Abstract
Hypothalamic tuberoinfundibular dopaminergic (TIDA) neurons secrete dopamine, which inhibits pituitary prolactin (PRL) secretion. PRL has demonstrated neurotrophic effects on TIDA neuron development in PRL-, GH-, and TSH-deficient Ames (df/df) and Snell (dw/dw) dwarf mice. However, both PRL and PRL receptor knockout mice exhibit normal-sized TIDA neuron numbers, implying GH and/or TSH influence TIDA neuron development. The current study investigated the effect of porcine (p) GH on TIDA neuron development in Ames dwarf hypothalamus. Normal (DF/df) and dwarf mice were treated daily with pGH or saline beginning at 3 d of age for a period of 42 d. After treatment, brains were analyzed using catecholamine histofluorescence, tyrosine hydroxylase immunocytochemistry, and bromodeoxyuridine (BrdU) immunocytochemistry to detect BrdU incorporation. DF/df males and df/df treated with pGH experienced increased (P ≤ 0.01) weight gain compared with those treated with saline. DF/df had greater (P ≤ 0.01) TIDA neuron numbers than df/df, regardless of treatment. TIDA neuron number in pGH-treated df/df was greater (P ≤ 0.01) than in saline-treated df/df. Zona incerta and periventricular dopamine neurons were not affected by treatment or genotype. There was no effect of genotype or treatment on BrdU incorporation in the arcuate nucleus, median eminence, or periventricular region surrounding the third ventricle. Saline-treated df/df experienced decreased (P ≤ 0.05) dentate gyrus BrdU incorporation compared with saline-treated DF/df. In the lateral ventricle, pGH-treated males had greater BrdU immunoreactivity than pGH-treated females. The results show an effect of pGH on TIDA neuron development, although this effect is less potent than that of PRL, and likely GH-induced preservation of TIDA neurons rather than generation of new TIDA neurons via neurogenesis.
Hypothalamic tuberoinfundibular dopaminergic (TIDA) neuron development primarily requires prolactin to achieve normal population size, but in hypopituitary mice lacking prolactin, treatment with the related pituitary hormone, growth hormone affects TIDA neuron development.
The tuberoinfundibular dopaminergic (TIDA) neurons are located in the arcuate nucleus (ARC) of the hypothalamus and extend to the external median eminence (ME). These neurons synapse on the pituitary portal vessels in which they release dopamine (DA), which inhibits prolactin (PRL) secretion (reviewed in1).
Ames (df/df) (2) and Snell (3) dwarf mice, which lack pituitary PRL, GH, and TSH due to mutation in transcription factor Prophet of Pituitary transcription factor-1 (4) and Pituitary transcription factor-1 (5), respectively, exhibit deficient TIDA neuron populations (6,7,8) and hypothalamic DA (9,10,11). Previous studies using Ames and Snell dwarf mice as a model for hormone replacement demonstrated neurotrophic actions of PRL on the development of TIDA neurons. Early postnatal PRL treatment for 30 d induces the maintenance of a normal-sized TIDA neuron number in Ames dwarf mice (12) and induces TIDA neuron differentiation in Snell dwarf mice (13). Long-term treatment of adult Snell dwarf mice with PRL also has been demonstrated to induce TIDA neuron differentiation (14).
However, PRL (15) and PRL receptor (16) knockout mice exhibit normal-sized TIDA neuron numbers (17,18) with diminished DA content (19). These observations indicate that although PRL has a significant neurotrophic effect on TIDA neurons, these PRL effects are not necessary for the development of a normal-sized TIDA population. Snell and Ames dwarf mice also lack GH and TSH, suggesting that GH and/or TSH can influence TIDA neuron differentiation in the absence of PRL. Because PRL and GH have high amino acid homology (20), GH is the most probable candidate for the maintenance of the TIDA neuron population in PRL and PRL receptor-deficient mice.
Further evidence for a possible regulatory effect of GH on TIDA neurons includes involvement of DA in GH regulation, which has been demonstrated to be both stimulatory and inhibitory (21). Release of GH from rat and human pituitaries is inhibited by DA and DA agonists in vitro (22,23). However, in vivo, DA stimulates GHRH and somatostatin release in the rat hypothalamus (24). DA agonists stimulate GH release in healthy individuals (25) and individuals with low baseline GH levels but inhibit GH secretion in cases of elevated GH levels (26). Deletion of the DA transporter, which abolishes DA reuptake, results in reduced somatotroph and lactotroph cell numbers (27). A lack of functional D2-type DA receptors in mice induces decreased GH levels in the first 2 months of life and impaired GH release (basal and GHRH stimulated) in the adult (28). GH may regulate TIDA neuron development because it may indirectly feed back to tyrosine hydroxylase (TH) neurons to regulate its own secretion because a subpopulation of TIDA neurons coexpress GHRH (29).
The present study investigated the hypothesis that GH has neurotrophic effects on TIDA neurons in Ames dwarf mice. It was hypothesized that GH would mimic the effect of PRL in maintaining a normal-sized TIDA neuron population during hypothalamic development in the absence of PRL. These studies were executed using the same methods as our previous studies, which showed the neurotrophic role of PRL on these TIDA neurons (30,31). To further delineate the role of GH, a possible mechanism of the anticipated TIDA neuron recruitment was examined using bromodeoxyuridine (BrdU) treatment to assess whether exogenous GH induced ARC cell proliferation to replenish the TIDA cell population.
Materials and Methods
Animals
Matings of normal (DF/df) females with Ames dwarf (df/df) males produced df/df and DF/df mice used in experimental procedures. Fertility was induced in dwarf males beginning at 6–8 wk of age through treatment with D/L-T4 (2 μg ip; Sigma, St. Louis, MO) three times a week, followed by renal capsule pituitary grafts from DF/df donors at 9 wk of age. Pituitary graft transplant surgeries were performed on isoflurane-anesthetized mice. Donors, which included DF/df males and females aged 2–12 months, were anesthetized with a rompin, acepromazine, ketamaine cocktail and decapitated for pituitary gland removal. Whole single glands were placed under the kidney capsule of recipient animals. Recipients were observed continuously and body temperature was maintained until recovery from anesthesia. The colony was maintained under controlled temperature (22 ± 2 C) and lighting (lights on from 0600 to 1800 h), with food and water available ad libitum. An Institutional Animal Care and Use Committee approved all procedures involving animals.
