Abstract
Four-and-a-half LIM 2 (FHL2) is a member of a family of LIM domain proteins which mediate protein-protein interactions. FHL2 acts as a coactivator and binds to important regulators of bone formation such as insulin-like growth factor binding protein (IGFBP)-5, androgen receptor, and β-catenin. We hypothesized that FHL2 is an important regulator of bone formation. We evaluated growth and skeletal parameters in FHL2 knockout (KO) and wild-type (WT) mice at 4, 8, and 12 weeks of age. At 4 weeks of age, lack of FHL2 reduced femur, tibia, and total bone mineral content (BMC) and body weight in all mice. A gender-by-treatment interaction (P ≤ 0.05) was observed for several parameters due to a greater reduction in females. Specifically, femur BMC was reduced 11–27% at 8 and 12 weeks of age and BMD was reduced 7–13% at all ages in female KO mice (P < 0.05). A similar reduction was observed in the tibias at 8 weeks of age. A 6% reduction (P = 0.07) in femur cortical thickness was observed at 12 weeks of age in female KO mice. Interestingly, a gender-specific reduction in IGFBP-5 expression was observed in the femurs of female KO mice. During differentiation of bone marrow stromal cells into osteoblasts, expression of osteocalcin, alkaline phosphatase, and bone sialoprotein was reduced 47–96% in FHL2 KO cells (P < 0.001). In conclusion, FHL2 is an important regulator of peak bone mass, lack of FHL2 produces gender- and site-specific effects on bone accretion and IGFBP-5 expression, and FHL2 is important for optimal osteoblast differentiation in vitro.
Keywords: Bone density, Female mice, FHL2, Osteoblast differentiation
Four-and-a-half LIM 2 (FHL2) is a LIM-only protein which consists of four and a half LIM domains and is a member of a family of LIM domain proteins that includes FHL 1–4 and activator of CREM in testis (ACT) [1, 2]. The lim domain proteins are characterized by a zinc figure motif [3], mediate protein-protein interactions, and bind to several proteins [4]. Specifically, FHL2 functions as a coactivator by binding to proteins such as the androgen receptor (AR), insulin-like growth factor binding protein (IGFBP)-5, cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), cAMP responsive element modulator (CREM) activator protein (AP)-1, β-catenin, CREB binding protein (CBP)/p300, breast cancer 1 (BRCA1), extracellular signal-regulated kinase 2 (ERK2), and α7β1 [5–12] and is a corepressor of mitogen/extracellular-regulated kinase (MEK1)-ERK1/2 signaling, by binding to ERK2, and of Hand1/E-protein transcriptional coactivation [13, 14]. FHL2 may also be important in tissue development by binding to factors such as CREB and CREM, which are involved in the regulation of proliferation and differentiation [10]. FHL2 binds to several factors which are important to the regulation of bone formation. Specifically, it increases the transcriptional activity of theAR[12], which is important in the processes of bone formation and resorption, as demonstrated in the AR knockout (KO) mouse model [15, 16]. In addition, we recently determined, using a yeast two-hybrid system, that FHL2 binds to IGFBP-5 and the two proteins translocate into the nucleus [11]. IGFBP-5 is the most abundant IGFBP stored in bone, and several in vivo and in vitro experiments have confirmed its important role in regulating bone formation [17–20].FHL2 has also been implicated as a modulator of the Wnt signaling pathway, thereby promoting differentiation of mouse myoblasts via its interaction with β-catenin andCBP/p300 [6, 8, 9]. Based on these findings and that FHL2 interacts with a number of key signaling molecules that regulate proliferation, differentiation, and apoptosis of bone-forming osteoblasts, we hypothesized that FHL2 is an important regulator of bone formation in vivo. To test this hypothesis, we evaluated growth and skeletal parameters in FHL2 KO mice.
During the preparation of our report, two studies were published that examined the consequence of FHL2 disruption on the skeleton. Gunther et al. [21] demonstrated that loss of FHL2 resulted in reduced bone formation rate and osteopenia, by histomorphometric analysis. They also demonstrated that FHL2 modulates transcription of Runx2, a key osteoblast differentiation factor. In contrast, Lai et al. [22] did not observe a significant difference in whole-body BMD in male and female mice; however, when females were ovariectomized, a greater reduction in total-body BMD was observed in the FHL2 KO mice. In the current study, we demonstrate that disruption of FHL2 reduced body weight and femur and tibia bone mineral density (BMD) in female, but not male, mice. In addition, expression of IGFBP-5 was reduced in the femurs of female, but not male, mice, suggesting a role for FHL2 in mediating the effects of estrogen.
