Abstract
The nuclear hormone receptor, REV-ERB, plays an essential role in adipogenesis. Rev-erbα expression is induced in 3T3-L1 cells during adipogenesis, and overexpression of this receptor leads to expression of adipogenic genes. We recently demonstrated that the porphyrin heme functions as a ligand for REV-ERB, and binding of heme is required for the receptor’s activity. We therefore hypothesized that REV-ERB ligands may play a role in regulation of adipogenesis. We detected an increase intracellular heme levels during 3T3-L1 adipogenesis that correlated with induction of aminolevulinic acid synthase 1 (Alas1) expression, the rate-limiting enzyme in heme biosynthesis. If the increase in Alas1 expression was blocked, adipogenesis was severely attenuated, indicating that induction of expression of Alas1 and the increase in heme synthesis is critical for differentiation. Inhibition of heme synthesis during adipogenesis leads to decreased recruitment of nuclear receptor corepressor to the promoter of a REV-ERB target gene, suggesting alteration of REV-ERB activity. Treatment of 3T3-L1 cells with a synthetic REV-ERB ligand, SR6452, resulted in induction of adipocyte differentiation to a similar extent as treatment with the peroxisomal proliferator-activated receptor-γ agonist, rosiglitazone. Combination of SR6452 and rosiglitazone had an additive effect on stimulation of adipocyte differentiation. These results suggest that heme, functioning as a REV-ERB ligand, is an important signaling molecule for induction of adipogenesis. Moreover, synthetic small molecule ligands for REV-ERB are effective modulators of adipogenesis and may be useful for treatment of metabolic diseases.
Ligands of the nuclear receptor, REV-ERB, play an essential role in regulation of adipogenesis.
Nuclear hormone receptors (NHRs) are transcription factors, and the majority of these transcription factors have been shown to have their activity modulated by small hydrophobic ligands. A significant number of receptors within this superfamily remain orphans with no characterized ligand. Alternatively, some NHR family members may function independent of ligands displaying behavior similar to classical transcription factors. Recently we and others identified the porphyrin, heme, as a ligand for REV-ERBα (1,2), an unusual member of the NHR superfamily that had originally been defined as a constitutive repressor of transcription (3,4,5,6). REV-ERBα requires heme to be bound for the receptor to recruit the nuclear receptor corepressor (NCoR) and to repress transcription (1,2). The affinity of REV-ERBα for heme is approximately 2–3 μm (1), and modulation of intracellular heme levels alters NCoR binding and transrepression activity of REV-ERBα (1,2). The requirement of the cell to maintain intracellular heme levels in this general concentration range explains why REV-ERBα had been characterized as a constitutive repressor of transcription. This leads to the question as to the role of REV-ERBα either as a true receptor for heme, sensing changes in intracellular heme levels and altering its activity appropriately, or alternatively as a protein that is constantly saturated with heme and exhibiting constitutive repressor activity.
REV-ERBα has been shown to play an important role in regulation of the circadian rhythm, metabolism, and adipogenesis. Heme has also been shown to play a role in circadian function (7,8), and previously we suggested that oscillations in intracellular heme levels might be associated with modulation of REV-ERBα’s role in regulation of the mammalian clock (9). Although it has been demonstrated that pharmacological or genetic manipulation of intracellular heme levels leads to alteration in expression of REV-ERBα target genes such as Bmal1 and G6pase (1,2), no clear physiological role has been identified in which alterations in intracellular heme levels modulate REV-ERBα activity.
One model in which the physiological role of REV-ERBα has been closely examined is differentiation of 3T3-L1 cells to adipocytes. Rev-erbα expression is induced during adipogenesis (10), and overexpression of REV-ERBα in 3T3-L1 preadipocytes results in increased expression of adipogenic markers including CCAAT/enhancer-binding protein-α, peroxisomal proliferator-activated receptor (PPAR)-γ, and adipocyte protein 2 (aP2) along with an increase in lipid accumulation (11). A more recent study indicated that the role of Rev-erbα in adipogenesis is more complex. Wang and Lazar (12) showed that Rev-erbα mRNA expression does not exactly mirror that of the protein expression. Rev-erbα mRNA increases during adipogenesis and during the initial stages of differentiation REV-ERBα protein also increases, but this is followed by a precipitous decrease in REV-ERBα protein levels (12). The initial increase in REV-ERBα expression is required for the early mitogenic event during 3T3-L1 adipogenesis, whereas at later stages proteasomal degradation of REV-ERBα is required for continued differentiation (12).
Here we demonstrate that intracellular heme levels significantly increase during adipogenesis in 3T3-L1 cells and that the increase is required for differentiation. The increase in heme levels is associated with altered REV-ERBα recruitment of the corepressor, NCoR. Consistent with the observation that heme induces differentiation via activation of REV-ERB, we found that a synthetic nonporphyrin REV-ERB ligand, 1,1-dimethylethyl N-[(4-chlorophenyl)methyl]N-[(5-nitro-2-thienyl)methyl]glycinate (SR6452), induced adipogenesis in 3T3-L1 cells. These data suggest that heme functions as a signaling molecule altering REV-ERBα activity important for adipogenesis and that synthetic REV-ERB ligands modulate adipogenesis and may be useful in the treatment of metabolic diseases.
Materials and Methods
RNA isolation and quantitative RT-PCR
RNA was isolated using the RNeasy kit (QIAGEN, Valencia, CA) and quantified. Gene expression was determined using an ABI PRISM 7700 sequence detector (Applied Biosystems, Foster City, CA) and a SYBR Green detection system (Bio-Rad, Hercules, CA). A standard curve was generated using cDNA pooled from the experimental samples. Relative expression levels were determined by normalization to cyclophilin B or glyceraldehyde-3-phosphate dehydrogenase and expressed as arbitrary units.
Cotransfection
Cotransfection assays were performed as previously described (13).