Treatments
Treatments of DF/df and df/df littermates were initiated at 3 d of age. Animals were injected with saline (0.03 m NaHCO3/0.15 m NaCl) or 50 μg porcine (p) GH daily (National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, Torrance, CA). The biopotency of pGH was 1.8 IU/mg in terms of International Standard for bovine GH. Litters treated with saline served as controls. For the first 5 treatment days, solutions were administered sc due to solution leakage out of the intraperitoneal space at this age. Thereafter treatment solutions were administered into the intraperitoneal space. All mice were treated with BrdU (50 μg/g, ip; Sigma) at 21, 31, 39, and 42 d of age and euthanized by perfusion at 45 d of age. Nine animals were used in each experimental group.
Tissue preparation and induction of catecholamine (CA) fluorescence
Mice were weighed and anesthetized with rompin, acepromazine, ketamaine cocktail (0.05 ml per 25 g body weight). Anesthesia was followed by transcardial perfusion with 0.9% NaCl and 4% paraformaldehyde-0.5% glutaraldehyde (faglu) fixative (32). Brains were postfixed overnight at 4 C, and ice crystal formation was prevented using a 30% sucrose-faglu solution. Brains were sectioned coronally at 30 μm using a sliding microtome (Riechert, now Leica, Heidelberg, Germany) once saturated with sucrose-faglu. Brain sections were divided into six serial sets, each representative of the entire brain, and postfixed overnight. Sections were stored in cryoprotectant antifreeze (33) at −20 C, except for those used for induction of CA fluorescence. Every sixth section was mounted out of faglu and examined for fixative-induced CA fluorescence using narrow-band excitation wavelengths (395–415 nm) and a violet barrier filter (460 nm) on a Nikon E800 microscope equipped for fluorescence epiillumination (Nikon, Tokyo, Japan).
Immunocytochemistry (ICC) for BrdU or TH
Tissue sections were pretreated with 0.1% hydrogen peroxide to inhibit endogenous peroxidase activity and 1% aqueous sodium borohydride to reduce glutaraldehyde-fixed linkages and allow antibody access. For BrdU ICC, pretreatment also included 6 n HCl for 15 min at room temperature to denature double-stranded DNA and allow BrdU epitope exposure. After extensive rinsing with PBS, sections were incubated in 1.5% normal rabbit serum for 1 h to reduce nonspecific staining. Sections were then incubated with sheep α-TH (Pel-Freeze Biologicals, Rogers, AR; 0.025 μg/ml) or sheep α-BrdU (Abcam Inc., Cambridge, MA; 0.05 μg/ml) for 48 h. Further processing included use of biotinylated rabbit α-sheep IgG (Vector Laboratories, Burlingame, CA; 1:200) and avidin-biotin complex solutions (Vectastain kit; Vector Laboratories). Visualization of immunoreactivity was accomplished through development with 0.02% diaminobenzedine tetrahydrochloride and 0.003% H2O2 in Tris buffer. BrdU immunoreactivity was visualized through development with a diaminobenzedine tetrahydrochloride solution containing 0.5% NiSO4. Sections were then mounted, dried, and coverslipped using DPX mountant for microscopy (Fisher Chemical Co., Pittsburgh, PA). Identical antiserum aliquots and reagents were used in each ICC run, and sections from all treatment groups were processed simultaneously.
TH cell counts
Neurons immunoreactive for TH were manually and visually quantified in hypothalamic dopaminergic areas A12 (TIDA), A13 (zona incerta), and A14 (periventricular nucleus) at 180-μm intervals, according to the classification of Björklund and Nobin (34). Cell counts were not corrected for recounted or missed cells because the section thickness (30 μm) and interval (180 μm) exceeded perikaryal diameter (35). Total cell counts were corrected for sectioning periodicity (×6 for every sixth section).
BrdU cell counts
Cells immunoreactive for BrdU incorporation were manually and visually quantified in the subventricular zone (SVZ) of the lateral ventricle (LV), dentate gyrus (DG), periventricular region surrounding the third ventricle (3V), ME, and ARC at 180-μm intervals. BrdU-immunoreactive cells in the entire SVZ were quantified beginning at the wall of the LV and extending approximately 25 μm into the surrounding tissue. BrdU-immunoreactive cells in the periventricular region surrounding the 3V were quantified beginning at the wall of the 3V and extending approximately 60 μm into the surrounding tissue, excluding the ARC. Sample sizes for BrdU cell counts are as follows: saline (six DF/df, six df/df), 50 μg pGH (six DF/df, six df/df). Counts were corrected for periodicity (×6 for every sixth section).
Imaging for illustrations
Images of endogenous CA fluorescence, TH immunoreactivity, and BrdU immunoreactivity were taken using a Nikon DX1200 color digital camera on a Nikon E800 microscope. Adobe Photoshop 7.0 (San Jose, CA) was used to composite images into multiphoto plates.
Statistical analysis
ANOVA (SuperANOVA; Abacus Concepts, Berkeley, CA) was used to statistically analyze data, followed by Student Newman-Keuls post hoc tests. Three- and two-way ANOVAs were initially used to identify significant effects. If significant effects were observed, one-way ANOVA was performed with post hoc tests. Differences were considered significant at or below the 5% probability level.