Materials and Methods
Animals
FHL2 KO mice were generated as previously described [13] in a Swiss black/129-Sv/J and C57BL/6 background. Breeding pairs heterozygous for deletion of the FHL2 gene were maintained and used to generate homozygous KO and wild-type (WT) littermates for experiments. Mice were weaned and genotyped at 3 weeks of age. Genotypes were determined using polymerase chain reaction (PCR) as previously described [13], with the following primer pairs: neo1, 5′-GGATCGGCCATTGAACAAGATG-3′; neo2, 5′-GAGCAAGGTGAGATGACAGGAG-3′; FHL2-P2, 5′-AGCATGACTGAACGCTTTGACT-3′; FHL2-P3, 5′-GGTAACCAGAACAGGGAGAGTG-3′. The experimental procedures were in compliance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the Animal Studies Subcommittee at the Jerry L. Pettis Memorial Veterans Affairs Medical Center. Bone densitometry, bone mineral content (BMC), and areal BMD (aBMD) were measured by dual X-ray absorptiometry (DXA) at 4, 8, and 12 weeks of age, using the PIXImus instrument (Lunar, Madison, WI). The precision for the BMC and BMD measurements was ±1% for repeat measurements of the same bones several times [23, 24]. Animals were anesthetized by a ketamine/xylazine (50/5 mg/kg body weight) injection prior to measurement. Following DXA analysis at 12 weeks of age, mice were killed by CO2 inhalation and decapitation. The femur and tibia from one leg were removed for volumetric BMD (vBMD) analysis, and the femur and tibia from the other leg (marrow was flushed from bones), liver, muscle, and kidney were collected, immediately frozen in liquid nitrogen, and stored at −70°C until RNA was extracted.
vBMD and Geometric Parameters
vBMD and geometric parameters at the mid-diaphysis of the femur of bones isolated at 12 weeks of age were determined by peripheral quantitative computed tomography (pQCT; Norland, Medical Systems, Inc., WI). Scans were analyzed using the manufacturer-supplied software program (Bone Density Software, version 5.40, Stratec Medzizintechnik, GmbH, Germany). Total vBMD and geometric parameters were estimated with loop analysis. The threshold was set at 230–630 mg/cm3. For femur analysis, nine scans per bone were measured, and the data are presented as the average of the fourth, fifth, and sixth scans (mid-diaphysis region). The coefficients of variation for total vBMD, periosteal circumference, and endosteal circumference for repeat measurements of four mouse femurs (two to five measurements) were <3%, <1%, and 2%, respectively [23–25]. The longitudinal lengths of the femurs were measured with a caliper.
Osteoblast Differentiation
To determine if lack of FHL2 altered osteoblast differentiation, bone marrow stromal cells were isolated from KO and WT mice and plated in α-minimal essential medium (α-MEM, containing 10% calf serum, 100 U/mL penicillin, 100 µg/mL streptomycin, 50 µg/mL ascorbic acid, and 10mM β-glycerol phosphate) to induce differentiation into osteoblast cells, as previously described [26]. At days 0, 6, and 18 after differentiation, medium was added and cells were washed with phosphate-buffered saline, extracted with 1 mL Tri-Reagent (Molecular Research Center, Cincinnati, OH), and stored at −70°C until RNA extraction was performed.
RNA Extraction
RNA was extracted from the tissues using the Lipid Tissue Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. RNA was extracted from cells using TriReagent and the RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. Following extraction, residual DNA was removed from up to 10 µg of RNA with a DNA-free kit (Ambion, Austin, TX). RNA quality was determined using a 2100 Bioanalyzer (Agilent, Palo Alto, CA), and RNA was quantified using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Gene Expression Analysis
Quantitative real-time reverse transcriptase (RT)-PCR analysis was used to determine the expression levels of FHL1 (F, 5′-GGCGATAAAGCTCCCACTGAGAC-3′; R, 5′-AGAAGGCGAGAAGGGAGTTGGAG-3′), FHL2 (F, 5′-AGCATGACTGAACGCTTTGACT-3′; R, 5′-TTGGCATAGAGTTCCTCAAAGC-3′), FHL3 (F, 5′-CACCATGAGCGAGGCATTTGACT-3′; R, 5′-GGTGTTGGCGAAGGTGTTGTCAT-3′), IGFBP-5 (F, 5′-ATACAACCCAGAACGCCAGCT-3′; R, 5′-ACCTGGGCTATGCACTTGATG-3′), osteocalcin (F, 5′-CTCTCTCTGCTCACTCTGCT-3′; R, 5′-TTTGTAGGCGGTCTTCAAGC-3′), alkaline phosphatase (ALP; F, 5′-ATGGTAACGGGCCTGGCTACA-3′; R, 5′-AGTTCTGCT CATGGACGCCGT-3′), bone sialoprotein (BSP; F, 5′-AACGGGTTTCAGCAGACAACC-3′; R, 5′-TAAGCTCGGTAAGTGTCGCCA-3′), and peptidylprolyl isomerase A (PPIA) (endogenous control; F, 5′-TCCTGGACCCAAAACGCTCC-3′; R, 5′-CCATGGCAAATGCTGGACCA-3′), as previously described [26, 27]. Primers were validated as previously described [26]. Delta CT values (comparative threshold) were determined (CT value for gene of interest minus CT value for control gene), and comparisons of CT values were used for relative quantification of gene expression [28].
