Abstract
Mitochondria do not exist as discrete, static entities. Rather, mitochondria form a network that continuously moves, divides and fuses. The structure of this dynamic network is in part maintained by a balance of division and fusion events (Hoppins et al., 2007). The ratio of division to fusion events that defines a proper balance is not universal but varies with developmental stage, cell type and biological circumstances. This is evident throughout the cell cycle in higher eukaryotes, where mitochondria elongate during the G1/S transition and fragment at the onset of mitosis, and when mitochondria fragment in response to certain cellular stimuli, such as increases in cytosolic calcium levels (Breckenridge et al., 2003; Cereghetti et al., 2008; Han et al., 2008; Mitra et al., 2009; Taguchi et al., 2007). The functional state and distribution of mitochondria are clearly influenced by its steady-state structure. When the normal balance of division and fusion is disrupted as a consequence of the inappropriate stimulation or inhibition of either process, problems arise at the cellular level that compromise the well-being of the organism as a whole. This is evident by the ever-increasing number of diseases in which abnormal mitochondrial dynamics have been etiologically implicated. In this context, the mitochondrial division and fusion machines are valuable and interesting targets of small molecule effectors as inhibition or activation of these processes may be able to restore the proper dynamic balance and function. A small molecule inhibitor of mitochondrial division, mdivi-1, has already been identified and characterized (Cassidy-Stone et al., 2008). This inhibitor has provided valuable insight into the mechanism of mitochondrial division and has shown great therapeutic promise in a wide array of disease models. This review will focus on small molecule effectors of mitochondrial division, discussing their value in basic biological research as well as their therapeutic potential.
Mitochondrial dynamics in healthy and disease states: a case for identifying small molecule inhibitors of mitochondrial division
Mitochondrial division and fusion are antagonistic activities whose fundamental roles are to create a compartment that is a connected conductor, able to mix its contents and have access to mtDNA and its products, but able to be distributed to distant cellular destinations via transport on actin or microtubule networks. In both yeast and higher eukaryotes, disruption of mitochondrial division leads to an extensively interconnected and collapsed mitochondrial network that leaves many areas of the cell devoid of the organelle. In contrast, defects in mitochondrial fusion cause extensive mitochondrial fragmentation and a complete or partial loss of mtDNA in yeast and mammalian cells, respectively (Hoppins et al., 2007). Disrupting division and fusion in yeast is not a lethal event. In contrast, the effects of disrupted division and fusion in higher eukaryotes are much more adverse to the organism. Mice lacking the dynamin related proteins (DRPs) that comprise the heart of mitochondrial division and fusion machines, Drp1 and MFN1/2 or OPA1, respectively, exhibit deleterious developmental defects (Chen et al., 2003; Davies et al., 2007; Ishihara et al., 2009; Wakabayashi et al., 2009). Mutations in human Drp1 cause early infant mortality, and mutations in MFN2 and OPA1 cause two distinct types of neurodegenerative diseases, Charcot Marie Tooth 2A (CMT2A) and dominant optic atrophy (DOA), underscoring the essential nature of mitochondrial dynamics in higher eukaryotes (Alexander et al., 2000; Delettre et al., 2000; Waterham et al., 2007; Zuchner et al., 2006).
Improper mitochondrial distribution and morphology as a result of attenuated division and fusion are likely to be a major cause of many adverse effects. However, disruption of mitochondrial dynamics also has negative effects on mitochondrial function that are not as easily explained by changes in the overall steady-state structure or content mixing of the organelle. In cells that lack MFN2 or express CMT2A-asscoiated disease alleles of MFN2, axonal transport of mitochondria is disrupted (Baloh et al., 2007). Interestingly this disruption in transport does not appear to be a consequence of attenuated fusion, suggesting a fusion-independent role for MFN2 in the regulation of mitochondrial motility (Misko et al., 2010). In the absence of ongoing mitochondrial division, general mitochondrial dysfunction, such as loss of membrane potential, increase in ROS, increase in oxidized proteins, and loss of mitochondrial DNA, are observed (Lee et al., 2007; Parone et al., 2008; Twig et al., 2008; Yoon et al., 2006). These sub-lethal stresses induce senescence-associated phenotypic changes in cells highlighting the intimate connection between mitochondrial function and cellular function (Lee et al., 2007; Yoon et al., 2006).
