Abstract
This report describes a 10 year old African American woman with sickle cell anemia (HbSS) who developed headaches and seizures associated with hypertension during hospitalization for a pulmonary abscess. Hypertension developed following multiple transfusions associated with a relatively abnormally high hematocrit and headache. Magnetic resonance imaging (MRI) was consistent with posterior leukoencephalopathy. Neurological symptoms, hypertension and high hematocrit resolved following erythrocytapheresis. An MRI one month after the episode was normal. Since reversible posterior leukoencephalopathy syndrome (RPLS) has only been reported in HbSS during severe acute chest syndrome, this report documents that milder illness can be associated with RPLS in HbSS. This report highlights the subtle symptoms that may herald serious neurological events in high-risk patients. Examination of the pathophysiology of RPLS in the context of HbSS suggests that patient with HbSS and subtle neurological symptoms should be treated with high vigilance.
Keywords: Reversible posterior leukoencephalopathy syndrome, Seizure, Headache, Sickle Cell Anemia, Hypertension
Introduction
Headache is the most common neurologic symptom in children with sickle cell anemia (HbSS). In one study 76.2% of a pediatric population with HbSS reported at least one headache within a 3 month period. More than 40% of this population had headaches that met criteria for migraine and 31% of this population reported having headaches at least once per week [1].
Headaches manifest with distinct patterns, and a careful history and examination can guide the diagnosis and management of headaches. For example, although the aura of migraine headaches may include neurologic changes such as a disturbance in sensation or speech, the aura should not include weakness and occur before the headache as a prodrome [2]. Headaches in adolescence without HbSS are commonly caused by insomnia, psychosocial factors, anxiety or tension, and are unlikely to be related to a pathologic etiology [3,4]. However, since serious neurological problems, such as stroke, are common in children with HbSS [5], and headache may be the presenting symptom for many neurological disorders, more serious causes of headaches need to be considered in pediatric patients with HbSS.
Reversible posterior leukoencephalopathy syndrome (RPLS) occurs as a consequence of acute hypertension and is a medical emergency [6]. RPLS is defined by headache, mental status change, vision loss and/or seizure with T2 and fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) changes in the cortex, primarily the posterior cerebral hemisphere [6]. RPLS has occurred in children with HbSS during severe acute chest syndrome (ACS) requiring intubation [7].
In the current case report, a patient with a milder acute pulmonary disease is reported to develop RPLS. This report demonstrates that, in sickle cell disease, neurologic complications can occur with more mild illness and suggests that subtle changes in hemodynamics and neurologic status should be monitored closely in hospitalized children with HbSS since neurological complications may occur more often than commonly appreciated.
Case Report
A 10 year-old African American woman with HbSS presented with subjective fever and a productive cough worsening over four days. Her oral temperature was 38.4° C, pulse was 137, blood pressure was 112/62, respiratory rate was 16 and saturation was 99%. She was well appearing but demonstrated a soft 1/6 systolic ejection murmur, occasional crackles over the right lung base and decreased breath sounds over the right apex. Otherwise the examination was unremarkable with no splenomegaly. Leukocyte was 21.7 cells/mm3 with 83% neutrophils, 13% lymphocytes and no bands. Hematocrit was 17.4% and Hemoglobin was 4.8 g/dL with a manual corrected reticulocyte count of 3.1% (baseline hematocrit 22%, hemoglobin 6.5 g/dL, reticulocytes 4%). The patient had a history of eight hospitalizations for pain crisis (first at 8 months of age), two episodes of splenic sequestration and a cholecystectomy.