Statistical Analysis
Bone parameters analyzed by DXA at 4, 8, and 12 weeks of age were analyzed by repeated measures analysis of variance (ANOVA). vBMD and geometric parameters determined by pQCT, gene expression at 12 weeks of age, and cell culture data were analyzed by ANOVA. Post hoc analysis was performed using the Newman-Keuls test. All data were analyzed using Statistica 6 software (StatSoft, Tulsa, OK) and presented as mean ± standard error. A significant difference was determined at P ≤ 0.05.
Results
Growth and Skeletal Parameters
Growth and skeletal parameters measured by DXA are listed in Table 1. For all FHL2 KO mice, 11% and 4% reductions (P < 0.01) in body weight were observed at 4 and 8 weeks of age, respectively; however, no difference in body length was observed compared with WT mice. Total-body BMD was not different between WT and KO mice (P ≥ 0.15) at all time points (data not shown). Reductions of 14% and 19% (P < 0.05) in femur and tibia BMC, respectively, were observed at 4 weeks of age by comparing mean BMC in the combined male and female KO mice versus control mice. Interestingly, a significant gender-by-treatment interaction (P ≤ 0.05) was observed for several parameters, and through further analysis we determined that lack of FHL2 had a greater effect on bone parameters in female mice when analyzed separately from males. Specifically, in female FHL2 KO mice, 27% and 11% reductions (P < 0.05) in femur BMC were observed at 8 and 12 weeks, respectively. Femur BMD was reduced 8%, 13%, and 7% (P< 0.05) at 4, 8, and 12 weeks, respectively. Tibia BMC and BMD were reduced 16% and 10% (P < 0.05), respectively, at 8 weeks of age. Although there was an 8% reduction in body weight in FHL2 KO female mice, the above parameters were still significant (P ≤ 0.05) when adjusted for body weight.
Table 1.
Growth and skeletal parameters at 4, 8, and 12 weeks of age
| Age (weeks) | ||||||
|---|---|---|---|---|---|---|
| 4 | 8 | 12 | ||||
| Parameter | WT | FHL2 KO | WT | FHL2 KO | WT | FHL2 KO |
| Body weight (g) | ||||||
| Female | 12.82±0.71 | 11.80±0.55*, ** | 19.28±0.37** | 17.76±0.28*, **, *** | 22.12±0.50** | 20.90±0.56** |
| Male | 15.28±0.43 | 13.17±0.82* | 23.96±0.39 | 23.23±0.62* | 27.80±0.91 | 27.25±0.93 |
| Body length (mm) | ||||||
| Female | 73.40±2.54 | 73.33±1.26 | 85.8±1.20** | 84.83±1.22** | 89.20±1.36** | 89.00±1.29** |
| Male | 77.60±1.21 | 73.67±1.52 | 93.20±0.37 | 90.83±1.78 | 95.00±0.63 | 95.00±1.75 |
| Femur BMC (mg) | ||||||
| Female | 6.54±0.49 | 5.83±0.31* | 16.6±0.93 | 12.07±0.83*** | 19.48±0.22 | 17.27±0.74*** |
| Male | 7.67±0.42 | 6.52±0.50* | 15.63±1.01 | 16.37±1.58 | 19.27±0.42 | 20.43±1.57 |
| Femur BMD (mg/cm2) | ||||||
| Female | 42.26±1.29 | 38.90±0.46 | 63.80±1.74** | 55.53±2.26**, *** | 72.02±1.03 | 67.12±1.49*** |
| Male | 41.20±2.15 | 40.17±1.28 | 65.03±1.95 | 67.18±4.56 | 68.72±1.92 | 77.02±5.24 |
| Tibia BMC (mg) | ||||||
| Female | 7.60±0.49 | 7.50±0.43* | 16.74±1.01 | 14.07±0.56*** | 18.90±0.61 | 16.88±1.12 |
| Male | 10.17±0.48 | 7.08±0.84* | 16.10±0.28 | 17.02±1.10 | 18.85±0.42 | 19.90±1.06 |
| Tibia BMD (mg/cm2) | ||||||
| Female | 34.58±1.13 | 33.00±1.16 | 53.00±2.09 | 47.82±0.95*** | 58.10±1.91 | 54.57±3.11 |
| Male | 35.85±1.30 | 33.28±1.95 | 51.62±1.94 | 53.57±2.14 | 58.38±1.52 | 60.57±2.82 |
Significant main effect for treatment at P < 0.05
Significant main effect for gender at P < 0.05
Significant treatment effect for female mice at P < 0.05; n = 5 or 6 in each treatment group (n = 23)
Bone Size and vBMD
Since differences in body weight can influence aBMD, we evaluated vBMD and bone size in the femurs of all mice at 12 weeks of age (Table 2). Although femur vBMD was reduced by 5% in female FHL2 KO mice, this difference was not statistically significant. Interestingly, a tendency (P = 0.09) for a treatment-by-gender interaction was observed for cortical thickness. Analysis of females demonstrated a 6% reduction (P = 0.07) in cortical thickness in the femurs of FHL2 KO mice.
