Introduction
The Gross Room is the area where pathology specimens from the operating rooms are transferred for pathological review and analysis. Thus, it serves as the bridge between the surgeon and diagnostic surgical pathologist in that a correct diagnosis or treatment of a patient depends upon the proper handling and processing of the specimens of tissue transferred to this very busy area. With the many new approaches to the management of Gross Rooms, including increased ancillary testing, the increased collection of tissues to support research, and new health care regulations such as HIPAA, it is useful to review the basic function and management of the Gross Room.
Gross Room Personnel
Anatomic pathologists are physicians who are trained in diagnosing disease based on gross and histologic examination of tissues. Anatomic pathologists are certified by the American Board of Pathology and in addition to their training for primary certification, often have additional fellowship training in surgical pathology or another pathology subspecialty (Bennett 2006). Surgical pathologists deal with many types of tissues and numerous specimens on a daily basis and each of these specimens must be evaluated carefully no matter how large or small the specimen. An accurate diagnosis by the pathologist cannot be rendered other than by handling and processing each specimen with great care with attention to proper specimen identification. The pathologist plays a vital role in patient care and his/her decisions are essential to the patient’s clinical outcome. The pathologist is aided by several health care professionals, including pathologists’ assistants and histotechnologists, and other gross room technicians, who come from a great variety of educational backgrounds. Their diligent work with the pathologist is also critical to a correct diagnosis. Each must pay close attention to the details of the specimen to ensure that the best diagnosis is rendered. Pathologists’ assistants play an increasingly important role in the modern Gross Room. They are allied health professionals with training programs accredited by the National Accrediting Agency for Clinical Laboratory Sciences (NACCLS) and contribute to the cost effective management of a pathology laboratory by working under the supervision of a pathologist to carry out tasks such as specimen accessioning, obtaining clinical data, dissection and gross examination of surgical specimens, preparing tissues for histologic processing, photography of specimens, submission of specimens for special studies, and administrative and teaching functions (Neri and Keshgegian 1986; Enriquez and Kelly 1991).
Specimen Identification
Most institutions have their own unique way of specimen identification giving each patient and tissue unique accession numbers which usually include the year the specimen was collected with varying prefixes for different types of specimens. For example, S06-1245 might represent the 1245th general surgical pathology specimen received in 2006. If multiple specimens are received on the same patient from the same operation/procedure, all specimens from one patient are usually given the same number followed by a numerical or alphabetical designation. For example, S05-20024-B3 may represent the third aliquot (tissue block) of the second tissue specimen received from the 20024th specimen collected in 2005. The specific number and letter designations for each case, specimen, and tissue sample will be used to label tissue blocks and the histologic sections obtained from them. Other letter prefixes might be used to designate the type of specimen, such as DP for dermatopathology specimens and G for gynecologic pathology specimens. These unique numbers are usually assigned by the pathology information system and to some extent the format may be determined or limited by the system in use.
The most important step in specimen handling is the correct identification of the specimen(s) with unique numbers so that an accurate link between the specimen and the patient from whom the specimen was removed is maintained. Each specimen container should include the patient’s name with age (birth date), a medical record number along with matching paper work (e.g., a surgical request form). These labels must be consistent and should be on the container so that the labels cannot be separated from the specimen (e.g., labels should not be attached just to the top of the container). The surgical request form provides the actual request for pathological services and the required relevant clinical history of the patient. Any discrepancies in specimen identification/labeling (e.g., a medical record number that does not match the patient name or a surgical request form which does not match the specimen) must be resolved with the clinician/surgeon or a supervisory nurse prior to processing any specimen and the discrepancy should be noted on the request form. Misidentification of any specimen can result in failure to make a proper diagnosis on one or more patients, incorrect treatment and possibly legal action. In one large study of medicolegal claims reported by an insurance company, misidentification of specimens accounted for 59% of identified operational errors (Troxel 2004). Since pathologists can easily differentiate different types of tissue histologically, most cases of misidentification are noted early and corrected prior to release of the pathology report. Problems arise when similar specimens are misidentified as can occur when tissues of the same type are accessioned sequentially. These errors most often involve breast and prostate biopsies (Troxel 2006). When possible, laboratories should avoid accessioning and grossing specimens of the same tissue type consecutively. This may not be possible in specialty laboratories or when large numbers of specimens arrive from a single specialty clinic at one time.
Most Gross Rooms currently use bar codes to identify specimens and hence reduce the chances of incorrect identification of specimens. Scanning the bar code permits the pathology information system to provide all needed patient information including name, age, sex and race/ethnicity. The basic demographics of age, sex and race/ethnicity are especially important in that diseases may vary with these demographic parameters such as osteosarcoma, retinoblastoma, colorectum, and melanoma (Manne et al. 2000).
When the labels of specimen containers contain patient identifiers such as name, initials, hospital number, surgical pathology number, etc., these containers constitute a source of protected health care information as per HIPAA (Anonymous 2002). All gross room personnel should be educated as to the importance of confidentiality regarding patient information which they encounter. Thus, pathologic specimens constitute both a potential biohazard as well as source of confidential medical information, and they should be disposed of appropriately.
Gross Examination of Specimens
Grossing of a specimen should proceed only after a decision is made on how the specimen should be processed; this is based on the specimen as well as the clinical history provided on the specimen request form. For some tissues, such as parathyroid, the gross parameters of size and weight may be more important than the histologic findings in determining the correct diagnosis (Bell 2005). Tissue specimens may range from tiny biopsies to large complete resections. They may be small pieces of bladder, bone marrow, breast, or larynx. These can be unidentifiable as to anatomic site; therefore gross descriptions are very important. The number of fragments must be documented, the type of biopsy, e.g., shave, needle or core biopsies, and whether the specimens represent tissue or even foreign material. In processing the contents of a container, one should record the number of fragments and try not to use terms such as multiple or numerous. If one sees only a few fragments, the dimensions of each can be specified. This will help in sign-out to ensure all tissue has been examined histologically. The features of the biopsy should be recorded for color and consistency and it should be noted if these appear to be tissue or other material such as mucous or blood clot.
In processing tissues, especially if they are fresh, the tissues should never be placed on or be placed in contact with dry, absorbent material. Dry paper towels, sponges, or cloth towels immediately desiccate tissues and desiccated tissues lose nuclear detail. This is especially the case with hematopoietic tissues such as lymph nodes. Cut tissues on non-absorbent surfaces (e.g., metal or plastic) or wet paper or cloth moistened with physiological saline.
The Concept of Stage in Gross Pathology
For malignant processes, it is important to understand the concept of “stage.” In general, stage represents the extent of a neoplastic process in a patient. The range of stage is from Stage 0, a pre-invasive neoplastic process, e.g., in situ carcinoma within a colorectal adenoma, to Stage IV, a distant metastasis, e.g., colorectal metastasis to the liver. The subcomponents of stage are the local characteristics of the primary tumor (tumor size and local features of spread), designated, pT; the extent of metastases to lymph nodes (pN) and the state of distant metastasis (pM). The stages were originally derived to aid in predicting the clinical outcome of specific cancers, but actually are more of a measure of time of progression of the neoplastic process and are used clinically to determine appropriate therapy. Molecular biomarkers are more and more likely to replace stage in determining prognosis; however, it is essential that the gross processing of specimens permits the accurate pathological staging of neoplastic lesions. The American Joint Committee on Cancer has developed the staging criteria for malignancies of all organs, and the AJCC Staging Manual is used to determine stage for all cancers in the United States (AJCC 2002). For example, in the case of colorectal cancers the following considerations apply: pT is a measure of the depth of invasion of a tumor into or through the colorectal wall and into adjacent tissues; pN is a nodal metastasis of the tumor (pN0 = none, pN1 = 1–3 nodes involves, and pN2 = 4 or more nodes involved). pM indicates whether or not there are distant metastases. The pathologist frequently cannot determine pM unless the surgeon biopsies distant lesions; however, the dissector should carefully identify lymph nodes since pN > 0 changes the stage of the lesion.