A chest computerized tomography (CT) scan confirmed a lung abscess. The patient underwent drainage and chest tube placement. Intravenous vancomycin and ceftriaxone was given initially but then changed to intravenous ceftriaxone and oral clindamycin once microaerophilic Streptococcus was identified in the aspirate. Pack red blood cells (PRBCs) 10cc/kg/day were transfused during the first three hospitalization days, raising her hematocrit and hemoglobin to 27.5% and 8.6 g/dL, respectively. Episodes of hypertension occurred over the next three days, with maximums of 142/81, 162/84 and 141/88 on the fourth, fifth and sixth days of admission, respectively, requiring treatment with sublingual nifedipine. Her blood pressure ranged from 94/40 to 123/68 on previous days. Hematocrit and hemoglobin increased to 39.3% and 12.5 g/dL,
The patient complained of a headache during the first two episodes of hypertension. One hour after the last episode of hypertension she developed a two minute generalized seizure. Head CT was negative and she was transferred to the PICU where her blood pressure was 112/71. Two hours later her blood pressure increased to 131/86, at which time she developed a headache, change in mental status and focal neurological symptoms manifested as left eye deviation, tonic posturing of her right arm and automatisms. She was treated with intravenous lorazepam. A second head CT scan was negative. She was loaded with 20mg/kg fosphenytoin equivalents.
Erythrocytapheresis reduced the hematocrit and hemoglobin to 31.4% and 11.2 g/dL, respectively. An electroencephalogram (EEG) demonstrated a poorly modulated disorganized slightly slow background with independent multifocal sharp transient waves. MRI of the brain performed approximately 24 hours after the 1st seizure demonstrated cortical edema in the right parietal, occipital and temporal lobes without restricted diffusion (See Figure 1). Magnetic resonance angiography of the Circle of Willis and magnetic resonance venography (MRV) of the cerebral sinuses were normal. Her neurological examination showed diffuse hyperreflexia without focal abnormalities. Oral phenytoin 5mg/kg/day was started. For the remainder of the admission, the patient’s blood pressure remained stable and she did not have any other neurological dysfunction. The patient was discharged on hospital day number 16.
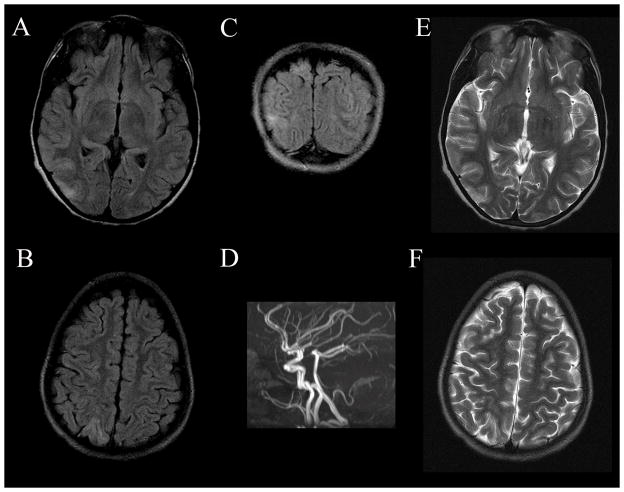
Figure 1.
Reversible Posterior Leukoencephalopathy Syndrome (RPLS). T2 and fluid attenuated inversion recovery (FLAIR) hyperintensities in the grey and white matter of the posterior cortex in this patient were transient and were absent on imaging three weeks after hospital discharge. Axial FLAIR images demonstrate occipital (A) and parietal (B) hyperintensities better than T2 imaging of the same area (E and F, respectively). The hyperintensities are also present on the coronal FLAIR image (C). A magnetic resonance angiogram (D) demonstrated no abnormalities.
Three weeks after discharge the patient’s follow-up brain MRI had normalized. Neurologically, the patient was asymptomatic with a normal examination. The phenytoin was tapered completed off at that time. At her 6 month neurological follow-up, the patient remained symptom-free.
Discussion
This case report describes a young woman with HbSS who developed RPLS during treatment for a lung abscess. Her neurological symptoms occurred during episodes of labile blood pressure. Control of the hypertension quickly improved her neurological symptoms. The diagnosis of RPLS did not occur until the patient developed seizure activity, but probably started two days earlier when she experienced headaches associated with the hypertension. The increase in her post-transfusion hematocrit was associated with worsening neurological symptoms over a three day period and erythrocytapheresis resulted in a reduction in the hematocrit and resolution of her neurological symptoms.