Table 2.
Bone size and vBMD of femurs at 12 weeks of age
| Females | Males | P | |||||
|---|---|---|---|---|---|---|---|
| WT (n = 6) |
KO (n = 6) |
WT (n = 5) |
KO (n = 7) |
Treatment | Gender | Treatment × Gender |
|
| Total density (mg/cm3) | 627±15 | 597±11 | 613±8 | 612±18 | 0.3103 | 0.9829 | 0.3444 |
| Periosteal circumference (mm) |
5.02±0.02 | 4.96±0.06 | 5.21±0.07 | 5.28±0.08 | 0.9815 | 0.0007 | 0.3286 |
| Endosteal circumference (mm) |
3.22±0.05 | 3.26±0.04 | 3.41±0.06 | 3.42±0.06 | 0.6014 | 0.0034 | 0.7973 |
| Cortical thickness (mm) | 0.2866±0.0066 | 0.2705±0.0044 | 0.2874±0.0035 | 0.2954±0.0090 | 0.5570 | 0.0740 | 0.0920 |
mRNA Expression of FHL Genes and IGFBP-5
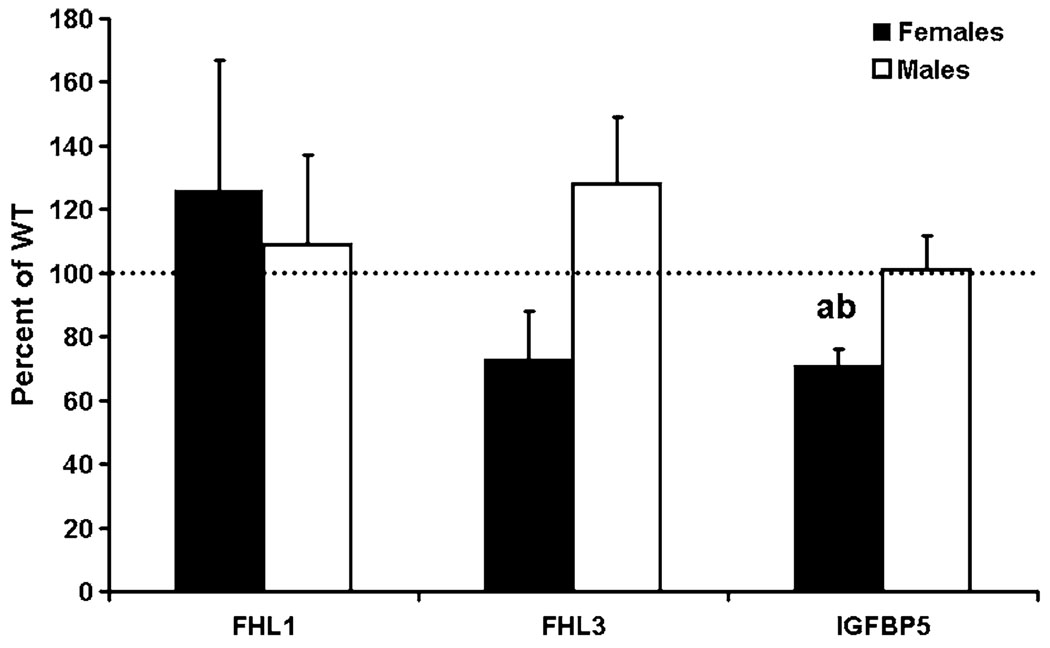
We confirmed the disruption of FHL2 expression in tissues of female and male FHL2 KO mice compared with WT mice by real-time RT-PCR (data not shown). To determine if expression of other FHL genes was altered by lack of FHL2, we determined their expression in bones of FHL2 KO and WT female and male mice. Accordingly, a difference in expression of FHL1 and -3 was not observed in male and female mice (Fig. 1). Interestingly, expression of IGFBP-5 was reduced 28% in the bones of female FHL2 KO mice, but no change was observed in FHL2 KO male mice compared with WT mice (Fig. 1). When the percent of control data was analyzed, a significant difference (P = 0.01) was observed between male and female mice for IGFBP-5 expression (Fig. 1). No difference in expression of FHL1, FHL3, and IGFBP-5 was observed in the muscle of FHL2 KO and WT mice (data not shown).
Fig. 1.
IGFBP-5 mRNA expression is reduced in bones of FHL2 KO female mice at 12 weeks of age. mRNA expression was determined by real-time RT-PCR. Data are presented as mean ± standard error (n = 7). A significant difference between WT and KO mice was not observed for FHL1 and -3 (P ≥ 0.4139). Expression of FHL2 was not detectable in KO mice. a Significant difference from WT mice at P < 0.05. b Significant difference from male mice at P = 0.01 when analyzed for percent control values.
