target gene by a peptide hormone (54). With regard to calbindin, the large induction of calbindin-D9k mRNA in intestine in response to 1,25(OH)2D3 has been shown to be due primarily to posttranscription mechanisms (55). Thus, it is possible that prolactin may enhance posttranscriptional mechanisms involved in an effect of 1,25(OH)2D3 on increasing calbindin-D9k mRNA half life. It is also possible that the effect of prolactin may be secondary to a primary effect of prolactin on other factors that in turn affect TRPV6 expression or 1,25(OH)2D3-induced calbindin-D9k or TRPV6 expression.
Although in this study we focused on duodenum and calcium transport proteins involved in active calcium transport, prolactin may also have an effect on intestinal calcium absorption via the paracellular path. An effect of prolactin on the paracellular path has previously been suggested (17,18,45). Recent studies have shown that 1,25(OH)2D3 can regulate tight junction and transmembrane proteins in the intestine, including claudin-2, claudin-12, and cadherin-17, suggesting a role for 1,25(OH)2D3 in the transjunctional movement of calcium (56,57). It will be of interest, in future studies, to determine whether prolactin can also affect the regulation of these proteins in the duodenum, as well as in other regions of the intestine.
Besides direct effects of prolactin on the intestine, an effect of prolactin on 1α(OH)ase expression and transcription in kidney cells was also observed. Early studies showed that circulating levels of 1,25(OH)2D3 are increased in pregnancy and during lactation (7,19,20). However, the mechanisms underlying the increase in serum 1,25(OH)2D3 and whether prolactin has a direct effect on 1α(OH)ase have been a matter of debate. During pregnancy and lactation, there is a significant flux of calcium to the fetus and the neonate, respectively. The drain on the plasma calcium pool results in a drop in serum calcium (19,20). Thus, it has been suggested that the drop in serum calcium in late pregnancy and lactation may stimulate the renal 1α(OH)ase via PTH (19,20). Parathyroidectomy of lactating rats caused a 70% inhibition of the 1,25(OH)2D3 increase (20). However, it has been reported that hypoparathyroid women during lactation treated with the usual dose of calcitriol develop hypercalcemia, and in the absence of treatment, serum 1,25(OH)2D3 levels remain within the normal range, suggesting that another factor besides PTH stimulates 1α(OH)ase during lactation (58,59,60). Previous studies suggested that prolactin can influence 1α(OH)ase. Early studies were done in chicks and showed that prolactin increased 1α(OH)ase activity after either long-term or short-term administration (61). In addition, prolactin was shown to act directly on chick renal cells to stimulate 1α(OH)ase activity (62,63). Further, in lactating rats, bromocriptine treatment significantly reduced 1,25(OH)2D3 levels (21). We now report that prolactin has a direct effect on the transcription of the 1α(OH)ase gene. Using the mouse 1α(OH)ase promoter, we report for the first time a STAT5-mediated regulation by prolactin of 1α(OH)ase transcription. Our findings indicate that prolactin regulation of 1α(OH)ase transcription involves JAK2 and STAT5 (Fig. 5B), similar to other genes regulated by prolactin in mammary gland, breast cancer cells, and in adipocytes (34,42,64,65). However, no consensus STAT5-binding sites were noted in the −1651/+22 region of the mouse 1α(OH)ase promoter, and mutations of regions that were similar to the consensus sequence did not affect activation of the 1α(OH)ase promoter by STAT5/prolactin. It is possible that STAT5 affects 1α(OH)ase transcription by binding to sites other than the STAT5 site [STAT5 has been reported to bind to sites that resemble the γ interferon-activated sequence (GAS TTNCNNNAA) (66,67)]. However, γ interferon-activated sequence sites were not identified within the region of the 1α(OH)ase promoter that responded to stimulation of transcription by prolactin/STAT5. Recent studies indicated that the majority of STAT5-binding sites are paired sites (68). In addition, it has been noted that the repertoire of potential binding sites for STAT5 is broader than expected (67). Thus, although more divergent, paired, nonconsensus sites may be involved in prolactin/STAT5 regulation, our findings nevertheless demonstrate a direct effect of prolactin via STAT5 and JAK2 on 1α(OH)ase transcription. Prolactin was also shown to enhance 1α(OH)ase protein expression. Therefore, it is likely that both PTH and prolactin have a physiological function to increase 1,25(OH)2D3 levels in lactation.