Results
Body weight
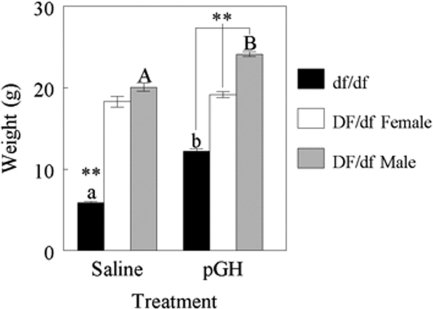
Body weights of Ames dwarf and normal mice treated with saline or pGH are shown in Fig. 1. In pGH-treated normal mice [F(1,7) = 73.3; P = 0.0001], body weight differed between genders: females (19.2 ± 0.4, n = 6) weighed less than (P ≤ 0.01) males (24.1 ± 0.3, n = 3). This effect of gender was not observed in saline-treated normal mice. Also, pGH treatment affected body weight in normal male mice [F(1,6) = 34.6; P = 0.0011] but not in normal female mice. Saline-treated normal males (20.1 ± 0.5, n = 5) weighed less than (P ≤ 0.01) pGH-treated normal males. Weight differences due to sex were not observed in dwarf mice.
Figure 1.
Body weights in Ames dwarf (df/df, solid bars) and normal (DF/df female, open bars, DF/df male, gray bars) mice treated with saline (left) or pGH (right) beginning at 3 d of age. **, Differences between dwarf and normal (P ≤ 0.01). Letters indicate differences between treatment (P ≤ 0.01).
Genotype affected body weight [F(1,34) = 135.9; P = 0.0001] in that dwarf mice (9.0 ± 0.8, n = 18) weighed less than (P ≤ 0.01) normal mice (20.1 ± 0.5, n = 18), regardless of treatment. An effect of treatment on body weight was observed in dwarf mice [F(1,16) = 370.2; P = 0.0001]. Dwarf mice treated with pGH (12.2 ± 0.3, n = 9) weighed more than (P ≤ 0.01) dwarf mice treated with saline (5.9 ± 0.2, n = 9).
CA fluorescence
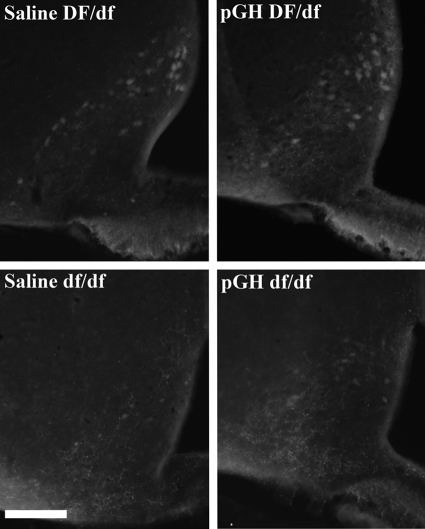
Figure 2 shows TIDA and ME DA fluorescence in Ames dwarf and normal mice treated with saline or pGH. Evaluation of TIDA and ME DA fluorescence was qualitative. In normal mice, TIDA and ME DA fluorescence was similar, regardless of treatment, and more intense than that in dwarf mice. In dwarf mice, ME DA fluorescence was more intense in pGH-treated animals, and TIDA fluorescence was slightly greater in pGH-treated animals. Fluorescence in nonhypophysiotropic regions, such as the substantia nigra, was qualitatively similar between genotypes and treatments (data not shown).
Figure 2.
Endogenous catecholamine fluorescence in ME and ARC of Ames dwarf (df/df, lower panels) and normal (DF/df, upper panels) mice treated with saline (left) or pGH (right) beginning at 3 d of age. Original objective magnification, ×20. For all panels, the horizontal bar at the lower left represents 100 μm.
TH immunoreactivity
Illustrations of TIDA and ME TH immunoreactivity in Ames dwarf and normal mice treated with saline or pGH are shown in Fig. 3. Normal mice exhibited similar TH immunoreactivity in TIDA and ME, regardless of treatment, which was qualitatively greater than that in dwarfs. Dwarf mice treated with pGH had higher TIDA TH immunostaining than dwarf mice treated with saline but similar ME TH immunostaining. Immunoreactivity in the substantia nigra was qualitatively similar between treatments and genotypes (data not shown).
Figure 3.
TH immunoreactivity in TIDA neurons and ME of Ames dwarf (df/df, lower panels) and normal (DF/df, upper panels) mice treated with saline (left) or pGH (right) beginning at 3 d of age. Original objective magnification, ×10. For all panels, the horizontal bar at the lower right indicates 100 μm.
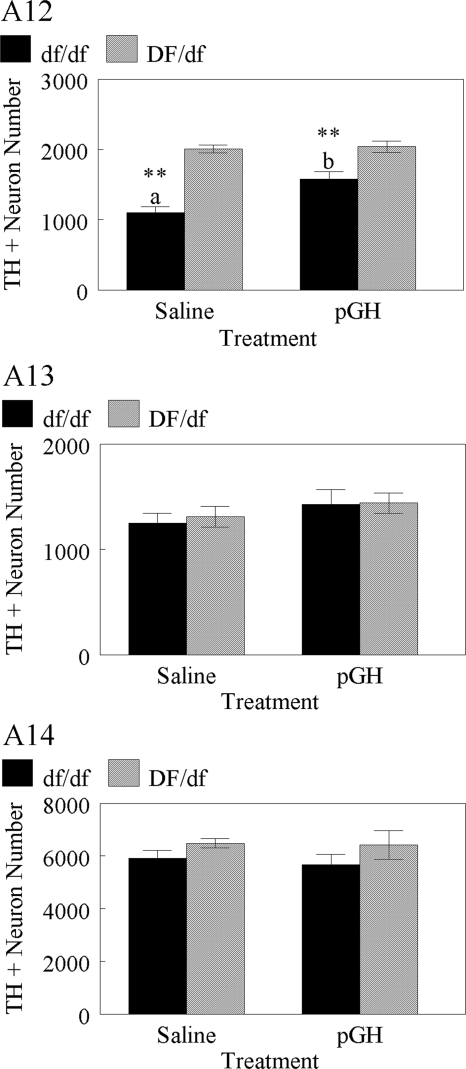
Figure 4 displays graphs of TH-immunoreactive cell numbers in dopaminergic areas A12, A13, and A14 of Ames dwarf and normal mice treated with saline or pGH. Genders were pooled because there was no effect of sex on area A12, A13, or A14 cell numbers. Genotype affected area A12 cell numbers [F(1,33) = 46.1; P = 0.0001], regardless of treatment. Normal mice had higher (P ≤ 0.01) TIDA neuron numbers (2026 ± 49, n = 17) than dwarf mice (1339 ± 87, n = 18). Area A12 cell numbers were affected by treatment in dwarf mice [F(1,16) = 12.6; P = 0.0026] but not in normal mice. Dwarf mice treated with pGH had higher (P ≤ 0.01) TIDA neuron numbers (1576 ± 106, n = 9) than dwarf mice treated with saline (1101 ± 83, n = 9). There was no effect of genotype or treatment on area A13 or A14 cell numbers.