Mitochondrial dynamics has also been proposed to play a role in the quality control of the organelle. Studies have shown that during a division event, functionally asymmetric daughter mitochondria with different membrane potentials can be produced. The functional daughter, which retains high membrane potential, can refuse with the mitochondrial network, while the dysfunctional daughter cannot refuse due to low membrane potential and is subsequently flagged for autophagic degradation (Parone et al., 2008; Twig et al., 2008). This is consistent with recent work on the Pink1/Parkin pathway, which demonstrates that the selective, Pink1-dependent recruitment of Parkin, an E3-ubiquitin ligase, to mitochondria with low membrane potential targets the damaged organelles for degradation (Matsuda et al.; Narendra et al., 2008; Narendra et al., 2010). Thus, it is possible that the increase in dysfunctional mitochondria in the absence of division may be due to the loss of a role for division in the quality control of the mitochondrial compartment.
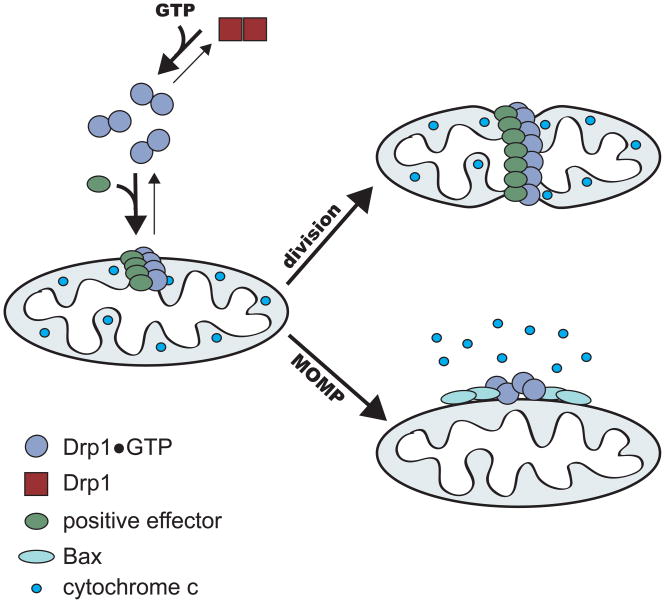
Current data suggest that Drp1 also plays an independent role in the regulation of intrinsic apoptosis, specifically via the control of outer membrane permeabilization by the pro-apoptotic Bcl2 protein, Bax (Figure 1). Concomitant with mitochondrial outer membrane permeabilization during apoptosis, Drp1 self-assembly and its recruitment to mitochondria are increased, resulting in an enhanced rate of Drp1-dependent mitochondrial division and mitochondrial fragmentation (Breckenridge et al., 2003; Frank et al., 2001; Wasiak et al., 2007). Inhibition of Drp1-dependent mitochondrial division delays and partially inhibits apoptosis suggesting a functional link between the progression of apoptosis and Drp1-mediated mitochondrial fragmentation. Mitochondrial fusion also plays a role in the regulation of apoptosis (Frank et al., 2001; Jagasia et al., 2005; Lee et al., 2004). In contrast to Drp1, MFN1 and MFN2 are thought to play a protective role against apoptosis in cells as inhibition of fusion makes cells more sensitive to apoptotic stimuli.
Figure 1.