3T3-L1 adipocyte differentiation
Murine 3T3-L1 preadipocytes were cultured in DMEM high glucose containing 10% calf serum and antibiotics (100 U/ml penicillin G and 100 μg/ml streptomycin). To obtain fully differentiated adipocytes, the 3T3-L1 preadipocytes were plated and grown to 2 d after confluence and induced to differentiate using a standard induction cocktail of 0.5 mm 3-isobutyl-1-methylxanthine, 1 μm dexamethasone, 1.7 μm insulin (MDI). After 48 h this medium was replaced with DMEM high glucose supplemented with 10% fetal bovine serum, and cells were maintained in this medium. In each case, the medium was changed every 48 h and the cells were maintained in a humidified chamber at 37 C and 5% CO2. Experiments were performed at least three times.
Lipid measurement
Adipocyte differentiation was assessed by Oil Red O staining. Cells were fixed for 1 h with 10% formalin and stained for 2 h with Oil Red O in 60% isopropanol. After washes with double-distilled H2O, Oil Red O was extracted from cells with 100% isopropanol. Lipid content was measured by absorbance at 510 nm. MG-132 was used as previously described (12).
Measurement of cellular heme content
Heme levels were measured using a modified Quantichrom heme assay (BioAssay Systems, Hayward, CA) as previously described (1,9). Briefly, 3T3-L1 cells were lysed in 1× cell lysis buffer (Invitrogen, Carlsbad, CA) and subjected to a freeze/thaw at −80/37 C. After centrifugation to remove insoluble debris, lysates were incubated at room temperature for 5 min in a chemical reaction, which converts heme to a uniform colored form. Absorbance at 400 nm is directly proportional to the heme concentration of the sample. The amount of heme in each sample was calculated using a calibrator solution of known heme concentration and expressed as micromole heme per milligram of total protein.
Inhibition of heme biosynthesis
Aminotriazole (ATZ; Sigma, St. Louis, MO) at a concentration of 5 mm was added to confluent 3T3-L1 cultures at the onset of differentiation. Heme (Sigma) was added at 60 μm with or without ATZ as previously described (14). Both ATZ and heme were replaced with each feeding every 2 d for the duration of differentiation. Protein expression, lipid content, and heme measurement was performed 4 or 5 d after initiation of differentiation. Aminolevulinic acid synthase 1 (Alas1) short hairpin RNA (shRNA) and scrambled control shRNA adenovirus was kindly provided by Dr. Bruce Spiegelman (Dana Farber Cancer Institute, Boston, MA), and Alas1 expression was suppressed by infection of cells with this virus as previously described (15).
Chromatin immunoprecipitation (ChIP)
HepG2 cells were treated with SR6452 (10 μm) for 24 h followed by assessment of NCoR occupancy of the BMAL1 promoter by ChIP. 3T3-L1 cells were treated under differentiation conditions (MDI) with or without supplementation (ATZ or ATZ + heme as described above) followed by ChIP assessment of NCoR occupancy of the Pai-1 promoter by ChIP. ChIP assays were performed according to the manufacturer’s instructions (Millipore, Bedford, MA). Immunoprecipitation with the following antibodies was performed at 4 C overnight: antimouse IgG, antiacetyl histone H3 anti-NCoR (Santa Cruz Biotechnology, Santa Cruz, CA). The BMAL1 primers used were AGCGGATTGGTCGGAAAGTAG (forward) and GGCCTGACACTCACCGTG (reverse). The Pai-1 primers used were GGGTGTGGTGCTGTGTACC (forward) and TTCTGTCCTTAGACCTGTGG (reverse).
Synthesis of SR6452
The REV-ERB agonist SR6452 (16) was synthesized using standard organic synthesis techniques.
REV-ERB-NCoR peptide interaction assay
A 33-residue coregulator peptide designated NCoR1 (Biotin-KGGVPRTHRLITLADHICQIITQDFARNQVSSQ) was synthesized by Anaspec, Inc. (San Jose, CA). The amino acid peptide sequence design was based on the known amphipathic helical core (CoRNR box) motif-1 from the corepressor protein, corepressor/nuclear receptor (NCoR). Low-capacity StreptAvidin beads were purchased from Radix Biosolutions (Georgetown, TX). To couple peptides to beads, 50 μg/ml working concentrations of peptides were prepared in distilled H2O and used to couple to streptavidin beads overnight at 4 C. All bead/peptide conjugates were washed twice with PBS/BSA buffer [10 mm NaH2PO4; 150 mm NaCl; 0.1% (wt/vol) BSA; 2 mm dithiothreitol, pH 7.4] and resuspended in 600 μl of PBS/BSA buffer. All bead/peptide conjugates were mixed into a single homogenous bead mix before addition to the nuclear receptor protein/PentaHis-Alexa532 complex. PentaHis Alexa532 antibody (QIAGEN, Valencia, CA) was diluted to a final concentration of 0.8 μg/ml in 1× Luminex buffer (25 mm HEPES; 100 mm NaCl; 0.1% BSA; 2 mm dithiothreitol, pH 7.4). For each 96-well Luminex reaction, 10 μl of PentaHis-Alexa532 antibody was added to 10 μl of 25× His-tagged-REV-ERB ligand-binding domain proteins being studied. REV-ERB/antibody complexes were allowed to form for a minimum of 30 min before addition of 220 μl peptide bead mix. Ten microliters of 25× SR6452 at each respective concentration were added to the appropriate wells. REV-ERB/NCOR1 peptide interactions were allowed to proceed for approximately 3 h with shaking at room temperature. REV-ERB/NCOR1 interaction data were obtained by xMAP technology using the Bio-Plex 200 system and suspension array platform (Bio-Rad Laboratories). Dose-dependent effects of SR6452 treatment on REV-ERB /NCoR1 interaction data were plotted using GraphPad Prism (GraphPad Inc., La Jolla, CA).
Western blots
Western blots were performed using standard procedures using the following antibodies to PPARγ (Santa Cruz), REV-ERBα (Cell Signaling, Beverly, MA), STAT3 (Santa Cruz), tubulin (Cell Signaling), and PPAR-γ coactivator 1 (PGC-1)-α (Abcam, Cambridge, MA).