Figure 4.
TH-immunoreactive neuron number in areas A12, A13, and A14 of Ames dwarf (df/df, solid bars) and normal (DF/df, gray bars) mice treated with saline (left) or pGH (right) beginning at 3 d of age. **, Differences between genotypes (P ≤ 0.01). Differences between treatments are denoted by letters (P ≤ 0.01).
BrdU immunoreactivity
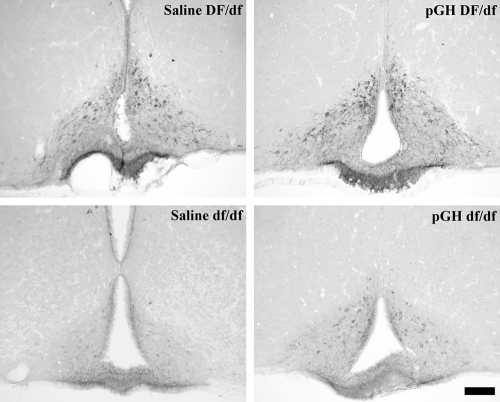
Figure 5 shows photomicrographs of BrdU immunoreactivity in the DG of Ames dwarf and normal mice treated with saline. Saline-treated normal animals had qualitatively higher BrdU-immunoreactive cell numbers than saline-treated dwarf animals.
Figure 5.
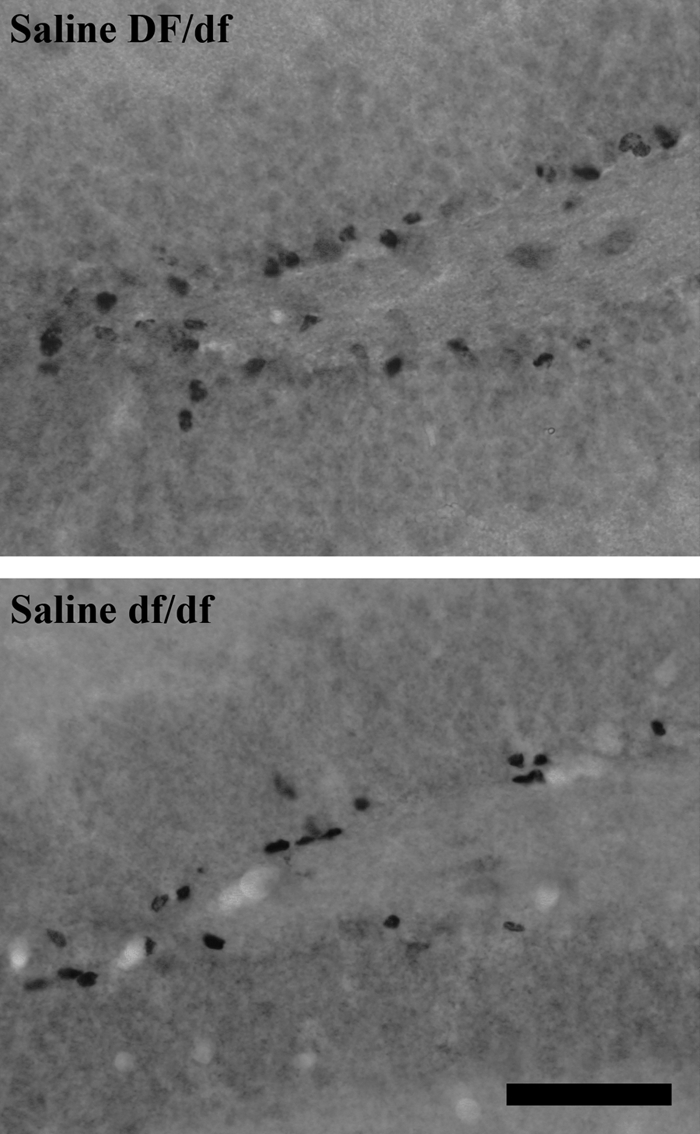
BrdU immunoreactivity in the DG of Ames dwarf (df/df, lower panels) and normal mice (DF/df, upper panels) treated with saline beginning at 3 d of age. Original objective magnification, ×40. The horizontal bar at the lower right represents 50 μm for all panels.
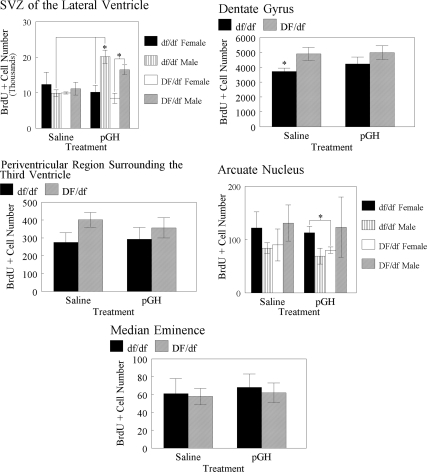
Cell numbers immunoreactive for BrdU in the SVZ of the LV, DG, the periventricular region surrounding the 3V, ARC, and ME of Ames dwarf and normal mice treated with saline or pGH are graphed in Fig. 6. Genders were pooled for analysis in the DG, ME, and periventricular region surrounding the 3V because sex did not affect BrdU incorporation in these three areas. In the DG, BrdU incorporation was affected by genotype in saline-treated animals [F(1,10) = 5.6; P = 0.0391]; normal mice had higher (P ≤ 0.05) BrdU-immunoreactive cell numbers (4897 ± 445, n = 6) than dwarf mice (3718 ± 223, n = 6). BrdU immunoreactivity in the ME and periventricular region surrounding the 3V was not affected by treatment or genotype.