Reduced Expression of Markers of Osteoblast Differentiation in FHL2 KO Cells
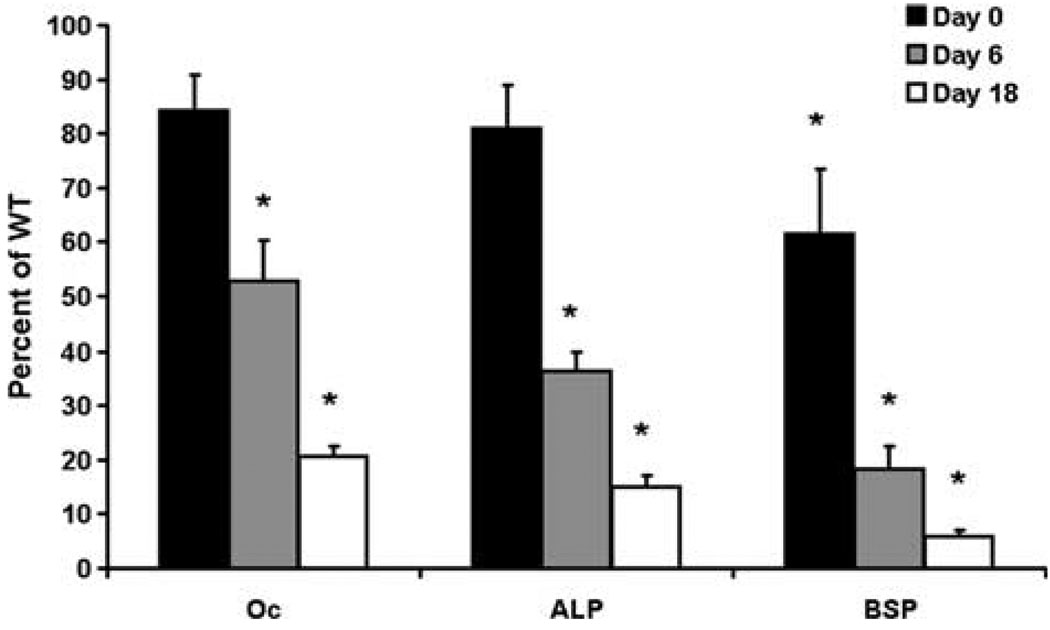
To determine if the reduced BMD was due to reduced differentiation of osteoblast cells, we looked at expression of markers of osteoblast differentiation during the differentiation of bone marrow stromal cells into osteoblasts in FHL2 KO and WT mice. Accordingly, expression of osteocalcin, ALP, and BSP was reduced 47–96% at days 6 and 18 of culture in FHL2 KO cells compared with WT cells (P < 0.001, Fig. 2). Expression of osteocalcin and ALP was similar between FHL2 KO and WT cells at day 0of cell culture (P ≥ 0.25). Since bone marrow stromal cells were pooled from male and female mice for our in vitro studies, we could not assess the functions of osteoblasts in male versus female KO mice.
Fig. 2.
mRNA expression of markers of osteoblast differentiation in FHL2-deficient cells. mRNA expression was determined by real-time RT-PCR. Oc, osteocalcin. Data are presented as mean ± standard error (n = 6/treatment/day). * Significant difference from WT (P < 0.001).
Discussion
FHL2 is an important regulator of growth and bone formation, as demonstrated by our novel findings that body weight and femur and tibia aBMD were reduced in female KO mice. This is not surprising since we know that FHL2 binds several factors which are important regulators of osteoblast proliferation and differentiation [6, 9, 11, 20]. In addition, our current findings are similar to those of a recent study in which mice deficient in FHL2 displayed characteristics of osteopenia [21]. Those authors also demonstrated that overexpression of FHL2 increased bone mass, as evaluated by histomorphometric analysis [21]. The degree of reduced bone formation and density observed between the two studies may be explained by the different methods employed (histological analysis vs. DXA and pQCT), later time points (1, 3, 6, and 9 vs. 1, 2, and 3 months of age), and different genetic backgrounds (C57BL/6 vs. Swiss black/129-SV/J and C57BL/6). Importantly, both studies demonstrate an important role for FHL2 in regulating bone formation.
During preparation of the current report, other results were published in which ovariectomized bone loss was accelerated in the absence of FHL2 [22]. Interestingly, those authors did not detect a reduction in total-body aBMD in control female mice between 1 and 4 months of age. Similarly, we did not observe a difference in total-body aBMD in female FHL2 KO mice (data not shown); however, we did observe a reduction in femur and tibia aBMD at 8 weeks of age in female mice, which was not reported in the previous study. Interestingly, in female mice, reductions in BMC and BMD were greater at 8 weeks than at 4 weeks of age. Puberty is a period of rapid growth, and these data suggest that FHL2 may be required for optimal bone development during this period. The deficit in BMD at some, but not other, skeletal sites in FHL2 KO mice may be explained by recent evidence that mechanisms that regulate bone accretion may vary depending on the skeletal site. Accordingly, it has been shown that the genetic loci that contribute to BMD variation are site-specific [29–34]. There are several potential explanations for the site-specific differences in the FHL2 effect: (1) FHL2 expression may vary depending on the site; (2) factors that bind to FHL2 may be expressed differently at various skeletal sites; and (3) other mechanisms could compensate for the loss of FHL2 at some, but not other, skeletal sites.