Results
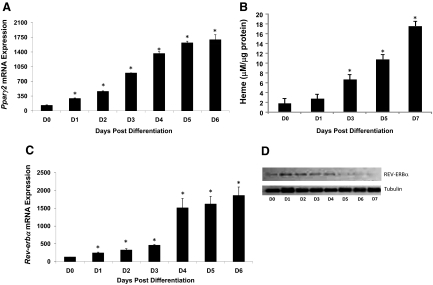
Previous studies in 3T3-L1 cells indicated that heme treatment led to enhanced adipocyte differentiation and inhibition of heme synthesis blocked differentiation (14). Based on our recent discovery that heme is a ligand for the nuclear receptor REV-ERBα (and -β) (1,2,3) as well as the well-characterized role for these receptors in adipogenesis, we sought to determine whether heme may be functioning as a physiological ligand for REV-ERBα during the adipogenic process. Adipocyte differentiation was induced in 3T3-L1 cells by treatment with MDI media (as indicated in Materials and Methods), and induction of Pparγ2 mRNA was measured as a marker of differentiation over approximately 1 wk. As shown in Fig. 1A, Pparγ mRNA level increased on d 1 of treatment to 2.3-fold that of uninduced cells (d 0), and mRNA levels continued to rise with the highest induction noted on d 6 (13.2-fold vs. d 0). PPARγ protein expression was also elevated and remained elevated during adipogenesis (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Because no previous studies examined intracellular heme levels during adipogenesis, we ran a parallel experiment in which we treated 3T3-L1 cells identically but retained the cells for determination of intracellular heme concentration ([heme]i). A significant increase in [heme]i was noted on d 3 after induction, and [heme]i remained significantly elevated through d 7 (the last day tested) (Fig. 1B). We also measured Rev-erbα mRNA levels during differentiation and noted a small but significant increase in expression beginning on d 1 (1.9-fold vs. d 0) that continued to rise, reaching a maximum induction of 14.4-fold (Fig. 1C). In parallel, REV-ERBα protein expression was shown by Western blot, and we observed a similar pattern to that recently described by Wang and Lazar (12) in which protein levels initially mirrored the mRNA expression, but later in the differentiation process REV-ERBα protein levels decreased, whereas mRNA levels remained elevated. We noted an increase in protein expression on d 1 and 2 followed by a precipitous decrease in expression beginning on d 3 (Fig. 1D), which was previously shown to be mediated by proteosomal degradation (12). We confirmed that the degradation was mediated by the proteosome because treatment of the cells with MG-132 prevented the degradation of REV-ERBα protein in the late stages of adipogenesis (Supplemental Fig. 2).
Figure 1.
Examination of intracellular heme levels in 3T3-L1 cells during adipogenesis. A, Pparγ2 mRNA levels measured by quantitative PCR during adipogenesis. B, Intracellular heme levels measured at various points after initiation of adipocyte differentiation. C, Rev-erbα mRNA levels measured by real-time PCR during adipogenesis. D, REV-ERBα protein measured by Western blot during adipogenesis. Tubulin loading controls are indicated. The numbers below each well indicate the intensity vs. undifferentiated cells (d 0). *, P < 0.05 vs. undifferentiated cells.
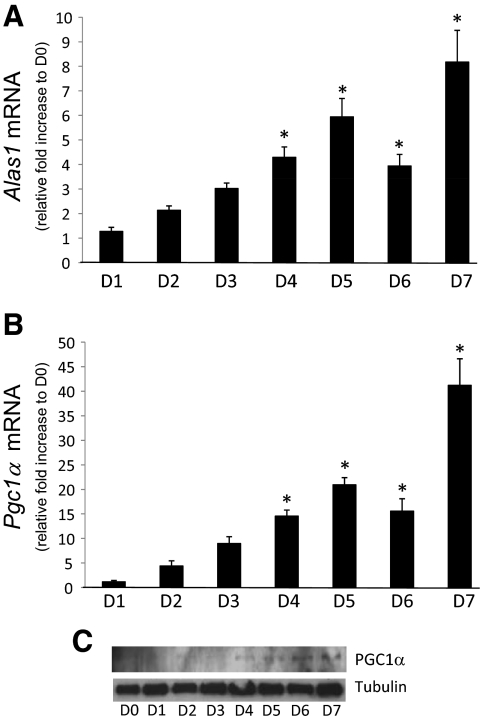
Given the increase in [heme]i beginning on d 3 after induction of differentiation shown in Fig. 1B, we speculated that this increase was a result of either increased synthesis or decreased degradation of heme. We monitored the expression of the rate-limiting enzyme in heme biosynthesis (Alas1) as well as the key enzyme involved in regulated heme degradation [heme oxygenase 1 (Hmox1)] throughout the differentiation process. No significant alterations in Hmox1 mRNA expression were observed (data not shown). Interestingly, Alas1 expression was found to be induced in the same time frame as we observed elevated [heme]i. Figure 2A shows that Alas1 mRNA levels begin to increase on d 2 (∼2-fold) and reach levels as much as 8-fold greater than undifferentiated cells on d 7. Alas1 mRNA remained elevated for the remainder of the differentiation process, which is consistent with the elevated [heme]i. Expression of the coactivator PGC-1α is induced during adipogenesis in 3T3-L1 cells (17) and has been demonstrated to be a key regulator of Alas1 expression (18). We examined the expression of Pgc1α mRNA during adipogenesis to determine whether PGC-1α might be leading to the increase in Alas1 expression and thus the increased [heme]i. We observed an increase in Pgc1α mRNA expression beginning on d 2 that followed the pattern of Alas1 expression through the differentiation process, suggesting that PGC-1α may be involved in the increase in Alas1 expression and thus in the increase in [heme]i (Fig. 2B). An increase in PGC-1α protein expression was also observed during adipogenesis (Fig. 2C).
Figure 2.
Expression of the rate-limiting enzyme for heme synthesis, Alas1, increases during adipogenesis. A, Alas1 mRNA levels measured by quantitative PCR during adipogenesis. B, Pgc1α mRNA levels measured by quantitative PCR during adipogenesis. C, Western blot illustrating PGC-1α protein levels during adipogenesis. For Alas1 and Pgc1α, expression levels were normalized to undifferentiated controls. *, P < 0.05.