Modulation of Drp1-mediated mitochondrial division and potentiation of apoptosis by negative and positive effectors. Negative effectors may block Drp1 assembly, as is the case for mdivi-1, or other critical aspects of Drp1 function and thus attenuate mitochondrial division and/or mitochondrial outer membrane permeabilization (MOMP). Positive effectors may stimulate Drp1 assembly and/or function, and depending on the distinct set of effectors, which are likely defined by biological circumstances, mitochondrial division and/or MOMP can be stimulated.
Given the importance of mitochondrial dynamics for cellular function, regulation and cell death, it is perhaps not surprising that aberrant mitochondrial dynamics/morphology have been associated with numerous and prevalent human diseases, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and diabetes (Poole et al., 2008; Wang et al., 2009; Zorzano et al., 2009). In these cases, fragmentation of the mitochondrial network has been observed in disease patient samples and/or in cell culture disease models, suggesting a shift in the division/fusion balance towards division. The cause of the shift in each case is unclear and could be a result of direct activation of mitochondrial division, direct inhibition of mitochondrial fusion or both, or as an indirect result of cellular stress, which can affect the division/fusion balance. In the cases of the mitochondrial fusion-linked diseases DOA and CMT2A, it is clear that the disease-associated mitochondrial morphologies are at least in part due to a direct shift towards division as a result of attenuated fusion. The link between increased mitochondrial division and disease states and mitochondrial division and apoptosis makes the mitochondrial division machine an attractive target for therapeutics that could treat an impressive array of diseases from neurodegeneration to more acute conditions, such as stroke, myocardial infarction and drug toxicity.
The mitochondrial division machine: a deep target for small molecule effectors
The heart of the mitochondrial division machine is the dynamin-related GTPase Dnm1 (yeast)/Drp1 (mammals). While cytological analysis, which places Dnm1/Drp1 at sites of mitochondrial division, provides compelling support for the role of Dnm1/Drp1 as the master regulator of mitochondrial division, biochemical and structural analyses of the yeast mitochondrial division dynamin have provided the insight needed for a mechanistic understanding of how Dnm1/Drp1 does the work of mitochondrial division (Ingerman et al., 2005; Legesse-Miller et al., 2003; Naylor et al., 2006; Sesaki and Jensen, 1999). These analyses have suggested a model in which GTP binding to Dnm1 dimers produces conformational changes that facilitate the assembly of Dnm1 into helical structures (Ingerman et al., 2005). These helical structures possess diameters that match the diameters of mitochondrial constriction sites in vivo, indicating that Dnm1 self-assembly drives mitochondrial constriction (Figure 1). Dnm1 self-assembly also stimulates GTP hydrolysis, which is required for mitochondrial division, likely by producing additional structural changes in the Dnm1 helix and by promoting disassembly (Ingerman et al., 2005; Naylor et al., 2006). Interestingly, Dnm1 self-assembly proceeds via a rate limiting, Dnm1 concentration-dependent nucleation event, which may be exploited as a means to regulate the assembly and thus the function of Dnm1 in vivo (Ingerman et al., 2005).
The mammalian mitochondrial division dynamin Drp1 also assembles into helical structures that can tubulate liposomes in vitro, albeit with smaller diameters than those formed by Dnm1, and thus likely functions via a similar mechanism (Smirnova et al., 2001; Yoon et al., 2001). While the basic mechanism of membrane division is likely conserved, regulation of Drp1 assembly, however, is more complex and regulated likely because Drp1 self-assembly and/or assembly associated conformational changes have been harnessed in mammalian cells to integrate mitochondrial division with cellular physiology. This is evident when comparing the steady-state cellular distributions of the two proteins. In contrast to Dnm1, which predominantly exists in assembled structures, Drp1 is mainly distributed in a diffuse manner throughout the cytosol in cultured cells, which suggests that it is predominantly unassembled (Labrousse et al., 1999; Otsuga, 1998; Sesaki and Jensen, 1999; Smirnova et al., 2001). However, in response to certain cellular stimuli, such as increases in cytosolic calcium levels or the initiation of apoptosis, mitochondrial targeting and assembly of Drp1 is greatly enhanced and consequently mitochondrial division is increased (Breckenridge et al., 2003; Frank et al., 2001; Germain et al., 2005). While the mechanisms underlying the regulation of Drp1 assembly are unclear, it is still evident that regulation of assembly can be used as a means to regulate function.