Another interesting finding in this study is the loss of FHL2-induced bone accretion in female, but not male, mice. Based on the findings that FHL2 binds to the AR, colocalizes with the AR in the nucleus of prostate epithelial cells, and increases the transcriptional activity of the AR [12], we anticipated that loss of FHL2 would affect bone accretion in male mice; however, that was not the case. In this regard, it is possible that androgen action in bone cells may not require FHL2. Further studies are needed to understand the molecular basis for gender-specific differences in FHL2 action.
FHL2 is highly homologous to FHL1 and -3, and the expression patterns of the three genes often overlap [13]. Therefore, we anticipated that lack of FHL2 would result in a compensatory overexpression of FHL1 and/or -3. However, similar to heart tissues, a difference in FHL1 and -3 expression was not observed in the bone and muscle tissues of KO compared to WT mice.
FHL2 is an important regulator of osteoblast function, as demonstrated by the reduced expression of markers of osteoblast differentiation. Our findings are consistent with the recent report by Lai et al. [22] as well as previous findings that FHL2 deficiency reduces osteoblast activity [21]. In addition, FHL2 overexpression increased osteoblast proliferation and differentiation [21]. FHL2 may regulate osteoblast activity by binding to factors involved in bone formation and acting as a coactivator. For example, we previously demonstrated that FHL2 binds to IGFBP-5 and that both translocate into the nucleus [11]. IGFBP-5 is an important regulator of osteoblast proliferation and differentiation; therefore, FHL2 may mediate bone formation through IGFBP-5 [20]. In addition, FHL2 binds to β-catenin and modulates the Wnt signaling pathway, an important mediator of bone development. However, if FHL2 is the sole intracellular mediator of these factors, we would anticipate a greater deficit in BMD in FHL2 KO mice. Therefore, FHL2 does not appear to be the sole mediator of these factors. However, based on the data presented here, FHL2 is required for optimal bone formation in female mice.
To identify the mechanistic basis for the gender-specific effect, we focused on IGFBP-5 expression in FHL2 KO mice based on our recent findings that transgenic overexpression of IGFBP-5 exerts gender-specific effects on bone density and bone formation parameters [35] and findings that estrogen regulates IGFBP-5 expression in multiple cell types [36–38]. Therefore, we tested the possibility that IGFBP-5 expression may be differentially affected in male versus female FHL2 KO mice, which could in part explain the observed gender difference. Our finding that IGFBP-5 expression was reduced in FHL2 female mice suggests that FHL2 action may be important in mediating estrogen effects. While FHL2 has been shown to interact with the AR [12], no such interaction between FHL2 and the estrogen receptor has been reported. Thus, whether FHL2 interacts with the estrogen receptor or components of the estrogen receptor signaling pathway to modulate IGFBP-5 expression and bone formation remains an interesting possibility which needs to be investigated.
Acknowledgment
This work was supported by National Institutes of Health grant AR31062. The authors thank Erica Winter and Catrina Raynor for technical assistance and Sean Belcher for secretarial assistance.
Footnotes
The information contained in this publication does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred. All work was performed in facilities provided by the Department of Veterans Affairs.
References
- 1.Genini M, Schwalbe P, Scholl FA, Remppis A, Mattei MG, Schafer BW. Subtractive cloning and characterization of DRAL, a novel LIM-domain protein down-regulated in rhabdomyosarcoma. DNA Cell Biol. 1997;16:433–442. doi: 10.1089/dna.1997.16.433. [DOI] [PubMed] [Google Scholar]
- 2.Chan KK, Tsui SK, Lee SM, Luk SC, Liew CC, Fung KP, Waye MM, Lee CY. Molecular cloning and characterization of FHL2, a novel LIM domain protein preferentially expressed in human heart. Gene. 1998;210:345–350. doi: 10.1016/s0378-1119(97)00644-6. [DOI] [PubMed] [Google Scholar]
- 3.Bach I. The LIM domain: regulation by association. Mech Dev. 2000;91:5–17. doi: 10.1016/s0925-4773(99)00314-7. [DOI] [PubMed] [Google Scholar]
- 4.Dawid IB, Breen JJ, Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 1998;14:156–162. doi: 10.1016/s0168-9525(98)01424-3. [DOI] [PubMed] [Google Scholar]
- 5.Morlon A, Sassone-Corsi P. The LIM-only protein FHL2 is a serum-inducible transcriptional coactivator of AP-1. Proc Natl Acad Sci USA. 2003;100:3977–3982. doi: 10.1073/pnas.0735923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei Y, Renard CA, Labalette C, Wu Y, Levy L, Neuveut C, Prieur X, Flajolet M, Prigent S, Buendia MA. Identification of the LIM protein FHL2 as a coactivator of beta-catenin. J Biol Chem. 2003;278:5188–5194. doi: 10.1074/jbc.M207216200. [DOI] [PubMed] [Google Scholar]