Post-transfusion RPLS was reported in a 45 year-old woman with severe anemia but without hemoglobinopathy two days after four PRBC transfusions increased her hematocrit from 9% to 31% [8]. It would be interesting to speculate that the blood transfusion per se contributed to RPLS. However, only one of the three cases reported by Henderson et al. [7] developed RPLS after PRBC transfusion, while the other two cases developed RPLS after erythrocytapheresis. Miller and Rao [9] commented on Henderson et al. [7] suggesting that simple transfusions rather than erythrocytapheresis may have been beneficial. Clearly, transfusion, in the current case, led to an increased hematocrit and was associated with hypertension, and erythrocytapheresis reduced the hematocrit and was associated with a resolution of the hypertension. As clearly discussed by Boyd and DeBaun in their response to Miller and Rao [9] neither erythrocytapheresis nor simple transfusion have been found to be superior in the treatment of HbSS associated complications. Since neurological complications have been associated with both treatments, neurological complications are probably not the result of a specific transfusion protocol, but due to dynamic changes in hemodynamic parameters such as blood pressure, blood viscosity or hematocrit.
The general characteristics of RPLS are well described, but the extent and scope of the syndrome has evolved over the past decade [6]. The pathophysiology of RPLS is controversial with two major theoretical mechanisms proposed. The cytotoxic theory suggests that a sudden increase in blood pressure results in vasoconstriction and cerebral ischemia, while the vasogenic theory suggests that hypertension overcomes autoregulation, partially through endothelial dysfunction, resulting in vasodilation. Since endothelial dysfunction is believed to be an important contributor to cerebrovascular disease in HbSS, HbSS patients may be at an increased risk and susceptibility to RPLS [10].
The evolution of the hypertension, hematocrit and neurological symptoms in the current patient is similar to a syndrome of hypertension, convulsion and cerebral hemorrhage follow blood-transfusions in thalassaemia patients first reported by Wasi et al. [11]. These patients demonstrated a delayed increase in hematocrit and blood pressure and onset of neurological symptom up to 15 days following blood transfusion. Pathology from autopsies in nine fatal cases demonstrated marked edema and congestion, consistent with hypertensive encephalopathy, as well as changes consistent with hypertensive cerebral hemorrhage [12]. If this represents a milder form of RPLS in HbSS, these autopsy findings would support the pathological mechanisms associated with the vasogenic theory of RPLS.
In order to study neurological complications in HbSS, we must ensure that definitions of neurological complications remain consistent throughout the literature. Henderson et al. [7] described a “silent infarct” as an infarct seen on MRI associated with neurological symptoms lasting less than 24 hours. However, this definition is not consistent with the neurological or hematology literature. A transient ischemic attack is defined as neurological signs or symptoms lasting less than 24 hours attributable to cerebral ischemia. An infarct associated with neurological symptoms is a symptomatic stroke regardless of symptom duration while an infarct without associated symptoms is an asymptomatic stroke or “silent infarcts.” It should be noted that even asymptomatic strokes or “silent infarcts” are not really asymptomatic or “silent” since sickle cell patients with such infarcts usually have subtle cognitive or neuropsychological abnormalities [13].
The current case illustrates the wide spectrum of neurological disorders in patients with HbSS. Although cerebrovascular dysfunction has been clearly documented in HbSS, the pathophysiology is still unclear. This is the second report documenting RPLS in a hospitalized l patient with HbSS. Mild, non-specific, neurological symptoms of RPLS (e.g. headache, nausea, lethargy and confusion) can be subtle and are commonly encountered in hospitalized patients. This is especially true in the sickle cell population where analgesics can obscure the symptoms of RPLS. Thus, this report should raise our index of suspicion when hypertension accompanies any of these otherwise non-specific symptoms. As suggested by Henderson et al. [7] MRI with diffusion weighted imaging may be helpful in diagnosis of RPLS. In addition, MRV is essential to rule out superior saggital sinus thrombosis in this setting.