Although DRPs are the heart of the division machine, mitochondrial division is strictly dependent on additional non-DRP factors. The roles of these factors are best characterized in yeast where it is clear that two additional proteins, Fis1 and Mdv1, are essential for Dnm1-mediated mitochondrial division (Cerveny et al., 2001; Fekkes et al., 2000; Mozdy et al., 2000; Tieu and Nunnari, 2000). Fis1 is anchored to the mitochondrial outer membrane via a C-terminal transmembrane domain. The N-terminus of the protein is exposed to the cytosol and interacts directly with Mdv1 (Dohm et al., 2004; Karren et al., 2005; Mozdy et al., 2000; Suzuki et al., 2003; Suzuki et al., 2005; Tieu and Nunnari, 2000; Tieu et al., 2002). Mdv1 functions as a molecular bridge between mitochondrial-anchored Fis1 and soluble Dnm1, and together Fis1 and Mdv1 function to target Dnm1 to the mitochondrial surface (Griffin et al., 2005; Karren et al., 2005; Tieu and Nunnari, 2000; Tieu et al., 2002). Recent work has demonstrated that Mdv1 also functions post-targeting to nucleate the assembly of Dnm1 on the mitochondrial surface. Indeed, biochemical and cytological evidence suggests that the native yeast division machine is a structure comprised of co-assembled Dnm1 and Mdv1 (Lackner et al., 2009). Two additional Dnm1 interacting proteins Caf4, which is an Mdv1 paralog, and Num1, which is a coiled-coil protein localized to the cell cortex, have been identified, but are not essential for division (Cerveny et al., 2007; Griffin et al., 2005). How these proteins act together combinatorially with Fis1 and Mdv1 to modulate division and potentially other processes in the cell will provide additional insight into the regulation and functions of the division DRP.
In mammalian cells, several non-DRP proteins have been identified as important regulators of mitochondrial division, however, their precise functions have not yet been determined. For example, a mammalian Fis1 ortholog has been identified, and likely plays a role in division, but it is not essential for Drp1 targeting, suggesting that additional pathways for Drp1 activation have evolved in mammalian cells (Lee et al., 2004; Stojanovski et al., 2004; Suzuki et al., 2003). Indeed, a structural or functional ortholog of Mdv1 has not yet been identified in higher eukaryotes. Given division is more integrated and regulated in mammalian cells, it seems likely that Mdv1 has been replaced by several adaptor and/or nucleator type components that perform similar essential functions in division, but may be present in a cell type specific manner and/or respond to different cellular signaling pathways (Lackner and Nunnari, 2008). Candidates for Drp1 effectors in mammalian cells include the mitochondrial outer membrane proteins, hFis1, Mff and GDAP1 (ganglioside-induced differentiation associated protein 1), which when mutated causes CMT, the inner membrane space protein MTP18 and the BAR domain protein, Endophilin B1 (Gandre-Babbe and van der Bliek, 2008; Karbowski et al., 2004; Lee et al., 2004; Niemann et al., 2005; Stojanovski et al., 2004; Suzuki et al., 2003; Tondera et al., 2005; Tondera et al., 2004). Also, during apoptosis, both Bax and Drp1 translocate to the mitochondrial outer membrane and co-localize in foci on mitochondria, suggesting that Bax and potentially other Bcl2 family member proteins also function as effectors of Drp1 activity, as suggested by the finding that Bcl-xL overexpression positively regulates Drp1 to stimulate synapse formation (Karbowski et al., 2002; Li et al., 2008). In addition to these potential protein regulators of mitochondrial division, the function of Drp1 is also influenced by post-translational modification. Phosphorylation, nitrosylation, ubiquitination, and sumolyation have all been identified as post-translational modifications that can positively or negatively affect the function of Drp1 (Cereghetti et al., 2008; Chang and Blackstone, 2007; Cho et al., 2009; Cribbs and Strack, 2007; Han et al., 2008; Harder et al., 2004; Nakamura et al., 2006; Taguchi et al., 2007; Yonashiro et al., 2006; Zunino et al., 2007). It is likely that a distinct combination of protein effectors and post-translational modifications regulate the activity of Drp1 in different physiological contexts (Figure 1).