Evolving Concepts Regarding Lymph Node Involvement
For malignant processes which metastasize via lymph nodes such as breast, colon, and melanoma, the concept of monitoring spread of the tumor using metastasis to sentinel lymph nodes has evolved. The sentinel node is the first lymph node draining lymphatic fluid from the tumor, and therefore if tumor cells are metastasizing through the lymphatics, the sentinel node is usually the first lymph node involved. The sentinel lymph node is identified by lymphoscintigraphy which involves injecting the tumor with dye and a radioactive isotope. The radioactive dye travels to the sentinel node which can be detected by color change and/or by gamma probe (Hunt et al. 2002; Bertagnolli et al. 2004; Thompson and Uren 2004). Processing of sentinel nodes varies from institution to institution and depends on site of the primary tumor. General recommendations are to serially section the nodes for histologic processing and examine multiple levels histologically. In some cases immunohistochemical stains may be used to highlight small foci of tumor within sentinel nodes (ADASP 2001; Hunt et al. 2002; Bertagnolli et al. 2004; Rivera et al. 2004; Spanknebel et al. 2005; Redston et al. 2006).
Additionally, data are emerging regarding the minimal number of nodes which should be examined in order for nodal status to be determined accurately. Current data suggest 12 lymph nodes should be considered the minimum acceptable harvest from a colorectal carcinoma specimen for adequate staging (Compton 2006). Of course, the standard is that all nodes that can be identified should be submitted from such specimens. Clearing agents may be useful in grossly identifying small mesenteric lymph nodes embedded in fat.
Gross Description and Dissection
Most institutions have developed standardized formats for dictating the gross description of specific specimens/lesions. Additionally there are several reference texts with protocols for grossing specimens of all types (Westra et al. 2003; Lester 2005), guidelines from the Association of Directors of Anatomic and Surgical Pathology for many organs(ADASP 1995a,1995b,1996c,1996b,1996a,1997,2000a,2000b,2001,2003,2005), and excellent independent reviews on the approach to gross dissection and description of specific organs (Young and Castro 2002; Young et al. 2005). In following such formats of gross description, it becomes fairly clear how specific specimens are to be grossed in order to match their dictation. However, dissecting a large specimen can be confusing. If proper orientation is not achieved, the specimen could be grossed incorrectly, margins may be confused, and inaccurate diagnoses rendered. Thus, use of drawings and photographs to indicate the source of sections can be useful. Similarly, it may be useful to contact the surgeon/clinician to ensure proper orientation of complex specimens. It is important to remember that each specimen no matter how large or small should lead to a further treatment or cure.
There are seven major components in processing a gross specimen:
Reliable and rapid transfer of the specimen from surgery to pathology
Accurate identification of all specimens
Accurate description of original specimens
Accurate description of additional specimens received from the same patient - operation
Recording normal and abnormal features of the specimen including markers (e.g., sutures) which orientate the specimens.
Special studies requested and/or needed
The location from which specific sections of tissue are taken for histologic evaluation
If and only if specimens are properly grossed, can a surgical pathologist expect proper processing. In today’s busy medical care system, it is not only the pathologist but also residents, pathologists’ assistants, and trained histotechnologists who may be grossing specimens; all work together to achieve a common goal of optimal patient care.
General Approaches to Common Surgical Specimens
Small Biopsies
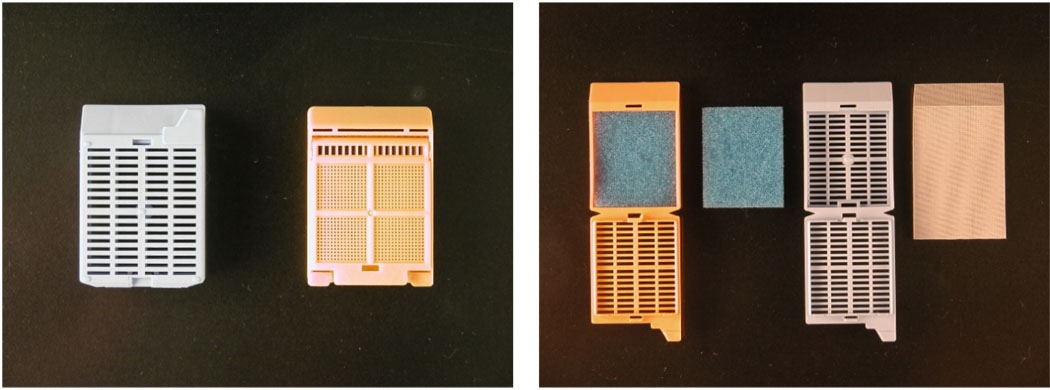
Very small specimens should not be cut or bisected while fresh because the accurate cutting of fresh specimens can be very difficult and an irregular cut may cause problems in embedding. Small specimens must be processed either in cassettes with a fine mesh, or in lens paper or a “tea bag” so that the specimen cannot be lost in processing, especially during the transit of the cassette through the tissue processor (Figure 1). Sponges are an alternative, but the sponge may dry tissues and tiny fragments of tissue may dry, harden, and stick to the sponge. However, if the biopsy is large enough, such as a colon or skin biopsy, processing the specimen using sponges may help with orientation. For all processing aids, make sure the papers and sponges are wet with the fixative of choice.
Figure 1.
This figure demonstrates some of the supplies that can be used for processing small specimens.Panel A demonstrates a standard cassette used in tissue processing as compared to a cassette with very small holes permitting fluid exchange but minimizing the likelihood of loss of small specimens. Note that air bubbles may form in this type of cassette and air bubbles may cause inhomogeneous processing of tissue. Panel B demonstrates the use of sponges in a standard cassette and a “tea bag” to minimize the likelihood of specimen loss during tissue processing.
Dermatologic Specimens
Skin specimens, both biopsies and resections, represent some of the most frequent and surprisingly complex specimens regarding attention to detail received in the surgical pathology gross room. In the gross examination of dermatology specimens, four major issues should be considered. These include size in that the dimensions of some specimens may be small, so great care should be taken to prevent their loss either during processing and embedding or after inappropriate embedding. Second, specimens require careful orientation to determine the depth of invasion of specific lesions and the margins of resection. Core biopsies deserve special attention to cutting and to orientation because it may be difficult to visualize some lesions on core biopsies, so the. Pigmented lesions may represent melanomas so they should be processed carefully to demonstrate the maximum thickness of the lesions.
Dermatology specimens may be excisional biopsies, shave biopsies, core biopsies, re-excision specimens, or specimens that represent a complete excision. Each type of specimen should be handled differently. As with other small biopsies, very small specimens of skin should not be bisected; instead, the whole specimen should be embedded in total on edge. The personnel handling the grossing of the specimen should note the small dimensions of the specimen on the gross sheet and should note that the specimen was embedded on edge.
Punch Biopsies
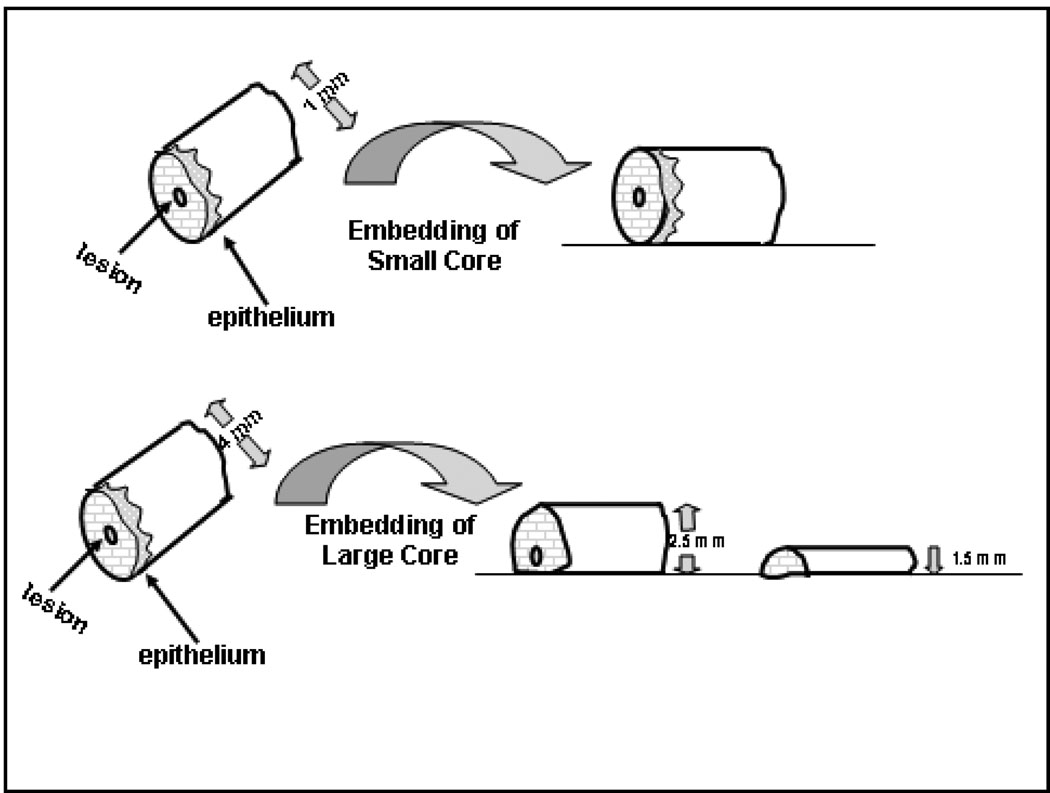
Punch biopsies usually are taken of a larger lesion or of a diffuse inflammatory or other disease process. The punch biopsy should be taken so that the center of the punch biopsy is the lesion of interest. Thus, for larger cores, e.g., ≥4mm, the biopsy should be bisected eccentrically, perhaps 2/3 and 1/3 and the specimen embedded totally with cut surfaces down. This permits the initial paraffin sections from the specimen to sample the center of the core and ensures lesions at the center of the core are not missed. For small core biopsies, ≤ 2mm, bisecting the core may damage it (e.g., the surface epithelium may be lost) so that such cores are embedded totally without cutting (Figure 2). Occasionally large punch biopsies may be submitted in total on edge (as with bullous lesions) or even en face as in the case of alopecia studies.