Acknowledgments
Dr. Richard Frye is supported by K23 NS046565 from NINDS
Abbreviations
- ACS
acute chest syndrome
- CT
computerized tomography
- EEG
electroencephalogram
- FLAIR
fluid attenuated inversion recovery
- HbSS
sickle cell anemia
- MRI
magnetic resonance imaging
- MRV
magnetic resonance venography
- PICU
pediatric intensive care unit
- PRBCs
pack red blood cells
- RPLS
reversible posterior leukoencephalopathy syndrome
Footnotes
There are not conflicts of interest or financial disclosures.
References
- 1.Palermo TM, Platt-Houston C, Kiska RE, et al. Headache symptoms in pediatric sickle cell patients. J Pediatr Hematol Oncol. 2005;27:420–424. doi: 10.1097/01.mph.0000175408.27180.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 2nd Edition. Cephalalgia. 2004;24(suppl 1):1–159. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 3.Karli N, Akgoz S, Zarifoglu M, Akis N, Erer S. Clinical characteristics of tension-type headache and migraine in adolescents: a student-based study. Headache. 2006;46:399–412. doi: 10.1111/j.1526-4610.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JN, Camargo CA, Jr, Pelletier AJ, Edlow JA. Headache in United States emergency departments: demographics, work-up and frequency of pathological diagnoses. Cephalalgia. 2006;26:684–690. doi: 10.1111/j.1468-2982.2006.01093.x. [DOI] [PubMed] [Google Scholar]
- 5.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 6.Stott VL, Hurrell MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Int Med J. 2005;35:83–90. doi: 10.1111/j.1445-5994.2004.00750.x. [DOI] [PubMed] [Google Scholar]
- 7.Henderson JN, Noetzel MJ, McKinstry RC, et al. Reversible posterior leukoencephalopathy syndrome and silent cerebral infarcts are associated with severe acute chest syndrome in children with sickle cell disease. Blood. 2003;101:415–419. doi: 10.1182/blood-2002-04-1183. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Niwa H, Idida T, et al. Post-transfusion reversible posterior leukoencephalopathy syndrome with cerebral vasoconstriction. Neurology. 1997;49:1174–1175. doi: 10.1212/wnl.49.4.1174. [DOI] [PubMed] [Google Scholar]
- 9.Miller ST, Roa SP. Acute chest syndrome, transfusion and neurologic events in children with sickle cell disease. Blood. 2003;102:1556. doi: 10.1182/blood-2003-04-1077. [DOI] [PubMed] [Google Scholar]
- 10.Kato GJ, Hsieh M, Machado R, et al. Cerebrovascular disease associated with sickle cell pulmonary hypertension. Am J Hematol. 2006;81:503–510. doi: 10.1002/ajh.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasi P, Na-Nakorn S, Pootrakul P, Sonakul D, Piankijagum A, Pacharee P. A syndrome of hypertension, convulsion, and cerebral haemorrhage in thalassaemic patients after multiple blood-transfusions. Lancet. 1978;2(8090):602–604. doi: 10.1016/s0140-6736(78)92824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiwanitkit V. Brain pathology in a syndrome of hypertension, convulsion, and cerebral haemorrhage in thalassaemic patients after multiple blood-transfusions: a summary in reported Thai autopsy cases. J Hypertens. 2006;24:601. doi: 10.1097/01.hjh.0000203844.49561.fd. [DOI] [PubMed] [Google Scholar]
- 13.Bernaudin F, Verlhac S, Freard F, et al. Multicenter prospective study of children with sickle cell disease: radiographic and psychometric correlation. J Child Neurol. 2000;15:333–343. doi: 10.1177/088307380001500510. [DOI] [PubMed] [Google Scholar]