While the mitochondrial division machine is comprised of and influenced by many factors, the mitochondrial division dynamins, Dnm1 and Drp1, are the most obvious targets of small molecule modulators of division. They are the highly conserved, core mechanical component of the division machine and are the best understood mechanistically. In addition, the division DRP is a deep target for small molecule effectors in the sense that there are many functionally critical features of the protein, such as GTP binding, GTP hydrolysis and self-assembly, which is mediated by conformational changes linked to the GTPase cycle. Therefore, small molecule effectors that target different functional facets of Dnm1/Drp1 and thus either positively or negatively affect distinct stages of Dnm1/Drp1-driven mitochondrial division can be identified. Finally, given the increasing number of Drp1 interacting proteins that have been implicated in division, it may be possible to identify tissue specific or context-specific inhibitors of mitochondrial division by identifying small molecules that target and modulate Drp1-effector interactions.
Mdivi-1: the first inhibitor of the mitochondrial division DRP
Using a simple growth based assay in yeast, the first small molecule effector of the mitochondrial division DRP, Dnm1, was identified and was demonstrated to also target Drp1 in mammalian cells (Cassidy-Stone et al., 2008), speaking to the conserved primary mechanism of mitochondrial division. Mechanistically, mdivi-1 acts as a mixed type inhibitor to attenuate the early stages of division DRP assembly by preventing the polymerization of higher order structures. Mdivi-1 selectively targets the unassembled pool of the mitochondrial division dynamin, and its binding creates and/or stabilizes an assembly-deficient conformation (Figure 1). Assembly of the division DRP is critical for function; thus, mdivi-1 acts as an efficacious inhibitor of mitochondrial division in cells. Given the conservation of the DRP super family members, it is remarkable that mdivi-1 also displays a high degree of selectively for the mitochondrial division dynamins. It has no effect on other DRPs, such as the endocytic dynamin or the mitochondrial fusion dynamins. This selectivity is encouraging for the division dynamin as a therapeutic target (see below). Systems are in place to screen for additional small molecule effectors of the mitochondrial division dynamin, as both cellular and biochemical assays of Dnm1/Drp1 activity and function are well established. In addition, these assays can be used, as they were for mdivi-1, to precisely identify the cellular and biochemical activities of the division DRP.
The identification of mdivi-1 and likely other inhibitors of the mitochondrial division dynamin has and will continue to prove beneficial in dissecting the exact roles mitochondrial division or Drp1, specifically, play in the maintenance of proper cellular function. These small molecule effectors can extend the resolution of genetic, cytological and biochemical approaches by allowing discrete steps in mitochondrial division or Drp1 activities to be modulated selectively, rapidly and reversibly. This modulation can occur in an otherwise wild-type background without the need for the generation of mutant or knockout cell lines or the depletion of protein levels by techniques such as RNAi. Thus, complications from second site suppressors or indirect effects can be reduced. In addition, the effects of increased or decreased Drp1 activity can be monitored immediately. Thus the evolution of the cellular defects associated with loss of Drp1 activity can be tracked over time, providing a clearer picture of the primary and secondary consequences of aberrant Drp1 function. Also, when used in combination with biochemical assays, biochemical activity can be correlated with cellular function. Indeed mdivi-1 has already proven to be useful as a tool to identify the role of Drp1 in intrinsic apoptosis. Specifically, in cell-free MOMP assays where mitochondrial division does not occur, mdivi-1 blocks cytochrome-c release (Cassidy-Stone et al., 2008). Thus Drp1 has a positive regulatory role in MOMP that is independent of its role in mitochondrial division, demonstrating that Drp1 possesses multiple independent roles in mammalian cells (Figure 1). In addition, the use of mdivi-1 for the acute inhibition of Drp1 activity in cultured mammalian cells has revealed a regulatory role for a hyperfused mitochondrial state in the regulation of cyclin E levels and consequently in cell cycle progression (Mitra et al., 2009).