Figure 2.
This figure represents an approach to processing punch biopsies which are typical cores ranging in diameter from 1mm to 5mm. The center of the punch usually represents the lesion. Small punches (top) should not be cut, but should be embedded on their side because cutting prior to processing is likely to result in missing the lesion. Larger punches (bottom) should be cut eccentrically and both parts embedded, cut side down, to best ensure that the central lesion is captured in initial levels.
Shave biopsies
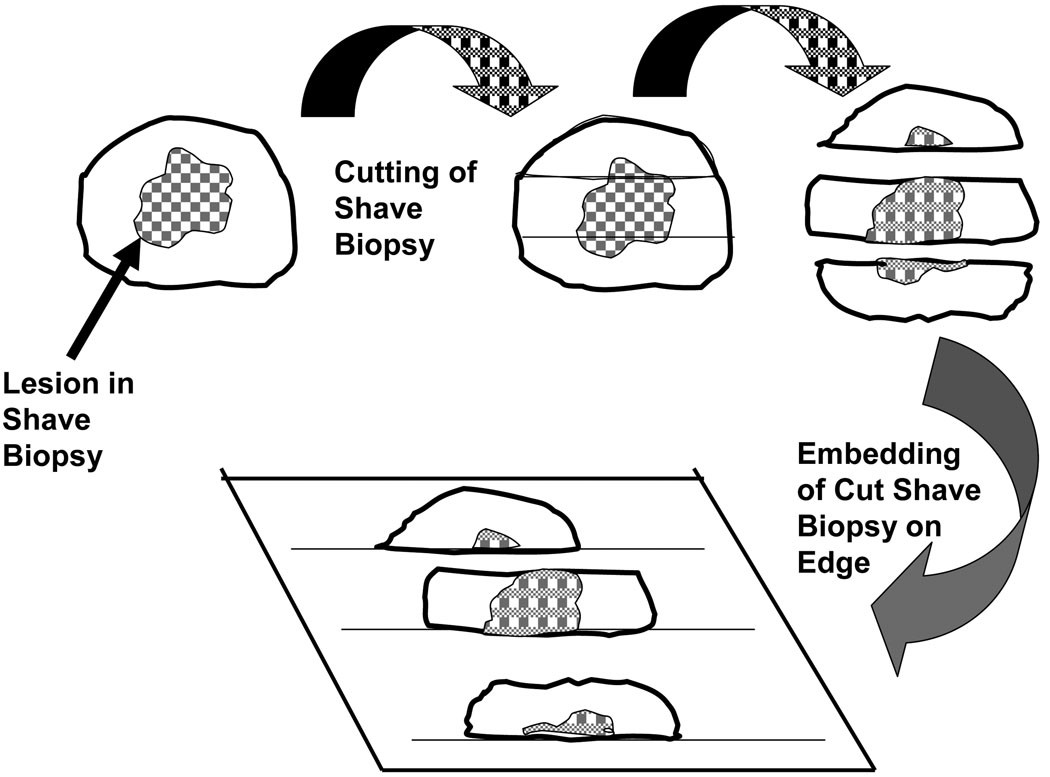
Shave biopsies are usually obtained to remove and/or sample specific lesions of the skin. Frequently, after the lesion is “cut away” the base of the lesion is treated further, for example, by cauterization. Thus, even if the lesion extends throughout the shave biopsy, the lesion may have been treated effectively. Nevertheless, shave biopsies are not equivalent to excisional biopsies. Depending upon the size of the shave biopsy, it may be bisected, trisected or cut into more sections. In general most specimens of skin or other relatively thin epithelial surfaces should be cut so that all aliquots are easily embedded on edge (Figure 3).
Figure 3.
This figure demonstrates one approach to a shave biopsy. Un-orientated shave biopsies usually are not taken to demonstrate margins. A large shave biopsy can be trisected and embedded on edge to best demonstrate the whole lesion.
Great care should be taken with any pigmented lesions of the skin. Although excision rather than shave biopsy is the method of choice for surgical removal of melanomas, sometimes melanomas are removed by shave biopsies. Because the width of the melanoma and depth of invasion are of prognostic importance, the shave biopsy should be processed to demonstrate the thickness of the lesion. Great care should be taken to cut the specimen eccentrically so that the thickest part of the lesion can be evaluated.
Skin Excisions
Excisional biopsies or wide excisions of the skin or other epithelial surfaces (e.g., oral mucosa) are obtained to ensure the lesion of interest is removed completely and correctly diagnosed. Excisional biopsies may be orientated using sutures, dyes, or other means. When the specimen is orientated, the margins should be taken and labeled with respect to the orientation and the margins should be marked prior to grossing with indelible ink. This will be useful if a tumor comes close but does not involve a margin. It also is useful to draw or photograph the specimen to maintain a record of orientation (Figures 4 and 5).
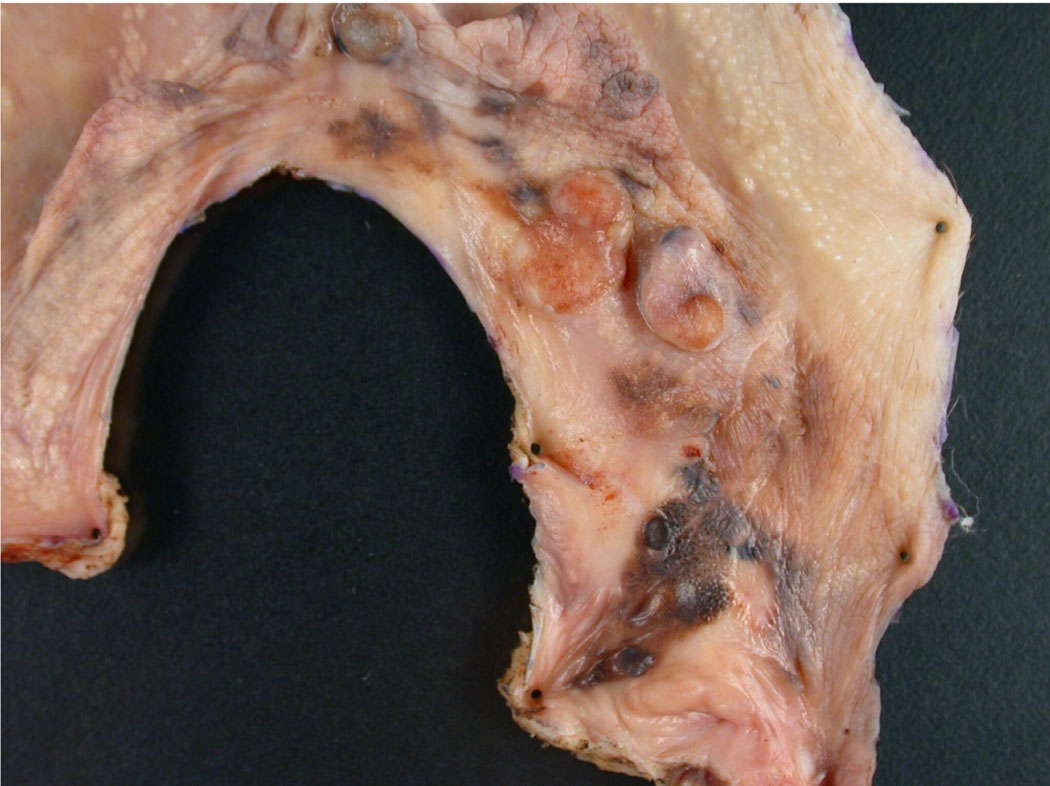
Figure 4.