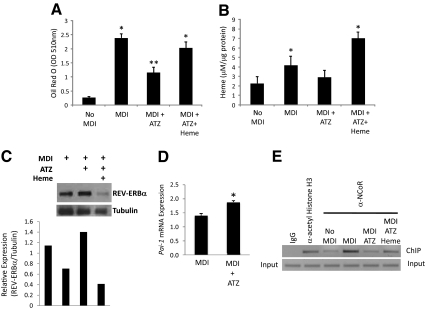
Our data illustrated in Fig. 2A suggests that the elevation of [heme]i is due to increased synthesis and not decreased degradation of this porphyrin. To examine the requirement of heme for adipogenesis and the association of REV-ERBα in this process, we induced adipogenesis in 3T3-L1 cells with MDI in the presence and absence of an inhibitor of heme biosynthesis, ATZ. ATZ is an inhibitor of aminolevulinic acid dehydratase (the enzymatic step after ALAS1) and has been shown to significantly decrease heme synthesis in a variety of cells and tissues (19,20,21,22). Additionally, another control group was added in which the cells were treated with ATZ, but additional heme was added to mimic a replete state. We allowed the cells to differentiate to an intermediate state in which [heme]i levels were significantly elevated, but differentiation had not progressed to the point at which detection of REV-ERBα was limiting. Lipid content (Oil Red O staining) and [heme]i were measured under these conditions.
Figure 3A demonstrates that MDI treatment resulted in significant accumulation of lipid as indicated by the increased Oil Red O staining consistent with differentiation. This was associated with a significant increase in [heme]i (Fig. 3B). Reduction of heme biosynthesis with ATZ caused inhibition of the MDI-dependent increase [heme]i (Fig. 3A). Addition of heme to the cells treated with ATZ resulted in recovery of the lipid accumulation to that observed in the absence of inhibitor (Fig. 3A), which is consistent with the increased [heme]i observed in these cells (Fig. 3B). These data suggest that heme biosynthesis is required for the 3T3-L1 cells to realize their full adipogenic potential. These results are consistent with studies of Chen and London (14) performed in 3T3-F442 cells; however, our studies offer insight into the function of heme during adipogenesis. Degradation of REV-ERBα protein during the late stages of adipogenesis is mediated by the proteasome (12) (Supplemental Fig. 2). Because many NHRs are degraded by the proteasome in response to ligand binding (23,24,25,26) and heme levels increase concurrently with the increase in REV-ERBα degradation (Fig. 1, B and D), we hypothesized that the degradation may be heme dependent. To examine this, we assessed REV-ERBα protein levels at d 3 after induction of differentiation by MDI treatment to determine the effect of heme on proteasomal degradation of the receptor. As shown in Fig. 3C, inhibition of heme synthesis with ATZ treatment resulted in increased levels of REV-ERBα protein, and treatment of cells with ATZ plus heme was associated with a significant decrease in REV-ERBα protein expression. Thus, degradation of REV-ERBα appears to be heme dependent.
Figure 3.
Heme functions as a REV-ERBα ligand during adipogenesis. A, Lipid accumulation in 3T3-L1 cells as measured by the Oil Red O method after MDI treatment, MDI + ATZ and MDI + ATZ/heme as described in Materials and Methods. B, Intracellular heme levels after MDI treatment, MDI + ATZ and MDI + ATZ/heme. C, Western blot illustrating relative REV-ERBα protein expression under MDI, MDI + ATZ and MDI + ATZ + heme conditions. Normalized (relative to tubulin) is indicated below the Western blot. D, ATZ treatment results in increased Pai-1 expression in 3T3-L1 cells during differentiation. Day 3 of treatment was examined. E, ChIP analysis of NCoR occupancy of the REV-ERBα target gene, Pai-1, promoter. IgG and antiacetyl histone H3 were used as negative and positive controls, respectively. MDI, MDI + ATZ, and MDI + ATZ + heme treatments are described in Materials and Methods, and d 3 of differentiation was examined. *, P < 0.05 vs. control cells at the identical day of treatment; **, P < 0.05 vs. the indicated group.
The function of REV-ERBα was assessed in this experiment by examining alterations in corepressor NCoR recruitment to a REV-ERBα target gene promoter by ChIP. Heme stabilizes the interaction between REV-ERBα and NCoR (1,2); thus, by examining the relative level of NCoR occupancy of a REV-ERBα target gene promoter, we can determine whether the alterations in [heme]i levels are sufficient to alter REV-ERBα activity. We examined the plasminogen activator inhibitor type I gene (Pai-1), which is a well-characterized REV-ERBα target gene (27,28). As would be expected for a REV-ERBα target gene, reduction of heme levels using ATZ resulted in increased expression of Pai-1 (Fig. 3D). ChIP analysis of NCoR occupancy of the Pai-1 promoter is shown in Fig. 3E. MDI treatment results in an increase in [heme]i (Figs. 1B and 3A), and as shown in Fig. 3E, this is associated with increased NCoR recruitment. This is consistent with previous observations that heme is required for REV-ERBα recruitment of NCoR (1,2). Blocking the increase in [heme]i with ATZ results in a decrease in NCoR occupancy of the promoter, whereas addition of heme to the ATZ-treated cells resulted in recovery an increase in NCoR recruitment to the promoter (Fig. 3E). These data indicate that the increases in [heme]i associated with MDI treatment are also associated with increased recruitment of NCoR to a REV-ERBα target gene promoter and thus are consistent with heme functioning as a REV-ERBα ligand during this differentiation process.