The therapeutic potential of small molecule effectors of mitochondrial division: putting mdivi-1 to the test
While the etiology of many diseases in which aberrant mitochondrial morphology is observed is uncertain, inhibiting mitochondrial division with mdivi-1 in Parkinson’s disease cell culture disease models or a dominant negative form of Drp1 in Alzheimer’s and Huntington’s disease cell culture models attenuates disease associated phenotypes (Barsoum et al., 2006; Cho et al., 2009; Cui et al., 2010; Lutz et al., 2009; Wang et al., 2009) (Table 1). These results demonstrate the therapeutic potential of small molecule inhibitors of mitochondrial division to disease. The role of Drp1 in the facilitation of apoptosis has also been exploited for its therapeutic potential (Table 1). The reperfusion of ischemic cells as a result of events such as stroke or myocardial infarction induces apoptotic cell death, which can lead to severe tissue and organ damage. In these cases, small molecule inhibitors of Drp1 may serve to protect against apoptotic cell death following such a temporary insult. Indeed, this approach seems promising as the therapeutic potential of Drp1 inhibition using genetic approaches and also with mdivi-1 has now been tested with good success in experimental models of cardiac and renal ischemia/reperfusion (Brooks et al., 2009; Ong et al., 2010). In addition, it has been shown that mdivi-1 is efficacious in rodent models of cisplatin induced renal damage, also a result of apoptotic cell death (Brooks et al., 2009). Thus inhibitors of Drp1 have potential therapeutic application for a wider array of drug toxicity-induced tissue damage.
Table 1.
The therapeutic potential of genetic and chemical inhibition of mitochondrial division
| Disease/Injury | Effects of inhibited mitochondrial division | Method of inhibition | References |
|---|---|---|---|
| Parkinson’s disease | attenuation of the adverse effects Pink1 and Parkin mutants have on mitochondrial function and morphology in PD disease models | genetic and chemical |
Lutz et al., 2009 Cui et al., 2010 |
| Alzheimer’s disease | attenuation of β-amyloid protein- or nitric oxide-induced mitochondrial fragmentation and neuronal cell damage | genetic |
Barsoum et al., 2006 Cho et al., 2009 |
| Huntington’s disease | attenuation of the adverse effects of Htt mutations on mitochondrial function and morphology | genetic | Wang et al., 2009 |
| Ischemia/reperfusion injury | protection against cardiac injury in a murine model of ischemia/reperfusion | genetic and chemical | Ong et al., 2010 |
| protection against renal injury in a rodent model of inschemia/reperfusion | genetic and chemical | Brooks et al., 2009 | |
| Drug toxicity-induced tissue damage | protection against cisplatin-induced renal damage | genetic and chemical | Brooks et al., 2009 |
Although not yet put to the test, it seems likely that mdivi-1 or other inhibitors of mitochondrial division will prove beneficial the mitochondrial fusion-linked neuropathies CMT2A and DOA, where the detrimental effects of ongoing division in the absence or attenuation of mitochondrial fusion are clearly evident (Alexander et al., 2000; Delettre et al., 2000; Kijima et al., 2005). Interestingly, recent work has shown that partial restoration of mitochondrial fusion in mammalian cells can rescue the longer term defects associated with loss of fusion: decreased respiratory capacity, reduction in mtDNA levels, and increased rates of mtDNA mutation (Chen et al., 2005; Chen et al., 2010). Thus, small molecule inhibitors of mitochondrial division, which can serve to restore the connectivity of the mitochondrial network, may also rescue the defects associated with loss/attenuation of fusion. Inhibitors of Drp1 are also logical potential therapeutics in cases of human diseases caused by heteroplasmic mtDNA mutations. Inhibition of mitochondrial division would increase connectivity of mitochondria and enhance access to wild type products of mtDNA genes to allow for complementation of respiratory or other dysfunction. It is important to