This is a photograph of a primary vulvar melanoma. Grossly, the lesion shows variable pigmentation in an irregular distribution with focal polypoid tumor growth. Due to the irregular borders in this specimen it would be essential to diagram on a photograph or drawing the location from which sections are taken so that margins can be fully assessed and the exact location of any positive margins can be effectively communicated to the surgeon. Also important in this case is adequate sampling for measurement of maximal depth of invasion which will determine the pT for the melanoma.
Figure 5.
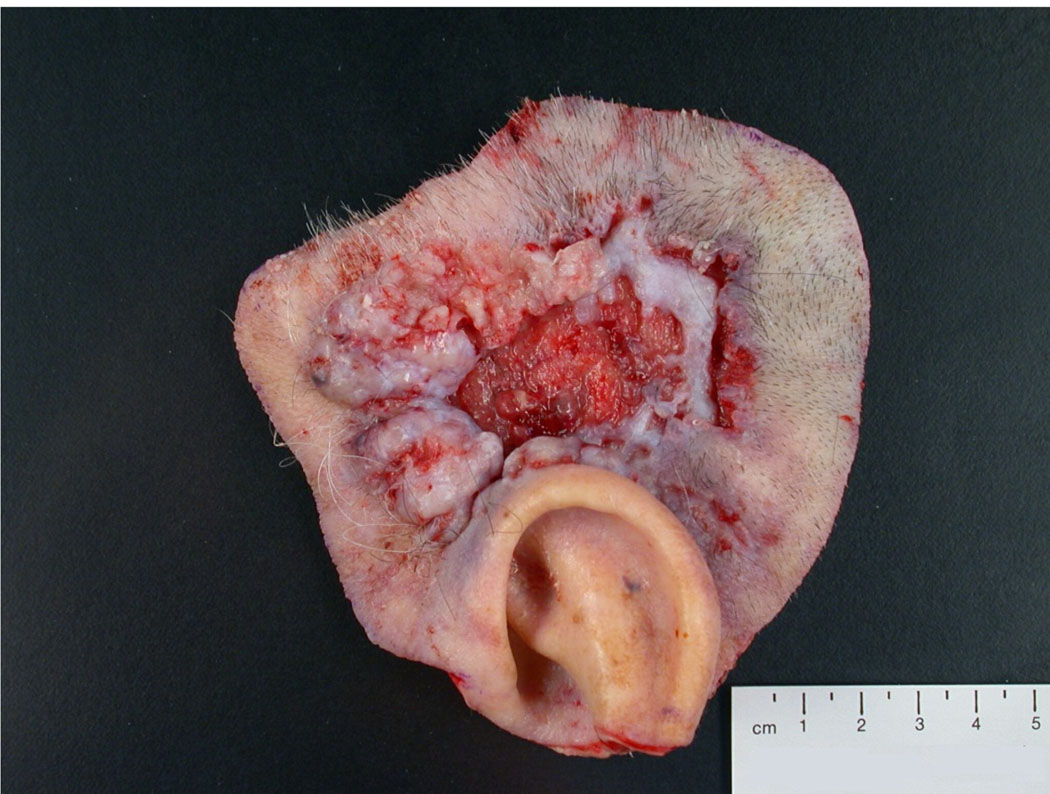
This is a photograph of a wide local excision of a large ulcerated basal cell carcinoma above the ear. The specimen can be properly oriented based on the anatomic marker provided by the upper ear. For this specimen, inking of margins and thorough sampling of the skin and deep margins is necessary to assure complete removal of this large tumor.
Re-excision Specimens
If margins are involved by tumor at the initial excision or for close margins for melanomas, the original area of the lesion may be re-excised. In such re-excisions, the area of the scar is of interest as are the new margins. In the case of a diagnosis of melanoma via biopsy, a wide re-excision is performed even if the margins of the original excision are histopathologically free of tumor. Re-excision is performed because there is rapid and extensive radial spread from the original primary tumor and this radial spread may not be observed in the original description of the lesion. The radial spread may even produce satellite lesions. In all re-excisions, the scar is evaluated carefully to determine if residual disease is present. Similarly, the new margins are evaluated carefully for both surface satellite and metastatic lesions.
Non-Skin Specimens
Excisional Specimens
Excision specimens from areas other than skin may be quite complex. In most cases, depth of invasion is important as well as determining the overall size of the tumor. Other features to evaluate are involvement of lymph nodes, metastases to peritoneal or pleural surfaces, invasion of bones, depth of invasion into walls or through walls, and involvement of the surgical margins. It is critical to understand the orientation of the specimen including determining what are the true margins of the specimen. Also, one should understand the disease process as it is important to know the pattern of metastases and how various cancers typically cause the death of patients. For example, breast cancer typically metastasizes to local lymph nodes prior to spreading to bone and/or brain which are typically terminal sites. In contrast, while colorectal cancer also metastasizes to local lymph nodes, metastases to the liver result in patient mortality via liver failure or by peritoneal metastases and subsequent intestinal obstruction (Figure 6). Ovarian epithelial cancers also kill patients via peritoneal spread, malignant ascites and intestinal obstruction but rarely do ovarian epithelial cancers metastasize outside the peritoneal space (abdomen and pelvis). Understanding the disease process permits the characterization of such features of the specimen which are important to the disease process. Thus, the importance of identifying peritoneal implants or malignant cells in peritoneal fluid in women with ovarian cancer becomes clear.
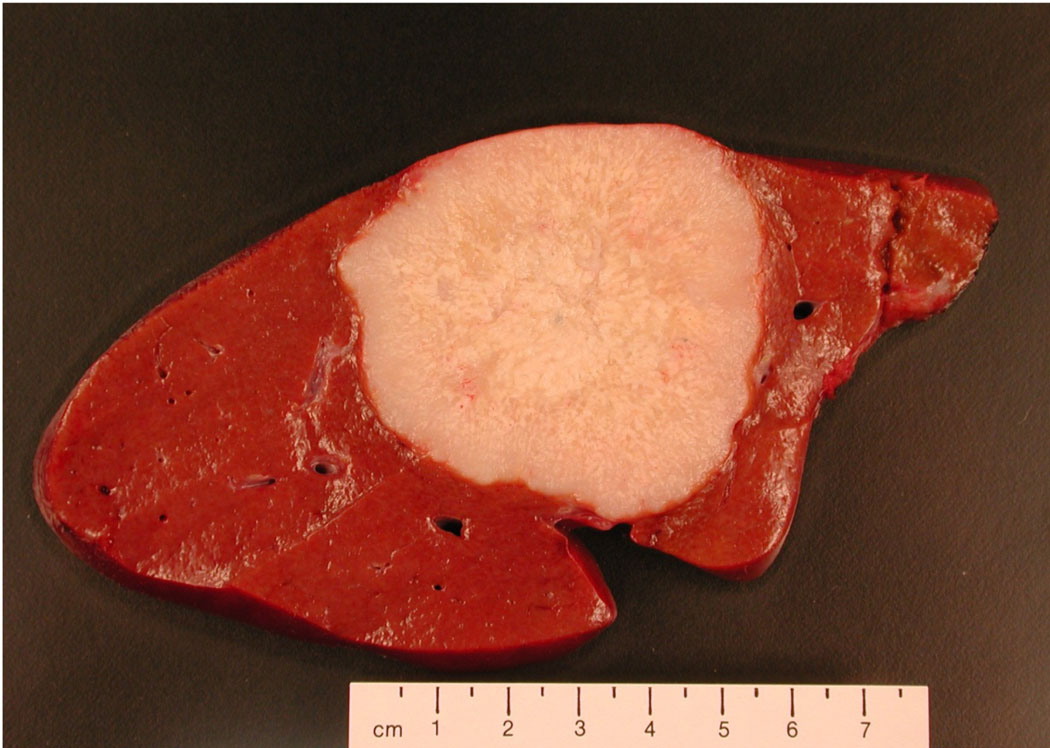
Figure 6.