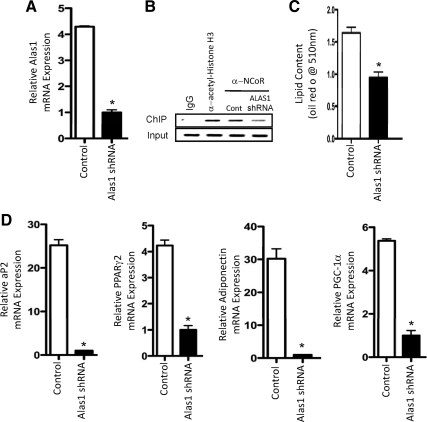
We examined the requirement for induction of Alas1 mRNA expression for adipocyte differentiation by suppressing the expression of this enzyme by infection of the 3T3-L1 cells with an Alas1 shRNA adenovirus (15), whereas the cells were treated with MDI media. After the 7-d differentiation assay, lipid content and gene expression was analyzed. As shown in Fig. 4A, Alas1 mRNA was suppressed nearly 80% relative to cells infected with scrambled shRNA adenovirus. Furthermore, when we assessed the recruitment of NCoR to the Pai-1 promoter, the results were consistent with reduced recruitment of the corepressor by REV-ERBα due to lower levels of heme (Fig. 4B). Lipid content was significantly reduced in cells in which Alas1 mRNA expression was reduced (Fig. 4C) as well as the expression of several adipogenic genes (Fig. 4D). These data indicate that induction of Alas1 expression is a critical event mediating efficient adipogenesis.
Figure 4.
Induction of Alas1 expression is required for efficient adipogenesis. A, Alas1 mRNA expression after knockdown of Alas1 expression by Alas1 shRNA adenovirus. Control cells received scrambled shRNA adenovirus in all the experiments shown in the figure. Data reflect expression at d 7 of the differentiation assay. B, ChIP experiment illustrating that NCoR recruitment to the REV-ERBα target gene promoter (Pai-1) is reduced when Alas1 expression is reduced. IgG and antiacetyl histone H3 were used as negative and positive controls, respectively. C, Lipid content of 3T3-L1 cells after 7 d of treatment with MDI and treatment with control adenovirus or the Alas1 shRNA virus. D, Expression of adipogeneic genes after 7 d of treatment with MDI and treatment with control adenovirus or the Alas1 shRNA virus. *, P < 0.05 vs. control cells.
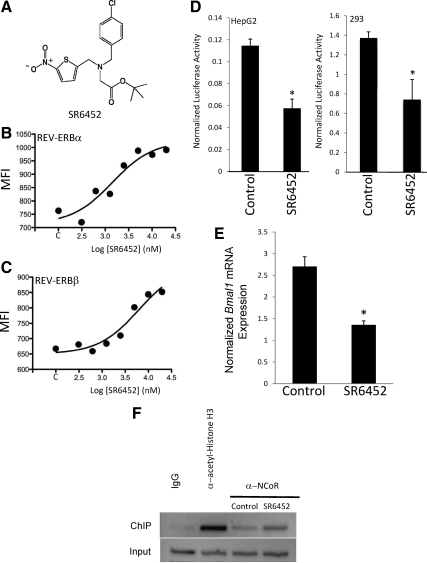
Heme is a prosthetic group essential for the function of many proteins and enzymes, and although we found that the elevation of [heme]i during adipogenesis was associated with an alteration in REV-ERBα-NCoR binding activity, it is unclear how important a role this REV-ERB ligand plays during adipogenesis. To address this, we used a recently described nonporphyrin synthetic REV-ERB ligand (16) to examine the effects of activation of REV-ERB on adipogenesis. The REV-ERBα ligand, SR6452 (Fig. 5A), was shown to dose-dependently increase the interaction of REV-ERBα with a CoRNR box peptide derived from the transcriptional corepressor NCoR as well as modulate circadian rhythm in cell culture (16). These authors indicated that SR6452 was not active on other NHRs (liver X receptor-α and -β, retinoic acid receptor-related orphan receptor α, liver receptor homolog-1, steroidogenic factor-1, or farnesoid X receptor) (16), and we subsequently examined PPARγ in a Gal4-PPARγ cotransfection assay and noted no activity (data not shown).
Figure 5.
Characterization of SR6452, a REV-ERBα/REV-ERBβ agonist. A, Structure of SR6452. B, Luminex assay illustrating the ability SR6452 to dose-dependently increase the interaction of REV-ERBα with a CoRNR box peptide of NCoR. C, Luminex assay illustrating the ability SR6452 to dose-dependently increase the interaction of REV-ERBβ with a CoRNR box peptide of NCoR. D, Cotransfection of HepG2 or human embryonic kidney 293 cells with an expression vector for REV-ERBα along with a luciferase reporter under the control of the Bmal1 promoter. SR6452 increased the transcriptional repressor activity of REV-ERBα. E, SR6452 treatment decreases BMAL1 expression in HepG2 cells. F, ChIP analysis of NCoR occupancy of the BMAL1 promoter in HepG2 cells. IgG and antiacetyl histone H3 were used as negative and positive controls, respectively. *, P < 0.05 vs. control cells. SR6452 was used at a concentration of 10 μm unless otherwise indicated. MFI, Median fluorescence intensity.
We examined the pharmacology of SR6452 in more detail by testing its ability to modulate the interaction of either REV-ERBα or REV-ERBβ with an NCoR CoRNR box peptide using Luminex technology (1). As shown in Fig. 5, B and C, SR6452 dose-dependently increases the interaction of both REV-ERBα and REV-ERBβ with the NCoR peptide, indicating that the ligand modulates the activity of both REV-ERB subtypes. We also examined the ability of SR6452 to modulate the activity of REV-ERBα in a cotransfection experiment in which we cotransfected HepG2 or human embryonic kidney 293 cells with an expression vector for REV-ERBα along with a Bmal1 promoter-luciferase construct. Bmal1 is a well-characterized REV-ERB target gene with a REV-ERB response element in the proximal promoter (3). Consistent with our observation that SR6452 increases REV-ERB recruitment of NCoR, treatment of these cells with SR6452 resulted in significantly greater repressive activity of REV-ERBα (Fig. 5D). Treatment of HepG2 cells with SR6452 resulted in decreased expression of Bmal1 mRNA (Fig. 5E) and increased recruitment of NCoR to the Bmal1 promoter as detected by ChIP (Fig. 5F). These data clearly indicate that SR6452 functions as a REV-ERB ligand mimicking the action of heme.