note, however, that mitochondrial division is an essential event in cells (Ishihara et al., 2009; Wakabayashi et al., 2009; Waterham et al., 2007). Indeed, stimulation of mitochondrial division enhances mitochondrial mass and distribution in neurons and stimulates synapse formation (Li et al., 2008). Mitochondrial division may also be an essential event for mitochondrial quality control. In this context, the mechanism of mdivi-1 inhibition is advantageous as it can act much like a dimmer switch on a light bulb, to cause increasing degrees of mitochondrial connectivity, which provide for efficacy while allowing for distribution and other Drp1 related functions. Finally, although not yet discovered, small molecule activators of Drp1 and inhibitors of mitochondrial fusion DRPs are attractive as anti-cancer therapies as they have potential to stimulate apoptotic cell death.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander C, Votruba M, Pesch UEA, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nature Genetics. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveny KL, McCaffery JM, Jensen RE. Division of mitochondria requires a novel DNM1-interacting protein, Net2p. Mol Biol Cell. 2001;12:309–321. doi: 10.1091/mbc.12.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveny KL, Studer SL, Jensen RE, Sesaki H. Yeast mitochondrial division and distribution require the cortical num1 protein. Dev Cell. 2007;12:363–375. doi: 10.1016/j.devcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Tang X, Christian WV, Yoon Y, Tieu K. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem. 2010;285:11740–11752. doi: 10.1074/jbc.M109.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, Nicols PP, Boulton ME, Votruba M. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet. 2007;16:1307–1318. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nature Genetics. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Dohm JA, Lee SJ, Hardwick JM, Hill RB, Gittis AG. Cytosolic domain of the human mitochondrial fission protein fis1 adopts a TPR fold. Proteins. 2004;54:153–156. doi: 10.1002/prot.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekkes P, Shepard KA, Yaffe MP. Gag3p, an outer membrane protein required for fission of mitochondrial tubules. J Cell Bio. 2000;151:333–340. doi: 10.1083/jcb.151.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner W, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. Embo J. 2005;24:1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EE, Graumann J, Chan DC. The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria. J Cell Biol. 2005;170:237–248. doi: 10.1083/jcb.200503148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H, Matsushita M. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004;14:340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol. 2004;166:1027–1039. doi: 10.1083/jcb.200407046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karren MA, Coonrod EM, Anderson TK, Shaw JM. The role of Fis1p-Mdv1p interactions in mitochondrial fission complex assembly. J Cell Biol. 2005;171:291–301. doi: 10.1083/jcb.200506158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima K, Numakura C, Izumino H, Umetsu K, Nezu A, Shiiki T, Ogawa M, Ishizaki Y, Kitamura T, Shozawa Y, et al. Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth neuropathy type 2A. Hum Genet. 2005;116:23–27. doi: 10.1007/s00439-004-1199-2. [DOI] [PubMed] [Google Scholar]
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- Lackner LL, Horner JS, Nunnari J. Mechanistic analysis of a dynamin effector. Science. 2009;325:874–877. doi: 10.1126/science.1176921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner LL, Nunnari JM. The molecular mechanism and cellular functions of mitochondrial division. Biochim Biophys Acta. 2008;1792:1138–1144. doi: 10.1016/j.bbadis.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse-Miller A, Massol RH, Kirchhausen T. Constriction and dnm1p recruitment are distinct processes in mitochondrial fission. Mol Biol Cell. 2003;14:1953–1963. doi: 10.1091/mbc.E02-10-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, McNay EC, Yu T, Schwarzenbacher R, Bossy B, et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:2169–2174. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert AS, et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem. 2009;284:22938–22951. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A. 2009;106:11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151:367–379. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.00210.1001371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor K, Ingerman E, Okreglak V, Marino M, Hinshaw JE, Nunnari J. Mdv1 interacts with assembled dnm1 to promote mitochondrial division. J Biol Chem. 2006;281:2177–2183. doi: 10.1074/jbc.M507943200. [DOI] [PubMed] [Google Scholar]
- Niemann A, Ruegg M, La Padula V, Schenone A, Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting Mitochondrial Fission Protects the Heart Against Ischemia/Reperfusion Injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- Otsuga D, Keegan BR, Brisch E, Thantcher JW, Hermann GJ, Bleazard W, Shaw J. The dynamin GTPase, Dnm1p, controls mitochondrial morphology in yeast. J Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. DOI 3210.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol. 1999;147:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van Der Bliek AM. Dynamin-related protein drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Jeong SY, Karbowski M, Youle RJ, Tjandra N. The solution structure of human mitochondria fission protein Fis1 reveals a novel TPR-like helix bundle. J Mol Biol. 2003;334:445–458. doi: 10.1016/j.jmb.2003.09.064. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Neutzner A, Tjandra N, Youle RJ. Novel structure of the N terminus in yeast Fis1 correlates with a specialized function in mitochondrial fission. J Biol Chem. 2005;280:21444–21452. doi: 10.1074/jbc.M414092200. [DOI] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- Tieu Q, Nunnari J. Mdv1p is a WD repeat protein that interacts with the dynamin-related GTPase, Dnm1p, to trigger mitochondrial division. J Cell Biol. 2000;151:353–365. doi: 10.1083/jcb.151.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu Q, Okreglak V, Naylor K, Nunnari J. The WD repeat protein, Mdv1p, functions as a molecular adaptor by interacting with Dnm1p and Fis1p during mitochondrial fission. J Cell Biol. 2002;158:445–452. doi: 10.1083/jcb.200205031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondera D, Czauderna F, Paulick K, Schwarzer R, Kaufmann J, Santel A. The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J Cell Sci. 2005;118:3049–3059. doi: 10.1242/jcs.02415. [DOI] [PubMed] [Google Scholar]
- Tondera D, Santel A, Schwarzer R, Dames S, Giese K, Klippel A, Kaufmann J. Knockdown of MTP18, a novel phosphatidylinositol 3-kinase-dependent protein, affects mitochondrial morphology and induces apoptosis. J Biol Chem. 2004;279:31544–31555. doi: 10.1074/jbc.M404704200. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- Yonashiro R, Ishido S, Kyo S, Fukuda T, Goto E, Matsuki Y, Ohmura-Hoshino M, Sada K, Hotta H, Yamamura H, et al. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. Embo J. 2006;25:3618–3626. doi: 10.1038/sj.emboj.7601249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Pitts KR, McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol Biol Cell. 2001;12:2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M, Malka F, Jou MJ, Martinou JC, Yoon G. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol. 2006;209:468–480. doi: 10.1002/jcp.20753. [DOI] [PubMed] [Google Scholar]
- Zorzano A, Liesa M, Palacin M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol. 2009;41:1846–1854. doi: 10.1016/j.biocel.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Zuchner S, De Jonghe P, Jordanova A, Claeys KG, Guergueltcheva V, Cherninkova S, Hamilton SR, Van Stavern G, Krajewski KM, Stajich J, et al. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol. 2006;59:276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120:1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]