Photograph of colonic carcinoma metastatic to the liver. Knowledge of the patient’s clinical history and the natural history of disease are important for development of an appropriate differential diagnosis at the gross level and in arriving at the correct final diagnosis.
Colorectal Carcinoma
An adequate pathological examination of an adenocarcinoma of colorectum should involve orientation of the specimen using markers such as suture placed by the surgeon or anatomic markers such as ileocaecal valve and appendix for cecal resections (Figure 7). Margins, including resection margins and the peritoneum beneath the tumor should be examined for tumor involvement. The size and depth of tumor invasion, especially invasion into adjacent tissues, and all lymph nodes and areas of peritoneal involvement should be evaluated. At least two sections and one section per cm of tumor size (up to 5 cm) including the area of deepest invasion should be taken to determine the histopathology (cellular grade) of the tumor.
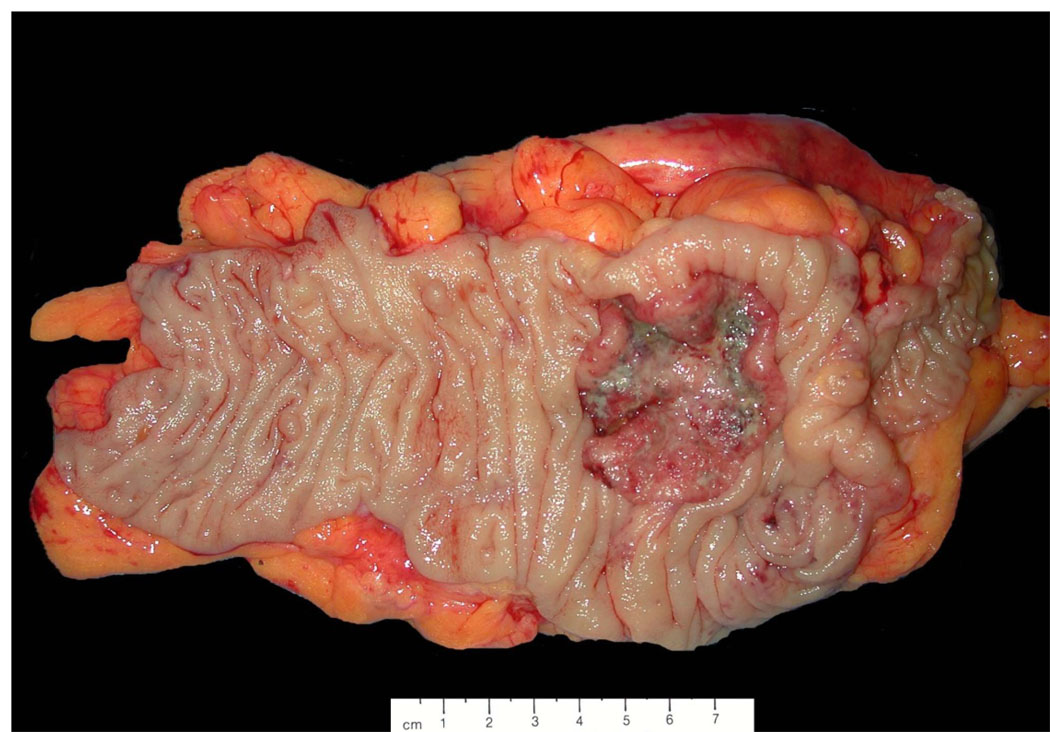
Figure 7.
Photograph of a primary cecal carcinoma. The specimen can be oriented based on gross identification of the terminal ilium (far right), ileocaecal valve, and appendix if present. Sections should be taken for histologic analysis to demonstrate the depth of invasion through the wall of the cecum (pT) and marginal status, and the associated adipose tissue should be carefully searched to find all lymph nodes for histologic examination (pN).
Breast Carcinoma
In ductal carcinoma of the breast, similar parameters of stage including location, size, skin or nipple involvement, and marginal and nodal status are important in determining prognosis. Additionally, biomarkers such as estrogen receptor (ER) and progesterone (PR) status, proliferation (Ki67-MIB-1) and p185 erbB-2 may also be important in determining prognosis and therapy for a particular patient. These markers may be affected by the method and length of fixation, and it is important at the time of grossing to be aware of these issues (Arnold et al. 1996; Arber 2002; Selvarajan et al. 2002; Hashizume et al. 2003).
Lung Carcinoma
Another common cancer encountered in surgical resections is non-small cell lung carcinoma. Small cell carcinoma is unsually diagnosed by biopsy and treated with chemotherapy rather than with surgical resection. In grossing lung carcinoma, in addition to tumor size and status of lymph nodes, it is also important to document whether the overlying pleura is involved by tumor and to sample the bronchial margin (Figure 8). Careful examination should be conducted to determine if there is more than one lesion.
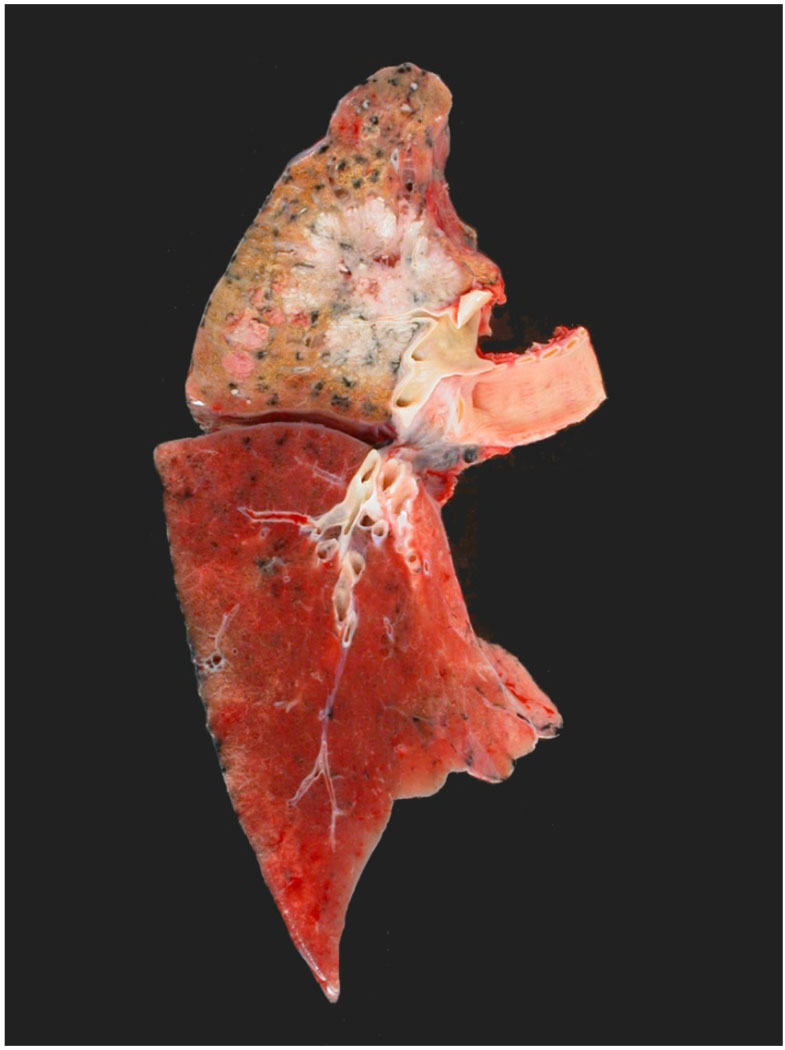
Figure 8.
Photograph of an upper lobe non-small cell carcinoma. Staging considerations include the size of the tumor, presence or absence of pleural involvement, and lymph node status.
Prostate Carcinoma
One of the major issues in evaluating prostatic adenocarcinoma (PCa) is that PCa frequently cannot be distinguished easily from benign prostatic tissues on gross examination. Also, PCa readily infiltrates benign prostatic tissue and adjacent tissues (e.g., seminal vesicles). Thus, it is difficult to ensure that sections taken to demonstrate PCa actually contain tumor. To insure adequate sampling, initially sections should be obtained at multiple sites in each lobe. In addition, the extent of involvement of each lobe is an important component of prostate cancer staging as is the extension of tumor beyond the prostate. Sections should include the capsule which has ink applied to mark the margins. When the location of the tumor is identified, additional sections can be obtained to further clarify the lateral margins. Sections may be taken for research away from the margins. These can be held until diagnosis is confirmed. Also, the quality control slides from the research specimens may be provided to aid in diagnosis. Other margins include the bladder, urethra, vas deferens, and seminal vesicle. Nodal involvement and bone involvement usually are evaluated before a radial prostatectomy is performed.