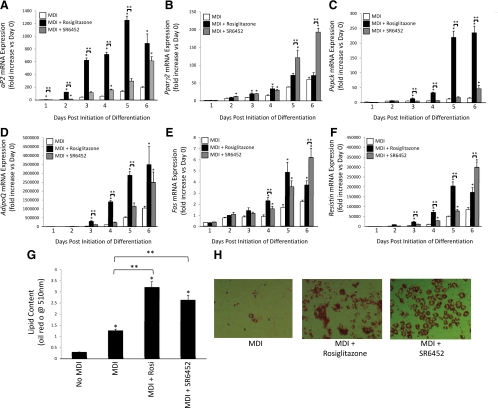
We compared the ability of the PPARγ agonist, rosiglitazone, and the REV-ERB agonist, SR6452, to induce adipogenesis in 3T3-L1 cells. Adipogenesis was induced in 3T3-L1 cells as described in Materials and Methods except that the media were supplemented with MDI, rosiglitazone (1 μm), or SR6452 (10 μm of either) (and compared with MDI treatment alone). Gene expression [aP2, Pparγ2, Pepck, adiponectin (AdipoQ), fatty acid synthase (Fas), and resistin] was monitored each day for 6 d of differentiation. Figure 6A illustrates the increase in expression of aP2 during differentiation. Rosiglitazone supplementation clearly accelerates the rate of expression of aP2 during the process as well as amplifies the level of induction vs. control media alone. Interestingly, SR6452 also accelerates the rate of expression of aP2 and amplifies the level of induction vs. media alone but does so at a slower rate than does the PPARγ agonist. On the last day of measurement, the level of aP2 expression is equivalent in the rosiglitazone- and SR6452-treated cells. When we examine expression of Pparγ2, we find that both rosiglitazone and SR6452 significantly increase the expression of this gene early in differentiation (d 3 and 4), but after d 5, SR6452 treatment is the only treatment that is significantly greater than control media-treated cells (Fig. 6B). At d 6, SR6452 treatment results in Pparγ2 expression that is approximately 3-fold greater than either control or rosiglitazone-treated cells (Fig. 6B). However, a clearly different pattern was observed whether we examine the expression of Pepck in these cells in response to these treatments. Pepck expression increases during adipogenesis, and either rosiglitazone or SR6452 treatment increases the level of induction relative to control adipocytes. However, rosiglitazone is a much more efficacious activator of expression of Pepck reaching levels that are approximately 10-fold higher that control adipocytes and 5-fold greater than SR6452-treated cells (Fig. 6C).
Figure 6.
The REV-ERB ligand SR6452 induces adipogenesis in 3T3-L1 cells. Assessment of aP2 (A), Pparγ2 (B), Pepck (C), AdipoQ (D), Fas (E), or resistin (F) mRNA expression during induction of adipogenesis in 3T3-L1 cells either with differentiation media (MDI) or MDI supplemented with rosiglitazone (10 μm) or SR6452 (10 μm). G, Treatment of 3T3-L1 cells with rosiglitazone or SR6452 induces adipogenesis as detected by lipid accumulation (Oil Red O). H, Photomicrographs of Oil Red O-stained 3T3-L1 cells illustrating the effects on lipid accumulation due to treatment with rosiglitazone or SR6452. *, P < 0.05 vs. control cells at the identical day of treatment; **, P < 0.05 vs. the indicated group.
Adiponectin expression was induced by both drugs, but the kinetics were distinct with rosiglitazone inducing expression before SR6452 (Fig. 6D). Fas and resistin displayed similar patterns of expression with both drugs increasing expression above MDI alone. Rosiglitazone induced expression earlier than SR6452, but for both of these genes, the effect of SR6452 was significantly greater at d 6 (Fig. 6, E and F). After 7 d of treatment, the 3T3-L1 cells were examined for lipid content by Oil Red O staining, and the results are shown in Fig. 6, G and H. As would be predicted based on the gene expression studies, both activation of PPARγ or REV-ERB led to a significant increase in lipid content vs. MDI treatment alone. We also examined the effect of SR6452 on REV-ERBα protein expression during adipogenesis. As shown in Supplemental Fig. 3, the pattern of REV-ERBα protein expression is similar to that without SR6452, but the transition from increased expression to degradation appears to be much more abrupt and effective (Fig. 1D). Furthermore, the decrease in REV-ERBα protein expression in the later stages is also mediated by proteasomal degradation based on sensitivity to MG-132 (Supplemental Fig. 3). These results indicate that the REV-ERB agonist, SR6452, is adipogenic and also suggest that increases in intracellular heme during adipogenesis are likely to play a role in inducing REV-ERB activity required for the differentiation process.
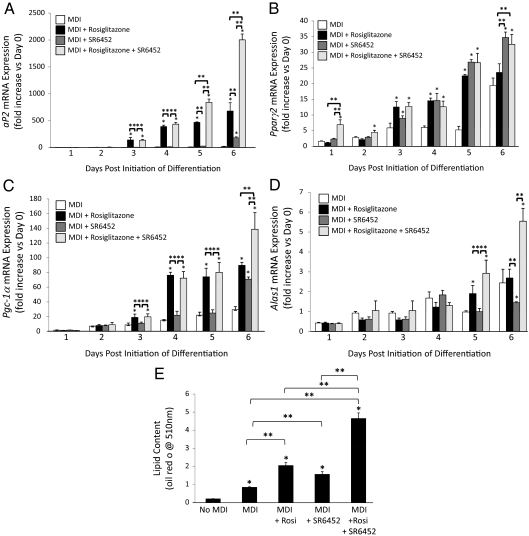
Based on the appearance that PPARγ and REV-ERB agonists exhibit unique patterns of effects on gene expression during adipogenesis, we hypothesized that combination of the two drugs (rosiglitazone and SR6452) would induce additive or synergistic adipogenesis activity. An additional set of experiments was performed in which we treated 3T3-L1 cells with MDI, MDI + rosiglitazone, MDI + SR-6452, or MDI + both drugs followed by assessment of gene expression and lipid accumulation. As shown in Fig. 7A, the drugs displayed a synergistic effect on the expression of aP2 expression. Interestingly, the effects of combination of the drugs was dependent on which genes were examined. Pparγ2 expression showed no synergy or even additivity (Fig. 7B), whereas additivity was shown in other cases [Pgc-1α (Fig. 7C) and Alas1 (Fig. 7D)]. When lipid accumulation was measured (d 7), we noted that combination of the two drugs resulted in additivity in which clearly the most efficacious effect was seen in the MDI + rosiglitazone + SR6452 group (Fig. 7E).