In summary, our findings indicate for the first time mechanisms whereby prolactin modulates vitamin D-mediated calcium homeostasis. Prolactin can regulate the intestinal epithelial calcium channel TRPV6 and can cooperate with 1,25(OH)2D3 in regulating TRPV6 and calbindin-D9k. In addition, prolactin has a direct effect on the 1α(OH)ase gene, which is mediated by STAT5 and JAK2. These mechanisms may have physiological importance during lactation when plasma levels of prolactin are markedly elevated and there is increased calcium requirement for the neonate.
Footnotes
This work was supported by the National Institutes of Health Grant DK-38961-21 (to S.C.) and by a United Negro College Fund Merck Graduate Science Research Dissertation Fellowship (D.V.A.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 12, 2010
Abbreviations: bw, Body weight; C/EBP β, CCAAT enhancer-binding protein-β; DN, dominant negative; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; h, human; JAK2, Janus kinase 2; 1α(OH)ase, 25-hydroxyvitamin D3 1α hydroxylase; 1,25(OH)2D3, 1,25-Dihydroxyvitamin D3; PTHrP, PTH related peptide; STAT, signal transducer and activator of transcription; TRPV6, transient receptor potential vanilloid type 6; VDR, vitamin D receptor.
References
- Carafoli E, Santella L, Branca D, Brini M 2001 Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol 36:107–260 [DOI] [PubMed] [Google Scholar]
- Bikle D, Adams J, Christakos S 2008 Vitamin D production, metabolism, mechanism of action, and clinical requirements In: Rosen C, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 7th ed. Washington, DC: American Society for Bone and Mineral Research; 141–149 [Google Scholar]
- Christakos S 2008 Vitamin D gene regulation. In: Bilezikian J, Raisz L, Martin T, eds. Principles of bone biology. 3rd ed. San Diego, CA: Elsevier; 779–794 [Google Scholar]
- DeLuca HF 2008 Evolution of our understanding of vitamin D. Nutr Rev 66(10 Suppl 2):S73–S87 [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ 2005 Calcium absorption across epithelia. Physiol Rev 85:373–422 [DOI] [PubMed] [Google Scholar]
- Wasserman RH, Fullmer CS 1995 Vitamin D and intestinal calcium transport: facts, speculations and hypotheses. J Nutr 125(7 Suppl):1971S–1979S [DOI] [PubMed] [Google Scholar]
- Halloran BP, DeLuca HF 1980 Calcium transport in small intestine during pregnancy and lactation. Am J Physiol 239:E64–E68 [DOI] [PubMed] [Google Scholar]
- Boass A, Toverud SU, Pike JW, Haussler MR 1981 Calcium metabolism during lactation: enhanced intestinal calcium absorption in vitamin D-deprived, hypocalcemic rats. Endocrinology 109:900–907 [DOI] [PubMed] [Google Scholar]
- Brommage R, Baxter DC, Gierke LW 1990 Vitamin D-independent intestinal calcium and phosphorus absorption during reproduction. Am J Physiol 259:G631–G638 [DOI] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Rummens K, Stockmans I, van Herck E, Dijcks FA, Ederveen AG, Carmeliet P, Verhaeghe J, Bouillon R, Carmeliet G 2003 Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res 18:1725–1736 [DOI] [PubMed] [Google Scholar]
- Charoenphandhu N, Krishnamra N 2007 Prolactin is an important regulator of intestinal calcium transport. Can J Physiol Pharmacol 85:569–581 [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA 1998 Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268 [DOI] [PubMed] [Google Scholar]
- Gouilleux F, Wakao H, Mundt M, Groner B 1994 Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J 13:4361–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW 2002 Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285:1–24 [DOI] [PubMed] [Google Scholar]