Figure 6.
BrdU-immunoreactive cell numbers in the SVZ of the LV, DG, the periventricular region surrounding the 3V, and ME of Ames dwarf and normal mice. *, Differences noted are between genotypes unless indicated otherwise (P ≤ 0.05).
An effect of sex on BrdU incorporation in the SVZ of the LV was observed in pGH-treated animals [F(1,10) = 22.0; P = 0.0009], regardless of genotype; males exhibited greater (P ≤ 0.01) BrdU-immunoreactive cell numbers (18312 ± 1424, n = 4) than females (9313 ± 1150, n = 8). Treatment affected BrdU incorporation in the SVZ of the LV in male dwarf mice [F(1,3) = 30.8; P = 0.0115] but not in female dwarf mice or normal mice. In male dwarf mice, pGH treatment resulted in greater (P ≤ 0.05) SVZ BrdU-immunoreactive cell numbers (20139 ± 1875, n = 2) than saline treatment (9886 ± 936, n = 3). Otherwise, there was no effect of genotype or treatment on BrdU-immunoreactive cell numbers in the SVZ of the LV.
ARC BrdU incorporation was affected by sex in dwarf mice [F(1,10) = 5.4; P = 0.0419]; females had higher (P ≤ 0.05) BrdU-immunoreactive cell numbers (117 ± 13, n = 7) than males (78 ± 8, n = 5). However, sex affected ARC BrdU immunoreactivity in dwarf mice only when treatments were grouped. Thus, no sex differences were observed in pGH-treated dwarf mice or in saline-treated dwarf mice. An effect of genotype was observed in pGH-treated females [F (1,6) = 7.0; P = 0.0378]. In pGH-treated females, dwarf mice exhibited greater (P ≤ 0.05) ARC BrdU-immunoreactive cell numbers (113 ± 12, n = 4) than normal mice (80 ± 6, n = 4). Otherwise, there was no effect of treatment or genotype on ARC BrdU immunoreactivity.
Discussion
We have been investigating the role of pituitary hormones in the development of the PRL inhibitory TIDA neurons. This laboratory has previously shown a significant neurotrophic effect of PRL on this neuron population in that early postnatal PRL treatment induces maintenance of a normal-sized TIDA neuron number in Ames dwarf mice (12) and induces TIDA neuron differentiation in Snell dwarf mice (13). However, PRL and PRL receptor knockout mice show that PRL is not necessary for development of a normal-sized TIDA neuron population. Also, although PRL induces differentiation of TIDA neurons in GH-, PRL-, and TSH-deficient Snell dwarf mice, a normal-sized TIDA population is not induced. These findings indicate that other factors are important in TIDA neuron development. The current studies examined the developmental effect of GH on TIDA neurons in GH-, PRL-, and TSH-deficient Ames dwarf mice.
Examination of GH effect on TIDA neurons was carried out using pGH. Weight gain was recorded to establish effectiveness of pGH in mouse. pGH treatment induced weight gain in dwarf mice and normal male mice, confirming an expected GH effect. However, weight gain was not observed in normal female mice (Fig. 1). Lack of weight gain in normal female mice also was not accompanied by changes in TIDA neuron numbers. Absence of weight gain in normal female mice was an unexpected result because GH-induced body weight increases have been reported in other studies. For example, Ueki et al. (36) demonstrated GH-induced body weight gain, regardless of gender. However, this study used bovine GH (80.6% amino acid homology to mouse GH) rather than porcine GH (88.9% amino acid homology to mouse GH), a different mouse model and a different treatment paradigm. Ueki et al. administered 3 μg/g twice daily, whereas in the current study, mice received 50 μg once a day. It is possible that the long duration of GH treatment, as well as the high amount of pGH given, induced formation of antibodies to pGH, which may explain some lack of exogenous GH effect on body weight. This lack of weight gain may alternatively be due to pGH-negative feedback on endogenous GH secretion or to interaction with sex hormones.
The current investigation has shown that daily, early postnatal pGH induces increased TIDA neuron numbers in dwarf, but not normal, mice (Fig. 4). Also, qualitative assessment of endogenous hypothalamic DA fluorescence showed that pGH-treated dwarf mice exhibited increased TIDA DA content compared with saline-treated dwarf mice (Fig. 2). Although this increased TIDA neuron number and DA content was reduced compared with that in normal mice, these results indicate that GH induced increased numbers of functional TIDA neurons in the dwarf hypothalamus.
The observed increase in TIDA neurons was lower than the maximum TIDA neuron numbers seen in Ames dwarf development, which are comparable with those in normal animals. The maximum TIDA neuron population in Ames dwarf mice is established by 21 d of age, after which TIDA neuron numbers decrease to the adult deficit (37). Therefore, pGH did not induce TIDA neuron differentiation or even maintain the maximum population seen in Ames dwarf development. Furthermore, pGH did not mimic the effect of PRL on TIDA neuron development in Ames dwarf mice, which if administered daily, beginning at 12 d of age, maintains a normal-sized TIDA neuron number (12). Treatment with pGH in the present study showed a lesser effect on TIDA neuron development than PRL, although treatment was initiated at an earlier time point (3 d of age). However, pGH did induce an increase in TIDA neuron number and TIDA DA content in dwarf mice, which demonstrates a minimal effect of pGH on TIDA neurons. Thus, pGH affects TIDA neuron development, although it is less potent than PRL in this action.