Pediatric Specimens
The gross examination and processing of pediatric tumors also requires special care(Debski et al. 2004). Because many pediatric tumors appear histologically similar (small round blue cell tumors), they must be separated diagnostically based upon ancillary studies (Devoe and Weidner 2000; Peydro-Olaya et al. 2003). Pediatric tumors are rare and unless practicing in a specialized pediatric hospital, the personnel who staff the gross room may not have experience with them, and may not be familiar with the required ancillary studies that are usually necessary to aid in their diagnosis and to help in determining prognostic factors. Ancillary studies may include immunohistochemistry, electron microscopy, flow cytometry, cytogenetics and molecular genetics. Such studies also may be necessary to determine therapy and/or to enter the patient into clinical protocols. These ancillary/special studies may require fresh/frozen and/or specially processed tissues. Also, excellent photography of the gross specimen is required with clear demarcations as to where specimens are obtained for diagnostic examination and for ancillary studies.
Prior to resection, a working diagnosis should be made based on histology and demographics of the patient, clinical presentation, laboratory results and radiographic features. An approach to identifying ancillary studies should be developed, especially the requirements of special studies needed for clinical trials under which the patient may be treated. Specifically, the laboratories performing these special studies should be contacted as to optimal tissue handling.
Specific protocols developed by the College of American Pathologists have been published for some pediatric tumors including hepatoblastoma, neuroblastoma, Ewing’s sarcoma, Wilms tumor, retinoblastoma, and rhabdomyosarcoma (Albert and Syed 2001; Qualman et al. 2003a; Qualman et al. 2003b; Carpentieri et al. 2005; Qualman et al. 2005; Finegold et al. 2006). (Devoe and Weidner 2000; Peydro-Olaya et al. 2003).
Gross Only Examination
Some specimens are not examined microscopically and therefore are only examined grossly (Zarbo and Nakhleh 1999). Examples of non-tissue components include bullets, implants, foreign bodies, etc., and in some institutions may include tissues which are unlikely to require a detailed diagnosis (e.g., tonsils, traumatic injuries and incidental resections such as ribs, fat, and vessels). For some non-tissue specimens, e.g., bullets, it may be necessary to maintain a chain of evidence and there should be a written procedure to ensure a chain of evidence is maintained as well as proper security during stroage. All organizations should develop guidelines to deal with non-diagnostic specimens and these must be followed as well as rules and regulations in accordance with CAP, JCHO, HIPAA, OSHA and any other regulatory guidelines under which your institution may fall. In the case of tissues removed secondary to traumatic injuries, photographic documentation of the tissues may be very important. The requirements of institutions are quite variable; thus one should be aware of his own institutional rules and regulations. These should be clearly documented in standard operating procedures (SOPs) and all changes should be documented in SOPs before the changes are instituted.
Fixation
After grossing any specimen, the tissue must be placed in the appropriate fixative which usually starts with a 10% dilution of concentrated formaldehyde (about 37% formaldehyde) which is called formalin. This is buffered to a neutral pH to form 10% Neutral Buffered Formalin (NBF). Fixation will preserve the morphology, minimize the loss of molecular components into solution, prevent decomposition and autolysis, and to minimize microbial/fungal growth. 10% NBF as well as most of the other fixatives used today maximize desirable properties and minimize undesirable properties of the other microscopic examination of the tissue (Eltoum et al. 2001a; Eltoum et al. 2001b). It is important to ensure adequate fixation by covering the specimen with fixative that is at least 10 times the volume of the specimen. For bloody specimens it may be necessary to replace the fixative as needed with fresh fixative. Fixatives will penetrate very slowly, about 1mm per hour. In some cases when large specimens cannot be sampled promptly, the specimen may sliced at 1 cm intervals and the specimen may be covered with fixative plus fixative soaked gauze or cloth to help keep the surface dry and to aid in penetration. On such large specimens, (e.g., brains) over a week may be necessary for proper fixation, and allowing fixation for less time may render poor results on subsequent processing of the tissue (Eltoum et al. 2001a; Eltoum et al. 2001b; Grizzle et al. 2001; Jones et al. 2001). Thus, thin sections need to be cut as soon as practicable to speed fixation provided the tissue is firm enough to cut. Subsequent to fixation, specimens are transferred for processing, embedding, cutting and staining (Grizzle et al. 2001; Jones et al. 2001).
Biohazards and Gross Room Safety
The Gross Room and associated areas may be one of the most dangerous areas of the hospital/university in which to work even though safety-risks and hazards have been minimized (Grizzle and Fredenburgh 2001; Grizzle et al. 2005). Personnel in the gross room encounter physical, infectious, flammable, toxic, carcinogenic, allergic, electrical and other risks that include cuts, needle sticks, and chemical fumes.
Safety of an organization is the prevue of a safety committee which develops a safety plan that is administered by a safety officer (Grizzle and Fredenburgh 2001; Grizzle et al. 2005). Each institution has different safety rules based on the safety plan but the most commonly used in all areas are as follows):
Treat all specimens with universal precautions
Always wear gloves, aprons or disposable gowns as well as face masks, goggles, or both when handling or processing tissue specimens
Clean all instruments with a disinfectant
Do not touch “clean” areas (e.g., telephone receiver) with gloves
Dispose of gowns, face masks or eye protection as well as gloves in a designated area for proper pick up and/or disposal before one leaves any potentially contaminated areas.
Wash hands frequently
Minimize skin exposures to chemicals as well as respiratory exposure to chemical vapors; understand the toxicities of chemicals used in the Gross Room and in related areas (e.g., tissue processors).
Have material safety data sheets readily available to all chemicals to which personnel are exposed.
Understand and train personnel in all OSHA regulations concerning safety.
By following these simple steps, obtaining proper training in safety and following the requirements of the safety manual, employees of a pathology department can minimize their exposure to dangerous pathogens, chemicals, and other safety-risks. Training in safety also may be necessary for non-pathology personnel who enter the gross room, especially janitorial personnel. Safety for all employees should be the most important concern with any pathologist or institution.
Obtaining Tissues to Support Research
To support future developments in clinical medicine it is critical that human tissues be available to support biomedical research (LiVolsi et al. 1993; Grizzle et al. 1996; Grizzle et al. 1998; Grizzle et al. 1999; Grizzle and Sexton 1999; Qualman et al. 2004). Such tissues usually are obtained from the gross room. Tissues taken for research should never compromise the diagnostic usefulness of the specimen. Most importantly, the margins of the specimen should not be compromised by obtaining samples for research at the surgical margins. Similarly, measurements of the thickness of the pigmented and other neoplastic lesions should not be compromised. Thus, for small lesions, one may be limited to obtaining small samples away form the margins but toward the edge of lesions. This should not be a difficult or time consuming process.
In obtaining tissues to support biomedical research, time after removal of the tissue from the body is an important parameter (Huang et al. 2001; Dash et al. 2002; Jewell et al. 2002; Spruessel et al. 2004) as is obtaining tissues which meet the needs of investigator experimental protocols (size and quantity).
To supply tissues to investigators requires review and approval of the Institutional Review Board (IRB) and review and approval by the HIPAA privacy board (Grizzle et al. 1996;Anonymous 2002). In general if the 18 HIPAA identifiers (e.g., name, social security number, dates of birth and treatment, hospital number, surgical pathology number, etc.) are not supplied with the specimen and associated medical information, the transfer is exempt from HIPAA regulations. Similarly, if these identifiers are removed from the tissue specimen, research by an investigator using such a specimen does not constitute “human research” and such research does not fall under the common rule or the Code of Federal Regulations (CFR) (45 CFR 46). However, such research proposals should be reviewed by the local IRB as well as the local Privacy Board to ensure they agree with the human subjects approach. In contrast, if tissues are collected as part of a diagnostic archival collection and/or a separate bank to support research, and the specimens are identified by any of the 18 HIPAA identifiers, the tissue resource requires approvals by both the IRB and Privacy Board. Such approval may require obtaining informed consent and HIPAA authorization or waiver of informed consent and authorization.
Summary
This review is not intended to serve as a procedure manual for operation of a gross room; but provides discussion of some of the more important issues related to the gross room function. Each laboratory should develop written standards and standard operating procedures for their gross room based on the types of specimens received, personnel involved in grossing, ancillary testing performed, and involvement in tissue collection for research. Following standard operating procedures will minimize risks and enhance ones ability to turn out the very best results possible for patients who should always remain the primary concern.