- 7.Yan J, Zhu J, Zhong H, Lu Q, Huang C, Ye Q. BRCA1 interacts with FHL2 and enhances FHL2 transactivation function. FEBS Lett. 2003;553:183–189. doi: 10.1016/s0014-5793(03)00978-5. [DOI] [PubMed] [Google Scholar]
- 8.Labalette C, Renard CA, Neuveut C, Buendia MA, Wei Y. Interaction and functional cooperation between the LIM protein FHL2, CBP/p300, and beta-catenin. Mol Cell Biol. 2004;24:10689–10702. doi: 10.1128/MCB.24.24.10689-10702.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin B, Schneider R, Janetzky S, Waibler Z, Pandur P, Kuhl M, Behrens J, von der MarkK, Starzinski-Powitz A, Wixler V. The LIM-only protein FHL2 interacts with beta-catenin and promotes differentiation of mouse myoblasts. J Cell Biol. 2002;159:113–122. doi: 10.1083/jcb.200202075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fimia GM, De Cesare D, Sassone-Corsi P. A family of LIM-only transcriptional coactivators: tissue-specific expression and selective activation of CREB and CREM. Mol Cell Biol. 2000;20:8613–8622. doi: 10.1128/mcb.20.22.8613-8622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amaar YG, Thompson GR, Linkhart TA, Chen ST, Baylink DJ, Mohan S. Insulin-like growth factor-binding protein 5 (IGFBP-5) interacts with a four and a half LIM protein 2 (FHL2) J Biol Chem. 2002;277:12053–12060. doi: 10.1074/jbc.M110872200. [DOI] [PubMed] [Google Scholar]
- 12.Muller JM, Isele U, Metzger E, Rempel A, Moser M, Pscherer A, Breyer T, Holubarsch C, Buettner R, Schule R. FHL2, a novel tissue-specific coactivator of the androgen receptor. EMBO J. 2000;19:359–369. doi: 10.1093/emboj/19.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu PH, Bardwell WM, Gu Y, Ross J, Jr, Chen J. FHL2 (SLIM3) is not essential for cardiac development and function. Mol Cell Biol. 2000;20:7460–7462. doi: 10.1128/mcb.20.20.7460-7462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell NH, Darwis D, Bueno OF, Muller JM, Schule R, Molkentin JD. Extracellular signal-regulated kinase 2 interacts with and is negatively regulated by the LIM-only protein FHL2 in cardiomyocytes. Mol Cell Biol. 2004;24:1081–1095. doi: 10.1128/MCB.24.3.1081-1095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci USA. 2003;100:9416–9421. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govoni KE, Baylink DJ, Mohan S. The multifunctional role of insulin-like growth factor binding proteins in bone. Pediatr Nephrol. 2005;20:261–268. doi: 10.1007/s00467-004-1658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan S, Nakao Y, Honda Y, Landale E, Leser U, Dony C, Lang K, Baylink DJ. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem. 1995;270:20424–20431. doi: 10.1074/jbc.270.35.20424. [DOI] [PubMed] [Google Scholar]
- 19.Andress DL. IGF-binding protein-5 stimulates osteoblast activity and bone accretion in ovariectomized mice. Am J Physiol Endocrinol Metab. 2001;281:E283–E288. doi: 10.1152/ajpendo.2001.281.2.E283. [DOI] [PubMed] [Google Scholar]
- 20.Richman C, Baylink DJ, Lang K, Dony C, Mohan S. Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology. 1999;140:4699–4705. doi: 10.1210/endo.140.10.7081. [DOI] [PubMed] [Google Scholar]
- 21.Gunther T, Poli C, Muller JM, Catala-Lehnen P, Schinke T, Yin N, Vomstein S, Amling M, Schule R. Fhl2 deficiency results in osteopenia due to decreased activity of osteoblasts. EMBO J. 2005;24:3049–3056. doi: 10.1038/sj.emboj.7600773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai CF, Bai S, Uthgenannt BA, Halstead LR, McLoughlin P, Schafer BW, Chu PH, Chen J, Otey CA, Cao X, Cheng SL. Four and half Lim protein 2 (FHL2) stimulates osteoblast differentiation. J Bone Miner Res. 2006;21:17–28. doi: 10.1359/JBMR.050915. [DOI] [PubMed] [Google Scholar]
- 23.Stabnov L, Kasukawa Y, Guo R, Amaar Y, Wergedal JE, Baylink DJ, Mohan S. Effect of insulin-like growth factor-1 (IGF-1) plus alendronate on bone density during puberty in IGF-1-deficient MIDI mice. Bone. 2002;30:909–916. doi: 10.1016/s8756-3282(02)00738-x. [DOI] [PubMed] [Google Scholar]
- 24.Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest. 2001;107:73–81. doi: 10.1172/JCI10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohan S, Kutilek S, Zhang C, Shen HG, Kodama Y, Srivastava AK, Wergedal JE, Beamer WG, Baylink DJ. Comparison of bone formation responses to parathyroid hormone(1–34), (1–31), and (2–34) in mice. Bone. 2000;27:471–478. doi: 10.1016/s8756-3282(00)00355-0. [DOI] [PubMed] [Google Scholar]
- 26.Govoni KE, Amaar YG, Kramer A, Winter E, Baylink DJ, Mohan S. Regulation of insulin-like growth factor binding protein-5, four and a half lim-2, and a disintegrin and metalloprotease-9 expression in osteoblasts. Growth Horm IGF Res. 2006;16:49–56. doi: 10.1016/j.ghir.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govoni KE, Lee SK, Chadwick RB, Yu H, Kasukawa Y, Baylink DJ, Mohan S. Whole genome microarray analysis of growth hormone induced gene expression in bone: T-box3, a novel transcription factor, regulates osteoblast proliferation. Am J Physiol Endocrinol Metab. 2006;291:E128–E136. doi: 10.1152/ajpendo.00592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorak MT. Real-time PCR. 2005 http://dorakmt.tri-pod.com/genetics/realtime.html.