- Pahuja DN, DeLuca HF 1981 Stimulation of intestinal calcium transport and bone calcium mobilization by prolactin in vitamin D-deficient rats. Science 214:1038–1039 [DOI] [PubMed] [Google Scholar]
- Charoenphandhu N, Limlomwongse L, Krishnamra N 2001 Prolactin directly stimulates transcellular active calcium transport in the duodenum of female rats. Can J Physiol Pharmacol 79:430–438 [PubMed] [Google Scholar]
- Kraidith K, Jantarajit W, Teerapornpuntakit J, Nakkrasae LI, Krishnamra N, Charoenphandhu N 2009 Direct stimulation of the transcellular and paracellular calcium transport in the cecum by prolactin. Pflugers Arch 458:993–1005 [DOI] [PubMed] [Google Scholar]
- Charoenphandhu N, Nakkrasae LI, Kraidith K, Teerapornpuntakit J, Thongchote K, Thongon N, Krishnamra N 2009 Two-step stimulation fo intestinal Ca2+ absorption during lactation by long-term prolactin exposure and suckling induced prolactin surge. Am J Physiol Endocrinol Metab 297:E609–E619 [DOI] [PubMed] [Google Scholar]
- Halloran BP, Barthell EN, DeLuca HF 1979 Vitamin D metabolism during pregnancy and lactation in the rat. Proc Natl Acad Sci USA 76:5549–5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike JW, Parker JB, Haussler MR, Boass A, Toverud SV 1979 Dynamic changes in circulating 1,25-dihydroxyvitamin D during reproduction in rats. Science 204:1427–1429 [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Spanos E, James MF, Pike JW, Haussler MR, Makeen AM, Hillyard CJ, MacIntyre I 1982 Role of prolactin in vitamin D metabolism and calcium absorption during lactation in the rat. J Endocrinol 94:443–453 [DOI] [PubMed] [Google Scholar]
- Omdahl JL, DeLuca HF 1972 Rachitogenic activity of dietary strontium. I. Inhibition of intestinal calcium absorption and 1,25-dihydroxycholecalciferol synthesis. J Biol Chem 247:5520–5526 [PubMed] [Google Scholar]
- Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S 2003 Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology 144:3885–3894 [DOI] [PubMed] [Google Scholar]
- Tong Y, Pelletier G 1992 Prolactin regulation of pro-opiomelanocortin gene expression in the arcuate nucleus of the rat hypothalamus. Neuroendocrinology 56:561–565 [DOI] [PubMed] [Google Scholar]
- Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S 2008 Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 149:3196–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter D, Rosen SM 1959 Active transport of Ca45 by the small intestine and its dependence on vitamin D. Am J Physiol 196:357–362 [DOI] [PubMed] [Google Scholar]
- Armbrecht HJ, Zenser TV, Gross CJ, Davis BB 1980 Adaptation to dietary calcium and phosphorus restriction changes with age in the rat. Am J Physiol 239:E322–E327 [DOI] [PubMed] [Google Scholar]
- Armbrecht HJ, Zenser TV, Davis BB 1980 Effect of vitamin D metabolites on intestinal calcium absorption and calcium-binding protein in young and adult rats. Endocrinology 106:469–475 [DOI] [PubMed] [Google Scholar]
- Bringhurst FR, Juppner H, Guo J, Urena P, Potts Jr JT, Kronenberg HM, Abou-Samra AB, Segre GV 1993 Cloned, stably expressed parathyroid hormone (PTH)/PTH-related peptide receptors activate multiple messenger signals and biological responses in LLC-PK1 kidney cells. Endocrinology 132:2090–2098 [DOI] [PubMed] [Google Scholar]
- Friedman PA, Gesek FA, Morley P, Whitfield JF, Willick GE 1999 Cell-specific signaling and structure-activity relations of parathyroid hormone analogs in mouse kidney cells. Endocrinology 140:301–309 [DOI] [PubMed] [Google Scholar]
- Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW 2006 The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20:1447–1461 [DOI] [PubMed] [Google Scholar]