REFERENCES
- ADASP. Recommendations for the reporting of breast carcinoma. Association of Directors of Anatomic and Surgical Pathology. American journal of clinical pathology. 1995a;104(6):614–619. doi: 10.1093/ajcp/104.6.614. [DOI] [PubMed] [Google Scholar]
- ADASP. Recommendations for the reporting of resected primary lung carcinomas. Association of Directors of Anatomic and Surgical Pathology. American journal of clinical pathology. 1995b;104(4):371–374. doi: 10.1093/ajcp/104.4.371. [DOI] [PubMed] [Google Scholar]
- ADASP. Recommendations for the reporting of resected large intestinal carcinomas. Association of Directors of Anatomic and Surgical Pathology. Modern Pathology. 1996a;9(1):73–76. see comment. [PubMed] [Google Scholar]
- ADASP. Recommendations for the reporting of resected neoplasms of the kidney. Association of Directors of Anatomic and Surgical Pathology. Human pathology. 1996b;27(10):1005–1007. [PubMed] [Google Scholar]
- ADASP. Recommendations for the reporting of urinary bladder specimens containing bladder neoplasms. Association of Directors of Anatomic and Surgical Pathology. American journal of clinical pathology. 1996c;106(5):568–570. doi: 10.1093/ajcp/106.5.568. [DOI] [PubMed] [Google Scholar]
- ADASP. Recommendations for the reporting of larynx specimens containing laryngeal neoplasms. Association of Directors of Anatomic and Surgical Pathology. Pathology international. 1997;47(11):809–811. doi: 10.1111/j.1440-1827.1997.tb04463.x. [DOI] [PubMed] [Google Scholar]
- ADASP. Recommendations for the reporting of surgical specimens containing uterine cervical neoplasms. American journal of clinical pathology. 2000a;114(6):847–851. doi: 10.1309/n1aw-6adr-8qgm-nrxj. [DOI] [PubMed] [Google Scholar]
- ADASP. Recommended reporting format for thyroid carcinoma. American journal of clinical pathology. 2000b;114(5):684–686. doi: 10.1309/JX6N-K5A8-EE8T-PEHW. [DOI] [PubMed] [Google Scholar]
- ADASP. ADASP recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Mod Pathol. 2001;14(6):629–632. doi: 10.1038/modpathol.3880362. [DOI] [PubMed] [Google Scholar]
- ADASP. Recommendations for the reporting of lymphoid neoplasms. American journal of clinical pathology. 2003;119(2):185–189. doi: 10.1309/r52gh32ma8b4794v. [DOI] [PubMed] [Google Scholar]
- ADASP. Recommendations for the reporting of tissues removed as part of the surgical treatment of malignant liver tumors. American journal of clinical pathology. 2005;123(4):494–498. doi: 10.1309/lgc6g3yel1lu195x. [DOI] [PubMed] [Google Scholar]
- AJCC. AJCC Cancer Staging Manual. Sixth Edition. New York: Springer-Verlag; 2002. [Google Scholar]
- Albert D, Syed N. Protocol for the examination of specimens from patients with retinoblastoma: a basis for checklists. Archives of pathology & laboratory medicine. 2001;125(9):1183–1188. doi: 10.5858/2001-125-1183-PFTEOS. [DOI] [PubMed] [Google Scholar]
- Anonymous. Standards for privacy of individually identifiable health information. Final rule: Fed Regist. 2002:53181–53273. [PubMed] [Google Scholar]
- Arber DA. Effect of prolonged formalin fixation on the immunohistochemical reactivity of breast markers. Appl Immunohistochem Mol Morphol. 2002;10(2):183–186. doi: 10.1097/00129039-200206000-00015. [DOI] [PubMed] [Google Scholar]
- Arnold MM, Srivastava S, Fredenburgh J, Stockard CR, Myers RB, Grizzle WE. Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotech Histochem. 1996;71(5):224–230. doi: 10.3109/10520299609117164. [DOI] [PubMed] [Google Scholar]
- Bell WC. Surgical pathology of the parathyroid glands. Advances in experimental medicine and biology. 2005;563:1–9. doi: 10.1007/0-387-32025-3_1. [DOI] [PubMed] [Google Scholar]
- Bennett BD. Certification from the American Board of Pathology: getting it and keeping it. Human pathology. 2006;37(8):978–981. doi: 10.1016/j.humpath.2006.02.023. [see comment] [DOI] [PubMed] [Google Scholar]
- Bertagnolli M, Miedema B, Redston M, Dowell J, Niedzwiecki D, Fleshman J, Bem J, Mayer R, Zinner M, Compton C. Sentinel node staging of resectable colon cancer: results of a multicenter study. Annals of surgery. 2004;240(4):624–628. doi: 10.1097/01.sla.0000140753.41357.20. discussion 628–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentieri DF, Qualman SJ, Bowen J, Krausz T, Marchevsky A, Dickman PS. Protocol for the examination of specimens from pediatric and adult patients with osseous and extraosseous ewing sarcoma family of tumors, including peripheral primitive neuroectodermal tumor and ewing sarcoma. Archives of pathology & laboratory medicine. 2005;129(7):866–873. doi: 10.5858/2005-129-866-PFTEOS. [DOI] [PubMed] [Google Scholar]
- Compton CC. Key issues in reporting common cancer specimens: problems in pathologic staging of colon cancer. Archives of pathology & laboratory medicine. 2006;130(3):318–324. doi: 10.5858/2006-130-318-KIIRCC. [DOI] [PubMed] [Google Scholar]
- Dash A, Maine IP, Varambally S, Shen R, Chinnaiyan AM, Rubin MA. Changes in differential gene expression because of warm ischemia time of radical prostatectomy specimens. The American journal of pathology. 2002;161(5):1743–1748. doi: 10.1016/S0002-9440(10)64451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debski RF, Rutledge JC, Kapur FP. A plea for the masses: A gross room approach to pediatric tumors. J Histotechnology. 2004;24(3):173–190. [Google Scholar]
- Devoe K, Weidner N. Immunohistochemistry of small round-cell tumors. Semin Diagn Pathol. 2000;17(3):216–224. [PubMed] [Google Scholar]
- Eltoum I, Fredenburgh J, Grizzle WE. Advanced concepts in fication: effects of fixation on immunohistochemistry, reversibility of fixation and recovery of proteins, nucleic acids, and other molecules from fixed and processed tissues, developmental methods of fixation. J Histotechnology. 2001a;24(3):201–210. [Google Scholar]
- Eltoum I, Fredenburgh J, Myers RB, Grizzle WE. Introduction to the theory and practice of fixation of tissues. J Histotechnology. 2001b;24(3):173–190. [Google Scholar]
- Enriquez RE, Kelly LJ. The varied and useful role of the pathologists' assistant. MLO: medical laboratory observer. 1991;23(3):33–34. 36,38. [PubMed] [Google Scholar]
- Finegold MJ, Lopez-Terrada D, Bowen J, Washington MK, Qualman SJ. Protocol for the examination of specimens from patients with hepatoblastoma. Archives of pathology & laboratory medicine. 2006 doi: 10.5858/2007-131-520-PFTEOS. (In Press) [DOI] [PubMed] [Google Scholar]
- Grizzle W, Grody WW, Noll WW, Sobel ME, Stass SA, Trainer T, Travers H, Weedn V, Woodruff K. Recommended policies for uses of human tissue in research, education, and quality control. Ad Hoc Committee on Stored Tissue, College of American Pathologists. Archives of pathology & laboratory medicine. 1999;123(4):296–300. doi: 10.5858/1999-123-0296-RPFUOH. [DOI] [PubMed] [Google Scholar]
- Grizzle WE, Aamodt R, Clausen K, LiVolsi V, Pretlow TG, Qualman S. Providing human tissues for research: how to establish a program. Archives of pathology & laboratory medicine. 1998;122(12):1065–1076. [PubMed] [Google Scholar]
- Grizzle WE, Bell W, Fredenburgh J. Safety in Biomedical and Other Laboratories, Chapter 33. In: Patrinos G, Ansorg W, editors. Molecular Diagnostics. 2005. pp. 421–428. [Google Scholar]