Figure 7.
Combination of rosiglitazone and SR6452 additively increases adipogenesis. Assessment of aP2 (A), Pparγ2 (B), Pgc-1α (C), AdipoQ (D), or Alas1 (E) mRNA expression during induction of adipogenesis in 3T3-L1 cells either with differentiation media (MDI) or MDI supplemented with rosiglitazone (10 μm), SR6452 (10 μm), or both drugs (10 μm each). F, Treatment of 3T3-L1 cells with a combination of rosiglitazone or SR6452 additively increases lipid accumulation (Oil Red O). *, P < 0.05 vs. control cells at the identical day of treatment; **, P < 0.05 vs. the indicated group.
Discussion
Given the important role of REV-ERBα in adipogenesis and our recent observation that heme functions as a REV-ERBα ligand regulating its ability to modulate target gene transcription, we reasoned that heme might function as an essential ligand for this receptor during this differentiation process. More than 27 yr ago, Chen and London (14) observed that 3T3-F442A cells showed enhanced adipogenesis in the presence of heme and that reducing heme biosynthesis reduced the adipogenic potential of the cells. However, the mechanism by which heme modulated the adipogenic process was unknown. Here we demonstrated that [heme]i increases during adipogenesis in 3T3-L1 cells and that the increase is associated with an alteration in REV-ERBα function. Blocking the accumulation of heme interferes with the adipogenesis and also blocks recruitment of NCoR to a REV-ERBα target gene promoter. Our data indicate that the increase in heme is mediated by an increase in expression of Alas1 and whether the increase in Alas1 expression is blocked, adipogenesis is significantly attenuated. Activation of REV-ERB with a synthetic agonist, SR6452, led to increased adipogenesis, which clearly illustrates the ability of a REV-ERB agonist to promote adipocyte differentiation. Our previous report (1) along with that of Yin et al. (2) identified heme as a REV-ERB agonist and demonstrated that pharmacological and genetic manipulation of [heme]i altered the ability or REV-ERBα to interact with NCoR and modulate target gene transcription. Our current study clearly indicates that physiological alterations in [heme]i are sufficient to alter REV-ERBα activity and pharmacological activation of REV-ERB is sufficient to induce adipogenesis, and thus, our results are consistent with heme functioning as a physiological REV-ERB ligand.
O'Malley and Conneely (29) hypothesized in 1992 that some of the novel orphan NHRs that were being rapidly identified at that time might function via intracrine mechanism in which the ligand acts on receptors within the same cell in which the ligand is synthesized. We believe that REV-ERBα functions in this manner; here adipogenic signals induce an increase in heme biosynthesis, and the resulting abundance of intracellular heme can then modulate REV-ERBα function required for later adipogenic events. Adipogenesis is a complex process involving the temporal induction of expression of an array of transcription factors including CCAAT/enhancer-binding protein-α/β/γ, PPARγ, adipocyte determination and differentiation-1/sterol regulatory element-binding protein, and Krüppel-like factor-5 (30,31). Interestingly, REV-ERBα protein expression is necessary for early events, but its degradation is also required for the later events, even in the presence of very high levels of Rev-erbα mRNA (12). We noted that the point at which we begin to detect REV-ERBα protein degradation is also the point at which we observe a significant increase in [heme]i (d 3). Thus, heme appears to be required for degradation of REV-ERBα protein. The degradation of REV-ERBα protein during adipogenesis is mediated by the 26S proteaosome and is required for adipogenesis because maintenance of REV-ERBα expression results in inhibition of expression of Pparγ2, a key regulator of adipocyte differentiation (12). Interestingly, we observed that treatment of cells with the REV-ERB agonist resulted in greater induction of Pparγ2 expression in the later stages of adipogenesis. One might expect that SR6452 would result in greater repression of Pparg2 expression via increased REV-ERB activity, but the ligand may actually be causing very efficient degradation of REV-ERB, an event that is required for later stages of adipogenesis (12). Consistent with previous studies (14), we found that heme is required for adipogenesis. Furthermore, because heme is required for degradation of REV-ERBα protein, we would expect that this degradation step is required for adipogenesis as a ligand-dependent 26S proteasome-mediated event. This model is in agreement with the fact that several NHRs have been demonstrated to be degraded by the 26S proteasome in response to ligand binding (23,24,25,26).
The process of adipogenesis has garnered increasing attention as the epidemic of obesity and type 2 diabetes has rapidly progressed. The adipocyte and the process of adipogenesis are becoming more attractive targets for treatment of these disorders as we begin to understand more about adipocyte physiology (32). One NHR that is a key regulator of adipogenesis, PPARγ, is already a well-known drug target for type 2 diabetes. Because REV-ERBα also plays a key role in adipogenesis, our data indicating that ligand regulation of this receptor is important in this process suggest that REV-ERB may also serve as a target for the development of drugs for the treatment of metabolic syndrome and obesity. Our results demonstrating an additive effect of a REV-ERB agonist with a PPARγ agonist suggest that combination of these two classes of drugs may offer a clinical advantage in treatment of type 2 diabetes.
Supplementary Material
Acknowledgments
We thank Dr. Bruce Spiegelman for providing the Alas1 shRNA adenovirus.