GH is known to stimulate IGF-I production and secretion (38,39), so the effect of pGH on TIDA neurons, if not direct, could be mediated by IGF-I. IGF-I receptors have been localized to the ARC, so it is possible that IGF-I could act as a direct mediator of GH on TIDA neurons (40). However, due to constraints on this work by the destruction of Hurricane Katrina, IGF-I levels in these mice were not measured.
Although neurogenesis is not conventionally known to occur in the adult hypothalamus, reports have indicated that hypothalamic BrdU-immunoreactive neurons can be detected in response to factors, such as brain-derived neurotrophic factor (41) or ciliary neurotrophic factor (42). These new neurons may be derived locally or migrate from other regions. Evidence for local generation of new hypothalamic neurons includes basic fibroblast growth factor-induced neurogenesis in the ependymal layer of the 3V, contributing to new hypothalamic neurons (43) and detection of constitutive hypothalamic neurogenesis after intracerebroventricular delivery of BrdU (44).
Because Ames dwarf mice experience a normal-sized TIDA neuron number during development, the pGH-induced increase in TIDA neurons is not likely due to pGH-induced neurogenesis. In this investigation, pGH treatment was initiated at 3 d of age, suggesting that pGH induced preservation of TIDA neurons, rather than recruitment of new TIDA neurons through neurogenesis. The BrdU results from the ARC and periventricular region of the 3V support pGH-induced preservation of TIDA neurons. No differences in BrdU incorporation in these areas was observed between treatments (Fig. 6), indicating a lack of pGH-induced cell proliferation. The reduction in TIDA neuron number observed after 21 d of age in untreated Ames dwarf mice may be a result of cell death and/or phenotype change. Thus, pGH-induced preservation of TIDA neurons may be accomplished through inhibition of cell death and/or inhibition of phenotype change.
BrdU incorporation in brain regions in which neurogenesis is known to occur in adults, such as the SVZ of the LV and DG, was also assessed. There was a nonsignificant trend of increased cell proliferation in the SVZ of the LV due to treatment with pGH in males but not in females, which was significant in dwarf mice. However, sample sizes were small when the BrdU data were divided by gender. Regardless, this trend is in agreement with previous findings demonstrating a neurogenic effect of GH in adult central nervous system (45,46). In the DG, saline-treated dwarf mice had reduced cell proliferation compared with saline-treated normal mice. This result is inconsistent with the findings of Sun et al. (47), who showed increased DG neurogenesis in 3-month-old Ames dwarf mice. These inconsistencies may be due to differences in BrdU treatment (daily for 7 d vs. four times over 3 wk) and age of mice (3 vs. 1.5 months).
PRL has been shown to have a significant neurotrophic effect on TIDA neuron development, although this PRL effect is not necessary for the development of a normal-sized TIDA cell population, as evidenced by studies using PRL- or PRL receptor-deficient mice (17,18,19). The current study examined the possible role of GH in the development of TIDA neurons using a PRL-, GH-, and TSH-deficient mouse model. We observed a role, although minimal, of GH in TIDA cell development. However, the GH effects do not result in the TIDA neuron population observed in PRL or PRL receptor knockout mice, suggesting that TSH and GH act synergistically in the absence of PRL.
The effect of GH on TIDA neuron development, observed in this study, appears to occur only in the absence of PRL. Data from transgenic mice that express human GH or bovine GH (48) and models of isolated GH deficiency, such as the dw dwarf rat (49) and the little mouse (Phelps, C. J., unpublished data) support this concept. Mice transgenic for human GH serve as models of excess PRL as well as GH because primate GH binds the PRL receptor; these mice have increased TIDA neuron numbers. No change in TIDA neuron number occurs in mice expressing bovine GH, which does not bind to the PRL receptor (48). Therefore, PRL, but not GH, stimulates TIDA neuron differentiation in the absence of PRL deficiency. The dw dwarf rat exhibits elevated TIDA neuron numbers (49), and preliminary data from little mice show no reduction in TIDA neurons (Phelps, C. J., unpublished data).
It would be interesting to examine the effect of GH treatment in the GH-, PRL-, and TSH-deficient Snell dwarf mice, which exhibit a more severe TIDA deficit than Ames dwarf mice. However, our colony of Snell dwarf mice was destroyed by Hurricane Katrina, and we were not able to fund replacement for these studies. Because Snell dwarf mice exhibit a more severe TIDA neuron deficit than Ames dwarf mice, it is likely that GH treatment would have a lesser effect on TIDA neuron development in Snell dwarf mice compared with Ames dwarf mice.
The present study has demonstrated the ability of pGH to support TIDA neuron development, which was an unproven premise from previous studies of TIDA neurons in transgenic PRL and PRL receptor knockout mice, thus providing a clear avenue for further investigation of the cross talk of these evolutionary related hormones on different neuronal populations. We have shown that treatment with GH did not induce TIDA neuron differentiation but preserved a portion of TIDA neurons that would normally be absent in dwarf hypothalamus. Thus, it is likely that some aspect of GH signaling inhibited cell death or phenotype change in this portion of neurons, rather than induced recruitment of new TIDA neurons through neurogenesis. The presence of GH receptors or the connections of these TIDA neurons to other neurons with GH receptors remains to be fully elucidated to determine the mechanism by which pGH affects the developmental population. Learning more of such connections or interconnections will aid in understanding the nature of developmental processes in this complex population of ARC neurons.
Acknowledgments
This manuscript is lovingly dedicated to the memory of Dr. Carol J. Phelps, Professor of Structural and Cellular Biology at Tulane University Medical School and wife of David Hurley. Dr. Phelps started the dwarf mouse project more than 20 yr ago and contributed zeal and drive until the day of her death. We all greatly miss her intellect and humor. We acknowledge the San Antonio Nathan Shock Animal Core for their generosity and expert care of the mice as well as Dr. James Roberts (University of Texas Health Science Center at San Antonio) for his generosity and help in allowing these projects to progress during the aftermath of Hurricane Katrina. Porcine GH was provided by the National Hormone and Pituitary Program (Dr. A. F. Parlow, Torrance, CA).