- Brenza HL, DeLuca HF 2001 Analysis of basal regulatory elements in the 25-hydroxyvitamin D3 1α-hydroxylase gene promoter. Arch Biochem Biophys 388:121–126 [DOI] [PubMed] [Google Scholar]
- Wyszomierski SL, Rosen JM 2001 Cooperative effects of STAT5 (signal transducer and activator of transcription 5) and C/EBPβ (CCAAT/enhancer-binding protein-β) on β-casein gene transcription are mediated by the glucocorticoid receptor. Mol Endocrinol 15:228–240 [DOI] [PubMed] [Google Scholar]
- Brockman JL, Schroeder MD, Schuler LA 2002 PRL activates the cyclin D1 promoter via the Jak2/Stat pathway. Mol Endocrinol 16:774–784 [DOI] [PubMed] [Google Scholar]
- Shen Q, Christakos S 2005 The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J Biol Chem 280:40589–40598 [DOI] [PubMed] [Google Scholar]
- Dhawan P, Peng, X, Sutton AL, MacDonald PN, Croniger CM, Trautwein C, Centrella M, McCarthy TL, Christakos S 2005 Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Mol Cell Biol 25:472–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Zhong Y, Armbrecht HJ, Christakos S 2009 Calcitonin, a regulator of the 25-hydroxyvitamin D3 1α-hydroxylase gene. J Biol Chem 284:11059–11069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau DL, Freier EF 1967 Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS). Clin Chem 13:101–114 [PubMed] [Google Scholar]
- Pinto M, Robine-Leon S, Appay MD, Kedinger M, Triadow N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffer K, Fogh J Zweibaum M 1983 Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell 47:323–330 [Google Scholar]
- Fleet JC, Wood RJ 1994 Identification of calbindin D-9k mRNA and its regulation by 1,25-dihydroxyvitamin D3 in Caco-2 cells. Arch Biochem Biophys 308:171–174 [DOI] [PubMed] [Google Scholar]
- White UA, Coulter AA, Miles TK, Stephens JM 2007 The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes 56:1623–1629 [DOI] [PubMed] [Google Scholar]
- Mainoya JR 1975 Effects of bovine growth hormone, human placental lactogen and ovine prolactin on intestinal fluid and ion transport in the rat. Endocrinology 96:1165–1170 [DOI] [PubMed] [Google Scholar]
- Charoenphandhu N, Limlomwongse L, Krishnamra N 2006 Prolactin directly enhanced Na+/K+- and Ca2+-ATPase activities in the duodenum of female rats. Can J Physiol Pharmacol 84:555–563 [DOI] [PubMed] [Google Scholar]
- Jantarajit W, Thongon N, Pandaranandaka J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N 2007 Prolactin-stimulated transepithelial calcium transport in duodenum and Caco-2 monolayer are mediated by the phosphoinositide 3-kinase pathway. Am J Physiol Endocrinol Metab 293:E372–E384 [DOI] [PubMed] [Google Scholar]
- Fudge NJ, Kovacs CS 2010 Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology 151:886–895 [DOI] [PubMed] [Google Scholar]
- Meites J, Lu KH, Wuttke W, Welsch CW, Nagasawa H, Quadri SK 1972 Recent studies on functions and control of prolactin secretion in rats. Recent Prog Horm Res 28:471–526 [PubMed] [Google Scholar]
- Wu W, Zanello L, Walker AM 2007 S179D prolactin sensitizes human prostate cancer cells such that physiological concentrations of 1, 25 dihydroxy vitamin D3 result in growth inhibition and cell death. Prostate 67:1498–1506 [DOI] [PubMed] [Google Scholar]
- Huang YC, Lee S, Stolz R, Gabrielides C, Pansini-Porta A, Bruns ME, Bruns DE, Miffin TE, Pike JW, Christakos S 1989 Effect of hormones and development on the expression of the rat 1,25-dihydroxyvitamin D3 receptor gene. Comparison with calbindin gene expression. J Biol Chem 264:17454–17461 [PubMed] [Google Scholar]