- 29.Deng HW, Shen H, Xu FH, Deng HY, Conway T, Zhang HT, Recker RR. Tests of linkage and/or association of genes for vitamin D receptor, osteocalcin, and parathyroid hormone with bone mineral density. J Bone Miner Res. 2002;17:678–686. doi: 10.1359/jbmr.2002.17.4.678. [DOI] [PubMed] [Google Scholar]
- 30.Deng HW, Stegman MR, Davies KM, Conway T, Recker RR. Genetic determination of variation and covariation of peak bone mass at the hip and spine. J Clin Densitom. 1999;2:251–263. doi: 10.1385/jcd:2:3:251. [DOI] [PubMed] [Google Scholar]
- 31.Akhter MP, Iwaniec UT, Covey MA, Cullen DM, Kimmel DB, Recker RR. Genetic variations in bone density, histomorphometry, and strength in mice. Calcif Tissue Int. 2000;67:337–344. doi: 10.1007/s002230001144. [DOI] [PubMed] [Google Scholar]
- 32.Bouxsein ML, Rosen CJ, Turner CH, Ackert CL, Shultz KL, Donahue LR, Churchill G, Adamo ML, Powell DR, Turner RT, Muller R, Beamer WG. Generation of a new congenic mouse strain to test the relationships among serum insulin-like growth factor I, bone mineral density, and skeletal morphology in vivo. J Bone Miner Res. 2002;17:570–579. doi: 10.1359/jbmr.2002.17.4.570. [DOI] [PubMed] [Google Scholar]
- 33.Bouxsein ML, Uchiyama T, Rosen CJ, Shultz KL, Donahue LR, Turner CH, Sen S, Churchill GA, Muller R, Beamer WG. Mapping quantitative trait loci for vertebral trabecular bone volume fraction and microarchitecture in mice. J Bone Miner Res. 2004;19:587–599. doi: 10.1359/JBMR.0301255. [DOI] [PubMed] [Google Scholar]
- 34.Beamer WG, Shultz KL, Donahue LR, Churchill GA, Sen S, Wergedal JR, Baylink DJ, Rosen CJ. Quantitative trait loci for femoral and lumbar vertebral bone mineral density in C57BL/6J and C3H/HeJ inbred strains of mice. J Bone Miner Res. 2001;16:1195–1206. doi: 10.1359/jbmr.2001.16.7.1195. [DOI] [PubMed] [Google Scholar]
- 35.Salih DA, Mohan S, Kasukawa Y, Tripathi G, Lovett FA, Anderson NF, Carter EJ, Wergedal JE, Baylink DJ, Pell JM. Insulin-like growth factor-binding protein-5 induces a gender-related decrease in bone mineral density in transgenic mice. Endocrinology. 2005;146:931–940. doi: 10.1210/en.2004-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasu M, Sugimoto T, Kaji H, Chihara K. Estrogen modulates osteoblast proliferation and function regulated by parathyroid hormone in osteoblastic SaOS-2 cells: role of insulin-like growth factor (IGF)-I and IGF-binding protein-5. J Endocrinol. 2000;167:305–313. doi: 10.1677/joe.0.1670305. [DOI] [PubMed] [Google Scholar]
- 37.Coutts A, Murphy LJ, Murphy LC. Expression of insulin-like growth factor binding proteins by T-47D human breast cancer cells: regulation by progestins and antiestrogens. Breast Cancer Res Treat. 1994;32:153–164. doi: 10.1007/BF00665766. [DOI] [PubMed] [Google Scholar]
- 38.Krywicki RF, Figueroa JA, Jackson JG, Kozelsky TW, Shimasaki S, Von Hoff DD, Yee D. Regulation of insulin-like growth factor binding proteins in ovarian cancer cells by oestrogen. Eur J Cancer. 1993;29A:2015–2019. doi: 10.1016/0959-8049(93)90464-q. [DOI] [PubMed] [Google Scholar]