- Grizzle WE, Fredenburgh J. Avoiding biohazards in medical, veterinary and research laboratories. Biotech Histochem. 2001;76(4):183–206. [PubMed] [Google Scholar]
- Grizzle WE, Sexton KS. Development of a facility to supply human tissues to aid in medical research. Chapter 24. In: Srivastava S, Henson DE, Gazdar A, editors. Molecular Pathology of Early Cancer. Amsterdam, Netherlands: IOS Press; 1999. pp. 371–383. [Google Scholar]
- Grizzle WE, Stockard CR, Billings PE. The effects of tissue processing variables other than fixation on histochemical staining and immunohistochemical detection of antigens. J Histotechnology. 2001;24(3):213–219. [Google Scholar]
- Grizzle WE, Woodruff KH, Trainer TD. The pathologist's role in the use of human tissues in research--legal, ethical, and other issues. Archives of pathology & laboratory medicine. 1996;120(10):909–912. [PubMed] [Google Scholar]
- Hashizume K, Hatanaka Y, Kamihara Y, Kato T, Hata S, Akashi S, Kato T, Koyatsu J, Tani Y, Tsujimoto M, Tsuda H. Interlaboratory comparison in HercepTest assessment of HER2 protein status in invasive breast carcinoma fixed with various formalin-based fixatives. Appl Immunohistochem Mol Morphol. 2003;11(4):339–344. doi: 10.1097/00129039-200312000-00011. [DOI] [PubMed] [Google Scholar]
- Huang J, Qi R, Quackenbush J, Dauway E, Lazaridis E, Yeatman T. Effects of ischemia on gene expression. The Journal of surgical research. 2001;99(2):222–227. doi: 10.1006/jsre.2001.6195. [DOI] [PubMed] [Google Scholar]
- Hunt JL, Baloch ZW, LiVolsi VA. Sentinel lymph node evaluation for tumor metastasis. Semin. 2002;19(4):263–277. [PubMed] [Google Scholar]
- Jewell SD, Srinivasan M, McCart LM, Williams N, Grizzle WH, LiVolsi V, MacLennan G, Sedmak DD. Analysis of the molecular quality of human tissues: an experience from the Cooperative Human Tissue Network. American journal of clinical pathology. 2002;118(5):733–741. doi: 10.1309/VPQL-RT21-X7YH-XDXK. [DOI] [PubMed] [Google Scholar]
- Jones WT, Stockard CR, Grizzle WE. Effects of time and temperature during attachment of sections to microscope slides on immunohistochemical detection of antigens. Biotech Histochem. 2001;76(2):55–58. [PubMed] [Google Scholar]
- Lester SC. Manual of Surgical Pathology. New York: Churchill Livingstone; 2005. [Google Scholar]
- LiVolsi VA, Clausen KP, Grizzle W, Newton W, Pretlow TG, 2nd, Aamodt R. The Cooperative Human Tissue Network. An update. Cancer. 1993;71(4):1391–1394. doi: 10.1002/1097-0142(19930215)71:4<1391::aid-cncr2820710434>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Manne U, Weiss HL, Grizzle WE. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res. 2000;6(10):4017–4025. [PubMed] [Google Scholar]
- Neri RA, Keshgegian AA. The pathologists' assistant. Distribution, use, and employer perceptions. American journal of clinical pathology. 1986;85(1):87–89. doi: 10.1093/ajcp/85.1.87. [DOI] [PubMed] [Google Scholar]
- Peydro-Olaya A, Llombart-Bosch A, Carda-Batalla C, Lopez-Guerrero JA. Electron microscopy and other ancillary techniques in the diagnosis of small round cell tumors. Semin Diagn Pathol. 2003;20(1):25–45. [PubMed] [Google Scholar]
- Qualman SJ, Bowen J, Amin MB, Srigley JR, Grundy PE, Perlman EJ. Protocol for the examination of specimens from patients with Wilms tumor (nephroblastoma) or other renal tumors of childhood. Archives of pathology & laboratory medicine. 2003a;127(10):1280–1289. doi: 10.5858/2003-127-1280-PFTEOS. [DOI] [PubMed] [Google Scholar]
- Qualman SJ, Bowen J, Fitzgibbons PL, Cohn SL, Shimada H. Protocol for the examination of specimens from patients with neuroblastoma and related neuroblastic tumors. Archives of pathology & laboratory medicine. 2005;129(7):874–883. doi: 10.5858/2005-129-874-PFTEOS. [DOI] [PubMed] [Google Scholar]
- Qualman SJ, Bowen J, Parham DM, Branton PA, Meyer WH. Protocol for the examination of specimens from patients (children and young adults) with rhabdomyosarcoma. Archives of pathology & laboratory medicine. 2003b;127(10):1290–1297. doi: 10.5858/2003-127-1290-PFTEOS. [DOI] [PubMed] [Google Scholar]
- Qualman SJ, France M, Grizzle WE, LiVolsi VA, Moskaluk CA, Ramirez NC, Washington MK. Establishing a tumour bank: banking, informatics and ethics. British journal of cancer. 2004;90(6):1115–1119. doi: 10.1038/sj.bjc.6601678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redston M, Compton CC, Miedema BW, Niedzwiecki D, Dowell JM, Jewell SD, Fleshman JM, Bem J, Mayer RJ, Bertagnolli MM. Analysis of micrometastatic disease in sentinel lymph nodes from resectable colon cancer: results of Cancer and Leukemia Group B Trial 80001. J Clin Oncol. 2006;24(6):878–883. doi: 10.1200/JCO.2005.03.6038. [DOI] [PubMed] [Google Scholar]
- Rivera M, Merlin S, Hoda RS, Gopalan A, Hoda SA. Controversies in surgical pathology: minimal involvement of sentinel lymph node in breast carcinoma: prevailing concepts and challenging problems. Int J Surg Pathol. 2004;12(4):301–306. doi: 10.1177/106689690401200402. [see comment] [DOI] [PubMed] [Google Scholar]
- Selvarajan S, Bay BH, Choo A, Chuah KL, Sivaswaren CR, Tien SL, Wong CY, Tan PH. Effect of fixation period on HER2/neu gene amplification detected by fluorescence in situ hybridization in invasive breast carcinoma. J Histochem Cytochem. 2002;50(12):1693–1696. doi: 10.1177/002215540205001215. [DOI] [PubMed] [Google Scholar]
- Spanknebel K, Coit DG, Bieligk SC, Gonen M, Rosai J, Klimstra DS. Characterization of micrometastatic disease in melanoma sentinel lymph nodes by enhanced pathology: recommendations for standardizing pathologic analysis. American Journal of Surgical Pathology. 2005;29(3):305–317. doi: 10.1097/01.pas.0000152134.36030.b7. [see comment] [DOI] [PubMed] [Google Scholar]
- Spruessel A, Steimann G, Jung M, Lee SA, Carr T, Fentz AK, Spangenberg J, Zornig C, Juhl HH, David KA. Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. BioTechniques. 2004;36(6):1030–1037. doi: 10.2144/04366RR04. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Uren RF. Teaching points on lymphatic mapping for melanoma from the Sydney Melanoma Unit. Semin Oncol. 2004;31(3):349–356. doi: 10.1053/j.seminoncol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Troxel DB. Error in surgical pathology. The American journal of surgical pathology. 2004;28(8):1092–1095. doi: 10.1097/01.pas.0000126772.42945.5c. [DOI] [PubMed] [Google Scholar]
- Troxel DB. Medicolegal aspects of error in pathology. Archives of pathology & laboratory medicine. 2006;130(5):617–619. doi: 10.5858/2006-130-617-MAOEIP. [DOI] [PubMed] [Google Scholar]
- Westra WH, Hruban RH, Phelps TH, Isacson C, Askin FB. Surgical Pathology Dissection: An Illustrated Guide. Springer. 2003 [Google Scholar]
- Young ES, Castro CY. The pancreaticoduodenectomy. Annals of diagnostic pathology. 2002;6(3):188–193. doi: 10.1053/adpa.2002.32382. [DOI] [PubMed] [Google Scholar]
- Young ES, Diaz-Arrastia C, Castro CY. The hysterectomy. Annals of diagnostic pathology. 2005;9(4):202–208. doi: 10.1016/j.anndiagpath.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Zarbo RJ, Nakhleh RE. Surgical pathology specimens for gross examination only and exempt from submission: a College of American Pathologists Q-Probes study of current policies in 413 institutions. Archives of pathology & laboratory medicine. 1999;123(2):133–139. doi: 10.5858/1999-123-0133-SPSFGE. [DOI] [PubMed] [Google Scholar]