- Healy KD, Frahm MA, DeLuca HF 2005 1,25-Dihydroxyvitamin D3 up-regulates the renal vitamin D receptor through indirect gene activation and receptor stabilization. Arch Biochem Biophys 433:466–473 [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW, Wagner KU, Liu W 1997 Prolactin signaling in mammary gland development. J Biol Chem 272:7567–7569 [DOI] [PubMed] [Google Scholar]
- Guyette WA, Matusik RJ, Rosen JM 1979 Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell 17:1013–1023 [DOI] [PubMed] [Google Scholar]
- Choi KM, Barash I, Rhoads RE 2004 Insulin and prolactin synergistically stimulate β-casein messenger ribonucleic acid translation by cytoplasmic polyadenylation. Mol Endocrinol 18:1670–1686 [DOI] [PubMed] [Google Scholar]
- Zierold C, Mings JA, DeLuca HF 2001 Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc Natl Acad Sci USA 98:13572–13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret JM, Brun P, Perret C, Lomri N, Thomasset M, Cuisinier-Gleizes P 1987 Transcriptional and post-transcriptional regulation of vitamin D-dependent calcium-binding protein gene expression in the rat duodenum by 1,25-dihydroxycholecalciferol. J Biol Chem 262:16553–16557 [PubMed] [Google Scholar]
- Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H 2008 Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 19:1912–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzova GD, DeLuca HF 2004 Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys 432:152–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghe J, Bouillon R 1992 Calciotropic hormones during reproduction. J Steroid Biochem Mol Biol 41:469–477 [DOI] [PubMed] [Google Scholar]
- Cundy T, Haining SA, Guilland-Cumming DF, Butler J, Kanis JA 1987 Remission of hypoparathyroidism during lactation: evidence for a physiological role for prolactin in the regulation of vitamin D metabolism. Clin Endocrinol 26:667–674 [DOI] [PubMed] [Google Scholar]
- Caplan RH, Beguin EA 1990 Hypercalcemia in a calcitriol-treated hypoparathyroid woman during lactation. Obstet Gynecol 76:485–489 [PubMed] [Google Scholar]
- Spanos E, Pike JW, Haussler MR, Colston KW, Evans IM, Goldner AM, McCain TA, MacIntyre I 1976 Circulating 1α,25-dihydroxyvitamin D in the chicken: enhancement by injection of prolactin and during egg laying. Life Sci 19:1751–1756 [DOI] [PubMed] [Google Scholar]
- Spanos E, Brown DJ, Stevenson JC, MacIntyre I 1981 Stimulation of 1,25-dihydroxycholecalciferol production by prolactin and related peptides in intact renal cell preparations in vitro. Biochim Biophys Acta 672:7–15 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Spencer EM, Burke WH, Rost CR 1980 Prolactin but not growth hormone stimulates 1,25-dihydroxyvitamin D3 production by chick renal preparations in vitro. Endocrinology 107:81–84 [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L 1995 Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA 92:8831–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan JC, Stephens JM 2005 The regulation of fatty acid synthase by STAT5A. Diabetes 54:1968–1975 [DOI] [PubMed] [Google Scholar]
- Ooi GT, Hurst KR, Poy MN, Rechler MM, Boisclair YR 1998 Binding of STAT5a and STAT5b to a single element resembling a γ-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Mol Endocrinol 12:675–687 [DOI] [PubMed] [Google Scholar]
- Soldaini E, John S, Moro S, Bollenbacher J, Schindler U, Leonard WJ 2000 DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a larger repertoire of divergent tetrameric Stat5a binding sites. Mol Cell Biol 20:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laz EV, Sugathan A, Waxman DJ 2009 Dynamic in vivo binding of STAT5 to growth hormone-regulated genes in intact rat liver. Sex-specific binding at low but not high affinity STAT5 sites. Mol Endocrinol 23:1242–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]