Footnotes
This work was supported by Louisiana BORSF Board of Regents Support Fund grant (to C.E.K.), National Science Foundation Grant IBN-0350537 (to D.L.H.), and Public Health Service Grant NS25987 (to C.J.P.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 12, 2010
Abbreviations: ARC, Arcuate nucleus; BrdU, bromodeoxyuridine; CA, catecholamine; DA, dopamine; DG, dentate gyrus; faglu, paraformaldehyde-glutaraldehyde; ICC, immunocytochemistry; LV, lateral ventricle; ME, median eminence; p, porcine; PRL, prolactin; SVZ, subventricular zone; TH, tyrosine hydroxylase; TIDA, tuberoinfundibular dopaminergic; 3V, third ventricle.
References
- Ben-Jonathan N, Hnasko R 2001 Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22:724–763 [DOI] [PubMed] [Google Scholar]
- Schaible R, Gowen JW 1961 A new dwarf mouse. Genetics 46:896 [Google Scholar]
- Snell GD 1929 Dwarf, a new mendelian recessive character of the house mouse. Proc Natl Acad Sci USA 15:733–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carrière C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG 1996 Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384:327–333 [DOI] [PubMed] [Google Scholar]
- Li S, Crenshaw 3rd EB, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG 1990 Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533 [DOI] [PubMed] [Google Scholar]
- Phelps CJ 1987 Isolated deficiency of tyrosine hydroxylase immunoreactivity in tuberoinfundibular neurons in pituitary prolactin-deficient Snell dwarf mice. Brain Res 416:354–358 [DOI] [PubMed] [Google Scholar]
- Phelps CJ, Carlson SW, Vaccarella MY 1994 Hypothalamic dopaminergic neurons in prolactin-deficient Ames dwarf mice: localization and quantification of deficit by tyrosine hydroxylase immunocytochemistry. J Neuroendocrinol 6:145–152 [DOI] [PubMed] [Google Scholar]
- Phelps CJ 2004 Postnatal regression of hypothalamic dopaminergic neurons in prolactin-deficient Snell dwarf mice. Endocrinology 145:5656–5664 [DOI] [PubMed] [Google Scholar]
- Morgan WW, Bartke A, Pfeil K 1981 Deficiency of dopamine in the median eminence of Snell dwarf mice. Endocrinology 109:2069–2075 [DOI] [PubMed] [Google Scholar]
- Phelps CJ, Sladek Jr JR, Morgan WW, Bartke A 1985 Hypothalamic catecholamine histofluorescence in dwarf mice. Cell Tissue Res 240:19–25 [DOI] [PubMed] [Google Scholar]
- Phelps CJ, Carlson SW, Vaccarella MY, Felten SY 1993 Developmental assessment of hypothalamic tuberoinfundibular dopamine in prolactin-deficient dwarf mice. Endocrinology 132:2715–2722 [DOI] [PubMed] [Google Scholar]
- Romero MI, Phelps CJ 1993 Prolactin replacement during development prevents the dopaminergic deficit in hypothalamic arcuate nucleus in prolactin-deficient Ames dwarf mice. Endocrinology 133:1860–1870 [DOI] [PubMed] [Google Scholar]
- Khodr CE, Hurley DL, Phelps CJ 2009 Prolactin induces tuberoinfundibular dopaminergic neurone differentiation in Snell dwarf mice if administered beginning at 3 days of age. J Neuroendocrinol 21:558–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodr CE, Clark SM, Hurley DL, Phelps CJ 2008 Long-term, homologous prolactin, administered through ectopic pituitary grafts, induces hypothalamic dopamine neuron differentiation in adult Snell dwarf mice. Endocrinology 149:2010–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K 1997 Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16:6926–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA 1997 Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 11:167–178 [DOI] [PubMed] [Google Scholar]
- Phelps CJ, Horseman ND 2000 Prolactin gene disruption does not compromise differentiation of tuberoinfundibular dopaminergic neurons. Neuroendocrinology 72:2–10 [DOI] [PubMed] [Google Scholar]
- Phelps CJ, Estrada IJ, Evdemon-Hogan M, Binart N, Kelly PA, Hypothalamic dopaminergic neurons in prolactin receptor-null mice. Program of the 84th Annual Meeting of The Endocrine Society, San Francisco, CA, 2002 (Abstract P1-163) [Google Scholar]
- Steger RW, Chandrashekar V, Zhao W, Bartke A, Horseman ND 1998 Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology 139:3691–3695 [DOI] [PubMed] [Google Scholar]
- Goffin V, Binart N, Touraine P, Kelly PA 2002 Prolactin: the new biology of an old hormone. Ann Rev Physiol 64:47–67 [DOI] [PubMed] [Google Scholar]
- Müller EE, Locatelli V, Cocchi D 1999 Neuroendocrine control of growth hormone secretion. Physiol Rev 79:511–607 [DOI] [PubMed] [Google Scholar]
- Cronin MJ, Hewlett EL, Evans WS, Thorner MO, Rogol AD 1984 Human pancreatic tumor growth hormone (GH)-releasing factor and cyclic adenosine 3′-5′-monophosphate evoke GH release from anterior pituitary cells: the effects of pertussis toxin, cholera-toxin, forskolin, and cyclohexamide. Endocrinology 114:904–913 [DOI] [PubMed] [Google Scholar]
- Marcovitz S, Goodyer CG, Guyda H, Gardiner RJ, Hardy J 1982 Comparative study of human fetal, normal adult and somatotropic adenoma pituitary function in tissue culture. J Clin Endocrinol Metab 54:6–16 [DOI] [PubMed] [Google Scholar]
- Kitajima N, Chihara K, Abe H, Okimura Y, Fujii Y, Sato M, Shakutsui S, Watanabe M, Fujita T 1989 Effect of dopamine on immunoreactive growth hormone-releasing hormone and somatostatin secretion from rat hypothalamic slices perfused in vitro. Endocrinology 124:69–76 [DOI] [PubMed] [Google Scholar]