Footnotes
This work was supported by National Institutes of Health Grants DK080201 (to T.P.B.), MH084512 (to P.R.G.), and P20 RR02195 (to Z.E.F.) and by the Pennington Biomedical Research Foundation (to J.M.G.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 28, 2010
Abbreviations: Alas1, Aminolevulinic acid synthase 1; aP2, adipocyte protein 2; ATZ, aminotriazole; ChIP, chromatin immunoprecipitation; Fas, fatty acid synthase; [heme]i, intracellular heme concentration; NCoR, nuclear receptor corepressor; NHR, nuclear hormone receptor; PGC-1, PPAR-γ coactivator 1; PPAR, peroxisomal proliferator-activated receptor; SR6452, 1,1-dimethylethyl N-[(4-chlorophenyl)methyl]N-[(5-nitro-2-thienyl)methyl]glycinate; shRNA, short hairpin RNA.
References
- Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F 2007 Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol 14:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA 2007 Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 318:1786–1789 [DOI] [PubMed] [Google Scholar]
- Burris TP 2008 Nuclear hormone receptors for heme: REV-ERBα and REV-ERBβ are ligand-regulated components of the mammalian clock. Mol Endocrinol 22:1509–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teboul M, Guillaumond F, Gréchez-Cassiau A, Delaunay F 2008 The nuclear hormone receptor family round the clock. Mol Endocrinol 22:2573–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummasti S, Tontonoz P 2008 Adopting new orphans into the family of metabolic regulators. Mol Endocrinol 22:1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez H, Staels B 2008 Rev-erbα gives a time cue to metabolism. FEBS Lett 582:19–25 [DOI] [PubMed] [Google Scholar]
- Kaasik K, Lee CC 2004 Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature 430:467–471 [DOI] [PubMed] [Google Scholar]
- Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, McKnight SL 2002 NPAS2: a gas-responsive transcription factor. Science 298:2385–2387 [DOI] [PubMed] [Google Scholar]
- Rogers PM, Ying L, Burris TP 2008 Relationship between circadian oscillations of Rev-erbα expression and intracellular levels of its ligand, heme. Biochem Biophys Res Commun 368:955–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Lazar MA 1993 Induction of REV-ERBA-α, an orphan receptor encoded on the opposite strand of the α-thyroid hormone-receptor gene, during adipocyte differentiation. J Biol Chem 268:16265–16269 [PubMed] [Google Scholar]
- Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J, Staels B 2003 The orphan nuclear receptor Rev-Erbα is a peroxisome proliferator-activated receptor (PPAR) γ target gene and promotes PPARγ-induced adipocyte differentiation. J Biol Chem 278:37672–37680 [DOI] [PubMed] [Google Scholar]
- Wang J, Lazar MA 2008 Bifunctional role of Rev-erbα in adipocyte differentiation. Mol Cell Biol 28:2213–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rogers PM, Su C, Varga G, Stayrook KR, Burris TP 2008 Regulation of cholesterologenesis by the oxysterol receptor, LXRα. J Biol Chem 283:26332–26339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, London IM 1981 Hemin enhances the differentiation of mouse 3t3 cells to adipocytes. Cell 26:117–122 [DOI] [PubMed] [Google Scholar]
- Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Maratos-Flier E, Shulman GI, Spiegelman BM 2009 PGC-1α negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erbα axis. Proc Natl Acad Sci USA 106:22510–22515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, McMaster A, Beesley S, Lu WQ, Gibbs J, Parks D, Collins J, Farrow S, Donn R, Ray D, Loudon A 2008 Ligand modulation of REV-ERBα function resets the peripheral circadian clock in a phasic manner. J Cell Sci 121:3629–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanlidis G, Karamitri A, Docherty K, Hazlerigg DG, Lomax MA 2007 C/EBPβ reprograms white 3T3-L1 preadipocytes to a brown adipocyte pattern of gene expression. J Biol Chem 282:24660–24669 [DOI] [PubMed] [Google Scholar]
- Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, Meyer UA, Spiegelman BM 2005 Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1α. Cell 122:505–515 [DOI] [PubMed] [Google Scholar]
- Mager D, Bernstein A 1979 Role of heme in the regulation of the late program of friend-cell erythroid-differentiation. J Cell Physiol 100:467–479 [DOI] [PubMed] [Google Scholar]
- Tschudy DP, Collins A 1957 Effect of 3-amino-1,2,4-triazole on Δ-aminolevulinic acid dehydrase activity. Science 126:168 [DOI] [PubMed] [Google Scholar]
- Baron J, Tephly TR 1969 Effect of 3-amino-1,2,4-triazole on stimulation of hepatic microsomal heme synthesis and induction of hepatic microsomal oxidases produced by phenobarbital. Mol Pharmacol 5:10–20 [PubMed] [Google Scholar]
- Dabney BJ, Beaudet AL 1977 Increase in globin chains and globin messenger-RNA in erythroleukemia cells in response to hemin. Arch Biochem Biophys 179:106–112 [DOI] [PubMed] [Google Scholar]
- Dace A, Zhao L, Park KS, Furuno T, Takamura N, Nakanishi M, West BL, Hanover JA, Cheng SY 2000 Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proc Natl Acad Sci USA 97:8985–8990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM 2000 Degradation of the peroxisome proliferator-activated receptor γ is linked to ligand-dependent activation. J Biol Chem 275:18527–18533 [DOI] [PubMed] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O'Malley BW 2000 The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F 2003 Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Mol Cell 11:695–707 [DOI] [PubMed] [Google Scholar]
- Wang J, Yin L, Lazar MA 2006 The orphan nuclear receptor Rev-erbα regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem 281:33842–33848 [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Varcoe TJ, Mau VJ 2003 Rhythmic expression of clock and clock-controlled genes in the rat oviduct. Mol Hum Reprod 9:503–507 [DOI] [PubMed] [Google Scholar]
- O'Malley BW, Conneely OM 1992 Orphan receptors—in search of a unifying hypothesis for activation. Mol Endocrinol 6:1359–1361 [DOI] [PubMed] [Google Scholar]
- Farmer SR 2006 Transcriptional control of adipocyte formation. Cell Metab 4:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA 2006 Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896 [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Scherer PE 2005 The adipocyte as a drug discovery target. Drug Discovery Today 10:1219–1230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.