- Vance ML, Kaiser DL, Frohman LA, Rivier J, Vale W, Thorner MO 1987 Role of dopamine in the regulation of growth hormone secretion: dopamine and bromocriptine augment growth hormone (GH) releasing hormone stimulated GH secretion in normal men. J Clin Endocrinol Metab 61:1136–1141 [DOI] [PubMed] [Google Scholar]
- Bazán MC, Barontini M, Domené H, Stefano FJ, Bergadá C 1981 Effect of α-bromocriptine on pituitary hormone secretion. J Clin Endocrinol Metab 52:314–318 [DOI] [PubMed] [Google Scholar]
- Bossé R, Fumagalli F, Jaber M, Giros B, Gainetdinov RR, Wetsel WC, Missale C, Caron MG 1997 Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron 19:127–138 [DOI] [PubMed] [Google Scholar]
- Díaz-Torga G, Feierstein C, Libertun C, Gelman D, Kelly MA, Low MJ, Rubinstein M, Becú-Villalobos D 2002 Disruption of the D2 dopamine receptor alters GH and IGF-I secretion and causes dwarfism in male mice. Endocrinology 143:1270–1279 [DOI] [PubMed] [Google Scholar]
- Meister B, Hökfelt T, Vale WW, Sawchenko PE, Swanson L, Goldstein M 1986 Coexistence of tyrosine hydroxylase and growth hormone-releasing factor in a subpopulation of tubero-infundibular neurons of the rat. Neuroendocrinology 42:237–247 [DOI] [PubMed] [Google Scholar]
- Phelps CJ 1994 Pituitary hormones as neurotrophic signals: anomalous hypophysiotrophic neuron differentiation in hypopituitary dwarf mice. Proc Soc Exp Biol Med 206:6–23 [DOI] [PubMed] [Google Scholar]
- Phelps CJ, Hurley DL 1999 Pituitary hormones as neurotrophic signals: update on hypothalamic differentiation in genetic models of altered feedback. Proc Soc Exp Biol Med 222:39–58 [DOI] [PubMed] [Google Scholar]
- Furness JB, Heath JW, Costa M 1978 Aqueous aldehyde (Faglu) methods for the fluorescence histochemical localization of catecholamines for ultrastructural studies of central nervous tissue. Histochemistry 57:285–295 [DOI] [PubMed] [Google Scholar]
- Watson RE, Wiegand SJ, Clough RW, Hoffman GE 1986 Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- Björklund A, Nobin A 1973 Fluorescence histochemical and microspectrofluorometric mapping of dopamine and noradrenaline cell groups in the rat diencephalon. Brain Res 51:193–205 [DOI] [PubMed] [Google Scholar]
- Selemon LD, Sladek Jr JR 1981 Aging of tuberoinfundibular (A-12) dopamine neurons in the C57B1/6N male mouse. Brain Res Bull 7:585–594 [DOI] [PubMed] [Google Scholar]
- Ueki I, Giesy SL, Harvatine KJ, Kim JW, Boisclair YR 2009 The acid-labile subunit is required for full effects of exogenous growth hormone on growth and carbohydrate metabolism. Endocrinology 150:3145–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps CJ, Vaccarella MY, Romero MI, Hurley DL 1994 Postnatal reduction in number of hypothalamic tuberoinfundibular dopaminergic neurons in prolactin-deficient dwarf mice. Neuroendocrinology 59:189–196 [DOI] [PubMed] [Google Scholar]
- Holder AT, Wallis M 1977 Actions of growth hormone, prolactin and thyroxine on serum somatomedin-like activity and growth in hypopituitary dwarf mice. J Endocrinol 74:223–229 [DOI] [PubMed] [Google Scholar]
- Strain AJ, Ingleton PM 1990 Growth hormone- and prolactin-induced release of insulin-like growth factor by isolated rat hepatocytes. Biochem Soc Trans 18:1206 [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Rodriguez JR, Torres-Aleman I 1997 Localization of the insulin-like growth factor I receptor in the cerebellum and hypothalamus of adult rats: an electron microscopic study. J Neurocytol 26:479–490 [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB 2001 Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci 21:6706–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS 2005 Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310:679–683 [DOI] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C 2005 Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 192:251–264 [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS 2007 Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol 505:209–220 [DOI] [PubMed] [Google Scholar]
- Aberg ND, Brywe KG, Isgaard J 2006 Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Scientific World J 6:53–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg ND, Johansson I, Aberg MA, Lind J, Johansson UE, Cooper-Kuhn CM, Kuhn HG, Isgaard J 2009 Peripheral administration of GH induces cell proliferation in the brain of adult hypophysectomized rats. J Endocrinol 201:141–150 [DOI] [PubMed] [Google Scholar]
- Sun LY, Evans MS, Hsieh J, Panici J, Bartke A 2005 Increased neurogenesis in dentate gyrus of long-lived Ames dwarf mice. Endocrinology 146:1138–1144 [DOI] [PubMed] [Google Scholar]
- Phelps CJ, Bartke A 1997 Stimulatory effect of human, but not bovine, growth hormone expression on numbers of tuberoinfundibular dopaminergic neurons in transgenic mice. Endocrinology 138:2849–2855 [DOI] [PubMed] [Google Scholar]
- Thomas GB, Phelps CJ, Robinson ICAF 1999 Differential regulation of hypothalamic tuberoinfundibular dopamine neurones in two dwarf rat models with contrasting changes in pituitary prolactin. J Neuroendocrinol 11:229–236 [DOI] [PubMed] [Google Scholar]